Abstract
Background
Putative phytogeographical links between America (especially North America) and the Hawaiian Islands have figured prominently in disagreement and debate about the origin of Pacific floras and the efficacy of long-distance (oversea) plant dispersal, given the obstacles to explaining such major disjunctions by vicariance.
Scope
Review of past efforts, and of progress over the last 20 years, toward understanding relationships of Hawaiian angiosperms allows for a historically informed re-evaluation of the American (New World) contribution to Hawaiian diversity and evolutionary activity of American lineages in an insular setting.
Conclusions
Temperate and boreal North America is a much more important source of Hawaiian flora than suggested by most 20th century authorities on Pacific plant life, such as Fosberg and Skottsberg. Early views of evolution as too slow to account for divergence of highly distinctive endemics within the Hawaiian geological time frame evidently impeded biogeographical understanding, as did lack of appreciation for the importance of rare, often biotically mediated dispersal events and ecological opportunity in island ecosystems. Molecular phylogenetic evidence for North American ancestry of Hawaiian plant radiations, such as the silversword alliance, mints, sanicles, violets, schiedeas and spurges, underlines the potential of long-distance dispersal to shape floras, in accordance with hypotheses championed by Carlquist. Characteristics important to colonization of the islands, such as dispersibility by birds and ancestral hybridization or polyploidy, and ecological opportunities associated with ‘sky islands’ of temperate or boreal climate in the tropical Hawaiian archipelago may have been key to extensive diversification of endemic lineages of North American origin that are among the most species-rich clades of Hawaiian plants. Evident youth of flowering-plant lineages from North America is highly consistent with recent geological evidence for lack of high-elevation settings in the Hawaiian chain immediately prior to formation of the oldest, modern high-elevation island, Kaua‘i.
Keywords: Angiosperms, adaptive radiation, disjunctions, flora, island biogeography, long-distance dispersal, ecological opportunity, Hawaiian Islands, North America, Pacific, phytogeography, sky islands
INTRODUCTION
Extreme isolation and endemicity of the Hawaiian flora, with 90 % of (approx. 1030) native angiosperm species found nowhere else (Wagner et al., 1999, 2005a), have made the effort to resolve original sources of founder lineages both fascinating and complex (Fig. 1). Engler (1879–1882; Fig. 2A), in his early analysis of Hawaiian phytogeography, and Hillebrand (1888a; Fig. 2B), who produced the first major account of the flora of the Hawaiian Islands, concluded that long-distance dispersal of great magnitude, although unlikely, must have been required for native plants to become established in the archipelago. They both noted the association between long-term isolation of the Hawaiian Islands and the exceptional proportion of endemic Hawaiian plants, as well as the importance of birds in plant dispersal and of ecological factors in diversification of the flora. Engler (1879) reasoned that the evolutionary potential of plants dispersed to the archipelago was high because ‘ … the newly developed varieties always encountered open terrain, and they had to fight with only few competitors’ (p. 130). Hillebrand (1888a) discussed the contribution of elevational and climatic diversity to ‘ … increase the variety of forms’ (p. XV). Hillebrand (1888a) also appreciated that the greater age of the western high-elevation islands, such as Kaua‘i, compared with the eastern high-elevation islands, such as Hawai‘i, was reflected by more single-island endemics and more extensive morphological differences between congeneric species on the older islands than on the younger islands. His questions about the relative importance of habitat diversity, evolutionary lability and paucity of founders in contributing to autochthonous development of the angiosperm flora still remain to be fully answered and demonstrate that his comprehension of island biology was well ahead of its time.
Fig. 1.
Map of the Pacific Basin. The distance from southern California to the closest Hawaiian Island (Hawai‘i) is approx. 3800 km to the south-west, and the distance from the Marquesas Islands (Eiao) to Hawai‘i is approx. 3400 km north across the equator. Map courtesy of D. T. Harbaugh.
Fig. 2.
Scientists who proposed hypotheses on the origins of Hawaiian flora. (A) Heinrich Gustav Adolf Engler (1844–1930), (B) Wilhelm (William) B. Hillebrand (1821–1886), (C) Alfred Russel Wallace (1823–1913), (D) Forest Buffen Harkness Brown (1873–1954), (E) Douglas Houghton Campbell (1859–1953), (F) Carl Johan Fredrik Skottsberg (1880–1963), (G) David Daniels Keck (1903–1995), (H) Francis Raymond Fosberg (1908–1993), (I) Sherwin Carlquist (1930–). A, B and D–H courtesy of the Hunt Institute for Botanical Documentation, Carnegie Mellon University. C courtesy of Smithsonian Institution Libraries, from Wallace (1905).
Drawing from early floristic work (e.g. Mann, 1869; Wawra, 1872–1875), Engler (1879–1882) presented a tabulation of Hawaiian vascular-plant taxa and the distributions of putative extra-Hawaiian relatives, mostly from tropical or subtropical areas, for well over one-third of the Hawaiian species and genera. Only 42 (20 %) of the indigenous Hawaiian angiosperm relationships proposed by Engler were exclusively with New World (mostly tropical) species, including all native Compositae and fleshy-fruited Campanulaceae (lobelioids). Over half of those native angiosperms proposed to have exclusively New World relatives were noted as having fleshy fruits, easily adhering seeds or fruits, or associations with wet habitats, all features favourable for dispersal by birds (see Carlquist, 1974, 1996).
Although Hillebrand (1888a) did not provide a synthetic account of his thoughts on the original source areas for native Hawaiian plants in his flora (the introduction was incomplete at the time of his death), he did suggest elsewhere that most Hawaiian plants have Australasian affinity, excluding the majority of Hawaiian Compositae, which appeared to have closest relatives in America (Hillebrand, 1888b). He also recognized that the ‘Hawaiian Islands (are) the only Polynesian Island group which contain a large proportion of indigenous plants with American affinities’ (Hillebrand, 1888a, p. XXIX). By ‘American’, Hillebrand (1888a) was referring at least in part to South America, based on his comment about ‘ … the important American element of the Andine regions which is apparent in the Hawaiian flora’ (p. XIV). His reference to American sources for Hawaiian flora evidently mostly did not refer to western North America north of Mexico: ‘ … the northwest coast of America, including California, has until quite recent times only contributed one or two inhabitants of the highest mountains, and has besides few so-called representative species’ (p. XIV).
Although various hypotheses were subsequently advanced to explain the arrival of native Hawaiian plants from source areas to the west and south of the islands, the difficulty of explaining a North American element in the Hawaiian flora by means other than long-distance dispersal of intercontinental magnitude (approx. 3800 km) led to major disagreement among botanists about the extent of North American–Hawaiian phytogeographical links. Attention to that disagreement serves to illuminate the intellectual obstacle during the early 20th century posed by the widespread conception of phenotypic evolution as too slow to account for highly divergent endemics on young islands. Here we provide historical perspective on some of those conflicting ideas and review modern evidence for angiosperm dispersal across the vast (and deep) oceanic barrier between North America and the Hawaiian Islands, with special focus on Hawaiian taxa from temperate and boreal North America, and the ecological obstacles and opportunities facing such plants in a tropical archipelago.
DISPARATE EARLY IDEAS ON ORIGINS OF HAWAIIAN FLORA
Endemism and antiquity of the Hawaiian flora
Early 20th century botanists presented a diversity of views on origins of the Hawaiian flora and differed about the importance of long-distance dispersal and particular source areas – especially America – as well as the age and timing of evolution and assembly of the flora. In general, the high level of Hawaiian floral and faunal endemism and distinctiveness of the endemic taxa was viewed as evidence for greater age of the islands and/or flora and fauna than is indicated by modern geological and molecular data (see Price and Clague, 2002; Clague et al., 2010) and, by some, as evidence for now-untenable past land connections between the Hawaiian Islands and areas to the south (e.g. Campbell, 1918). Alfred Russel Wallace's (1881; Fig. 1C) perspective on the high level of Hawaiian endemism in Island Life may have been influential: ‘These facts undoubtedly indicate an immense antiquity for this group of islands, or the vicinity of some very ancient land (now submerged), from which some portion of their peculiar fauna might be derived’ (p. 298).
Hypotheses of long-distance dispersal to the Hawaiian Islands
Guppy (1906) championed the idea of oversea long-distance dispersal by providing extensive evidence for dispersal ability of Hawaiian and other Pacific plants based on first-hand experimentation and detailed observations of fruit and seed characteristics. His conclusion that birds, in addition to physical forces, were an important agent of Pacific plant dispersal was corroborated and extended by subsequent studies (see Ridley, 1930; Carlquist, 1974, 1996), although Guppy's idea that high levels of floristic and avian endemism in Hawaiian forests meant that birds long ago ceased to move propagules to or from such habitats is at odds with documentation of frequent bird traffic to diverse sites in the archipelago from continental areas (see Ballard and Sytsma, 2000). Hillebrand's (1888a) earlier idea that the Pacific golden plover has been important in the distribution of Hawaiian plant species is still widely held (see Fosberg, 1951; Johnstone and McFarlane, 1967; Carlquist, 1974; Ballard and Sytsma, 2000; Lowrey et al., 2005; Harbaugh et al., 2009).
Guppy's (1906) suggestion that plant dispersal to the Hawaiian Islands occurred in distinct, successive waves, with an early and extremely ancient series of dispersals from America (during his early Tertiary ‘Age of Compositae’) and a much more recent (post-Pleistocene) wave from Indo-Malesia was attributed in part to Bentham (1873), who noted that the high level of endemism in Hawaiian Compositae shows ‘ … that the ancient connexion, of whatever nature it may have been, with America … has been so long severed as not to have left a single unmodified common form. The species are all either descendants altered by long isolation or possibly, in some instances, preserved remnants of types long since extinct elsewhere’ (p. 556). Guppy also may have been influenced by Wallace's (1881) extension of Bentham's (1873) views on the antiquity and origin of the morphologically unusual, endemic lineages of Hawaiian Compositae: ‘The great preponderance of American relations in the Compositae … is very interesting and suggestive … We may … look upon the Compositae as representing the most ancient portion of the existing flora of the Sandwich Islands, carrying us back to a very remote period when the facilities for communication with America were greater than they are now … (P)lants of North Temperate affinity may be nearly as old, but these may have been derived from Northern Asia by way of Japan and the extensive line of shoals which run northwestward from the Sandwich Islands … Those which exhibit Polynesian or Australian affinities, consisting, for the most part, of less highly modified species usually of the same genera, may have had their origin at a later, though still somewhat remote, period, when large islands, indicated by the extensive shoals to the south and southwest, offered facilities for the transmission of plants from the tropical portions of the Pacific Ocean’ (Wallace, 1881, pp. 303–304).
Guppy's (1906) ideas on origin and assembly of the Hawaiian flora also somewhat resemble the later speculations of Brown (1921, 1922; Fig. 2D) and Brown and Brown (1933), although not in mode of oversea dispersal or in timing of events. Brown (1922) proposed three dispersal waves for Hawaiian and South Pacific angiosperms, with the vast majority of founders arriving directly or indirectly via flotation in sea currents from the Isthmus region of Central America. He concluded that the earliest of those supposed dispersal waves included ancestors of modern endemic Hawaiian genera (‘ … characterized by Compositae and Lobelioideae’, p. 222) and occurred during the Mesozoic, possibly as early as the Jurassic and pre-dating the known angiosperm fossil record. Although Brown (1922) acknowledged a large Indo-Malesian element in the Hawaiian flora, he believed that even those plants, which he regarded as the latest natural arrivals, probably descended ultimately from Central American ancestors that dispersed west by oceanic rafting. Ridley (1930), in considering dispersal of plants worldwide, also noted a ‘distinct American element’ in the Hawaiian flora, although he found it ‘ … difficult to see how such plants as have American affinities could have crossed over 2,000 miles of sea’ (p. 687).
Vanished land connections and the American ‘stumbling-block’
In contrast to the ideas of Bentham, Wallace, Guppy and Brown, Campbell (1918, 1919, 1920, 1926, 1928, 1932, 1933; Fig. 2D) and Skottsberg (1925, 1939, 1941; Fig. 2F) regarded America as a relatively minor contributor to the Hawaiian flora compared with the South Pacific, Australasia and Indo-Malesia and did not believe that long-distance dispersal alone could account for the high floristic richness of the Hawaiian Islands. Bryan (1915) also concluded that ‘ … few indeed have been the representatives of the North American flora that have been brought to the islands’ (p. 191). Campbell subscribed to the Pacific snail expert Pilsbry's (1916) hypothesis of once-continuous land connections among the Hawaiian Islands and between those islands, the South Pacific, Australasia and Indo-Malesia, and Campbell suggested a southern connection with South America as well. Subsidence of the hypothetical block connecting those areas was suggested to have led to isolation of the Hawaiian volcanic peaks as islands, following occupation of those peaks by ancestors of most lineages of the modern flora. Skottsberg (1925) advanced an ‘Antarctic circuit theory’, based in part on evidence at that time for an ancient southern super-continent, with south-to-north overland migration from Antarctica into Polynesia and into South America to account for floristic similarities across those regions. He indicated that extending that land connection to the Hawaiian Islands, as suggested by Campbell, was ‘ … rather daring, but it is the only manner in which to get away from transpacific bridges, if we do not believe in oversea migration’ (Skottsberg, 1925, p. 34).
Campbell (1919, 1920, 1926, 1928, 1933) did believe in the possibility of oversea plant transport; he indicated that lack of North American diversity in the Hawaiian Islands was surprising in light of relatively favourable prospects for long-distance, trans-Pacific dispersal from north-west America as opposed to over-water dispersal from areas to the south. Although Campbell (1933) did not believe that over-water dispersal could account for the high representation of South Pacific, Malesian, Australasian or even South American plants in the Hawaiian flora, he believed that the relatively few founders from the Pacific coast of North America probably were ‘ … introduced … since the isolation of the islands’ (p. 181). Skottsberg (1925), who rejected Bryan's (1921) suggestion of a direct land connection across the deep Pacific abyss between America and the Hawaiian Islands and was highly sceptical about the efficacy of oversea dispersal, indicated that ‘ … the representatives in (the Hawaiian Islands) of American fauna and flora, even if they are few, are a stumbling-block’ (p. 35).
Shattering the American–Hawaiian floristic connection?
Keck (1936a; Fig. 2G), in his monograph of the Hawaiian silverswords (Argyroxiphium, Compositae), re-evaluated the evidence for American ancestry of Hawaiian vascular plants. He concluded that Skottsberg's (1925) ‘stumbling-block’ of trying to account for movement of plants between America and the Hawaiian Islands could be resolved in disfavour of phytogeographical affinities between the two areas except for as few as nine ‘very inconsequential’ late arrivals to the islands, with closely related congeners on the American mainland.
Keck (1936a) was principally concerned with the origins of endemic Hawaiian genera and the persistent suggestion that some of those highly distinctive groups – especially in Compositae – might be descendants of ancestors that arrived by long-distance dispersal, which was difficult to escape as an explanation for phytogeographical ties with America, especially North America. If the endemic Hawaiian genera of putatively American origin were instead found to be more closely related to plants from outside the New World, then ‘ … it is possible to follow one's inclinations and agree with Campbell, Skottsberg, and others that the endemic element in the Hawaiian flora, at least so far as the flowering plants are concerned, is for the most part of great age, and that it may have had its beginnings on nearby land masses in the Pacific even before the present archipelago became habitable’ (Keck, 1936a, p. 5). Like other early 20th century botanists, Keck (1936a) evidently viewed the high degree of endemism in the flora as indicative of greater antiquity than the islands: ‘Even the flora of the youngest Hawaiian mountains is very old, with 91 percent of the species endemic. This is not easily explained by those who regard the Hawaiian islands as purely oceanic in origin and the flora as carried there from great distances across the ocean … ’ (p. 4). He similarly noted that the species of endemic Hawaiian genera ‘ … are usually well-marked, rather static, and senile in character’ (p. 5) and that ‘ … many species appear to be on the verge of extinction because of their lack of plasticity’ (p. 4).
Keck's (1936a, b) focus on relationships of Hawaiian silverswords (Argyroxiphium; Fig. 3A) was well chosen to test an American–Hawaiian phytogeographical relationship; most botanists who had published on origins of the Hawaiian flora had pointed to Argyroxiphium as an example of an endemic genus of American origin (e.g. Bentham, 1873; Engler, 1879; Wallace, 1881; Hillebrand, 1888; Campbell, 1919; Brown, 1922) or at least possibly from America (Skottsberg, 1925; but see Skottsberg, 1931). Gray's (1852) taxonomic placement of Argyroxiphium (and the closely related Wilkesia) with the American tarweeds, in subtribe Madiinae, had largely held sway until Keck's (1936a) study, wherein he indicated that Argyroxiphium (including Wilkesia, in his classification) was most closely related to the endemic Hawaiian genus Dubautia (in which he included Railliardia) and that ‘… the affinities of these genera are to be found to the south or southwest in the Pacific’ (p. 11), with genera of tribe Senecioneae such as Robinsonia (Juan Fernandez Islands) or Brachionostylum (New Guinea), as earlier suggested by Skottsberg (1931), or Bedfordia (Australia) rather than with American Compositae. Keck's (1936a) suggestion that Argyroxiphium/Wilkesia and Dubautia/Railliardia represent a common Hawaiian group impacted two long-suggested phytogeographical links between endemic Hawaiian genera and American taxa (Gray, 1852, 1865, 1870): Argyroxiphium/Wilkesia to the mostly Californian tarweeds (Madiinae; Fig. 3B) and Railliardia to the mostly Californian Raillardella sensu lato (s.l.), which was then widely treated in tribe Senecioneae (following Bentham, 1873) and not yet recognized as a tarweed. Keck (1936a) concluded: ‘By thus divorcing Argyroxiphium from the American genera to which it has been thought related, the most persistently proposed connection between the ancient element in the Hawaiian flora and the New World has been shattered’ (p. 11).
Fig. 3.
Exemplars of Hawaiian angiosperm radiations (left) and American relatives (right) in Compositae–Madiinae (top) and Umbelliferae (bottom). (A) Argyroxiphium sandwicense subsp. macrocephalum. Image by W. L. Wagner. (B) Madia elegans. Image by B. G. Baldwin. (C) Sanicula mariversa. Image courtesy of J. K. Obata. (D) S. arctopoides. Image by B. G. Baldwin.
Evolutionary inactivity of American lineages in the Hawaiian flora?
Fosberg's (1948; Fig. 2H) tabulation of the source areas for Hawaiian vascular-plant lineages was the first comprehensive assessment of extra-Hawaiian affinities of the indigenous flora since Engler's (1879) and provided a detailed perspective on the importance of American founders. Although he drew in part on Skottsberg's ideas, Fosberg (1948) recognized that the Hawaiian flora was assembled by long-distance dispersal rather than by ancient land connections to other areas, and he wryly noted: ‘If we resort to land bridges or continents to account for the presence of the Hawaiian flora, then we may well have to build them in all directions’ (p. 119).
Of his estimated 272 angiosperm immigrants to the Hawaiian Islands, Fosberg (1948) concluded that regions to the west and south of the archipelago, widely believed at the time to be the most important sources for Hawaiian flora, contributed the majority of founder species, as corroborated subsequently (e.g. Wright et al., 2001; Lowrey et al., 2001; Gemmill et al., 2002; Howarth et al., 2003; Nepokroeff et al., 2003; Hao et al., 2004; Cronk et al., 2005; Costello and Motley, 2007; Harbaugh and Baldwin, 2007; Percy et al., 2008; Clark et al., 2009; Harbaugh et al., 2009). His table of flowering-plant founders included 109 from the ‘Indo-Pacific’ (‘Indonesia or southeastern Asia and attenuating out into the Pacific’) and 45 from the ‘Austral’ region (‘south Pacific, from Australia to Patagonia’). He also noted that temperate and tropical regions of the Americas were the source of more founder species (50) than expected, based on most contemporary views, and slightly exceeded the number of founder species from the ‘Austral’ region, which was believed by some to be the most important source area for Hawaiian plant lineages (e.g. Copeland, 1948).
Fosberg (1948, 1951) reconciled the high number of Hawaiian vascular-plant founders from temperate and tropical regions of the New World (50–51 of 272 angiosperms; 16–18 of 135 pteridophytes) with the widely held view of a relatively minor American element in the Hawaiian flora by noting that most indigenous Hawaiian plant lineages from the Americas had undergone minimal evolutionary change since arriving in the archipelago and probably represented recent arrivals, as Keck (1936a) also concluded. Based on Fosberg's (1948) tabulations, none of the vascular plant genera of exclusively American (and/or boreal) origin in the native Hawaiian flora had produced more than two species or infraspecific taxa per original immigrant except for Gunnera (Gunneraceae) and the endemic Hawaiian genera Hesperomannia (Compositae), Isodendrion (Violaceae) and Nothocestrum (Solanaceae). Founders of the three endemic genera and Hawaiian Psychotria (Rubiaceae) are the only plants of putatively American origin that he later noted as having ‘ … given rise to any significant number of evolutionary offshoots that still survive, and none has produced a large number. Furthermore, all these genera are among the more doubtfully American of the lot’ (Fosberg, 1951, pp. 204–205). Molecular phylogenetic studies have since overturned the hypotheses of American origins for Hesperomannia (Kim et al., 1998), Nothocestrum (Olmstead et al., 2008) and Hawaiian Psychotria (Nepokroeff et al., 2003), and upheld a tropical American ancestry for Isodendrion (Feng, 2005; Tokuoka, 2008) and Hawaiian Gunnera (Wanntorp et al., 2002; Wanntorp and Wanntorp, 2003), which is now treated as including only two species (see Wagner et al., 1990). Fosberg's (1948) suggestion of an American origin for the two species of Hawaiian raspberries (Rubus, Rosaceae) was confirmed by molecular phylogenetic studies, although his hypothesis of monophyly of Hawaiian Rubus was rejected (Howarth et al., 1997; Alice and Campbell, 1999; Morden et al., 2003); each of the two Hawaiian species evidently stems from distinct western North American ancestors, with R. hawaiiensis most closely related to the salmonberry (R. spectabilis) and R. macraei most closely related to the California blackberry (R. ursinus).
INVESTIGATING THE AMERICAN CONTRIBUTION TO HAWAIIAN FLOWERING PLANT DIVERSITY
Since the 1950s and especially over the last 20 years, intensive evolutionary studies have greatly improved understanding of the origins and relationships of Hawaiian angiosperms. Evidence from those efforts for ancestrally American lineages in the Hawaiian flora is detailed here.
Revisiting the silversword alliance
Carlquist's (1958a, b, 1959; Fig. 2I) comparative anatomical studies of the silverswords and relatives provided the first strong refutation of hypotheses of minimal evolution or lack of representation of American plants in the Hawaiian archipelago (e.g. Skottsberg, 1931; Keck, 1936a; Fosberg, 1951). Like Keck (1936a), Carlquist recognized that the true Hawaiian silverswords and greenswords (Argyroxiphium) belong to the same insular lineage as the Hawaiian na‘ene‘e or kupaoa group (Dubautia, including Railliardia), and not to a different tribe or subtribe of Compositae, as earlier treated (e.g. by Gray, 1870; Bentham, 1873). Unlike Keck (1936a), Carlquist (1957) recognized that the iliau (Wilkesia), a bizarre rosette-plant from Kaua‘i, is not more closely related to the rosette-plants in Argyroxiphium than to the trees, shrubs, mat-plants, cushion plants and vines in Dubautia, and, most importantly, that the insular lineage represented by Argyroxiphium, Dubautia and Wilkesia (i.e. the Hawaiian silversword alliance) belongs in subtribe Madiinae, with the primarily Californian tarweeds, including Raillardella s.l. (Carlquist, 1958a, b, 1959).
Carlquist's (1959) expansion of the tarweed subtribe Madiinae to include the Hawaiian silversword alliance and Raillardella united the two North American–Hawaiian phytogeographical links that Keck (1936a) rejected (i.e. Argyroxiphium/Wilkesia with the tarweeds; Dubautia/Railliardia with Raillardella). Carlquist's (1959) demonstration of a large suite of vegetative and reproductive characteristics uniting tarweeds and silverswords and his point-by-point refutation of Keck's (1936a) argument against a tarweed–silversword relationship provided a convincing case for a western North American origin of a major Hawaiian angiosperm radiation. In part, Keck's (1936a) rejection of a tarweed ancestry of the silversword alliance was based on the striking differences in habit between the mostly herbaceous tarweeds and the woody or semi-woody Hawaiian taxa. Carlquist (1959) suggested that those habital differences reflected evolution of woodiness in an insular setting in response to opportunities for year-round growth. Among the shared features of the tarweeds and silversword alliance, Carlquist (1965, 1974, 1980) noted that the sticky bracts associated with tarweed ray fruits and the pappus characteristics of tarweed disc fruits allow for ready attachment to animals, and that such external adhesion to bird feathers would have allowed for oversea transport from America to the Hawaiian Islands. Like St. John (1950), Carlquist (1959) could find no morphological support for Skottsberg's (1931) and Keck's (1936a) suggestions of a close relationship of the silversword alliance to genera of tribe Senecioneae from Australasia or the Juan Fernandez Islands.
Molecular phylogenetic studies confirmed Carlquist's (1958a, b, 1959) hypothesis of long-distance dispersal and subsequent adaptive radiation of tarweeds in the Hawaiian Islands by showing that the Hawaiian silversword alliance is a monophyletic group nested within the California tarweed lineage (Baldwin et al., 1991). Based on chloroplast DNA (cpDNA) and nuclear rDNA trees, the Hawaiian clade is more closely related to primarily Californian genera of the ‘Madia’ lineage than to other major lineages of Californian Madiinae (Baldwin, 1996, 2003). Molecular evidence for evolution of woodiness in the Hawaiian setting and ecological diversification of all modern members of the silversword alliance within the last 4–6 Myr (Baldwin and Robichaux, 1995; Baldwin and Sanderson, 1998) conforms to Carlquist's hypothesis of adaptive radiation of the group and contrasts with the once-prevalent view of endemic Hawaiian Compositae genera as ancient relicts that reflect an early Tertiary or even Mesozoic age of the Hawaiian chain (e.g. Guppy, 1906; Brown, 1921, 1922). Production of vigorous Hawaiian–Californian hybrids between members of the silversword alliance and tarweeds of the ‘Madia’ lineage (Baldwin et al., 1991; Carr et al., 1996) and hybrids between members of the two diploid Californian sublineages of the ‘Madia’ lineage implicated in an allotetraploid ancestry of the silversword alliance (Barrier et al., 1999) provided biological evidence for a level of retained genetic similiarity between tarweeds and silverswords consistent with a limited time frame for divergence from a common ancestor.
A Hawaiian offshoot of the Californian sanicles
The ecologically diverse Hawaiian sanicles (Sanicula, Umbelliferae; Fig. 3C) are another group judged to be of ‘obscure’ affinity by Fosberg (1948) that structural and molecular data have indicated are of western North American origin. Wallace (1881) noted that Hawaiian ‘ … Sanicula (is) allied to Oregon species’ (p. 302) and Hillebrand (1888) similarly indicated that the sole Hawaiian species of Sanicula recognized at the time, S. sandwicensis, ‘ … comes near S. menziesii [= S. crassicaulis] … from California and Oregon’ (p. 144). Froebe (1971) suggested that the Hawaiian species (sect. Sandwicenses) descended from two distinct lineages of the mostly Californian sect. Sanicoria (Fig. 3D), based in part on presence of a taproot in both groups. Subsequent molecular phylogenetic analyses indicated that the Hawaiian taxa constitute a monophyletic (rather than biphyletic) group that is nested within sect. Sanicoria (Vargas et al., 1998). Three Californian or mostly Californian species share a large (24-base pair) deletion in the nuclear rDNA internal transcribed spacer 2 (ITS-2) with the Hawaiian species; all of those taxa that share the deletion and their North American sister-group have prickly fruits that adhere readily to fur or feathers and were therefore inferred to have reached the Hawaiian Islands by external attachment to a bird (Vargas et al., 1998), as suggested earlier for the tarweed ancestor of the silversword alliance. Unlike silverswords, Hawaiian sanicles remain herbaceous and descended from diploid (not polyploid) continental ancestors, with diversification from a common ancestor less than 1 Mya based on an externally calibrated, rate-constant ITS tree.
Violets and boreal American plant diversification in the Hawaiian Islands
Ballard and Sytsma (2000) provided molecular evidence for a boreal, western North American origin of the Hawaiian violets (Viola, Violaceae; Fig. 4A), another group regarded by Fosberg (1948) to be of ‘obscure’ extra-Hawaiian affinity. Prevalence of woodiness and multi-flowered inflorescences among Hawaiian violets led Skottsberg (1940) to suggest that the Hawaiian members of Viola represent the most ancient lineage in this highly diverse, worldwide genus of mostly herbaceous, single-flowered taxa. Skottsberg (1940) and others (e.g. St. John, 1989; Wagner et al., 1990) suggested a closest relationship of Hawaiian violets to Latin American woody violets of sect. Leptidium or sect. Rubellium, although St. John (1989) regarded Hawaiian violets as polyphyletic, with separate introductions accounting for woody and herbaceous taxa in the islands. Ballard and Sytsma (2000) instead found that the Hawaiian violets constitute a young clade nested among lineages of boreal, mostly North American herbaceous taxa of boggy or otherwise wet habitats, like those where some of the Hawaiian species occur. As in the silversword alliance, molecular data were decisive in demonstrating that woodiness was derived in the Hawaiian setting from an ancestrally herbaceous condition.
Fig. 4.
Exemplars of Hawaiian angiosperm radiations (left) and American relatives (right) in Violaceae (top) and Labiatae (bottom). (A) Viola chamissoniana subsp. robusta. Image courtesy of G. D. Carr. (B) V. langsdorffii. Image courtesy of M. Goff. (C) Phyllostegia floribunda. Image courtesy of J. K. Obata. (D) Stachys chamissonis. Image courtesy of A. Brousseau, copyright © 1995 Saint Mary's College of California.
More specifically, Ballard and Sytsma (2000) showed that Hawaiian violets probably descended from an Arctic or far-north temperate American ancestor referable to Viola langsdorffii s.l. (‘Alaskan violet’; Fig. 4B), which occurs from northern California to Alaska in western North America and Kamchatka in the Russian Far East to northern Japan. They found that the sampled boreal American representative of V. langsdorffii and Hawaiian violets constituted a robust clade to the exclusion of a Japanese sample of V. langsdorffii. Experimental hydrolysis of seeds of V. langsdorffii and other violets to simulate conditions in a bird digestive tract demonstrated that violets could be avian dispersed from the American Arctic to the Hawaiian Islands without harm via internal transport. They also inferred a polyploid ancestry of Hawaiian violets based on chromosome counts consistent with hexaploidy, decaploidy and dodecaploidy of different populations of V. langsdorffii and with octoploidy of Hawaiian violets.
In addition to providing the first compelling evidence of a boreal origin for a diverse lineage of Hawaiian plants, Ballard and Sytsma (2000) pointed to evident niche conservatism of some Hawaiian violets to underline the importance of ecological opportunity in colonization of the Hawaiian Islands by plants from higher latitudes. They noted that lack of well-documented representation of boreal plant lineages in the Hawaiian flora ran counter to the frequent opportunities for such dispersal, given the wide diversity of Arctic- and sub-Arctic-breeding birds that migrate regularly to the islands, and suggested that transport to a suitable habitat within the tropical island setting may be a more important limitation for establishment of northern plants than crossing the oceanic expanse between the islands and boreal regions. That argument is consistent with earlier ideas expressed by Wallace (1881), Setchell (1935) and Carlquist (1974), who concluded that transport of plants to islands may be less of a limiting factor in long-distance dispersal than ecological considerations upon arrival. Ballard and Sytsma also noted that insufficient rigorous evidence about origins of other Hawaiian plants of possible northern ancestry may have resulted in underestimation of the importance of boreal lineages in the Hawaiian flora.
Hawaiian mints and American hedge nettles: another North American link to a major Hawaiian radiation
Lindqvist and Albert (2002) demonstrated that the highly diverse Hawaiian mints (approx. 59 species in Haplostachys, Phyllostegia and Stenogyne, Lamiaceae; Fig. 4C), previously regarded as Austral or south-east Asian in origin (e.g. Campbell, 1919; Fosberg, 1948), are phylogenetically nested within Stachys (‘hedge nettles’; Fig. 4D) and most closely related to temperate North American members of the nearly worldwide genus. Fleshy fruits of two of the three Hawaiian mint genera (Phyllostegia and Stenogyne) were in part responsible for their earlier association with other fleshy-fruited, mostly south-east Asian members of Lamioideae (Prasium, Bostrychanthera and Gomphostemma) in tribe Prasieae or subfamily Prasioideae (e.g. Bentham, 1832–1836; Briquet, 1895–1897; Wunderlich, 1967), rather than with dry-fruited lamioids. Pericarp analysis led Ryding (1994) to conclude instead that fleshy fruits evolved repeatedly in Lamioideae and that the Hawaiian mints, although probably monophyletic (including the dry-fruited Haplostachys), are not as closely related to Prasium or Gomphostemma as earlier suggested, in accordance with the subsequent molecular data.
In addition to representing an exceptionally diverse Hawaiian lineage of vascular plants in number of recognized species, Hawaiian mints have radiated across highly contrasting environmental settings, including dry shrublands and wet forests, and exhibit floral diversity associated with both insect pollination (in Haplostachys and Phyllostegia) and bird pollination (in Stenogyne). The presence of such diversity in pollination systems in the Hawaiian mints may reflect evolutionary lability present in their common ancestor with North American Stachys; among the closest relatives of Hawaiian mints in North American Stachys based on the molecular trees are the insect-pollinated S. quercetorum and the bird-pollinated S. chamissonis (Lindqvist and Albert, 2002). Polyploidy, possibly of hybrid origin, was inferred by Lindqvist and Albert (2002) to have been ancestral within the American founder lineage that gave rise to Hawaiian mints (2n = 64, 66) and suggested it to possibly account for the magnitude of their morphological diversification (see also Lindqvist et al., 2003), as proposed earlier for the silversword alliance (Barrier et al., 1999).
Hawaiian lineages of the carnation family
Schiedea
The most diverse lineage of Hawaiian Caryophyllaceae also appears to have probably descended from a North American founder, based on recent molecular results (Wagner et al., 2005b; Harbaugh et al., 2010). The endemic Hawaiian genus Schiedea (Fig. 5A) consists of 34 species of herbs, shrubs and vines that arguably represent the greatest diversification of reproductive traits in angiosperms of the islands, with lineages that are monomorphic and insect- or bird-pollinated (sometimes autogamous) or are dimorphic and wind-pollinated. A bird-pollinated lineage within Schiedea is so distinctive morphologically that it was until recently (Wagner et al., 2005b) treated as a distinct genus, Alsinidendron (Fig. 5A).
Fig. 5.
Exemplars of Hawaiian angiosperm radiations (left) and American relatives (right) in Caryophyllaceae. (A) Schiedea obovata. Image courtesy of G. D. Carr. (B) Wilhelmsia physodes. Image by L. Brothers. (C) Silene perlmanii. Image courtesy of J. K. Obata. (D) S. antirrhina. Image courtesy of G. Yatskievych.
Discerning relationships of Schiedea to other members of subfamily Alsinoideae outside the Hawaiian Islands has been complicated by extensive morphological divergence of the group from all living representatives of the subfamily. Skottsberg (1939) hesitantly suggested a boreal ancestry of Schiedea (including Alsinidendron) before concluding that the group is instead a southern (‘Austral’) element in the Hawaiian flora, derived ultimately from Antarctic ancestors (Skottsberg, 1941), as he concluded also for the silversword-alliance genera (see above). Fosberg (1948) treated Schiedea as of ‘obscure’ phytogeographical affinity. Extensive molecular phylogenetic sampling across Alsinoideae by Wagner et al. (2005b) and Harbaugh et al. (2010) led to the discovery that Schiedea is sister to two monotypic genera, Honckenya and Wilhelmsia (Fig. 5B), that are boreal, rather than austral, endemics. Although both boreal genera occur in Eurasia as well as North America, the founder of Schiedea appears most likely to have dispersed from North America, where three of the four taxa (subspecies) of Honckenya are restricted and where the most closely related continental relatives of Honckenya and Wilhelmsia are endemic (Harbaugh et al., 2010). Based on the phylogenetic findings, woodiness in Schiedea appears to be secondarily derived from an herbaceous habit, which is common to Honckenya, Wilhelmsia and their closest relatives (see Carlquist, 1995). Wagner et al. (2005b) noted that successful colonization of the Hawaiian Islands by the founder of Schiedea was not complicated by dioecy and the associated need for dispersal of multiple individuals from the mainland; floral hermaphroditism is unequivocally estimated as ancestral in Schiedea, based on occurrence of that condition throughout the closest continental relatives except Honckenya, which appears to have independently evolved a dimorphic sexual system.
Silene
The other endemic lineage of Hawaiian Caryophyllaceae was recently resolved as the sister group to a temperate American species (Eggens et al., 2007). The mostly Northern Hemisphere genus Silene (campions or catch-flies), with approx. 650 species, is represented in the islands by seven recognized woody species that belong to two lineages of sufficiently different habit, ecology and floral morphology to have been thought to represent descendants of distinct Hawaiian founders (see Wagner et al., 1990). One of these two groups contains subshrubs of often mesic habitats with white, notched petals (e.g. S. perlmanii; Fig. 5C); the other group comprises shrubs of dry habitats, with narrower leaves and green or cream-coloured to reddish, divided petals (e.g. S. hawaiiensis). Skottsberg (1941) included Hawaiian Silene in a group of temperate genera (including Rubus, Sanicula and Viola) that he regarded as being ‘ … so distinct and so distant from everything else that their history here (in the Hawaiian Islands) must have been a long one’ (p. 697). Fosberg (1948) regarded all members of Hawaiian Silene as of ‘obscure’ affinity. Chowdhuri (1957) classified Hawaiian members of the genus in a common section with two poorly known, putatively Japanese species, S. japonica and S. tanakae, possibly implying an East Asian origin of the Hawaiian taxa.
Based on molecular phylogenetic data from cpDNA and nuclear DNA (ITS) sequences, Eggens et al. (2007) found that the two morphological groups of Hawaiian Silene constitute a clade that is sister to S. antirrhina (Fig. 5D), a widespread, weedy annual of temperate North America and South America, with minimal molecular divergence between the American and Hawaiian taxa. Some uncertainty remains about the geographical affinities of Hawaiian campions because of the occurrence of S. antirrhina on both American continents and because of a sister-group relationship between the Hawaiian + American clade and a diverse Silene clade from Eurasia (specifically, the Mediterranean and Middle East), which conceivably could have been the source area for independent dispersal events to America and the Hawaiian Islands. Prevalence of herbaceousness in the continental lineages that are closely related to Hawaiian campions indicates that woodiness of the Hawaiian species probably evolved in the insular setting, as inferred for Schiedea, the silversword alliance and some lineages of Hawaiian mints and Viola.
Woody spurges from North America
The spectacular diversity of trees, shrubs and subshrubs in Hawaiian Euphorbia subg. Chamaesyce (16+ species; Fig. 6A) has been long suggested to represent an endemic lineage of Indo-Pacific origin (Degener and Croizat, 1936; Fosberg, 1948; Koutnik, 1987). Carlquist (1970, 1974, 1980) regarded the Hawaiian taxa as exemplary of adaptive radiation, based on anatomical, morphological and ecological considerations. Studies by Pearcy and colleagues (e.g. Robichaux and Pearcy, 1980; Pearcy et al., 1982; Pearcy and Fransceschi, 1986) demonstrated major ecophysiological differences among the ancestrally and uniformly C4 Hawaiian chamaesyces that correspond to the wide range of habitats occupied by different taxa, such as exposed, dry beaches and shaded, wet forests. Morden and Gregoritza (2005) noted levels of molecular variation and lineage divergence within the Hawaiian clade that are unusually high for an oceanic island group and that warrant species-level treatment for at least some of the ten endemic varieties that have been recognized recently (Koutnik, 1987, 1999).
Fig. 6.
Exemplars of Hawaiian angiosperm radiations (left) and American relatives (right) in Euphorbiaceae (top) and Geraniaceae (bottom). (A) Euphorbia rockii. Image courtesy of J. K. Obata. (B) E. leucantha. Image courtesy of V. Steinmann. (C) Geranium arboreum. Image courtesy of G. D. Carr. (D) G. richardsonii. Image courtesy of D. Fristrom.
Yang et al. (2009) presented molecular phylogenetic evidence for a south-western North American, rather than Indo-Pacific (Fosberg, 1948), origin of the Hawaiian chamaesyces. Analysis of both cpDNA and nuclear ribosomal ITS sequences from an extensive taxonomic and geographical sampling of Euphorbia subg. Chamaesyce indicated that the sister group of the Hawaiian taxa comprises four species of Chihuahuan Desert annuals from Texas and northern Mexico such as E. leucantha (Fig. 6B). Monophyly of the Hawaiian chamaesyces was established on the basis of molecular phylogenetic data in an earlier study by Raz et al. (1998), who contributed to Yang et al.'s effort (Y. Yang, University of Michigan, Ann Arbor, pers. comm.). Yang et al. (2009) noted that a possible hybrid origin of the Hawaiian ancestor might explain results from a low-copy nuclear gene (‘deadx9’ or EMB2765): one of three alleles of the sampled Hawaiian taxa was resolved as sister to the allele found in the four Chihuahuan Desert annuals; the other two alleles of the Hawaiian taxa were sister to the allele found in a fifth south-western North American herb, Euphorbia cinerascens. Coastal Hawaiian chamaesyces, which approach continental taxa in habit, have sticky seeds consistent with dispersal by birds from North America, with putative loss of such dispersal ability and increased woodiness in shrubs and trees of interior Hawaiian habitats (Carlquist, 1980).
Woody geraniums: another Hawaiian radiation of North American origin?
The morphologically anomalous, Hawaiian members of Geranium (Geraniaceae), with shrubby or arboreal life-forms, undivided leaves and free stamens (Fig. 6C), were regarded to be of ‘obscure’ origin (Fosberg, 1948; Wagner et al., 1990) until molecular data indicated that the taxa constitute a clade of American ancestry (Pax et al., 1997). Although these montane Hawaiian species can be assigned on the basis of morphology to Geranium sect. Geranium, ascertaining the source area of Hawaiian geraniums has been complicated by the great diversity (>250 species) and wide distribution of that section, as well as the highly disparate morphology of the Hawaiian taxa relative to the comparatively modest morphological variation among non-Hawaiian members of the group.
Phylogenetic analysis of cpDNA rbcL sequences from each of the six Hawaiian species, representatives of the three subgenera of Geranium, and a limited sampling of Asian, Australian and American members of sect. Geranium resolved a well-supported Hawaiian clade nested among montane North American herbaceous lineages from Mexico and the western United States, i.e. G. richardsonii (Fig. 6D), G. subulato-stipulatum and G. vulcanicola (Pax et al., 1997). The sole red- and zygomorphic-flowered, bird-pollinated species, G. arboreum (Fig. 6C), a tree-like Hawaiian endemic, was resolved as sister to the other, white- and actinomorphic-flowered, shrubby and subshrubby Hawaiian taxa. As noted by the authors, pinpointing the source of the Hawaiian lineage will require more extensive sampling of sect. Geranium and special attention to New World groups, such as alpine South American taxa sometimes treated in sect. Andinii, which have persistent leaf bases similar to those of the Hawaiian species. Lack of woody growth forms in Geranium apart from those of the Hawaiian taxa is consistent with a hypothesis of secondary woodiness evolving in the islands from an herbaceous founding lineage.
Temperate North American lineages of low diversity in the Hawaiian Islands
Engler (1879) and/or Fosberg (1948) noted some individual Hawaiian-endemic species of putative New World origin that have been confirmed as most closely related to American taxa, in addition to Rubus macraei and R. hawaiiensis (discussed above). Hawaiian purple mat, Nama sandwicensis (Boraginaceae s.l.), evidently has closest relatives among annuals of sect. Nama in south-western North America based on micromorphological and molecular data (see Wagner et al., 1999; S. Taylor and B. Simpson, University of Texas, Austin, pers. comm.). Hawaiian dodder, Cuscuta sandwichiana, was placed within different clades of primarily North American taxa in cpDNA trees versus nuclear ribosomal trees of Stefanovic and Costea (2008); they concluded that the Hawaiian ancestor was of hybrid origin and probably dispersed from south-western North America, where the two putatively parental clades co-occur. Argemone glauca (Papaveraceae), an endemic Hawaiian prickly poppy, was suggested by Ownbey (1961) to have descended from South American species; however, an ITS-1 tree for Argemone indicates that the South American taxa constitute a robust clade to the exclusion of North American and Hawaiian taxa (Schwarzbach and Kadereit, 1999). The Hawaiian nettle Hesperocnide sandwicensis (Urticaceae) has not been studied recently, but has only one congener, H. tenella, which is endemic to California.
Some individual Hawaiian endemic angiosperm species or species-pairs of uncertain relationship prior to molecular studies now appear to be of American origin, as well. The Hawaiian bog orchid, Platanthera holochila, one of three indigenous species of Orchidaceae in the Hawaiian flora – all suggested by Fosberg (1948) to be of Indo-Pacific origin – belongs to sect. Limnorchis and is nested among North American taxa in ITS trees (R. K. Lauri, Rancho Santa Ana Botanic Garden, Claremont, CA, pers. comm.; see also Bateman et al., 2009). Uncertainty about the relationships of two distinct groups of endemic Hawaiian sedges, Carex alligata + C. kauiensis and C. nealae [considered to be the Asian C. thunbergii by Koyama (1999)], was resolved by Dragon and Barrington (2009) in favour of separate, temperate North American origins of both lineages; they found that C. alligata and C. kauaiensis are sister to C. obnupta of western North America and C. nealae is sister to C. emoryi of central–eastern North America. Another Hawaiian endemic of uncertain origin, the bunchgrass Deschampsia nubigena, was strongly resolved in Chiapella's (2007) ITS tree as more closely related to a sample of D. cespitosa from Oregon (Hsiao et al., 1995; K. Jensen, US Department of Agriculture, pers. comm.) than to other samples of D. cespitosa from Europe and South America.
Neotropical ancestry of Hawaiian angiosperms
Some Hawaiian angiosperm lineages of neotropical origin or of uncertain life zone within the New World either appear to be from southern North America (including Central America and the Caribbean) or cannot be excluded as having North American ancestry. For example, Hawaiian Sicyos (Cucurbitaceae) contains three endemic sections (approx. 14 species) of monoecious climbing annuals and perennial vines that have been of obscure affinity (e.g. Fosberg, 1948); they were resolved from ITS data as a clade nested among primarily Mexican taxa (Sebastian et al., 2009; P. Sebastian and S. S. Renner, University of Munich, pers. comm.). The endemic Hawaiian, shrubby genus Isodendrion (four species; Violaceae) has been resolved as sister to Pombalia, from south-western North American and Latin America; Isodendrion and Pombalia are in turn sister to Mesoamerican members of Hybanthus (Ballard et al., 2009; H. E. Ballard, pers. comm.). Hawaiian Gunnera (approx. two species; Gunneraceae) was resolved as monophyletic and sister to a diverse South American clade, with the Hawaiian + South American group sister to North American G. mexicana (Wanntorp and Wanntorp, 2003). Although the ancestral area for the Hawaiian taxa was equivocal based on the molecular phylogeny, Wanntorp and Wanntorp (2003) entertained the hypothesis that the Hawaiian Gunnera clade dispersed from North America during the Tertiary, when subgenus Panke was widespread there, based on fossil evidence. Hawaiian-endemic Portulaca villosa (Portulacaceae), a putative sister-species of Hawaiian-endemic P. sclerocarpa (Wagner et al., 1999), also has been resolved within a clade of North American (including Caribbean) and South American taxa in molecular trees (Ocampo and Columbus, 2009a, b).
Examples of a northern neotropical connection to the Hawaiian flora include well-studied, monospecific coastal lineages that may have dispersed to the islands by oceanic flotation. Hawaiian-endemic cotton, Gossypium tomentosum (Malvaceae), is a coastal shrub most closely related to upland cotton (G. hirsutum) based on multiple lines of molecular evidence (DeJoode and Wendel, 1992; Wendel et al., 1995; Cronn et al., 1996; Seelanan et al., 1997; Small et al., 1998) and was suggested by DeJoode and Wendel (1992) to represent an example of long-distance dispersal by seed- or capsule-flotation in oceanic currents from Mesoamerica, where G. hirsutum is native. Although G. tomentosum is highly unusual morphologically, it is interfertile with G. hirsutum and is estimated to have diverged from upland cotton since the Pleistocene (DeJoode and Wendel, 1992; Cronn et al., 2002). The vine Jacquemontia sandwicensis (Convolvulaceae) is another coastal Hawaiian plant with closest relatives in subtropical North America. On the basis of cpDNA and nuclear trees, Namoff et al. (2010) found that J. obcordata of the Caribbean Basin is sister to J. sandwicensis and that the two species are in turn sister to J. ovalifolia of Africa. Lack of seed buoyancy in water in J. sandwicensis may reflect either evolutionary loss of such ability following oceanic dispersal to the Hawaiian Islands or a history of dispersal by birds (Guppy, 1906; Ridley, 1930; Robertson, 1974; Namoff et al., 2010).
Hawaiian lineages of indirect American ancestry?
Some highly diverse lineages of Hawaiian flowering plants have closest relatives in the South Pacific and cannot be excluded as having descended directly from ancestors there, although their closest continental relatives are evidently from the New World. Such Hawaiian groups may have instead originated directly from continental (New World) ancestors, either with subsequent out-of-Hawaii dispersal to the South Pacific, as resolved for various Hawaiian lineages from the Old World (e.g. Santalum, Harbaugh and Baldwin, 2007; Melicope, Harbaugh et al., 2009), or with independent dispersal to the South Pacific from the Americas. Any of the three scenarios for direct or indirect arrival in the Hawaiian Islands requires a hypothesis of long-distance dispersal of intercontinental magnitude from the New World.
Directly or indirectly, the only native crucifers in the Hawaiian Islands are evidently of temperate North American origin. A molecular phylogenetic analysis of worldwide diversity of Lepidium (pepperworts; Cruciferae) by Mummenhoff et al. (2001) indicated that the native, subshrubby or shrubby taxa of pepperworts in the islands (Fig. 7A) constitute a clade that is nested among North American lineages of mostly annual taxa, such as L. lasiocarpum, L. oblongum (Fig. 7B) and L. virginicum. Hawaiian pepperworts have been classified with Australian and New Zealand taxa (Thellung, 1906), although Carlquist (1967) proposed American ancestry for the Hawaiian plants, with long-distance dispersal by adhesion of the wetted, mucilaginous seeds to birds. Based on levels of cpDNA divergence between mainland and island taxa and geographical distribution of the American species, Mummenhoff et al. (2001) concluded that the Hawaiian lineage probably diverged from a Californian ancestor during the Pleistocene, as suggested for Hawaiian Sanicula by Vargas et al. (1998). Based on the taxa sampled, woodiness of the Hawaiian taxa appears to be have been derived from herbaceousness in an island setting, although the authors cautioned that sampling of additional taxa and molecular markers was needed to resolve the basis of insular woodiness.
Fig. 7.
Exemplars of Hawaiian angiosperm radiations (left) and American relatives (right) in Cruciferae (top) and Compositae–Coreopsideae (bottom). (A) Lepidium arbuscula. Image courtesy of J. K. Obata. (B) Lepidium oblongum. Image courtesy of J. M. DiTomaso. (C) Bidens cosmoides. Image courtesy of G. Diada. (D) B. pilosa. Image courtesy of J. M. DiTomaso.
One of the five species of native Hawaiian Lepidium recognized by Wagner et al. (1999), L. bidentatum, occurs in low-elevation, dry, rocky sites and is widespread across islands of the Pacific; L. bidentatum can be inferred to represent either the ancestral phenotype of the Hawaiian clade or an example of dispersal out of Hawai‘i and throughout the Pacific by a lineage that has undergone diversification in the Hawaiian Islands. Hawaiian diversification from an L. bidentatum-like ancestor would have involved a shift from xeric to mesic habitats, accompanied by upslope dispersal, to account for the ecological characteristics of the mesic-forest taxa, L. orbiculare and L. serra. Xeric ancestry also is consistent with ecological attributes of most of the closely related North American taxa sampled by Mummenhoff et al. (2001).
Hawaiian Bidens (Coreopsideae; Compositae), an adaptive radiation of approx. 19 species of interfertile annuals, perennial herbs and shrubs (Ganders and Nagata, 1984; Helenurm and Ganders, 1985), including the spectacular, putatively bird-pollinated B. cosmoides (Ganders and Nagata, 1983; Fig. 7C), has been resolved in ITS trees as most closely related to a South-east Pacific clade (Ganders et al., 2000). In turn, the Hawaiian + South-east Pacific clade is nested among American members of sect. Psilocarpaea, with the closest sampled relatives (B. alba and B. pilosa; Fig. 7D) from southern North America, including the Caribbean (Ganders et al., 2000). Fruits and pappus elements of the American relatives are barbed and readily dispersible by birds, with loss or change in dispersal ability in the Pacific clade (Carlquist, 1974). The Hawaiian taxa have been regarded as American (e.g. Engler, 1879; Hillebrand, 1888) or Austral (e.g. Fosberg, 1948) in origin and were suggested by Gillett (1972, 1973) to be ancestral to the less morphologically and ecologically diverse South-east Pacific taxa. Based on the molecular evidence for a nested phylogenetic position of the Hawaiian and South Pacific clade (sect. Campylotheca) among herbaceous American lineages of sect. Psilocarpaea, Ganders et al. (2000) concluded that woody and indeterminate habits evolved secondarily in the Pacific group.
Complex patterns of morphological and ecological diversity among the herbaceous and shrubby Hawaiian plantains (Plantago; Plantaginaceae) have been interpreted as evidence for two separate introductions from the south, one from New Zealand and one from the Juan Fernandez Islands (Rock, 1920; Fosberg, 1948; see Wagner et al., 1999). Dunbar-Co et al. (2008) instead resolved Hawaiian Plantago as a monophyletic group with closest relatives in either south-temperate Polynesia (Rapa), based on cpDNA trees, or temperate North America, based on ITS trees. They also found evidence from both lines of molecular evidence for more evolutionarily distinct lineages of Hawaiian plantains than have been recently recognized taxonomically. Both allopatric and ecological diversification across the archipelago and among wet, dry and bog habitats on individual islands appear to have occurred in the Hawaiian clade. A more extensive, worldwide sampling of Plantago indicates a temperate North American origin for Hawaiian Plantago, with P. tweedyi resolved as the closest relative (sister-species) of the Hawaiian clade (R. Hoggard, University of Oklahoma, Norman, OK, pers. comm.).
All indigenous palms of the Hawaiian Islands (approx. 23 species) constitute a clade within the monophyletic tropical-Pacific genus Pritchardia (Palmae), based on nuclear and cpDNA trees (Bacon et al., 2009). Molecular results also indicate that Pritchardia is most closely related to tropical and warm temperate New World fan palms in Copernicia and, probably, Washingtonia (Baker et al., 2009; C. Bacon, pers. comm.). Although the Hawaiian fan palms may well have descended from South Pacific ancestors in Pritchardia, as suggested earlier (e.g. Fosberg, 1948), the genus as a whole evidently stems from a dispersal event into the Pacific from America.
Molecular data indicate that various endemic or indigenous Hawaiian lineages of widespread genera and represented by only one or two insular species also arrived directly or indirectly from the New World. Lycium sandwicense (Solanaceae), known from Hawaiian and South Pacific islands, is a shrub of the littoral zone that was resolved in ITS trees as most closely related to the shrubby, North American L. carolinianum and part of a diverse North American clade with multi-seeded, orange or red berries (Miller, 2002), implicated in long-distance dispersal by birds (Symon, 1991; Miller et al., 2008). Ilex anomala (Aquifoliaceae), a tree or shrub of mesic to wet forests or bog habitats in the Hawaiian and South Pacific islands, was resolved in cpDNA trees as part of an otherwise New World clade including North American and South American taxa (Cuénoud et al., 2000). The ancestor of Portulaca lutea and P. molokiniensis (Portulacaceae) also appears either to have dispersed directly to the Hawaiian Islands from the New World, with subsequent dispersal of P. lutea throughout the Pacific, or to have arrived in the South Pacific, with subsequent dispersal to the Hawaiian Islands by P. lutea and divergence of P. molokiniensis (Ocampo and Columbus, 2009a, b). Similar alternative biogeographical scenarios are evident for Mucuna sloanei (Leguminosae), with var. sloanei in South America, the South-east Pacific and the Hawaiian Islands and var. persericea endemic to Maui (Wilmot-Dear, 1990).
Hawaiian angiosperms of possible New World ancestry that warrant further study include the endemic genus Lipochaeta and relatives (Compositae) and endemic taxa of Abutilon (Malvaceae), Calamagrostis (Gramineae), Chenopodium (Chenopodiaceae), Dissochondrus (Gramineae), Dodonaea (Sapindaceae), Eragrostis (Gramineae), Erythrina (Leguminosae), Ipomoea (Convolvulaceae), Luzula (Juncaceae), Myrsine (Myrsinaceae), Panicum (Gramineae), Perrottetia (Dipentodontaceae), Phytolacca (Phytolaccaceae), Poa (Gramineae), Ranunculus (Ranunculaceae), Rauvolfia (Apocynaceae), Rumex (Polygonaceae), Trisetum (Gramineae) and Vaccinium (Ericaceae).
REASSESSMENT OF THE AMERICAN CONTRIBUTION TO THE HAWAIIAN ANGIOSPERM FLORA
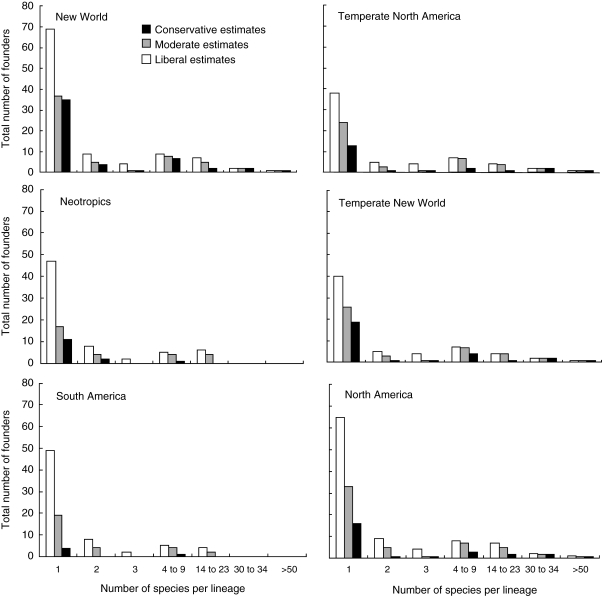
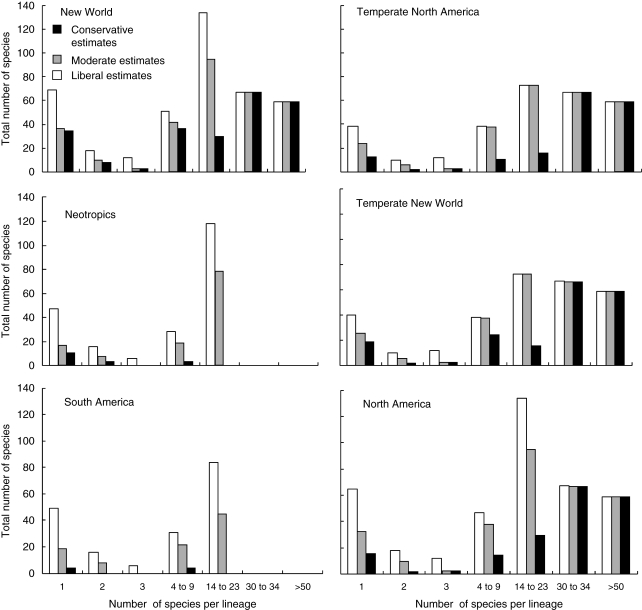
Reanalysis of the overall American contribution to the Hawaiian seed-plant flora, under either conservative or liberal assignment of lineages to source areas, yields results that require a new perspective from that of Fosberg (1948, 1951), who suggested 50–51 natural New World introductions to the islands, of which only four or five, of dubious American ancestry, resulted in more than two species (of these, only Isodendrion, with four species, is now regarded as American in origin; see above). Although most reassessed angiosperm founding lineages from the Americas are represented by only one endemic or indigenous Hawaiian species (Fig. 8), in keeping with such estimates for founding lineages from all Hawaiian source areas worldwide (W. Wagner and J. Price, unpubl. res.), founding lineages that gave rise to four or more endemic species include the majority of Hawaiian species of New World ancestry (Fig. 9). On a flora-wide scale, angiosperm clades of direct or indirect American origin represent at least six of 12 of the most species-rich lineages in the Hawaiian angiosperm flora (Table 1). In turn, those 12 lineages represent nearly half (approx. 47 %) of all native Hawaiian angiosperm species currently recognized. Founders from temperate North America, including desert regions, evidently spawned the majority of flowering-plant species of New World ancestry (Fig. 9), including the most species-rich clades, in contrast to earlier views that most or all New World plants in the Hawaiian flora descended from South American ancestors (e.g. Campbell, 1919; Skottsberg, 1925).
Fig. 8.
Total number of Hawaiian angiosperm founders from particular source areas in the New World that gave rise to lineages with different levels of species richness. Conservative estimates = taxa confidently assigned to area based on molecular, morphological and/or distributional data; moderate estimates = conservative estimate + taxa confidently assigned to New World and either equivocally assigned to subregion or of potentially indirect dispersal to the Hawaiian Islands, via the South Pacific; liberal estimates = moderate estimate + taxa possibly originating in the New World based on available data but not verified as New World in origin, such as pantropical taxa. See Appendix 2 for taxon assignments to regions.
Fig. 9.
Total number of Hawaiian angiosperm species resulting from lineages of particular New World source areas with different levels of species richness: Conservative estimates, moderate estimates and liberal estimates, as indicated. See legend to Fig. 8 for method of estimation and Appendix 2 for taxon assignments to regions.
Table 1.
Twelve most species-rich lineages of Hawaiian angiosperms
| Taxon or taxa constituting a lineage | Species number* | Source area | References |
|---|---|---|---|
| Brighamia, Cyanea, Clermontia, Delissea, Hawaiian Lobelia, Trematolobelia (Campanulaceae; Lobelioideae) | 126 | Africa, Polynesia, or southern Asia | Givnish et al. (2009) |
| Hawaiian Cyrtandra (Gesneriaceae) | 59 | South-east Asia directly or indirectly (via South Pacific islands) | Cronk et al. (2005), Clark et al. (2009) |
| Haplostachys, Phyllostegia, Stenogyne (Labiatae) | 59 | Temperate North America | Lindqvist and Albert (2002), Wood and Oppenhiemer (2008) |
| Melicope, Platydesma (Rutaceae) | 52 | Australia or New Guinea | Harbaugh et al. (2009) |
| Schiedea (Caryophyllaceae) | 34 | Temperate (boreal) North America | Wagner et al. (2005b), Harbaugh et al. (2010) |
| Argyroxiphium, Dubautia, Wilkesia (Compositae) | 33 | Temperate North America | Baldwin et al. (1991), Barrier et al. (1999), Baldwin and Carr (2005), Baldwin and Friar (2010) |
| Hawaiian Pritchardia (Palmae) | 23 | Warm temperate or tropical North America directly or indirectly (via South Pacific islands) | Bacon et al. (2009), Baker et al. (2009) |
| Hawaiian Kadua (Rubiaceae) | 22 | Australia, south-east Asia, or Africa directly or indirectly (via South Pacific islands) | Groeninckx et al. (2009), Lorence et al. (2010) |
| Hawaiian Peperomia (Piperaceae) endemic clade | 22 | Subtropical or tropical New World directly or indirectly (via South Pacific islands) | Bradley (2002), Wanke et al. (2006) |
| Lipochaeta/Melanthera (Compositae)† | 20 | Possibly of New World origin | Wagner and Robinson (2001), Chumley et al. (2002) |
| Hawaiian Bidens (Compositae) | 19 | Warm temperate or tropical North America directly or indirectly (via South Pacific) | Ganders et al. (2000) |
| Myrsine (Primulaceae) | 19 | Unknown (pantropical and subtropical) | see Wagner et al. (1999) |
*From the Flora of the Hawaiian Islands (http://botany.si.edu/pacificislandbiodiversity/hawaiianflora/) plus cited references.
†Included here based on molecular evidence that the closest relatives of Lipochaeta (2n = 26II), at least among sampled taxa, are Hawaiian members of Melanthera (2n = 15II).
Diversification of species-rich lineages of North American origin in the Hawaiian Islands may in part reflect factors likely also to have been important to insular colonization by American plants: timing of introduction, dispersal biology, ecological opportunity, and ancestral hybridization and/or polyploidization. Where sufficient phylogenetic resolution is available, highly diverse lineages of American origin mostly appear to date back to the oldest modern high-elevation island, Kaua‘i (see above), and may represent relatively old lineages of Hawaiian angiosperms, except by comparison with the evidently much older and more diverse lobelioids (Givnish et al., 2009) and the presumed relict Hillebrandia (Clement et al., 2004). Most Hawaiian angiosperm lineages of evident American origin, and all such clades of four or more species, have seed or fruit characteristics that have been implicated in ancestral long-distance dispersal by birds or have close extra-Hawaiian relatives with such features (Carlquist, 1974; Sakai et al., 1995; see Appendix 1). As discussed by Ballard and Sytsma (2000), opportunities for angiosperm dispersal by birds to the Hawaiian Islands appear to be ample for north temperate or boreal lineages, based on consideration of bird migration routes. As they noted, arriving in a suitable habitat in a tropical archipelago may be a more limiting factor in colonization and establishment than achieving oversea transport for bird-dispersed north temperate plants.
Once established, north temperate flowering plants (the vast majority evidently from North America; Appendix 1) would potentially have found conditions favourable for diversification in upper-elevation or dry, leeward habitats generally associated with new islands and generally less suitable for plants from tropical settings. Habitat diversity and lack of competition or interference from other plants on ‘sky islands’ of temperate or boreal climate in a tropical archipelago or in dry habitats in the rain shadow of major volcanoes could have allowed for ecological release, through both relaxed and redirected natural selection (see Schluter, 2000; Gillespie and Baldwin, 2009). Although inferring ancestral habitats for Hawaiian lineages often has been complicated by ecological shifts associated with adaptive radiation, climatic settings of closely related mainland clades and at least a subset of taxa in most diverse Hawaiian lineages of North American origin are consistent with initial insular colonization and diversification in situations resembling temperate or boreal habitats, e.g. in the silversword alliance, Hawaiian mints, Geranium, Sanicula, Schiedea, Silene and Viola. Presumed establishment of Hawaiian Euphorbia subg. Chamaesyce in dry coastal settings (see above) is consistent with a warm-temperate, xeric (south-west North American) ancestry for that clade, as for Hawaiian Argemone, Cuscuta and Nama.
Price and Wagner (2004) found that dispersal by birds, especially via external adhesion, is significantly associated with species-richness in Hawaiian angiosperm clades; they proposed that intermediate levels of vagility associated with bird dispersal resulted in sufficient isolation for divergence and sufficient dispersal ability to reach new islands and habitats as opportunities for colonization arose. Hybridization and/or polyploidization shortly before dispersal to the Hawaiian Islands may also have aided establishment or evolutionary success of some American lineages, such as the silversword alliance, Hawaiian mints, Cuscuta, Euphorbia subg. Chamaesyce and Viola, by elevating genetic or genomic variation and potentially allowing for extensive recombination and expression of diverse phenotypes on which natural selection could act (Crawford and Stuessy, 1997; Carr, 1998; Barrier et al., 1999; Ballard and Sytsma, 2000; Lindqvist and Albert, 2002; Stefanovic and Costea, 2008; Crawford et al., 2009). Floral attributes also may have contributed to diversification of American lineages; two of the three most species-rich clades of Hawaiian angiosperms of New World origin – the mints and Schiedea – also are among the most diverse Hawaiian lineages in pollination biology and, in Schiedea, breeding system (Sakai et al., 2006). The other major clade, the silversword alliance, is exceptional among Hawaiian angiosperms for sporophytic self-incompatibility in most tested taxa of the group (Carr et al., 1986; S. Bainbridge and B. Baldwin, unpubl.), which may have been beneficial to adaptive evolution by facilitating retention and recombination of genetic variation within populations.
CONCLUSIONS
Species-rich lineages of temperate North American origin in the Hawaiian flora mostly appear to represent special cases of adaptive diversification in equatorial highlands, but on ‘sky islands’ in a tropical oceanic archipelago (high-elevation ‘islands’ within true islands) rather than on ‘sky islands’ in a tropical continental setting, where examples of adaptive radiation of ancestrally temperate plant groups are well documented (e.g. in the Andes or East African Highlands; see Carlquist, 1974; Hughes and Eastwood, 2006). Prominence of temperate North America and the New World in general as a source area for the Hawaiian angiosperm flora adds substantially to the wealth of evidence from recent phylogenetic studies for a significant impact of long-distance dispersal in shaping plant (and animal) distributions on a global scale. With the rise of molecular methods for estimating divergence times between lineages, intercontinental plant disjunctions often appear to be much too recent to be explained by simple vicariance (see Donoghue and Moore, 2003; Givnish and Renner, 2004; Sanmartín and Ronquist, 2004; de Queiroz, 2005; Moore et al., 2006; Dick et al., 2007; Keppel et al., 2009), even within some clades presumed earlier to be of Gondwanan origin (Cook and Crisp, 2005; Won and Renner, 2006; Barker et al., 2007). Vicariance explanations for origins of Hawaiian biota in particular (e.g. Van Steenis, 1963; Melville, 1981) have relied on speculative geological histories that involve time frames more ancient than can be reasonably hypothesized for the vast majority of endemic plant lineages, based on molecular phylogenetic evidence. Attempts to incorporate dispersal more explicitly within biogeographical models and methodology are important steps toward progress in understanding plant distributions on continents and islands (e.g. Funk and Wagner, 1995; Ree et al., 2005; Sanmartín et al., 2008), in addition to the pursuit of much more empirical work on plant relationships worldwide.
ACKNOWLEDGEMENTS
We thank Sue Bainbridge and Rick Hanna for technical assistance; Gerry Carr, G. Diada, Joe DiTomaso, Dianne Fristrom, Matt Goff, John Obata, Ya Yang, George Yatskievych and Saint Mary's College for providing plant images, and Gerry Carr for providing the image files by Obata and Diada; Danica Harbaugh for providing the map in Fig. 1; Joachim Kadereit and Gudren Kadereit for German-to-English translations of Engler (1879–1882) and Hillebrand (1888b); John L. Strother and two anonymous reviewers for helpful comments on the manuscript; Alice Tangerini for preparing the plates; and the Hunt Institute for Botanical Documentation and the Smithsonian Institution Libraries for permission to reproduce images of people in Fig. 2. Special thanks to the many researchers who generously allowed us to cite and summarize their unpublished results on relationships of Hawaiian plants. This work was supported by the National Tropical Botanical Garden, Kaleheo, Hawaii (Senior McBryde Research Fellowship to B.G.B. and McBryde Chair to W.L.W.). Funding for images was obtained from the Walcott Botanical Publication Fund, Department of Botany, Smithsonian Institution.
APPENDIX 1
Native Hawaiian angiosperms of demonstrated or possible American origin
| Hawaiian taxa constituting a lineage | Recognized species* | Endemic or indigenous | Family | Source area estimate for New World | Closest American relatives | References | Dispersal mode† |
|---|---|---|---|---|---|---|---|
| Abutilon incanum | 1 | i | Malvaceae | Warm temperate North America | Abutilon incanum | Bates (1999) | df |
| Abutilon eremitopetalum and A. menziesii | 2 | e | Malvaceae | Possibly New World (warm and tropical) | Unknown Abutilon species | Bates (1999) | df |
| Abutilon sandwicense | 1 | e | Malvaceae | Possibly New World (warm and tropical) | Unknown Abutilon species | Bates (1999) | df |
| Acaena exigua | 1 | e | Rosaceae | Temperate South America | Acaena pumila | Marticorena (2006) | bb |
| Argemone glauca | 1 | e | Papaveraceae | Warm temperate North America | Mostly SW North American Argemone taxa | Schwarzbach and Kadereit (1999) | bi |
| Argyroxiphium/Dubautia/Wilkesia (endemic genera) | 33 | e | Compositae | Temperate North America | ‘Madia’ lineage (e.g. Anisocarpus, Carlquistia, Harmonia, Jensia, Madia) | Baldwin et al. (1991), Baldwin (1996), Barrier et al. (1999), Baldwin and Carr (2005), Baldwin and Friar (2010) | bv/bb |
| Bacopa monnieri | 1 | i | Plantaginaceae | Possibly New World (pantropical, and warm temperate North America) | Bacopa monnieri | Wagner et al. (1990) | bm |
| Bidens | 19 | e | Compositae | Directly or indirectly temperate or tropical North America | New World relatives in sect. Psilocarpaea: B. alba, B. pilosa (SE Pacific Bidens taxa are sister to Hawaiian taxa) | Ganders et al. (2000) | bb |
| Bobea (endemic genus) | 4 | e | Rubiaceae | Tropical South America | Guettarda crispiflora, G. hirsuta | Achille et al. (2006) | bi |
| Bonamia menziesii | 1 | e | Convolvulaceae | Neotropics | NW South/Central American Bonamia species | Austin (1999) | bv/dr |
| Bulboschoenus maritimus subsp. paludosus | 1 | i | Cyperaceae | Temperate North America | Bulboschoenus maritimus subsp. paludosus | Koyama (1999) | bm |
| Caesalpinia bonduc | 1 | i | Leguminosae | Possibly New World (pantropical, and warm temperate North America) | Caesalpinia boduc | Geesink et al. (1999) | df |
| Calamagrostis expansa | 1 | e | Gramineae | Possibly temperate or montane tropical America | Unknown Calamagrostis species | O'Connor (1999) | bb |
| Carex alligata and C. kauaiensis | 2 | e | Cyperaceae | Temperate North America | Carex obnupta | Dragon and Barrington (2009) | bm/bi |
| Carex echinata | 1 | i | Cyperaceae | Temperate Northern Hemisphere | Carex echinata | Koyama (1999), Reznicek (2002) | bm/bi |
| Carex nealae | 1 | e | Cyperaceae | Temperate North America | Carex emoryi | Dragon and Barrington (2009) | bm/bi |
| Carex subfusca | 1 | i | Cyperaceae | Temperate North America | Carex subfusca | Koyama (1999) | bm/bi |
| Cassytha filiformis | 1 | i | Lauraceae | Possibly New World (pantropical, including subtropical N America) | Cassytha filiformis | van der Werff (1999) | df |
| Chenopodium oahuense | 1 | e | Chenopodiaceae | Possibly New World | Unknown Chenopodium species | Wagner et al. (1999) | bm |
| Cladium jamaicense | 1 | i | Cyperaceae | Possibly New World (warm or tropical) | Cladium jamaicense | Koyama (1999) | bi |
| Colubrina oppositifolia | 1 | e | Rhamnaceae | Neotropics | Colubrina glandulosa | Johnston (1971) | df |
| Cressa truxillensis | 1 | i | Convolvulaceae | Temperate or tropical America | North/South American populations of Cressa truxillensis | Austin (1999) | df |
| Cuscuta sandwichiana | 1 | e | Convolvulaceae | Warm temperate North America | ‘B’ and ‘H’ clades of Cuscuta | Stefanovic and Costea (2008) | bi |
| Cyperus laevigatus | 1 | i | Cyperaceae | Possibly New World (warm or tropical) | Cyperus laevigatus | Koyama (1999) | bi/bm |
| Cyperus trachysanthos | 1 | e | Cyperaceae | Temperate or tropical America | Cyperus elegans and C. oxylepis | Koyama (1999) | bi/bm |
| Daucus pusillus | 1 | i | Umbelliferae | Temperate America | North/South American populations of Daucus pusillus | Constance and Affolter (1999) | bb |
| Deschampsia nubigena | 1 | e | Gramineae | Temperate North America | Deschampsia cespitosa | Chiapella (2007) | bb |
| Dichanthelium | 4 | e | Gramineae | Temperate or tropical America | Dichanthelium acuminatum, D. clandestinum, D. sabulorum | Aliscioni et al. (2003) | bm |
| Dodonaea viscosa | 1 | i | Sapindaceae | Possibly New World (pantropical, including warm temperate North America) | Dodonaea viscosa | Wagner et al. (1999) | df/dr |
| Drosera anglica | 1 | i | Droseraceae | Temperate Northern Hemisphere | Drosera anglica | Wagner et al. (1999), Rivadavia et al. (2003) | bm |
| Eleocharis erythropoda | 1 | i | Cyperaceae | Temperate North America | Eleocharis erythropoda | Koyama (1999) | bm |
| Eleocharis obtusa | 1 | i | Cyperaceae | Temperate North America | Eleocharis obtusa | Koyama (1999) | bm |
| Eragrostis | 9 | e | Gramineae | Possibly New World (warm or tropical) | Unknown Eragrostis species | O'Connor (1999) | bi |
| Erythrina sandwicensis | 1 | e | Leguminosae | Possibly New World (warm or tropical) | Unknown Erythrina species | Neill (1999) | dr/bi |
| Euphorbia subg. Chamaesyce | 16 | e | Euphorbiaceae | Warm temperate North America | Euphorbia cinerascens, other Chihuahuan Desert annuals (four species) | Yang et al. (2009) | bi |
| Fimbristylis cymosa | 1 | i | Cyperaceae | Possibly New World (warm or tropical) | Fimbristylis cymosa | Koyama (1999) | bm/bi |
| Fimbristylis dichotoma | 1 | i | Cyperaceae | Possibly New World (warm or tropical) | Fimbristylis dichotoma | Koyama (1999) | bm/bi |
| Fimbristylis hawaiiensis | 1 | e | Cyperaceae | Possibly New World (warm or tropical) | Possibly Fimbristylis ferruginea | Koyama (1999) | bm/bi |
| Fragaria chiloensis | 1 | i | Rosaceae | Temperate America | W North/S South American populations of Fragaria chiloensis | Wagner et al. (1999) | bi |
| Geranium | 7 | e | Geraniaceae | Temperate or montane tropical America | Species of Geranium subg. Geranium | Pax et al. (1997) | bb |
| Gossypium tomentosum | 1 | e | Malvaceae | Tropical North America | Gossypium hirsutum | DeJoode and Wendel (1992), Small et al. (1998) | dr |
| Gunnera | 2 | e | Gunneraceae | Neotropics | Central/South American Gunnera species; all plus Hawaiian taxa sister to G. mexicana | Wanntorp and Wanntorp (2003) | bi |
| Haplostachys/Phyllostegia/Stenogyne | 59 | e | Labiatae | Temperate North America | Stachys | Lindqvist & Albert (2002), Wood and Oppenheimer (2008) | bi |
| Heliotropium curassavicum | 1 | i | Boraginaceae | Possibly New World | Heliotropium curassavicum | Wagner et al. (1999) | df |
| Hesperocnide sandwicensis | 1 | e | Urticaceae | Temperate North America | Hesperocnide tenella | Wagner et al. (1999) | bb |
| Hibiscus furcellatus | 1 | i | Malvaceae | Neotropics | North/South American populations of Hibiscus furcellatus | Bates (1999) | dr |
| Ilex anomala | 1 | i | Aquifoliaceae | Directly or indirectly warm temperate or tropical America | All South American/most North American Ilex species sampled are sister to Hawaiian species (I. anomala also in S Pacific islands) | Cuénoud et al. (2000) | bi |
| Ipomoea imperati | 1 | i | Convolvulaceae | Possibly New World (pantropical, and warm temperate North America | Ipomoea imperati | Austin (1999) | df |
| Ipomoea pes-caprae subsp. brasiliensis | 1 | i | Convolvulaceae | Possibly New World (pantropical, and warm temperate North America) | Ipomoea pes-caprae subsp. brasiliensis | Austin (1999) | df |
| Ipomoea tuboides | 1 | e | Convolvulaceae | Possibly New World (warm or tropical) | Unknown Ipomoea species | Austin (1999) | df |
| Isodendrion (endemic genus) | 4 | e | Violaceae | Temperate or tropical North America | Pombalia (Hybanthus sensu lato) | Ballard et al. (2009), H. E. Ballard (pers. comm.) | bi/bm |
| Jacquemontia sandwicensis | 1 | e | Convolvulaceae | Tropical North America | Jacquemontia obcordata | Namoff et al. (2009) | dr |
| Kanaloa kahoolawensis | 1 | e | Leguminosae | Temperate or tropical America | Nested among mostly New World Mimosoideae genera (sister to Desmanthus and Schleinitzia) | Lorence and Wood (1994), Catalano et al. (2008) | |
| Lepechinia hastata | 1 | i | Labiatae | Tropical North America | North American (Baja California Sur, Mexico) populations of Lepechinia hastata | Wagner et al. (1999) | bv |
| Lepidium | 5 | e | Cruciferae | Directly or indirectly temperate North America | Lepidium austrinum, L. lasiocarpum, L. oblongum, L. virginicum (Hawaiian L. bidentatum also in S Pacific islands) | Mummenhoff et al. (2001) | bv |
| Lipochaeta (endemic genus)/Melanthera | 20 | e | Compositae | Possibly New World (warm or tropical) | Melanthera species | Wagner and Robinson (2001), Chumley et al. (2002) | bb |
| Luzula hawaiiensis | 1 | e | Juncaceae | Possibly temperate or montane tropical America | Species of Luzula sect. Luzula | Roalson (2005) | bm |
| Lycium sandwicense | 1 | i | Solanaceae | Directly or indirectly warm temperate North American | Lycium carolinianum (L. sandwicense also in S Pacific islands) | Miller (2002) | dr |
| Mucuna sloanei var. persericea and M. sloanei var. sloanei | 1 | e/i | Leguminosae | Tropical South America | South American populations of Mucuna sloanei var. sloanei | Wilmot-Dear (1990) | df |
| Myrsine | 19 | e | Myrsinaceae | Possibly New World (pantropical) | Unknown Myrsine species | Wagner et al. (1999) | bi |
| Nama sandwicensis | 1 | e | Boraginaceae | Warm temperate North America | Nama hispidum, N. stevensii, N. turneri, N. undulatum | Wagner et al. (1999) | bb |
| Panicum faurieri and P. nephelophilum | 2 | e | Gramineae | Possibly New World (warm or tropical) | Species of Panicum subg. Panicum | Aliscioni et al. (2003) | bi/bb |
| Peperomia (endemic spp. except P. remyi) | 22 | e | Piperaceae | Directly or indirectly New World (warm or tropical) | Species of Peperomia subg. Sphaerocarpidium (sister to other Pacific island taxa) | Wagner et al. (1999), Bradley (2002), Wanke et al. (2006) | bv |
| Peperomia tetraphylla | 1 | i | Piperaceae | Possibly New World (pantropical) | Peperomia tetraphylla | Wagner et al. (1999), Bradley (2002), Wanke et al. (2006) | bv |
| Perrottetia sandwicensis | 1 | e | Dipentodontaceae | Possibly New World (warm or tropical) | Unknown Perrottetia species | Wagner et al. (1999) | bi |
| Phytolacca sandwicensis | 1 | e | Phytolaccaceae | Possibly New World (warm or tropical) | Unknown Phytolacca species | Wagner et al. (1999) | bi |
| Pilea peploides | 1 | i | Urticaceae | Tropical South America | Pilea peploides | Wagner et al. (1999) | bm |
| Plantago | 3 | e | Plantaginaceae | Temperate North America | Plantago tweedyi | Dunbar-Co et al. (2008), R. Hoggard (pers. comm.) | bv |
| Platanthera holochila | 1 | e | Orchidaceae | Temperate North America | Species of Platanthera sect. Limnorchis | R. Lauri (pers. comm.), see Bateman et al. (2009) | a |
| Poa | 3 | e | Gramineae | Possibly temperate or montane tropical America | Unknown Poa species | O'Connor (1999) | bb |
| Portulaca lutea and P. molokiniensis | 2 | e/i | Portulacaceae | Directly or indirectly from warm temperate or tropical America | New World Portulaca species (Portulaca lutea also in SE Pacific islands) | Ocampo and Columbus (2009a, b) | df |
| Portulaca sclerocarpa and P. villosa | 2 | e | Portulacaceae | Temperate or tropical America | North/South American Portulaca species | Ocampo and Columbus (2009a, b) | df |
| Potamogeton foliosus | 1 | i | Potamogetonaceae | Temperate or tropical North America | Potamogeton foliosus | Wagner et al. (1999) | bi |
| Pritchardia | 23 | e | Palmae | Directly or indirectly warm temperate or tropical America | New World Copernicia (Pritchardia also in S Pacific islands) | Baker et al. (2009) | bi/dr |
| Pycreus polystachyos | 1 | i | Cyperaceae | Possibly New World (warm or tropical) | Pycreus polystachyos | Koyama (1999) | bi |
| Ranunculus | 2 | e | Ranunculaceae | Possibly temperate or montane tropical America | Unknown Ranunculus species | Duncan (1999) | bm |
| Rauvolfia sandwicensis | 1 | e | Apocynaceae | Possibly New World (warm or tropical) | Unknown Rauvolfia species | Wagner et al. (1999) | bi |
| Rhynchospora rugosa subsp. lavarum | 1 | i | Cyperaceae | Neotropics | North/South American populations of Rhynchospora rugosa subsp. lavarum | Koyama (1999) | bi/bb |
| Rubus hawaiiensis | 1 | e | Rosaceae | Temperate North America | Rubus spectabilis | Howarth et al. (1997) | bi |
| Rubus macraei | 1 | e | Rosaceae | Temperate North America | Rubus ursinus | Morden et al. (2003) | bi |
| Rumex | 3 | e | Polygonaceae | Possibly New World | Unknown Rumex species | Wagner et al. (1999) | dr/bi |
| Ruppia maritima | 1 | i | Ruppiaceae | Possibly New World (pantropical, and temperate North America) | Ruppia maritima | Wagner et al. (1999) | bm |
| Sanicula | 4 | e | Umbelliferae | Temperate North America | Sanicula arctopoides, S. arguta, S. laciniata | Vargas et al. (1998) | bb |
| Sapindus saponaria | 1 | i | Sapindaceae | Possibly New World (warm or tropical) | Sapindus saponaria | Wagner et al. (1999) | bi |
| Schiedea (including Alsinidendron) | 34 | e | Caryophyllaceae | Temperate North America | Honckenya, Wilhelmsia | Wagner et al. (2005b) | bm |
| Schoenoplectus lacustris | 1 | i | Cyperaceae | Possibly New World (warm or tropical) | Schoenoplectus lacustris | Wagner et al. (1999) | bm/bi |
| Scleria testacea | 1 | i | Cyperaceae | Neotropics | North/South American populations of Scleria testacea | Koyama (1999) | bm |
| Sesuvium portulacastrum | 1 | i | Aizoaceae | Possibly New World (pantropical, and warm temperate North America) | Sesuvium portulacastrum | Wagner et al. (1999) | df |
| Sicyos | 14 | e | Cucurbitaceae | Temperate or tropical North America | Mostly Mexican Sicyos species | Sebastian et al. (2009), P. Sebastian and S. S. Renner (pers. comm.) | bb |
| Silene | 7 | e | Caryophyllaceae | Temperate America | Silene antirrhina | Eggens et al. (2007) | bi/bm |
| Sisyrinchium acre | 1 | e | Iridaceae | Temperate or tropical America | Possibly S. tinctorium (Central America) | Goldblatt and Henrich (1999) | bm |
| Spermolepis hawaiiensis | 1 | e | Umbelliferae | Temperate America | North/S South American Spermolepis species | Constance and Affolter (1999) | bb |
| Sporobolus virginicus | 1 | i | Gramineae | Possibly New World (pantropical, and warm temperate North America) | Sporobolus virginicus | O'Connor (1999) | bv |
| Touchardia latifolia and Urera glabra | 2 | e | Urticaceae | Neotropics | Unknown Urera species | Wagner et al. (1999) | bi |
| Trisetum | 2 | e | Gramineae | Possibly temperate or montane tropical America | Unknown Trisetum species | O'Connor (1999) | bb |
| Uncinia brevicaulis | 1 | i | Cyperaceae | Temperate South America | S South American populations (also in Trintan de Cunha) of Uncinia brevicaulis | Koyama (1999) | bb |
| Urera kaalae | 1 | e | Urticaceae | Neotropics | Urera caracasana or relatives | Wagner et al. (1999) | bi |
| Vaccinium | 3 | e | Ericaceae | Temperate Northern Hemisphere | Species of Vaccinium sect. Myrtillus | Powell and Kron (2002) | bi |
| Vicia menziesii | 1 | e | Leguminosae | Temperate America | North/South American Vicia nigricans | Lassetter and Gunn (1979) | bi |
| Vigna marina | 1 | i | Leguminosae | Possibly New World (pantropical, including tropical West Indes, Panama) | Vigna marina | Geesink et al. (1999) | df |
| Viola | 7 | e | Violaceae | Temperate North America | Viola langsdorffii | Ballard and Sytsma (2000) | bi/bm |
| Waltheria indica | 1 | i | Sterculiaceae | Possibly New World (warm or tropical) | Waltheria indica | Wagner et al. (1999) | dr |
*From the Flora of the Hawaiian Islands (http://botany.si.edu/pacificislandbiodiversity/hawaiianflora/) plus cited references.
†Putative mode of dispersal from the New World to the Hawaiian Islands from Sakai et al. (1995); a = air, bb = bird external (barbs), bi = bird internal, bm = bird external (mud), bv = bird external (viscid), df = oceanic drift–frequent, dr = oceanic drift–rare.
APPENDIX 2
Assignments of native Hawaiian angiosperms to potential New World source areas under conservative (CE), moderate (ME) and liberal (LE) estimates (see Fig. 8 for methods of estimation). += assignment; −= non-assignment. See Appendix 1 for details of lineages.
| New World |
Neotropics |
North America |
Temperate North America |
Temperate New World |
South America |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hawaiian taxa constituting a lineage | LE | ME | CE | LE | ME | CE | LE | ME | CE | LE | ME | CE | LE | ME | CE | LE | ME | CE |
| Abutilon incanum | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Abutilon eremitopetalum and A. menziesii | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Abutilon sandwicense | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Acaena exigua | + | + | + | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + |
| Argemone glauca | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Argyroxiphium/Dubautia/ Wilkesia (endemic genera) | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Bacopa monnieri | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Bidens | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − |
| Bobea (endemic genus) | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | + | + | + |
| Bonamia menziesii | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Bulboschoenus maritimus subsp. paludosus | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Caesalpinia bonduc | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Calamagrostis expansa | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Carex alligata and C. kauaiensis | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Carex echinata | + | − | − | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − |
| Carex nealae | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Carex subfusca | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Cassytha filiformis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Chenopodium oahuense | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Cladium jamaicense | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Colubrina oppositifolia | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Cressa truxillensis | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Cuscuta sandwichiana | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Cyperus laevigatus | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Cyperus trachysanthos | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Daucus pusillus | + | + | + | − | − | − | + | + | − | + | + | − | + | + | + | + | + | − |
| Deschampsia nubigena | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Dichanthelium | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Dodonaea viscosa | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Drosera anglica | + | − | − | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − |
| Eleocharis erythropoda | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Eleocharis obtusa | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Eragrostis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Erythrina sandwicensis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Euphorbia subg. Chamaesyce | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Fimbristylis cymosa | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Fimbristylis dichotoma | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Fimbristylis hawaiiensis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Fragaria chiloensis | + | + | + | − | − | − | + | + | − | + | + | − | + | + | + | + | + | − |
| Geranium | + | + | + | + | + | − | + | + | − | + | + | − | + | + | + | + | + | − |
| Gossypium tomentosum | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − |
| Gunnera | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Haplostachys/Phyllostegia/ Stenogyne | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Heliotropium curassavicum | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Hesperocnide sandwicensis | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Hibiscus furcellatus | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Ilex anomala | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Ipomoea imperati | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Ipomoea pes–caprae subsp. brasiliensis | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Ipomoea tuboides | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Isodendrion (endemic genus) | + | + | + | + | + | − | + | + | + | + | + | − | + | + | − | − | − | − |
| Jacquemontia sandwicensis | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − |
| Kanaloa kahoolawensis | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Lepechinia hastata | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − |
| Lepidium | + | + | − | − | − | − | + | + | − | + | + | − | + | + | − | − | − | − |
| Lipochaeta (endemic genus)/Melanthera | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Luzula hawaiiensis | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Lycium sandwicense | + | + | − | − | − | − | + | + | − | + | + | − | + | + | − | − | − | − |
| Mucuna sloanei var. persericea and M. sloanei var. sloanei | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | + | + | + |
| Myrsine | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Nama sandwicensis | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Panicum faurieri and P. nephelophilum | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Peperomia (endemic spp. except P. remyi) | + | + | − | + | + | − | + | + | − | − | − | − | − | − | − | + | + | − |
| Peperomia tetraphylla | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Perrottetia sandwicensis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Phytolacca sandwicensis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Pilea peploides | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | + | + | + |
| Plantago | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Platanthera holochila | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Poa | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Portulaca lutea and P. molokiniensis | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Portulaca sclerocarpa and P. villosa | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Potamogeton foliosus | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | − | − | − |
| Pritchardia | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Pycreus polystachyos | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Ranunculus | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Rauvolfia sandwicensis | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Rhynchospora rugosa subsp. lavarum | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Rubus hawaiiensis | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Rubus macraei | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Rumex | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Ruppia maritima | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Sanicula | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Sapindus saponaria | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Schiedea (including Alsinidendron) | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Schoenoplectus lacustris | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Scleria testacea | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Sesuvium portulacastrum | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Sicyos | + | + | + | + | + | − | + | + | + | + | + | − | + | + | − | − | − | − |
| Silene | + | + | + | − | − | − | + | + | − | + | + | − | + | + | + | + | + | − |
| Sisyrinchium acre | + | + | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − |
| Spermolepis hawaiiensis | + | + | + | − | − | − | + | + | − | + | + | − | + | + | + | + | + | − |
| Sporobolus virginicus | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Touchardia latifolia and Urera glabra | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Trisetum | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| Uncinia brevicaulis | + | + | + | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + |
| Urera kaalae | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | + | + | − |
| Vaccinium | + | − | − | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − |
| Vicia menziesii | + | + | + | − | − | − | + | + | − | + | + | − | + | + | + | + | + | − |
| Vigna marina | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Viola | + | + | + | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − |
| Waltheria indica | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
LITERATURE CITED
- Achille F, Motley TJ, Lowry PP, II, Jérémie J. Polyphyly in Guettarda L. (Rubiaceae, Guettardeae) based on nrDNA ITS sequence data. Annals of the Missouri Botanical Garden. 2006;93:103–121. [Google Scholar]
- Alice LA, Campbell CS. Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. American Journal of Botany. 1999;86:81–97. [PubMed] [Google Scholar]
- Aliscioni SS, Giussani LM, Zuloaga FO, Kellogg EA. A molecular phylogeny of Panicum (Poaceae: Paniceae): Tests of monophyly and phylogenetic placement within the Panicoideae. American Journal of Botany. 2003;90:796–821. doi: 10.3732/ajb.90.5.796. [DOI] [PubMed] [Google Scholar]
- Austin DF. Convolvulaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 548–564. [Google Scholar]
- Bacon CD, Baker WJ, Simmons MP. Phylogeny and biogeography of the Trachycarpeae (Arecaceae: Palmae) Botany & Mycology 2009 (Snowbird, Utah, July 25–29) Abstract Book. 2009:195. (abstract 697; also see http://2009.botanyconference.org/engine/search/index.php?func=detail&aid=157 . Accessed 15 November 2009) [Google Scholar]
- Baker WJ, Savolainen V, Asmussen-Lange CB, et al. Complete generic-level phylogenetic analyses of palms (Arecaceae) with comparisons of supertree and supermatrix approaches. Systematic Biology. 2009;58:240–256. doi: 10.1093/sysbio/syp021. [DOI] [PubMed] [Google Scholar]
- Baldwin BG. Phylogenetics of the California tarweeds and the Hawaiian silversword alliance (Madiinae: Heliantheae sensu lato) In: Hind DJN, Beentje HJ, editors. Compositae: Systematics. Proceedings of the International Compositae Conference, Kew, 1994. vol. 1. Kew: Royal Botanic Gardens; 1996. pp. 377–391. [Google Scholar]
- Baldwin BG. A phylogenetic perspective on the origin and evolution of Madiinae. In: Carlquist S, Baldwin BG, Carr GD, editors. Tarweeds & silverswords: Evolution of the Madiinae (Asteraceae) St. Louis, MO: Missouri Botanical Garden Press; 2003. pp. 193–228. [Google Scholar]
- Baldwin BG, Carr GD. Dubautia kalalauensis, a new species of the Hawaiian silversword alliance (Compositae, Madiinae) from northwestern Kaua‘i, U.S.A. Novon. 2005;15:259–263. [Google Scholar]
- Baldwin BG, Friar EA. Dubautia carrii and D. hanaulaensis, new species of the Hawaiian silversword alliance (Compositae, Madiinae) from Moloka‘i and Maui. Novon. 2010;20:1–8. [Google Scholar]
- Baldwin BG, Robichaux RH. Historical biogeography and ecology of the Hawaiian silversword alliance (Asteraceae): New molecular phylogenetic perspectives. In: Wagner WL, Funk VA, editors. Hawaiian biogeography: Evolution on a hot spot archipelago. Washington, DC: Smithsonian Institution Press; 1995. pp. 259–287. [Google Scholar]
- Baldwin BG, Sanderson MJ. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proceedings of the National Academy of Sciences of the USA. 1998;95:9402–9406. doi: 10.1073/pnas.95.16.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin BG, Kyhos DW, Dvorak J, Carr GD. Chloroplast DNA evidence for a North American origin of the Hawaiian silversword alliance (Asteraceae) Proceedings of the National Academy of Sciences of the USA. 1991;88:1840–1843. doi: 10.1073/pnas.88.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard HE, Jr, Sytsma KJ. Evolution and biogeography of the woody Hawaiian violets (Viola, Violaceae): Arctic origins, herbaceous ancestry and bird dispersal. Evolution. 2000;54:1521–1532. doi: 10.1111/j.0014-3820.2000.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Ballard HE, Wahlert GA, De Paula-Souza J, Feng M. Molecular phylogenetic relationships, intrafamilial groups, and a proposed classification for the Violet family (Violaceae) Botany & Mycology 2009 (Snowbird, Utah, July 25–29) Abstract Book. 2009:188. (abstract 669; also see http://2009.botanyconference.org/engine/search/index.php?func=detail&aid=660 . Accessed 15 November 2009) [Google Scholar]
- Barker NP, Weston PH, Rutschmann F, Sauquet H. Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partially congruent with the timing of the break-up of Gondwana. Journal of Biogeography. 2007;34:2012–2027. [Google Scholar]
- Barrier M, Baldwin BG, Robichaux RH, Purugganan MD. Interspecific hybrid ancestry of a plant adaptive radiation: Allopolyploidy of the Hawaiian silversword alliance (Asteraceae) inferred from floral homeotic gene duplications. Molecular Biology and Evolution. 1999;16:1105–1113. doi: 10.1093/oxfordjournals.molbev.a026200. [DOI] [PubMed] [Google Scholar]
- Bateman RM, James KE, Luo Y-B, et al. Molecular phylogenetics and morphological reappraisal of the Platanthera clade (Orchidaceae: Orchidinae) prompts expansion of the generic limits of Galearis and Platanthera. Annals of Botany. 2009;104:431–445. doi: 10.1093/aob/mcp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DM. Malvaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 868–903. [Google Scholar]
- Bentham G. Labiatarum, genera et species. London: Ridgway; 1832–1836. [Google Scholar]
- Bentham G. Notes on the classification, history, and geographical distribution of Compositae. Journal of the Linnean Society (Botany) London. 1873;13:335–557. [Google Scholar]
- Bradley U. Biogeography and speciation of the genus Peperomia Ruiz and Pavón in Eastern Polynesia. 2002 PhD thesis, University of Dublin, Trinity College, Dublin, Ireland. [Google Scholar]
- Briquet J. Labiatae. In: Engler A, Prantl K, editors. Die Natürlichen Pflanzenfamilien, IV 3a. Leipzig: Engelmann; 1895–1897. pp. 183–375. [Google Scholar]
- Brown FBH. Origin of the Hawaiian flora. Special Publications of Bernice P. Bishop Museum. 1921;7:131–142. [Google Scholar]
- Brown FBH. The secondary xylem of Hawaiian trees. Occasional Papers of Bernice P. Bishop Museum. 1922;8:217–371. [Google Scholar]
- Brown FBH, Brown EDW. A discussion of representative Pacific genera with evidence bearing on their origin and migration (Abstract) Proceedings of the Hawaiian Academy of Sciences. 1933;8:17–18. [Google Scholar]
- Bryan WA. Natural history of Hawaii: Being an account of the Hawaiian people, the geology and geography of the islands, and the native and introduced plants and animals of the group. Honolulu: Hawaiian Gazette Co; 1915. [Google Scholar]
- Bryan WA. Hawaiian fauna and flora. Special Publications of Bernice P. Bishop Museum. 1921;7:153–158. [Google Scholar]
- Campbell DH. The origin of the Hawaiian flora. Memoirs of the Torrey Botanical Club. 1918;17:90–96. [Google Scholar]
- Campbell DH. The derivation of the Hawaiian flora. Stanford, CA: Stanford University Press; 1919. [Google Scholar]
- Campbell DH. Some botanical and environmental aspects of Hawaii. Ecology. 1920;1:257–269. [Google Scholar]
- Campbell DH. An outline of plant geography. New York: Macmillan; 1926. [Google Scholar]
- Campbell DH. The Australasian element in the Hawaiian flora. American Journal of Botany. 1928;15:215–221. [Google Scholar]
- Campbell DH. Some problems of the Hawaiian flora (Abstract) Science. 1932;76:544. [Google Scholar]
- Campbell DH. The flora of the Hawaiian Islands. Quarterly Review of Biology. 1933;8:164–184. [Google Scholar]
- Carlquist S. Leaf anatomy and ontogeny in Argyroxiphium and Wilkesia (Compositae) American Journal of Botany. 1957;44:696–705. [Google Scholar]
- Carlquist S. Wood anatomy of Heliantheae. Tropical Woods. 1958a;108:1–30. [Google Scholar]
- Carlquist S. Structure and ontogeny of glandular trichomes of Madinae (Compositae) American Journal of Botany. 1958b;45:675–682. [Google Scholar]
- Carlquist S. Studies on Madinae: Anatomy, cytology, and evolutionary relationships. Aliso. 1959;4:171–236. [Google Scholar]
- Carlquist S. Island life: A natural history of islands of the world. Garden City, NY: Natural History Press; 1965. [Google Scholar]
- Carlquist S. The biota of long-distance dispersal, V. Plant dispersal to Pacific Islands. Bulletin of the Torrey Botanical Club. 1967;94:129–162. [Google Scholar]
- Carlquist S. Wood anatomy of Hawaiian, Macaronesian, and other species of Euphorbia. Botanical Journal of the Linnean Society. 1970;63:181–193. supplement 1. [Google Scholar]
- Carlquist S. Island biology. New York: Columbia University Press; 1974. [Google Scholar]
- Carlquist S. Hawaii, a natural history: Geology, climate, native flora and fauna above the shoreline. Honolulu: Pacific Tropical Botanical Garden; 1980. [Google Scholar]
- Carlquist S. Wood anatomy of Caryophyllaceae: Ecological, habital, systematic, and phylogenetic implications. Aliso. 1995;14:1–17. [Google Scholar]
- Carlquist S. Plant dispersal and the origin of Pacific island floras. In: Keast A, Miller SE, editors. The origin and evolution of Pacific Island biotas, New Guinea to Eastern Polynesia: Patterns and processes. Amsterdam: SPB Academic Publishing; 1996. pp. 153–164. [Google Scholar]
- Carr GD. Chromosome evolution and speciation in Hawaiian flowering plants. In: Stuessy TF, Ono M, editors. Evolution and speciation of island plants. Cambridge: Cambridge University Press; 1998. pp. 5–47. [Google Scholar]
- Carr GD, Powell EA, Kyhos DW. Self-incompatibility in the Hawaiian Madiinae (Compositae): An exception to Baker's Rule. Evolution. 1986;40:430–434. doi: 10.1111/j.1558-5646.1986.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Carr GD, Baldwin BG, Kyhos DW. Cytogenetic implications of artificial hybrids between the Hawaiian silversword alliance and North American tarweeds (Asteraceae: Heliantheae—Madiinae) American Journal of Botany. 1996;83:653–660. [Google Scholar]
- Catalano SA, Vilardi JC, Tosto D, Saidman BO. Molecular phylogeny and diversification history of Prosopis (Fabaceae: Mimosoideae) Biological Journal of the Linnean Society. 2008;93:621–640. [Google Scholar]
- Chiapella J. A molecular phylogenetic study of Deschampsia (Poaceae: Aveneae) inferred from nuclear ITS and plastid trnL sequence data: Support for the recognition of Avenella and Vahlodea. Taxon. 2007;56:55–64. [Google Scholar]
- Chowdhuri PK. Studies in the genus Silene. Notes from the Royal Botanic Garden, Edinburgh. 1957;22:221–278. [Google Scholar]
- Chumley TW, Keeley SC, Panero JL, Jansen RK. A phylogeny of Lipochaeta (Asteraceae) inferred from the internal and external transcribed spacers of nuclear ribosomal DNA. Botany 2002 (Madison, Wisconsin, August 2–4), abstracts. 2002 (see http://bsa2002.scientific-conference.net/section12/abstracts/229.shtml . Accessed 15 November 2009) [Google Scholar]
- Clague DA, Braga JC, Bassi D, Fullagar PD, Renema W, Webster JM. The maximum age of Hawaiian terrestrial lineages: Geological constraints from Kōko Seamount. Journal of Biogeography. 2010 (in press). doi: 10·1111/j.1365-2699·2009·02235.x. [Google Scholar]
- Clark JR, Wagner WL, Roalson EH. Patterns of diversification and ancestral range reconstruction in the southeast Asian-Pacific angiosperm lineage Cyrtandra (Gesneriaceae) Molecular Phylogenetics and Evolution. 2009;53:982–994. doi: 10.1016/j.ympev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Clement WL, Tebbitt MC, Forrest LL, et al. Phylogenetic position and biogeography of Hillebrandia sandwicensis (Begoniaceae): A rare Hawaiian relict. American Journal of Botany. 2004;91:905–917. doi: 10.3732/ajb.91.6.905. [DOI] [PubMed] [Google Scholar]
- Constance L, Affolter J. Apiaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 198–213. [Google Scholar]
- Cook LG, Crisp MD. Not so ancient: The extant crown group of Nothofagus represents a post-Gondwanan radiation. Proceedings of the Royal Society of London Series B–Biological Sciences. 2005;272:2535–2544. doi: 10.1098/rspb.2005.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland EB. The origin of the native flora of Polynesia. Pacific Science. 1948;2:293–296. [Google Scholar]
- Costello A, Motley TJ. Phylogenetics of the Tetraplasandra group (Araliaceae) inferred from ITS, 5S-NTS, and morphology. Systematic Botany. 2007;32:464–477. [Google Scholar]
- Crawford DJ, Stuessy TF. Plant speciation on oceanic islands. In: Iwatsuki K, Raven PH, editors. Evolution and diversification of land plants. Tokyo: Springer; 1997. pp. 249–267. [Google Scholar]
- Crawford DJ, Lowrey TK, Anderson GJ, Barnardello G, Santos-Guerra A, Stuessy TF. Genetic diversity in Asteraceae endemic to oceanic islands: Baker's Law and polyploidy. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, editors. Systematics, evolution, and biogeography of Compositae. Vienna: International Association for Plant Taxonomy; 2009. pp. 139–151. [Google Scholar]
- Cronk QCB, Kiehn M, Wagner WL, Smith JF. Evolution of Cyrtandra (Gesneriaceae) in the Pacific Ocean: The origin of a supertramp clade. American Journal of Botany. 2005;92:1017–1024. doi: 10.3732/ajb.92.6.1017. [DOI] [PubMed] [Google Scholar]
- Cronn RC, Zhao X, Paterson AH, Wendel JF. Polymorphism and concerted evolution in a tandemly repeated gene family: 5S ribosomal DNA in diploid and allopolyploid cottons. Journal of Molecular Evolution. 1996;42:685–705. doi: 10.1007/BF02338802. [DOI] [PubMed] [Google Scholar]
- Cronn RC, Small RL, Haselkorn T, Wendel JF. Rapid diversification of the cotton genus (Gossypium: Malvaceae) revealed by analysis of sixteen nuclear and chloroplast genes. American Journal of Botany. 2002;89:707–725. doi: 10.3732/ajb.89.4.707. [DOI] [PubMed] [Google Scholar]
- Cuénoud M, Del Pero Martinez MA, Loizeau P-A, Spichiger R, Andrews S, Manen J-F. Molecular phylogeny and biogeography of the genus Ilex L. (Aquifoliaceae) Annals of Botany. 2000;85:111–122. [Google Scholar]
- Degener O, Croizat L. In: Chamaesyce. Degener O, editor. 1936. Flora Hawaiiensis. Privately published. [Google Scholar]
- DeJoode DR, Wendel JF. Genetic diversity and origin of the Hawaiian Islands cotton, Gossypium tomentosum. American Journal of Botany. 1992;79:1311–1319. [Google Scholar]
- Dick CW, Bermingham E, Lemes MR, Gribel R. Extreme long-distance dispersal of the lowland tropical rainforest tree Ceiba pentandra L. (Malvaceae) in Africa and the Neotropics. Molecular Ecology. 2007;16:3039–3049. doi: 10.1111/j.1365-294X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- Donoghue MJ, Moore BR. Toward an integrative historical biogeography. Integrative and Comparative Biology. 2003;43:261–270. doi: 10.1093/icb/43.2.261. [DOI] [PubMed] [Google Scholar]
- Dragon JA, Barrington DS. The systematics of the Carex aquatilis and Carex lenticularis lineages: Geographically and ecologically divergent sister clades of Carex section Phacocystis (Cyperaceae) American Journal of Botany. 2009;96:1896–1906. doi: 10.3732/ajb.0800404. [DOI] [PubMed] [Google Scholar]
- Dunbar-Co S, Wieczorek AM, Morden CW. Molecular phylogeny and adaptive radiation of the endemic Hawaiian Plantago species (Plantaginaceae) American Journal of Botany. 2008;95:1177–1188. doi: 10.3732/ajb.0800132. [DOI] [PubMed] [Google Scholar]
- Duncan T. Ranunculaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 1087–1090. [Google Scholar]
- Eggens F, Popp M, Nepokroeff M, Wagner WL, Oxelman B. The origin and number of introductions of the Hawaiian endemic Silene species (Caryophyllaceae) American Journal of Botany. 2007;94:210–218. doi: 10.3732/ajb.94.2.210. [DOI] [PubMed] [Google Scholar]
- Engler A. Versuch einer Entwicklungsgeschichte der Pflanzenwelt insbesondere der Florengebiete seit der Tertiärperiode. Leipzig: Wilhelm Engelmann; 1879–1882. [Google Scholar]
- Feng M. Floral morphogenesis and molecular systematics of the family Violaceae. 2005 PhD thesis, Ohio University, Athens, Ohio, USA. [Google Scholar]
- Fosberg FR. Derivation of the flora of the Hawaiian Islands. In: Zimmerman EC, editor. Insects of Hawaii. vol. 1. Honolulu: University of Hawaii Press; 1948. pp. 107–119. [Google Scholar]
- Fosberg FR. The American element in the Hawaiian flora. Pacific Science. 1951;5:204–206. [Google Scholar]
- Froebe HA. Wuchsform und infloreszenzgestaltung in den gattungen Sanicula, Hacquetia und Astrantia (Umbelliferae) Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1971;91:1–38. [Google Scholar]
- Funk VA, Wagner WL. Biogeographic patterns in the Hawaiian Islands. In: Wagner WL, Funk VA, editors. Hawaiian biogeography: Evolution on a hot spot archipelago. Washington, DC: Smithsonian Institution Press; 1995. pp. 379–419. [Google Scholar]
- Ganders FR, Nagata KM. Relationships and floral biology of Bidens cosmoides (Asteraceae) Lyonia. 1983;2:23–31. [Google Scholar]
- Ganders FR, Nagata KM. The role of hybridization in the evolution of Bidens on the Hawaiian Islands. In: Grant WF, editor. Biosystematics. Ontario: Academic Press Canada; 1984. pp. 179–194. [Google Scholar]
- Ganders FR, Berbee M, Pirseyedi M. ITS base sequence phylogeny in Bidens (Asteraceae): Evidence for the continental relatives of Hawaiian and Marquesan Bidens. Systematic Botany. 2000;25:122–133. [Google Scholar]
- Geesink R, Wagner WL, Herbst DR. Fabaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 629–721. [Google Scholar]
- Gemmill CEC, Allan GJ, Wagner WL, Zimmer EA. Evolution of insular Pacific Pittosporum (Pittosporaceae): Origin of the Hawaiian radiation. Molecular Phylogenetics and Evolution. 2002;22:31–42. doi: 10.1006/mpev.2001.1019. [DOI] [PubMed] [Google Scholar]
- Gillespie RG, Baldwin BG. Island biogeography of remote archipelagoes: Interplay between ecological and evolutionary processes. In: Losos JB, Ricklefs RE, editors. The theory of island biogeography revisited. Princeton, NJ: Princeton University Press; 2009. pp. 358–387. [Google Scholar]
- Gillett GW. Genetic affinities between Hawaiian and Marquesan Bidens (Asteraceae) Taxon. 1972;21:479–483. [Google Scholar]
- Gillett GW. Genetic relationships between Bidens forbesii and six species in the Hawaiian and the Marquesas islands. Brittonia. 1973;25:10–14. [Google Scholar]
- Givnish TJ, Renner SS. Tropical intercontinental disjunctions: Gondwana breakup, immigration from the boreotropics, and transoceanic dispersal. International Journal of Plant Sciences. 2004;165:S1–S6. [Google Scholar]
- Givnish TJ, Millam KC, Mast AR, et al. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae) Proceedings of the Royal Society of London Series B–Biological Sciences. 2009;276:407–416. doi: 10.1098/rspb.2008.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt P, Henrich JE. Iridaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 1444–1449. [Google Scholar]
- Gray A. Quarterly meeting, August 8, 1849. Proceedings of the American Academy of Arts and Sciences. 1852;2:159–160. [Google Scholar]
- Gray A. Characters of some new plants of California and Nevada, chiefly from the collections of Professor William H. Brewer, botanist of the State Geological Survey of California, and of Dr. Charles L. Anderson, with revisions of certain genera or groups. Proceedings of the American Academy of Arts and Sciences. 1865;6:519–556. [Google Scholar]
- Gray A. Botanical contributions. Proceedings of the American Academy of Arts and Sciences. 1870;9:187–218. [Google Scholar]
- Groeninckx I, Dessein S, Ochoterena H, et al. Phylogeny of the herbaceous tribe Spermacoceae (Rubiaceae) based on plastid DNA data. Annals of the Missouri Botanical Garden. 2009;96:109–132. [Google Scholar]
- Guppy HB. Observations of a naturalist in the Pacific between 1896 and 1899, vol. 2: Plant dispersal. London: Macmillan; 1906. [Google Scholar]
- Hao G, Yuan Y-M, Hu C-M, Ge X-J, Zhao N-X. Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast trnL-F and nuclear ITS sequences. Molecular Phylogenetics and Evolution. 2004;31:323–339. doi: 10.1016/S1055-7903(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Harbaugh DT, Baldwin BG. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): Repeated dispersals throughout the Pacific. American Journal of Botany. 2007;94:1028–1040. doi: 10.3732/ajb.94.6.1028. [DOI] [PubMed] [Google Scholar]
- Harbaugh D, Wagner W, Allan G, Zimmer E. The Hawaiian archipelago is a stepping-stone for dispersal in the Pacific: An example from the plant genus Melicope (Rutaceae) Journal of Biogeography. 2009;36:230–241. [Google Scholar]
- Harbaugh DT, Nepokroeff M, Rabeler RK, McNeill J, Zimmer EA, Wagner WL. A new lineage-based tribal classification of the family Caryophyllaceae. International Journal of Plant Science. 2010;171:185–198. [Google Scholar]
- Helenurm K, Ganders FR. Adaptive radiation and genetic differentiation in Hawaiian Bidens. Evolution. 1985;39:753–765. doi: 10.1111/j.1558-5646.1985.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Hillebrand W. Flora of the Hawaiian Islands: A description of their phanerogams and vascular cryptogams. Heidelberg: Carl Winter; 1888a. London: Williams & Norgate; New York: B. Westermann & Co. [Google Scholar]
- Hillebrand W. Die vegetationsformationen der Sandwich-Inseln. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1888b;9:305–314. [Google Scholar]
- Howarth DG, Gardner DE, Morden CW. Phylogeny of Rubus subgenus Idaeobatus (Rosaceae) and its implications toward colonization of the Hawaiian Islands. Systematic Botany. 1997;22:433–441. [Google Scholar]
- Howarth DG, Gustafsson MHG, Baum DA, Motley TJ. Phylogenetics of the genus Scaevola (Goodeniaceae): Implications for dispersal patterns across the Pacific Basin and colonization of the Hawaiian Islands. American Journal of Botany. 2003;90:915–923. doi: 10.3732/ajb.90.6.915. [DOI] [PubMed] [Google Scholar]
- Hsiao C, Chatterton NJ, Asay KH, Jensen KB. Molecular phylogeny of the Pooideae (Poaceae) based on nuclear rDNA (ITS) sequences. Theoretical and Applied Genetics. 1995;90:389–398. doi: 10.1007/BF00221981. [DOI] [PubMed] [Google Scholar]
- Hughes C, Eastwood R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the USA. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MC. Revision of Colubrina (Rhamnaceae) Brittonia. 1971;23:2–53. [Google Scholar]
- Johnstone DW, McFarlane RW. Migration and bioenergetics of flight in the Pacific golden plover. The Condor. 1967;69:156–168. [Google Scholar]
- Keck DD. The Hawaiian silverswords: Systematics, affinities, and phytogeographic problems of the genus Argyroxiphium. Bernice P. Bishop Museum Occasional Papers. 1936a;11:3–38. [Google Scholar]
- Keck DD. The silverswords of Hawaii. Carnegie Institution of Washington News Service Bulletin (School Edition) 1936b;4:75–78. [Google Scholar]
- Keppel G, Lowe AJ, Possingham HP. Changing perspectives on the biogeography of the tropical South Pacific: Influences of dispersal, vicariance and extinction. Journal of Biogeography. 2009;36:1035–1054. [Google Scholar]
- Kim H-G, Keeley SC, Vroom PS, Jansen RK. Molecular evidence for an African origin of the Hawaiian endemic Hesperomannia (Asteraceae) Proceedings of the National Academy of Sciences of the USA. 1998;95:15440–15445. doi: 10.1073/pnas.95.26.15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutnik DL. A taxonomic revision of the Hawaiian species of the genus Chamaesyce (Euphorbiaceae) Allertonia. 1987;6:331–388. [Google Scholar]
- Koutnik DL. Chamaesyce S. F. Gray. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press, 602–617; 1999. [Google Scholar]
- Koyama T. Cyperaceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 1381–1436. [Google Scholar]
- Lassetter JS, Gunn CR. Vicia menziesii Sprengel (Fabaceae) rediscovered: Its taxonomic relationships. Pacific Science. 1979;33:85–101. [Google Scholar]
- Lindqvist C, Albert VA. Origin of the Hawaiian endemic mints within North American Stachys (Lamiaceae) American Journal of Botany. 2002;89:1709–1724. doi: 10.3732/ajb.89.10.1709. [DOI] [PubMed] [Google Scholar]
- Lindqvist C, Motley TJ, Jeffrey JJ, Albert VA. Cladogenesis and reticulation in the Hawaiian endemic mints (Lamiaceae) Cladistics. 2003;19:480–495. doi: 10.1111/j.1096-0031.2003.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Lorence DH, Wood KR. Kanaloa, a new genus of Fabaceae (Mimosoideae) from Hawaii. Novon. 1994;4:137–145. [Google Scholar]
- Lorence DH, Wagner WL, Laidlaw WG. Kadua haupuensis (Rubiaceae: Spermacoceae), a new endemic species from Kaua‘i, Hawaiian Islands. Brittonia. 2010 in press. [Google Scholar]
- Lowrey TK, Quinn CJ, Taylor RK, Chan R, Kimball RT, De Nardi JC. Molecular and morphological reassessment of relationships within the Vittadinia group of Astereae (Asteraceae) American Journal of Botany. 2001;88:1279–1289. [PubMed] [Google Scholar]
- Lowrey TK, Whitkus R, Sykes WR. A new species of Tetramolopium (Asteraceae) from Mitiaro, Cook Islands: Biogeography, phylogenetic relationships, and dispersal. Systematic Botany. 2005;30:448–455. [Google Scholar]
- Mann H. Statistics and geographical range of Hawaiian (Sandwich Islands) plants. Journal of Botany, British and Foreign. 1869;12:171–183. [Google Scholar]
- Marticorena A. Revisión del género Acaena (Rosaceae) en Chile. Annals of the Missouri Botanical Garden. 2006;93:412–454. [Google Scholar]
- Melville R. Vicarious plant distributions and paleogeography of the Pacific region. In: Nelson G, Rosen DE, editors. Vicariance biogeography: A critique. New York: Columbia University Press; 1981. pp. 238–274. [Google Scholar]
- Miller JS. Phylogenetic relationships and the evolution of gender dimorphism in Lycium (Solanaceae) Systematic Botany. 2002;27:416–428. [Google Scholar]
- Miller JS, Levin RA, Feliciano NM. A tale of two continents: Baker's Rule and the maintenance of self-incompatibility in Lycium (Solanaceae) Evolution. 2008;62:1052–1065. doi: 10.1111/j.1558-5646.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Tye A, Jansen RK. Patterns of long-distance dispersal in Tiquilia subg. Tiquilia (Boraginaceae): Implications for the origins of amphitropical disjuncts and Galápagos Islands endemics. American Journal of Botany. 2006;93:1163–1177. doi: 10.3732/ajb.93.8.1163. [DOI] [PubMed] [Google Scholar]
- Morden CW, Gregoritza M. Population variation and phylogeny in the endangered Chamaesyce skottsbergii (Euphorbiaceae) based on RAPD and ITS analyses. Conservation Genetics. 2005;6:969–979. [Google Scholar]
- Morden CW, Gardner DE, Weniger DA. Phylogeny and biogeography of Pacific Rubus subgenus Idaeobatus (Rosaceae) species: Investigating the origin of the endemic Hawaiian raspberry R. macraei. Pacific Science. 2003;57:181–197. [Google Scholar]
- Mummenhoff K, Brüggemann H, Bowman JL. Chloroplast DNA phylogeny and biogeography of Lepidium (Brassicaceae) American Journal of Botany. 2001;88:2051–2063. [PubMed] [Google Scholar]
- Namoff S, Luke Q, Jiménez F, et al. Phylogenetic analyses of nucleotide sequences confirm a unique plant intercontinental disjunction between tropical Africa, the Caribbean, and the Hawaiian Islands. Journal of Plant Research. 2010;123:57–65. doi: 10.1007/s10265-009-0258-0. [DOI] [PubMed] [Google Scholar]
- Neill D. In: Erythrina. Wagner WL, Herbst DR, Sohmer SH, editors. Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 671–672. Manual of the flowering plants of Hawai‘i (revised edition). [Google Scholar]
- Nepokroeff M, Sytsma KJ, Wagner WL, Zimmer EA. Reconstructing ancestral patterns of colonization and dispersal in the Hawaiian understory tree genus Psychotria (Rubiaceae): A comparison of parsimony and likelihood approaches. Systematic Biology. 2003;52:820–838. [PubMed] [Google Scholar]
- O'Connor PJ. In: Calamagrostis. Wagner WL, Herbst DR, Sohmer SH, editors. Honolulu, HI: University of Hawaii Press and Bishop Museum Press; 1999. pp. 1508–1511. Manual of the flowering plants of Hawai‘i (revised edition). [Google Scholar]
- Ocampo G, Columbus JT. Systematics of Portulaca (Portulacaceae) Botany & Mycology 2009 (Snowbird, Utah, July 25–29) Abstract Book. 2009a:191. (abstract 680; also see http://2009.botanyconference.org/engine/search/index.php?func=detail&aid=381 . Accessed 15 November 2009) [Google Scholar]
- Ocampo G, Columbus JT. Phylogenetic relationships within Portulaca sect. Portulaca (Portulacaceae) Botany & Mycology 2009 (Snowbird, Utah, July 25–29) Abstract Book. 2009b:206. (abstract 738; also seehttp://2009.botanyconference.org/engine/search/index.php?func=detail&aid=385 . Accessed 15 November 2009) [Google Scholar]
- Olmstead RG, Bohs L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. A molecular phylogeny of the Solanaceae. Taxon. 2008;57:1159–1181. [Google Scholar]
- Ownbey GB. The genus Argemone in South America and Hawaii. Brittonia. 1961;13:91–109. [Google Scholar]
- Pax DL, Price RA, Michaels HJ. Phylogenetic position of the Hawaiian geraniums based on rbcL sequences. American Journal of Botany. 1997;84:72–78. [Google Scholar]
- Pearcy RW, Fransceschi VR. Photosynthetic characteristics and chloroplast ultrastructure of C3 and C4 tree species grown in high- and low-light environments. Photosynthetic Research. 1986;9:317–331. doi: 10.1007/BF00029797. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Osteryoung K, Randall D. Carbon dioxide exchange characteristics of C4 Hawaiian Euphorbia species native to diverse habitats. Oecologia (Berlin) 1982;55:333–341. doi: 10.1007/BF00376921. [DOI] [PubMed] [Google Scholar]
- Percy DM, Garver AM, Wagner WL, et al. Progressive island colonization and ancient origin of Hawaiian Metrosideros (Myrtaceae) Proceedings of the Royal Society, Biological Sciences, Series B. 2008;275:1479–1490. doi: 10.1098/rspb.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsbry HA. Mid-Pacific land snail faunas. Proceedings of the National Academy of Sciences of the USA. 1916;2:429–433. [Google Scholar]
- Powell EA, Kron KA. Hawaiian blueberries and their relatives—A phylogenetic analysis of Vaccinium sections Macropelma, Myrtillus, and Hemimyrtillus (Ericaceae) Systematic Botany. 2002;27:768–779. [Google Scholar]
- Price JP, Clague DA. How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proceedings of the Royal Society, Biological Sciences, Series B. 2002;269:2429–2435. doi: 10.1098/rspb.2002.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JP, Wagner WL. Speciation in Hawaiian angiosperm lineages: Cause, consequence, and mode. Evolution. 2004;58:2185–2200. doi: 10.1111/j.0014-3820.2004.tb01597.x. [DOI] [PubMed] [Google Scholar]
- de Queiroz A. The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology and Evolution. 2005;20:68–73. doi: 10.1016/j.tree.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Raz L, Albert VA, Motley TJ. Molecular systematics of Hawaiian Chamaesyce (Euphorbiaceae) American Journal of Botany. 1998;85:153–154. (supplement, abstracts) [Google Scholar]
- Ree RH, Moore BR, Webb CO, Donoghue MJ. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution. 2005;59:2299–2311. [PubMed] [Google Scholar]
- Reznicek AA Flora of North America Editorial Committee. Flora of North America north of Mexico, vol. 23 (Magnoliophyta: Commelinidae (in part): Cyperaceae. New York: Oxford University Press; 2002. Carex Linnaeus sect. Stellulatae Kunth; pp. 326–331. [Google Scholar]
- Ridley HN. The dispersal of plants throughout the world. Ashford: L. Reeve & Co; 1930. [Google Scholar]
- Rivadavia F, Kondo K, Kato M, Hasebe M. Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. American Journal of Botany. 2003;90:123–130. doi: 10.3732/ajb.90.1.123. [DOI] [PubMed] [Google Scholar]
- Roalson EH. Phylogenetic relationships in the Juncaceae inferred from nuclear ribosomal DNA internal transcribed spacer sequence data. International Journal of Plant Sciences. 2005;166:397–413. [Google Scholar]
- Robertson KR. Jacquemontia ovalifolia (Convolvulaceae) in Africa, North America, and the Hawaiian Islands. Annals of the Missouri Botanical Garden. 1974;61:502–513. [Google Scholar]
- Robichaux RH, Pearcy RW. Environmental characteristics, field water relations, and photosynthetic responses of C4 Hawaiian Euphorbia species from contrasting habitats. Oecologia (Berlin) 1980;47:99–105. doi: 10.1007/BF00541782. [DOI] [PubMed] [Google Scholar]
- Rock JF. The genus Plantago in Hawaii. American Journal of Botany. 1920;7:195–210. [Google Scholar]
- Ryding O. Pericarp structure in the tribe Prasieae (Lamiaceae–Lamioideae) and its systematic implications. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1994;116:391–399. [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. Origins of dioecy in the Hawaiian flora. Ecology. 1995;76:2517–2529. [Google Scholar]
- Sakai AK, Weller SG, Wagner WL, Nepokroeff M, Culley TM. Adaptive radiation and evolution of breeding systems in Schiedea (Caryophyllaceae), an endemic Hawaiian genus. Annals of the Missouri Botanical Garden. 2006;93:49–63. [Google Scholar]
- Sanmartín I, Ronquist F. Southern Hemisphere biogeography inferred by event-based models: Plant versus animal patterns. Systematic Biology. 2004;53:216–243. doi: 10.1080/10635150490423430. [DOI] [PubMed] [Google Scholar]
- Sanmartín I, van der Mark P, Ronquist F. Inferring dispersal: A Baysian approach to phylogeny-based island biogeography, with special reference to the Canary Islands. Journal of Biogeography. 2008;35:428–449. [Google Scholar]
- Schluter D. The ecology of adaptive radiation. Oxford: Oxford University Press; 2000. [Google Scholar]
- Schwarzbach AE, Kadereit JW. Phylogeny of prickly poppies, Argemone (Papaveraceae), and the evolution of morphological and alkaloid characters based on ITS nrDNA sequence variation. Plant Systematics and Evolution. 1999;218:257–279. [Google Scholar]
- Sebastian PM, Lira R, Cross HB, Telford IRH, Motley TJ, Renner SS. Biogeography of the Sicyos clade (Cucurbitaceae) — from the New World to Australia, New Zealand, and Hawaii. Botany & Mycology 2009 (Snowbird, Utah, July 25–29) Abstract Book. 2009:221. (abstract 792; also see http://2009.botanyconference.org/engine/search/index.php?func=detail&aid=185 . Accessed 15 November 2009) [Google Scholar]
- Seelanan T, Schnabel A, Wendel JF. Congruence and consensus in the cotton tribe. Systematic Botany. 1997;22:259–290. [Google Scholar]
- Setchell WA. Pacific insular floras and Pacific paleogeography. American Naturalist. 1935;69:289–310. [Google Scholar]
- Skottsberg C. Juan Fernandez and Hawaii. Bernice P. Bishop Museum Bulletin. 1925;16:1–47. [Google Scholar]
- Skottsberg C. Remarks on the flora of the high Hawaiian volcanoes. Acta Horti Gothoburgensis. 1931;6:47–65. [Google Scholar]
- Skottsberg C. Remarks on the Hawaiian flora. Proceedings of the Linnean Society of London. 1939;151:181–186. [Google Scholar]
- Skottsberg C. Observations on Hawaiian violets. Meddelanden från Göteborgs Botaniska Trädgård. 1940;13:451–528. [Google Scholar]
- Skottsberg C. The flora of the Hawaiian Islands and the history of the Pacific Basin. Proceedings of the 6th Pacific Science Congress, California. 1941;4:685–701. [Google Scholar]
- Small RL, Ryburn JA, Cronn RC, Seelanan T, Wendel JF. The tortoise and the hare: Choosing between noncoding plastome and nuclear Adh sequences for phylogeny reconstruction in a recently diverged plant group. American Journal of Botany. 1998;85:1301–1315. [PubMed] [Google Scholar]
- St. John H. The subgenera of Dubautia (Compositae): Hawaiian plant studies 18. Pacific Science. 1950;4:339–345. [Google Scholar]
- St. John H. Revision of the Hawaiian species of Viola (Violaceae). Hawaiian plant studies no. 135. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1989;111:165–204. [Google Scholar]
- van Steenis CGGJ. Transpacific floristic affinities, particularly in the tropical zone. In: Gressitt JL, editor. Pacific Basin biogeography: A symposium. Honolulu: Bishop Museum Press; 1963. pp. 219–231. [Google Scholar]
- Stefanovic S, Costea M. Reticulate evolution in the parasitic genus Cuscuta (Convolvulaceae): Over and over again. Botany. 2008;86:791–808. [Google Scholar]
- Symon DE. Gondwanan elements of the Solanaceae. In: Hawkes JG, Nee M, Lester RN, Estrada N, editors. Solanceae III: Taxonomy, chemistry, and evolution. Kew: Royal Botanic Gardens; 1991. pp. 139–150. [Google Scholar]
- Thellung A. Die Gattung Lepidium (L.) R. Br. Eine Monographische Studie. Neue Denkschriften der Schweizerischen Naturforschenden Gesellschaft. 1906;41:1–304. [Google Scholar]
- Tokuoka T. Molecular phylogenetic analysis of Violaceae (Malpighiales) based on plastid and nuclear DNA sequences. Journal of Plant Research. 2008;121:253–260. doi: 10.1007/s10265-008-0153-0. [DOI] [PubMed] [Google Scholar]
- Vargas P, Baldwin BG, Constance L. Nuclear ribosomal DNA evidence for a western North American origin of Hawaiian and South American species of Sanicula (Apiaceae) Proceedings of the National Academy of Sciences of the USA. 1998;95:235–240. doi: 10.1073/pnas.95.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner WL, Robinson H. Lipochaeta and Melanthera (Asteraceae: Heliantheae subtribe Ecliptinae): Establishing their natural limits and a synopsis. Brittonia. 2001;53:539–561. [Google Scholar]
- Wagner WL, Herbst DR, Sohmer SH. Manual of the flowering plants of Hawai‘i. Honolulu: University of Hawaii Press and Bishop Museum Press; 1990. [Google Scholar]
- Wagner WL, Herbst DR, Sohmer SH. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. [Google Scholar]
- Wagner WL, Herbst DR, Lorence DH. Flora of the Hawaiian Islands website. 2005a http://botany.si.edu/pacificislandbiodiversity/hawaiianflora/ . Accessed 15 November 2009. [Google Scholar]
- Wagner WL, Weller SG, Sakai A. Monograph of Schiedea (Caryophyllaceae-Alsinoideae) Systematic Botany Monographs. 2005b;72:1–169. [Google Scholar]
- Wallace AR. Island Life: or, The phenomena and causes of insular faunas and floras, including a revision and attempted solution of the problem of geological climates. New York: Harper; 1881. [Google Scholar]
- Wallace AR. My life: A record of events and opinions. London: Chatman & Hall ld; 1905. [Google Scholar]
- Wanke S, Samain M-S, Vanderschaeve L, Mathieu G, Goetghebeur P, Neinhuis C. Phylogeny of the genus Peperomia (Piperaceae) inferred from the trnK/matK region (cpDNA) Plant Biology. 2006;8:93–102. doi: 10.1055/s-2005-873060. [DOI] [PubMed] [Google Scholar]
- Wanntorp L, Wanntorp H-E. The biogeography of Gunnera L.: Vicariance and dispersal. Journal of Biogeography. 2003;30:979–987. [Google Scholar]
- Wanntorp L, Wanntorp H-E, Källersjö M. Phylogenetic relationships of Gunnera based on nuclear ribosomal DNA ITS region, rbcL and rps16 intron sequences. Systematic Botany. 2002;27:512–521. [Google Scholar]
- Wawra H. Beiträge zur Flora der Hawai'schen Inseln. Flora. 1872–1875;Vols. 55–58 [Google Scholar]
- van der Werff H. Lauraceae. In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawai‘i (revised edition). Honolulu: University of Hawaii Press and Bishop Museum Press; 1999. pp. 843–848. [Google Scholar]
- Wendel JF, Schnabel A, Seelanan T. Bidirectional interlocus concerted evolution following allopolyploid speciation in cotton (Gossypium) Proceedings of the National Academy of Sciences of the USA. 1995;92:208–284. doi: 10.1073/pnas.92.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot-Dear CM. A revision of Mucuna (Leguminosae: Phaseoleae) in the Pacific. Kew Bulletin. 1990;45:1–35. [Google Scholar]
- Wright SD, Yong CG, Wichman SR, Dawson JW, Gardener RC. Stepping stones to Hawaii: A trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS) Journal of Biogeography. 2001;28:769–774. [Google Scholar]
- Won H, Renner SS. Dating dispersal and radiation in the gymnosperm Gnetum (Gnetales)—Clock calibration when outgroup relationships are uncertain. Systematic Biology. 2006;55:610–622. doi: 10.1080/10635150600812619. [DOI] [PubMed] [Google Scholar]
- Wood KR, Oppenheimer H. Stenogyne kauaulaensis (Lamiaceae), a new species from Maui, Hawaiian Islands. Novon. 2008;18:544–549. [Google Scholar]
- Wunderlich R. Ein Vorschlag zu einer natürlichen Gliederung der Labiaten auf Grund der Pollenkörner, der Samenentwicklung und des reifen Samens. Oesterreichische Botanische Zeitschrift. 1967;114:383–483. [Google Scholar]
- Yang Y, Morden CW, Sack L, Sporck MJ, Berry PE. Phylogeny and adaptive radiation of woody Hawaiian Chamaesyce from herbaceous and annual ancestors in subtropical North America (Euphorbia-Euphorbiaceae) Botany & Mycology 2009 (Snowbird, Utah, July 25–29) Abstract Book. 2009:182. (abstract 649; also see http://2009.botanyconference.org/engine/search/index.php?func=detail&aid=861 . Accessed 15 November 2009) [Google Scholar]