Abstract
Background and Aims
Spring geophytes require a period of low temperature for proper flower development but the mechanism that underlies the relationship between cold treatment and flowering remains unknown. The present study aims to compare the developmental anatomy and carbohydrate content of the tuberous geophyte Corydalis bracteata growing under natural winter conditions from 10 to −10 °C (field-grown) and under a mild temperature regime of 18 °C (indoor-grown plants).
Methods
Samples were studied under light and electron microscopy. A histochemical test (periodic acid – Schiff's) was employed to identify starch in sectioned material. Sugars were analysed by capillary gas chromatography. Apoplastic wash fluid was prepared.
Key Results
Under natural conditions, shoots were elongated, and buds gained in dry mass and developed normally. For indoor-grown plants, these parameters were lower in value and, from December, a progressive necrosis of flower buds was observed. The tuber consisted of the new developing one, which was connected to the bud, and the old tuber with its starch reserve. Due to the absence of plasmodesmata between new and old tuber cells, sugar transport cannot be through the symplast. Thus, a potential apoplastic route is proposed from old tuber phloem parenchyma cells to the adjacent new tuber cells. Sugar content in buds during the autumn months (September–November) was lower for indoor-grown plants than control plants, whereas the sugar content in tubers during the same period was similar for plants from both temperature treatments. However, the amount of apoplastic sugars in tubers of field-grown plants was almost 15-fold higher than in indoor-grown tubers.
Conclusions
The results suggest that low temperature activates the apoplastic route of sugar transport in C. bracteata tubers and a consequent carbohydrate delivery to the bud. In the absence of cold treatment, the carbohydrate reserve is locked in old tuber cells so the nutrient supply to the buds is suppressed, possibly leading to bud abortion.
Keywords: Corydalis bracteata, geophytes, temperature treatment, bud abortion, anther, apoplast, TEM
INTRODUCTION
Ornamental geophytes are characterized by a particular rhythm of development which depends on a wide range of climatic conditions. Plants that exhibit active growth and flowering in spring generally lose their aerial parts as summer begins and enter dormancy without visible organogenesis. Their growth resumes in autumn, when the development of a flower bud begins. The flowering process involves three major stages: (1) initiation of floral meristem and differentiation of floral parts; (2) slow growth and maturation of the future flower; and (3) rapid shoot elongation and anthesis. In a temperate climate, these three stages proceed in the summer, winter and spring periods, respectively, and are known to be controlled by numerous environmental and internal factors.
For most of the year, ephemeroids are represented only by a specialized underground organ, exhibiting a reserve function and supporting the future plant development. It is likely that the initial response to a temperature regime occurs within this underground organ. These tubers, corms and bulbs have a great capacity to store and remobilize reserve metabolites, particularly carbohydrates, which all contribute to plant development. In the absence of photosynthesis during a long period of the year, the regulation and control of the metabolism of these constituents are essential for the survival of plants.
Temperature is considered to be one of the most important factors affecting geophyte growth and development and, for the majority of them, a ‘warm–cold–warm’ sequence is required to complete the life cycle. Different genera and species demand various temperature optima but, in general, the optimal temperature for the initial organogenesis ranges from 15 to 21 °С, while low positive temperature is required for final flower formation (De Hertogh and Le Nard, 1993; Le Nard and De Hertogh, 1993). Numerous studies have shown that the effects of the temperature surrounding the underground organs during storage can lead to important developmental modifications, particularly in flowering. For example, storage at a higher positive temperature (+17 − 19 °C) slows shoot elongation and leaf growth and can also cause the necrosis of flower buds (Lambrechts et al., 1994; Rebers et al., 1995; Van der Toorn et al., 2000; Kamenetsky et al., 2003).
At the heart of this phenomenon is the arrest of bud development, the physiological mechanism of which remains unknown. It has been suggested that bud abortion is a result of a disorder in the plant hormonal status (Aung and De Hertogh, 1979; Rebers et al., 1995; Rietveld et al., 2000) or in water changes during the storage period (Zemah et al., 1999; Van der Toorn et al., 2000; Van Kilsdonk et al., 2002). In addition to water balance, the entry of physiologically active substances into a bud, particularly carbohydrates, and the activity of enzymes involved in their metabolism are also temperature-dependent (Gorin and Heidema, 1985; Lambrechts et al., 1994; Kamenetsky et al., 2003). In the absence of low temperature, the resulting blockage of carbohydrate flow from the underground organ (or from the stem in the case of trees) toward a bud is also expected to lead to bud abortion (Gorin and Heidema, 1985; Lambrechts et al., 1994; Bonhomme et al., 2005). However, none of the studied parameters correlates significantly with the completion of chilling and flowering.
Despite the fact that carbohydrates are the most intensively studied class of metabolites in flower geophytes, knowledge of their distribution remains rather limited (Miller, 1992). Previous research has mainly used bulbs stored under dry conditions at different temperatures and aimed at studying starch and soluble sugar contents along with the activity of α-amylase, invertase and sucrose-synthase (e.g. Gorin and Heidema, 1985; Balk and De Boer, 1999; Kamenetsky et al., 2003). Of particular interest is the carbohydrate status of bulbs during the first stages of growth after planting (Lambrechts et al., 1994) and during their in vitro development (Taeb and Alderson, 1990; Vishnevetsky et al., 2000). It has been shown that different plant tissues react in different ways toward storage conditions. For tulip bulbs, starch hydrolysis was not influenced by low temperature treatment (Van der Toorn et al., 2000) whereas in anthers, α-amylase activity was higher in plants kept away from low temperature (Gorin and Heidema, 1985). Moreover, different species and even cultivars demonstrated varied reactions to temperature treatment (De Hertogh and Le Nard, 1993).
Corydalis bracteata (family Papaveraceae) and related species are early spring, ornamental, tuberous geophytes originating from Siberia that are widely used in alpine gardening. A blooming plant produces a dense raceme with beautifully coloured flowers. The growth cycle of this species is as follows. At the beginning of April, the leaves begin to sprout immediately followed by flowering. The reproductive period continues until the beginning of June, when seeds reach maturity and the leaves fade. Until the following spring, there is no visible growth and the plant is represented by only the underground organ with a bud. The tuberous underground organ is root-like in nature and its organogenesis occurs in such a way that every year the new tuber develops within the tissues of the older one and thus replaces it entirely (Ryberg, 1959). Little is known about the temperature requirements for C. bracteata development and the response of its tubers to different wintering conditions.
As mentioned above, a detailed and satisfactory explanation of the low temperature requirements for the further flowering of early spring geophytes is still being sought. There is also scarce information about the physiological and developmental processes that occur in bulbous and tuberous plants growing in natural conditions during a cold period lasting several months. The present study compares the effects of natural autumn and winter conditions (from +10 to −10 °C) and mild temperature (+18 °C) on the anatomy and carbohydrate distribution in buds and tubers of C. bracteata. A possible explanation of bud abortion under mild temperature wintering is suggested.
MATERIALS AND METHODS
Plant material and temperature treatments
Corydalis bracteata Pers. plants were collected in the Botanical Garden of the Russian Academy of Sciences in St-Petersburg, Russia. Voucher samples were deposited in the herbarium of the Botany Department of Saint-Petersburg State University (LECB).
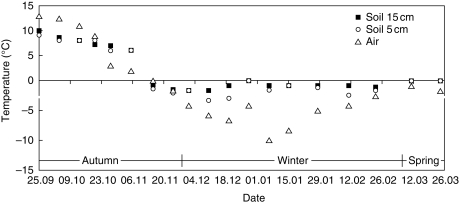
Two temperature treatments were applied to tubers. The first consisted of growing plants in natural autumn and winter conditions (outdoor-grown plants, or field-grown plants). Air and soil temperatures during the studied period are presented in Fig. 1. Field-grown material (100 tubers) was collected over a 2-year period (September 2006 to March 2008), once or twice per month. Only mature 3- to 4-year-old tubers (about 1·5 cm in diameter) were chosen for sampling. The second temperature treatment consisted of keeping tubers away from low temperature. For this experiment, 35 mature tubers were transferred on 15 September to a greenhouse (indoor-grown plants). The tubers were harvested with the natural soil, placed in pots and cultivated at +18 ± 2 °С (day and night temperature) with sufficient watering to keep the soil moist during the autumn/spring period (September–March). As the plants had no overground parts, the pots were placed only under natural light. During the whole experimental period, no fertilizing or treatment against diseases was applied to plants of both temperature treatments. For field-grown tubers, the sole source of water came from the natural environment. Indoor-grown plants were collected on the same date as the field-grown material.
Fig. 1.
Temperature conditions of air and soil for field-grown plants during the sample period.
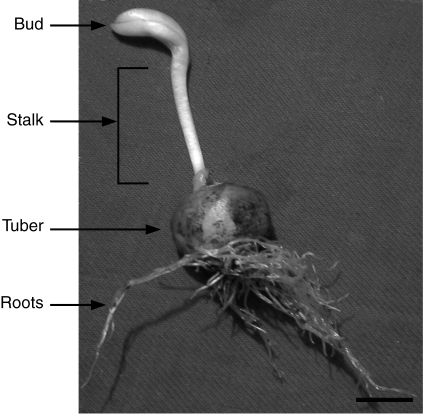
Three plants (Fig. 2) were sampled on the collection dates. Each individual plant was considered as an experimental unit.
Fig. 2.
The plant of C. bracteata in November. Scale bar = 1 cm.
Light and electron microscopy
For microscopic observations of tuber development, cells of phloem parenchyma (2–3 cell layers adjacent to the cambium) were analysed. Anther structure was studied in buds.
Immediately after collection, anthers and tuber pieces were fixed for 48 h with a mixture of 2·5 % paraformaldehyde and 2 % glutaraldehyde in 0·1 m phosphate buffer, pH 7·4, and then washed three times with fixative buffer followed by 12 h of fixation at 4 °C with 2 % osmium tetroxide in 0·1 m phosphate buffer, pH 8. Tuber pieces were then washed three times with fixative buffer, dehydrated using a 30, 50 and 70 % ethanol series and then stained with 2 % uranyl acetate for 2 h at room temperature. They were subsequently dehydrated using 95 and 100 % ethanol and 100 % acetone, and infiltrated in Epon-Araldit M resin (Fluka, Buchs, Switzerland) using a 1 : 5, 1 : 4, 1 : 3, 1 : 2 and 1 : 1 (resin/acetone) series with 1 h of incubation in each solution. Samples were transferred to pure resin, cast into 0·4-mL capsules and polymerized at 60 °C for 72 h.
Semi-thin and thin sections were cut on an Ultracut E (Reichert) ultratome using a diamond knife. Semi-thin sections were stained with 0·1 % toluidine blue and observed with an Olympus Cover-018 (Olympus, Hamburg, Germany) and photographed with an Olympus Color View II camera (Olympus). Thin sections were stained with 4 % uranyl acetate in ethanol and Reynold's lead citrate and examined at 60 eV in a Hitachi-H600 (Tokyo, Japan) transmission electron microscope.
For scanning electron microscopy, pollen grains were fixed with the same fixative mixture, placed onto specimen stubs, sputter-coated with gold and examined at low vacuum using a Jeol JSM35C (Tokyo, Japan) scanning electron microscope.
Partial volumes of starch granules and amyloplasts were calculated as a ratio between starch and amyloplast area and amyloplasts and cell area (Steer, 1981), respectively (10–15 photos). Amyloplast and cell areas were estimated on the micrographs using an image analysis program (UTHSCSA, Image Tool for Windows, version 3·00).
Staining for polysaccharides
The periodic acid – Schiff's procedure (PAS) was used for staining starch in sectioned materials. Sections (1 − 1·2 µm thick) were incubated in 1 % periodic acid for 30 min, washed and then incubated with Schiff's reagent (Sigma, Lille, France) for 30 min. After rinsing with running water, sections were ready for analysis by light microscopy. Starch and cell walls stained bright red, while other cell components (cytoplasm) remained unstained.
Preparation of apoplastic wash fluid
Sugars and total soluble proteins from apoplastic fluid were isolated by vacuum infiltration. Three tubers (3–6 g), previously washed with running water, were cut into pieces (0·5 cm2) and washed twice with ultrapure water. Subsequently, tuber pieces were infiltrated with either ultrapure water (for sugar extraction) or 50 mm Tris-HCl buffer, pH 7·8, supplemented with 3·3 mm MgCl2 (for protein extraction) in a vacuum desiccator for 5 min, at 1 kPa and 4 °C. In order to collect apoplastic fluids, tuber pieces were then quickly dried and centrifuged at 1000g for 5 min at 4 °C, in a 25-syringe barrel placed in a centrifuge tube. Tubers of plants subjected to both temperature treatments were analysed. Two independent apoplastic preparations were carried out for each temperature treatment.
Total soluble protein extraction from tubers
Fresh tubers were weighed and ground into fine powder in ice-cold buffer like that used to extract apoplastic proteins. Crude extracts were centrifuged at 14 000g for 3 min at 4 °C, and supernatants were assayed for glucose 6-phosphate dehydrogenase (G6PDH) activity.
G6PDH assay
Samples of intercellular washing fluid were analysed for possible contamination with symplastic constituents by measuring the activity of G6PDH, a cytoplasmic enzyme used as a specific marker for any plasma membrane damage that may occur during apoplast extraction (Vanacker et al., 1998). Its activity was assessed in tuber homogenate and in apoplastic fluid obtained from the same tuber.
G6PDH activity was assayed as described in Weimar and Rothe (1986). In brief, G6PDH was measured spectrophotometrically at 340 nm at 25 °C. The assay (1 mL) contained 50 mm Tris-HCl buffer, pH 7·8, with 3·3 mm MgCl2, 6 mm NADP, 100 mm glucose 6-phosphate and 20 µL of extract. An increase in A340 was measured as NADP was reduced to NADPH.
Analysis of soluble carbohydrates
For analysis of sugars, fresh tubers and buds were cut into pieces and soaked overnight in methanol. After filtration on filter paper, the solvent was evaporated at 40 °C under reduced pressure. Apoplastic fractions were lyophilized for sugar analysis. Both types of residue obtained were derivatized with a mixture of N-methyl-N-(trimethylsilyl)-trifluoroacetamide and pyridine (1 : 1, v/v) (Supelco, Bellefonte, PA, USA) in a hermetically closed tube for 15 min at 100 °C (Medeiros and Simoneit, 2007) and then analysed by capillary gas chromatography. The gas chromatograph (Carlo Erba 5300; Carla Erba, Milan, Italy) was equipped with a capillary column (15 m length of 0·53 mm i.d. capillary column SPB-5 coated with a 0·5-μm film) (Supelco) and an on-column detector. Helium was used as the carrier gas at a flow rate of 1 ml min−1. The column was operated at an initial temperature of 70 °C and adjusted to 326 °C at 4 °C min−1. The final temperature was held for 4 min. The flame ionization detector and the on-column detector were operated at 340 °C and room temperature, respectively. The injected volume was 1 µL. The internal standard used was naphthalene (Sigma-Aldrich, Paris, France).
Statistics
Where indicated, standard errors and standard deviations were determined. Differences in traits measured between treatments were established with ANOVA performed with Statistica 8·0 software (StatSoft, Inc., Tulsa, OK, USA). Tukey's HSD (honest significant difference) tests were performed for several parameters (partial volume of amyloplasts and number of amyloplasts). All analyses were performed at the 95 % significance level.
RESULTS
Bud morphology and development
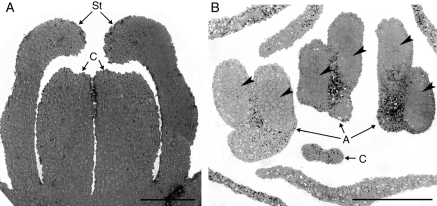
The inflorescence meristem of C. bracteata (Fig. 3A) was initiated in August, during the summer dormancy of the tuber. By the middle of September, before the transfer of plants to a greenhouse, both gynoecium and androecium were well formed with clearly differentiated carpels and anthers. In the latter, the mother microspore cells were present (Fig. 3B). The bud was covered with two yellow bracts. Subsequent microscopic observations of bud development were concentrated on the structure of anthers, known to be more sensitive to temperature treatment.
Fig. 3.
Light microscopy. (A) Longitudinal section of C. bracteata inflorescence meristem in August. Note the well-developed flower primordia. Scale bar = 100 µm. (B) Cross-section of the flower bud on 15 September. Note the mother microspore cells (arrowheads) in the locules of anthers. Scale bar = 100 µm. Abbreviations: A, anther; C, carpels; St, stamens.
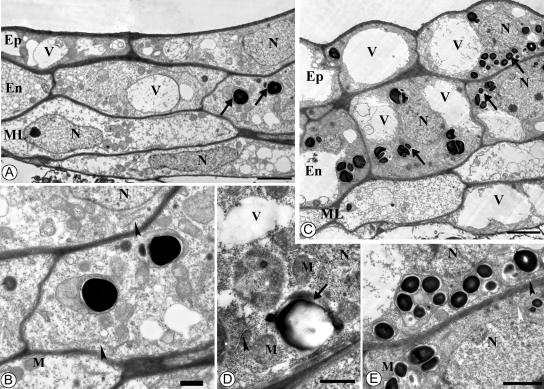
Electron microscopic observations showed that, in October, the anther walls of plants from the two temperature treatments developed without differences. In all cell layers of the anther wall plastids, mitochondria and endoplasmic reticulum were abundant (Fig. 4). Plastids of epidermal and endothecium cells accumulated starch (Fig. 4).
Fig. 4.
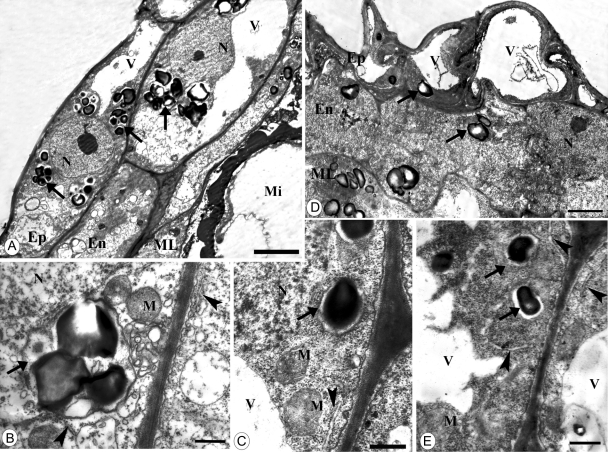
Transmission electron microscopy of C. bracteata anther on 12 October: outdoor-grown plants (A, B) and indoor-grown plants (C–E). (A, C) Anther wall. Scale bar = 2 µm. (B) Part of (A). Scale bar = 1 µm. (D) Fragment of endothecium cell. Scale bar = 0·5 µm. (E) Part of (B). Scale bar = 1 µm. Note the similar structure of anther wall of plants from both temperature treatments: large vacuoles, presence of starch in amyloplasts (arrows) and endoplasmic reticulum (arrowheads). Abbreviations: En, endothecium; Ep, epidermis; N, nucleus; M, mitochondria; ML, middle layer; V, vacuole.
At the beginning of November, anthers of field-grown plants were characterized by the same structure as described above (Fig. 5A, B). In anthers of indoor-grown plants, the structure of the anther wall was similar to those of control plants: amyloplasts, mitochondria and endoplasmic reticulum were observed (Fig. 5C, E). In the epidermis, however, large vacuoles were present and the epidermal cells had highly irregular shapes (Fig. 5D).
Fig. 5.
Transmission electron microscopy of C. bracteata anther on 1 November: outdoor-grown plants (A, B) and indoor-grown plants (C–E). (A, C) Anther wall. Scale bar = 5 µm. Note large vacuoles and irregular shape of epidermal cells in anthers of indoor-grown plants. (B, D) Fragment of epidermal cell. Scale bar = 0·5 µm. (E) Fragment of an endothecium cell. Scale bar = 0·5 µm. Note presence of amyloplasts (arrows) and endoplasmic reticulum (arrowheads) in the anthers of plants from both temperature treatments. Abbreviations: En, endothecium; Ep, epidermis; N, nucleus; M, mitochondria; Mi, microspore; ML, middle layer; N, nucleus; V, vacuole.
At the end of November, the anthers of field-grown plants had the same structure as on the previous collection date, and numerous amyloplasts were present in all layers of the anther (Fig. 6A). Microspores were rounded (Fig. 6B). By the same date, all cell layers of the anther wall were disrupted in indoor-grown plants, and no cellular structure was detectable (Fig. 6C). In parallel with the disruption of the anther wall, microspores also lost turgor and were squeezed (Fig. 6D).
Fig. 6.
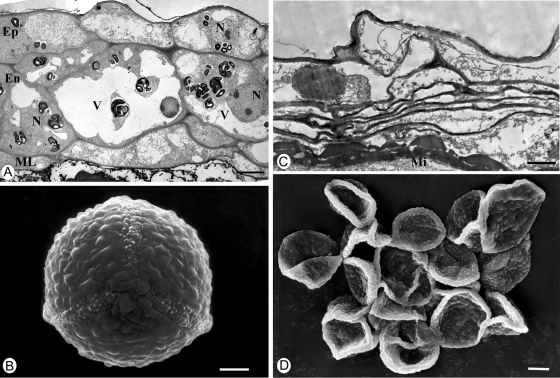
Transmission electron microscopy of C. bracteata anther on 23 November: outdoor-grown plants (A) and indoor-grown plants (C). (A, B) Anther wall. Note the well-developed anther wall with numerous amyloplasts (arrows) in outdoor-grown plants and the absence of cellular structure in anthers of indoor-grown plants. Scale bar = 2·5 µm. Scanning electron microscopy of pollen grains of outdoor-grown plants (B) and indoor-grown plants (D). Note the squeezed pollen grains of indoor-grown plants. Scale bar = 0·5 µm. Abbreviations: En, endothecium; Ep, epidermis; N, nucleus; M, mitochondria; ML, middle layer; N, nucleus; V, vacuole.
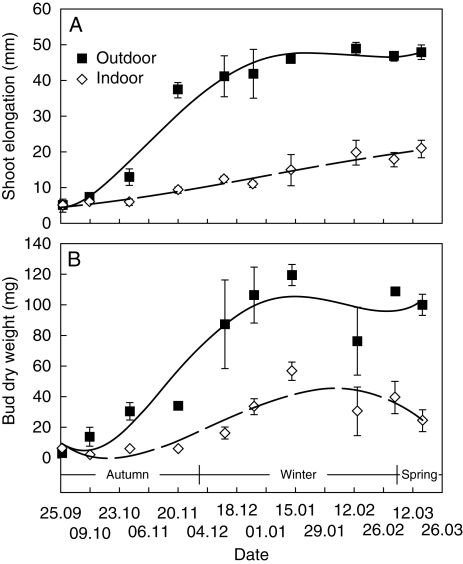
In the autumn and winter months, a progressive stalk and bud elongation and an increase in bud dry weight were observed for outdoor-grown plants. These measurements were lower for indoor-grown plants than for control plants (Fig. 7). The bracts of the former showed no visible morphological changes until February, and then were mortified. For indoor-grown plants, neither sprouting leaves nor flowering were observed.
Fig. 7.
Shoot elongation (A) and dry weight of buds (B) of C. bracteata outdoor-grown plants and indoor-grown plants, as indicated. Values are means ± s.d. of three experiments.
Tuber structure and starch content
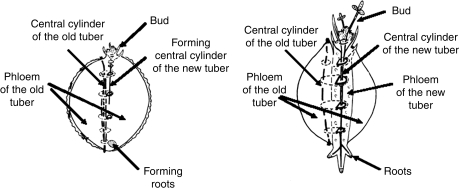
At the beginning of September, C. bracteata tubers consisted of the present year's tuber and the newly developing one (Fig. 8A) connected to the bud that will develop next spring. Being of secondary root nature, a fully developed tuber consists only of vascular tissues: the central xylem, several layers of cambium cells and highly parenchymatized phloem, which represents the major volume of the mature tuber and has an epidermal function (Fig. 8B).
Fig. 8.
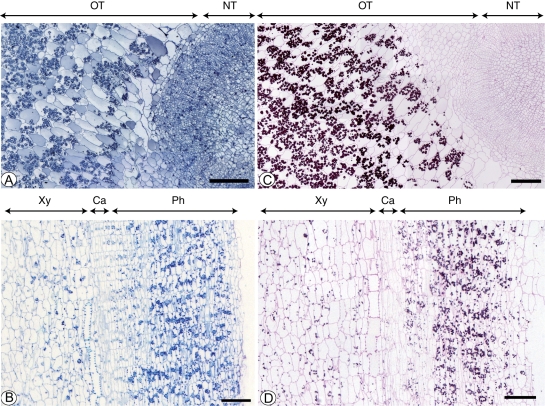
General anatomy (A, B) and starch localisation (C, D) in C. bracteata tubers. (A, C) Cross-section of a tuber on 15 September. (A) General view: cells of the old tuber are larger than cells of the new one. (C) PAS: note a significant amount of starch granules in phloem parenchyma cells of the old tuber. Scale bar = 250 µm. (B, D) Longitudinal section of a new tuber on 23 November. (B) General view. (D) PAS staining for polysaccharides. Note the progressive appearance of starch grains when cells are differentiated into phloem parenchyma. Scale bar = 250 µm. Abbreviations: Ca, cambium; NT, new tuber; OT, old tuber; Se, sieve elements; Ve, vessels.
As the formation of the new tuber was initiated under the bud, the vascular bundles of the latter were connected with the vascular tissues of the hypocotyl and the developing tuber. No bud structure had direct contact with the sheaths of the old tuber (data not shown). The new tuber developed under the lateral bud and, because it has a lateral position relative to the cambium cylinder of the old tuber, the vascular tissues of the old tuber were not included in the newly forming one. As a result, there was no direct contact between their vascular sheaths and they bordered each other only by a junction of phloem cell walls. Plasmodesmata were never observed between the cells of new and old tubers.
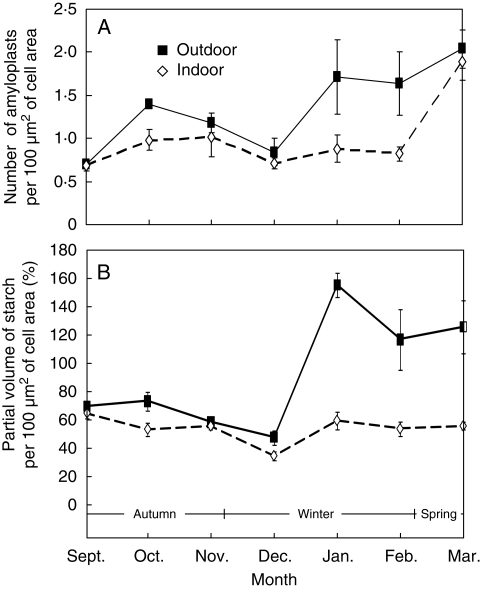
Early in autumn, the parenchyma cells of the old tuber possessed a large amount of densely packed starch granules accumulated within amyloplasts, while starch was almost absent in the new tuber (Fig. 8A). Later in October, the amyloplasts appeared in the cells of the new tuber as well, in plants from both temperature treatments. During October–December, a progressive decrease in the partial volume of amyloplasts and starch was observed in cells of field-grown tubers. In January, a marked increase in apparent starch content was seen followed by a progressive decrease during the following months (Fig. 9A).
Fig. 9.
Number of amyloplasts (A) and partial volume of starch per 100 µm2 of cell area (B) of C. bracteata new tuber parenchyma of outdoor-grown plants and indoor-grown plants, as indicated. Values are means ± s.e. of 10–15 photographs.
In tubers of indoor-grown plants, the dynamics of starch accumulation were different as compared with those of control plants: a decrease in the partial volume of amyloplasts and apparent starch content was observed during October–January, while in February–March these parameters increased slightly (Fig. 9A). During the whole study period, the amyloplasts were larger and more abundant, and the partial volume of starch was higher in cells of field-grown tubers than in tubers grown indoors (Fig. 9).
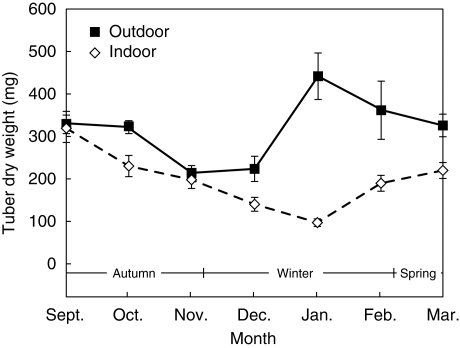
Together with decreasing starch content, the dry weight of tubers decreased, and increased in parallel with higher starch concentration. This correlation was observed for tubers from both temperature treatments (Fig. 10).
Fig. 10.
Dry weight of tubers of C. bracteata outdoor-grown plants and indoor-grown plants, as indicated. Values are means ± s.d. of three experiments.
Sugar content in tubers and buds
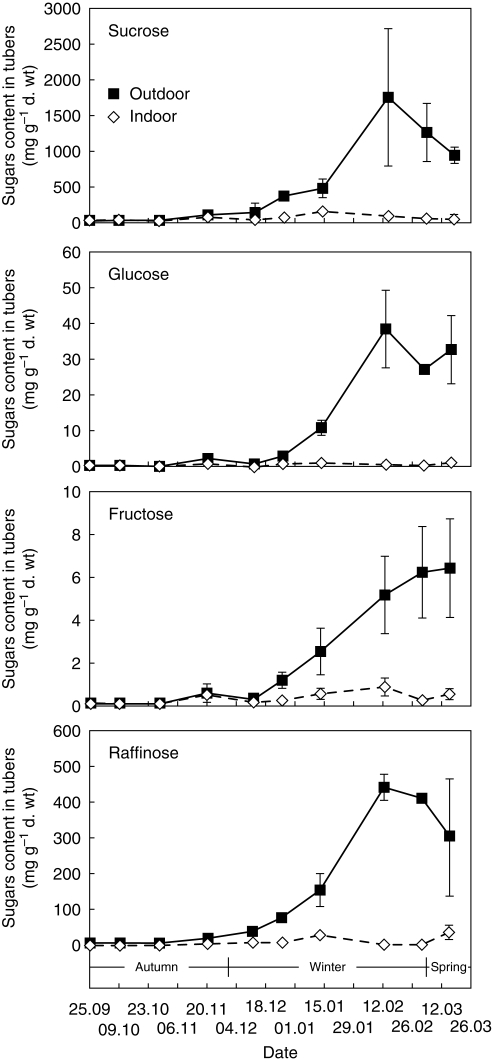
During the autumn period (September–November), sugar content was low in all tubers of plants grown either outdoors or indoors. There were no significant changes in October but the amount of sugars increased slightly in November. In field-grown tubers, a significant increase in all sugar levels was observed in the middle of December, and this rise continued up to the end of March (Fig. 11).
Fig. 11.
Sugar content in tubers of C. bracteata plants grown outdoors and indoors, as indicated. Values are means ± s.d. of three experiments.
In tubers of indoor-grown plants, the quantity of sugars remained almost the same during the winter–spring period. Only a slight increase in sucrose and raffinose content was observed in January, and in fructose in February, but their levels were much lower than those of tubers grown outdoors (Fig. 11).
The major detectable sugar in tubers grown under both temperature treatments was sucrose, whose amount was up to five-fold higher than that of other total sugars. The second most abundant sugar was raffinose; quantities of fructose and glucose were much lower.
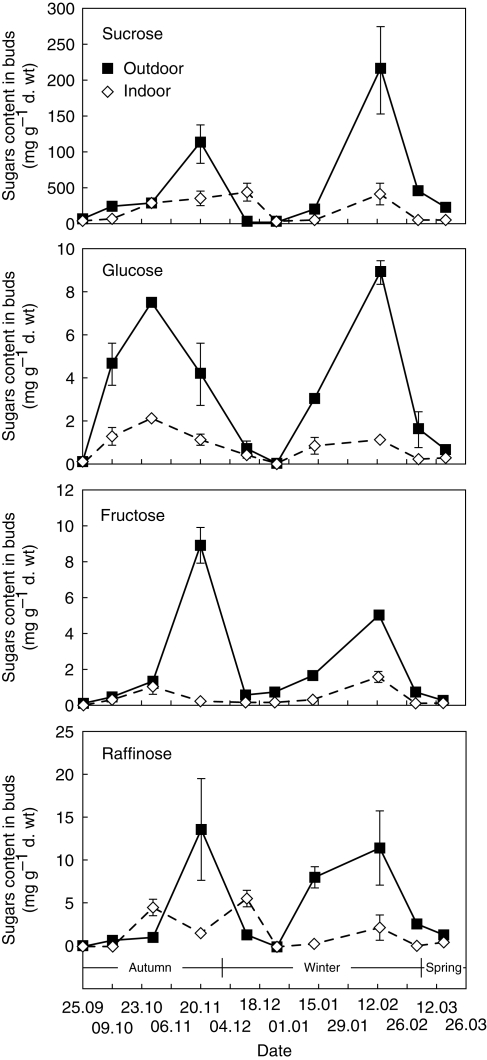
In buds of field-grown plants, an increase in the content of all detected sugars was observed in October–November, followed by a sharp decline in December and then a significant increase, with a maximum in the middle of February for sucrose, glucose and raffinose and in January for fructose (Fig. 12). After this peak was reached, a rapid return to the autumn level was observed for all sugars.
Fig. 12.
Sugar content in C. bracteata buds of outdoor-grown plants and indoor-grown plants, as indicated. Values are the means ± s.d. of three experimental units.
In buds of indoor-grown plants, an increase in sugar content was seen early in the autumn months followed by a decrease in the winter months. In October and November, the amount of sucrose and glucose was lower than that observed for tubers grown outdoors, while fructose had almost the same level for both temperature treatments, and the raffinose content was even higher than in control plants. During the winter months, the quantity of sugars stayed the same. In the middle of February, there was a slight increase followed by a decrease in March, but these levels were much less significant than those in control plants.
The major sugar in the buds of plants from both temperature treatments was sucrose; levels of hexoses and raffinose were lower.
Contamination of apoplastic extracts by cytoplasmic components
Before carrying out apoplastic extraction experiments, the apoplastic fluid was analysed for possible symplastic contamination and infiltration and vacuum conditions were then determined to obtain only the minor components. G6PDH, a cytoplasmic marker, was used to calculate symplastic contamination of the apoplastic extracts. On average, of all the samples collected under the vacuum conditions used, less than 0·5 % of total tuber G6PDH activity was observed in apoplastic fluid (total tuber G6PDH activity = 29·21 ± 11·67 nmol min−1 mg −1 f. wt, apoplast extract = 0·03 ± 0·02 %; means ± s.d.). This contamination was considered to be negligible and was ignored when calculating the metabolite content in the apoplast.
Sugar content of apoplastic extractions
The collection of material for apoplastic extraction was chosen according to the results of bud development and sugar content in tubers. The period selected was when the difference in the temperature surrounding the tubers was significant, but the anthers had not yet been damaged and the total amount of sugars in tubers from both temperature treatments was approximately the same. Thus, the plants were collected on 15 November.
The apoplastic fluid of tubers from both temperature treatments contained a significant quantity of sugars. The total sugar content of the apoplast of tubers from field-grown plants was more than 14-fold higher than that of indoor-grown plants (Table 1). Sucrose was the major sugar detected in tubers of plants grown either outdoors or indoors. Field-grown tubers also contained a significant amount of glucose and fructose but raffinose represented only a minor constituent. For tubers of indoor-grown plants, the hexoses/sucrose ratio was lower than in control plants, while the observed amount of raffinose was almost the same as that of glucose.
Table 1.
Sucrose, glucose, fructose and raffinose content in the apoplastic fluid in November from tubers of plants grown outdoors and indoors as compared with their total amount in tubers (means ± s.d., n = 2)
| Sugar | Outdoor-grown plants (nmol g f. wt−1) | Indoor-grown plants (nmol g f. wt−1) |
|---|---|---|
| Sucrose | 439·07 ± 22·68 (0·18 % of total sucrose content in tuber) | 36·61 ± 9·67 (0·01 % of total sucrose content in tuber) |
| Glucose | 253·68 ± 13·71 (5·05 % of total glucose content in tuber) | 5·51 ± 1·27 (0·11 % of total glucose content in tuber) |
| Fructose | 144·96 ± 26·17 (8·04 % of total fructose content in tuber) | 6·59 ± 2·77 (0·06 % of total fructose content in tuber) |
| Raffinose | 3·37 ± 1·04 (0·05 % of total raffinose content in tuber | 8·44 ± 0·58 (0·13 % of total raffinose content in tuber) |
| Total | 841·10 ± 44·81 (0·26 % of total sugar content in tuber) | 57·21 ± 11·32 (0·02 % of total sugar content in tuber) |
The sugar pool of the apoplast represented about 0·3 % of the total tuber sugar content for outdoor-grown plants, but only 0·02 % was detected in the apoplast of the indoor-grown plants (Table 1).
DISCUSSION
For most of the year, the Corydalis plant is represented by only an underground tuber that has a storage function and provides nutrients for the future development of the plant. The major part of the tuber volume is occupied by phloem parenchyma, in cells of which numerous amyloplasts accumulate starch. During a long non-photosynthetic period (end of June to beginning of April), the amyloplasts of the tuber supply the bud with sugars formed as a result of regulated starch degradation.
For C. bracteata, as shown by microscopic investigations (Ryberg, 1959), the activity of the apical meristem starts at the end of July, and flower development begins in August, but has to be completed during winter, which is a natural period of cold for tubers. For tubers of indoor-grown plants, flower bud development is initiated during the autumn–winter period but is completely stopped by the beginning of December when bud abortion appears. The first injured part of the flower is the androecium, known to be more sensitive to temperature treatment (De Munk, 1973). This is followed by a progressive necrosis of other parts of the flower and, finally, bud scales. Additionally, no sprouting of leaves could be observed for indoor-grown plants.
Although similar observations of flower bud necrosis in bulbous plants kept away from low temperature during the autumn–winter period have been reported by various authors (Le Nard and De Hertogh, 1993; Lambrechts et al., 1994; Rebers et al., 1995; Van der Toorn et al., 2000; Kamenetsky et al., 2003), there is still no adequate explanation for the principle that underlies this correlation between chilling and flowering. During the non-photosynthetic period, the bud and the stalk are sinks that require sucrose and starch and depend mainly on nutrition imported from a primary source organ. Consequently, in a previous study, we hypothesized that the arrest of bud development in Corydalis plants grown indoors might be the result of a temperature-induced inhibition of starch degradation leading to limited sugar availability for the developing buds (Khodorova et al., 2007). Moreover, it has been suggested that low sugar levels in stems could trigger flower bud abortion in trees under mild winter conditions (Marquat et al., 1999).
The role of carbohydrates in C. bracteata development
During the autumn months (September–November), the amount of sugars in buds of C. bracteata was lower for indoor-grown plants than for control plants. It was previously reported that hexoses, particularly glucose, contribute to stalk elongation in tulips (Lambrechts et al., 1994), and the lower bud elongation of indoor-grown C. bracteata plants in parallel with a lower glucose content in their buds compares well with this observation. Moreover, shoot elongation and dry weight of plant organs are parameters which could be a measure of the nutrient source–sink strength ratio (Marcelis et al., 2004; Mäkelä et al., 2005). Thus, the observed lower dry weight of buds of plants grown indoors may also be considered as an indicator of a suppressed supply from the source organ, which can block the development of reproductive organs (McLaughlin and Boyer, 2004; Mäkelä et al., 2005).
During the autumn months (September–November), sugar content in Corydalis tubers is low for plants from both temperature treatments. The significant difference in sugar levels in tuber tissues of plants from the two temperature treatments is revealed from the middle of December. The same results showing an increase in sugar content during the winter period, observed by Risser and Cottam (1968) for different geophyte species, were interpreted by the authors as evidence of a particular rhythm of their development. We suggest that the high sugar levels found in field-grown plants could also be explained as an adaptive function of sugars to prevent cells from dehydration at low temperatures (Levitt, 1980; Guy et al., 1992; Winter and Huber, 2000; Lennartsson and Ögren, 2003; Margesin et al., 2007). However, although starch hydrolysis is known to be activated by low temperature (Guy et al., 2008), the number of amyloplasts and the partial volume of starch per cell of the new tuber were lower for indoor-grown plants than for field-grown ones.
Similar results for sugar content during the autumn–winter period under different temperature conditions were observed for peach trees by Bonhomme et al. (2005). The buds of trees grown under mild winter conditions were necrotized, and their sugar content was lower than that of field-grown tree buds, whereas the sugar levels in stems were the same for plants from both temperature treatments. Unfortunately, the above authors did not provide a reliable explanation of these data, but they hypothesized that it is not the sugar content of stems that is disrupted, but the further transport of sugars to the developing bud, which may possibly lead to flower abortion.
Cold treatment makes sugars move into the apoplast
In spite of similar low sugar levels during September–November in Corydalis tubers from both temperature treatments, measured sugar contents in tuber apoplastic fluid in November showed a significant difference between field- and indoor-grown plants. The total apoplastic sugar content was almost 15-fold higher for outdoor-grown tubers. These data suggest that cold treatment influences sugar transport considerably in Corydalis plants.
It has previously been shown that the application of a cold jacket (+1 °C) on the petiole of a leaf leads to a dramatic increase in soluble sugar content in the apoplast (Ntsika and Delrot, 1986; Voitsekhovskaja et al., 2000). It is likely that in C. bracteata low temperature in the autumn months also activates sugar movement into the apoplastic domain of the tuber but this movement seems not to be induced in the indoor-grown plants.
It has been reported that sugars, stored in vacuoles (Pollock and Kingston-Smith, 1997; Winter and Huber, 2000), are effectively locked there due to the barrier function of the tonoplast (Marty, 1999; Gamalei et al., 2000; Voitsekhovskaja et al., 2000; Gamalei, 2007). The efflux of sugars into the apoplast is thus only possible following the loss of the tonoplast barrier function.
One of the ways of overcoming the tonoplast barrier function is cold treatment (Gamalei, 2007, and references therein). In parallel, low temperature leads to intensive activity of the enzymes of starch hydrolysis and the sucrose synthetic pathway (Stitt and Vaughan, 2002; Guy et al., 2008), which results in an increase in the sugar content of cells and, subsequently, an increase in hydrostatic pressure in vacuoles. The combined action of these factors in tubers of outdoor-grown plants may lead to the export of sugars from vacuome to apoplast. In the absence of low temperature, overcoming the tonoplast barrier function seems impossible and, as a result, the amount of sugars in the apoplastic fluid of indoor-grown plants is low.
For tubers from both temperature treatments, sucrose was the major sugar in the apoplastic fluid. Because sucrose is known to be the major transport sugar, its abundant presence in the apoplast may be evidence of future sugar transport throughout the plant (Turgeon, 1996; Turgeon and Medville, 2004).
Indeed, carbohydrate distribution within a plant can occur via both symplastic and apoplastic routes (Winter and Huber, 2000). However, for most vascular species, the apoplast compartment is considered as the alternative channel, which is activated only when the symplastic route through plasmodesmata is blocked (Gamalei, 2007). In this case, apoplastic assimilates are henceforth loaded to phloem in an energy-dependent manner (Gamalei et al., 2000; Voitsekhovskaja et al., 2000).
We have not observed plasmodesmata between cells of new and old tubers, suggesting that there is no symplastic flux between the two and only apoplastic transport is active.
Do tuber apoplastic sugars guarantee the supply of bud nutrition?
Corydalis bracteata and related species are characterized by a particular tuber organogenesis (Fig. 13), which implies a complex relationship in the distribution of nutrition between tuber and bud.
Fig. 13.
Diagram showing C. bracteata and related species development (adapted from Ryberg, 1959).
A new tuber is formed within the tissue of the previous year's tuber and replaces it entirely (Ryberg, 1959). Thus, tissues of the old tuber are not included in the new one. The formation of the new tuber starts at the end of July and is completed by October. This means that, from the beginning of October, the plant de facto consists of two tubers: a new developing one with a bud that gives the inflorescence in the following spring, and the old one, with a reserve of carbohydrates that accumulated during spring growth.
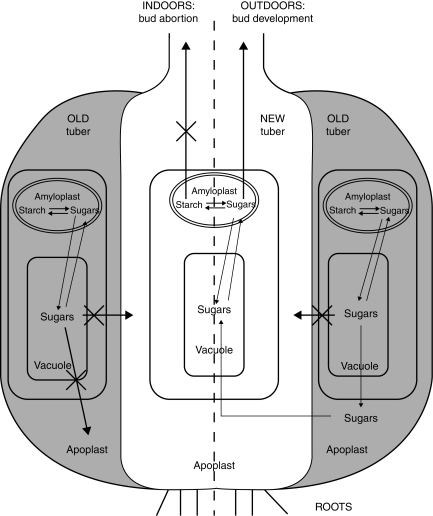
Figure 14 depicts a hypothetical model of sugar distribution between the C. bracteata tuber and bud of field-grown and indoor-grown plants. The present data and data from the literature suggest that cold treatment activates the efflux of sugars into the apoplast. In this case, sugars formed as a result of starch hydrolysis in old tuber cells may be loaded to the phloem of the new tuber and thus provide sufficient nutrients for bud development during the autumn–winter period, the release from dormancy (Marquat et al., 1999; Lennartsson and Ögren, 2003) and then overground growth of photosynthetic organs in spring.
Fig. 14.
Model of sugar distribution in C. bracteata plants grown outdoors (right half of the image) and indoors (left half of the image). In field-grown plants, low temperature activates the efflux of sugars from vacuoles of the old tuber into the apoplast and their consequent loading into the phloem of the new tuber, from which they are transported into the developing bud. In tubers of plants grown indoors, overcoming the barrier function of the tonoplast is impossible and sugars are locked in the vacuoles of cells of the old tuber. Thus, neither the new tuber nor the developing bud receives a nutritive supply, which results in necrosis of the latter.
In tubers of indoor-grown plants, there is no efflux of sugars into the apoplastic domain and thus no future loading to the new tuber phloem. As a result, the reserve of carbohydrates accumulated in cells of the old tuber is locked there and is not available for the developing plant. We have not observed any contact of the bud vascular sheaths with the old tuber. However, according to Ryberg (1959), phloem elements of the old tuber have contact with the sheaths of bud scales until the end of September. This suggests that during a short period (July–September) the bud can still receive the nutrient supply from the old tuber. Nevertheless, for indoor-grown plants, after disconnection of tuber vascular tissues, the direct flux of nutrients from the previous year's tuber to the new one (and to the bud) seems to be practically impossible. The new tuber's own reserve enables bud development to begin but apparently is insufficient to complete it.
In conclusion, low temperature (from +10 to −10 °C) during the autumn and winter months is an essential factor for the development of spring tuberous geophytes of temperate climate. From the example of C. bracteata, we hypothesize that the progressive decrease in temperature during the autumn period leads to the exit of the sugar pool from the phloem parenchyma cells of the old tuber into the apoplast and then to their loading into the phloem of the developing tuber, thus providing a carbohydrate supply for the developing bud. In the absence of low temperature, this activation does not occur and, as a result, the new plant is unable to use the stored carbohydrates of the mother tuber. When the reserves of the new tuber and the bud are exhausted, the lack of nutrients leads to bud abortion.
ACKNOWLEDGEMENTS
We thank Professor Yu. V. Gamalei (Photosynthesis Department of the Komarov Botanical Institute of the Russian Academy of Sciences, Saint-Petersburg, Russia) for his helpful discussions on this issue and Dr N. K. Koteyeva (Laboratory of Plant Anatomy, Komarov Botanical Institute of the Russian Academy of Sciences) for critical reading of the manuscript. We also thank Drs C. Jousse and Y. Huet (EA 3900 BioPI, Jules Verne University of Picardie, Amiens, France) for their assistance with GC-MS and enzyme assays. This work was partly funded by the Russian Fund for Fundamental Research [06-04-49028]. N.V.K. gratefully acknowledges the French government for financial support of her studies [2007–20].
LITERATURE CITED
- Aung LH, De Hertogh AA. Temperature regulation of growth and endogenous abscisic acid-like content of Tulipa gesneriana L. Plant Physiology. 1979;63:1111–1116. doi: 10.1104/pp.63.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk PA, De Boer AD. Rapid stalk elongation in tulip (Tulipa gesneriana L. cv. Apeldoorn) and the combined action of cold-induced invertase and the water-channel protein γTIP. Planta. 1999;209:346–354. doi: 10.1007/s004250050642. [DOI] [PubMed] [Google Scholar]
- Bonhomme M, Rageau R, Lacointe A, Gendraud M. Influences of cold deprivation during dormancy on carbohydrate contents of vegetative and floral primordia and nearby structures of peach buds (Prunus persica (L.) Batsch) Scientia Horticulturae. 2005;105:223–240. [Google Scholar]
- De Hertogh AA, Le Nard M. Physiological and biochemical aspects of flower bulbs. In: De Hertogh AA, Le Nard M, editors. The physiology of flower bulbs. Amsterdam: Elsevier Science Publishers; 1993. pp. 53–71. [Google Scholar]
- De Munk WJ. Flower-bud blasting in tulips caused by ethylene. Netherlands Journal of Plant Pathology. 1973;79:41–53. [Google Scholar]
- Gamalei YV. The role of mesophyll cell tonoplast in determining the route of phloem loading. Thirty years of the studies of phloem loading. Russian Journal of Plant Physiology. 2007;54:1–9. [Google Scholar]
- Gamalei YV, Pakhomova MV, Syutkina AV, Voitsekhovskaja OV. Compartmentation of assimilate fluxes in leaves. I. Ultrastructural responses of mesophyll and companion cells to the alteration of assimilate transport export. Plant Biology. 2000;2:98–106. [Google Scholar]
- Gorin N, Heidema FT. Starch contents of freeze-dried anthers and α-amylase activity of their extracts as criteria that dry-stored bulbs (Tulipa gesneriana L.) cultivar ‘apelddorn’ have been exposed to 5 °C. Scientia Horticulturae. 1985;26:183–189. [Google Scholar]
- Guy CL, Huber JLA, Huber SC. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiology. 1992;100:502–508. doi: 10.1104/pp.100.1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C, Kaplan F, Kopka J, Selbig J, Hincha DK. Metabolomics of temperature stress. Physiologia Plantarum. 2008;132:220–235. doi: 10.1111/j.1399-3054.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- Kamenetsky R, Barzilay A, Erez A, Halevy AH. Temperature requirements for floral development of herbaceous peony cv. ‘Sarah Bernhardt. Scientia Horticulturae. 2003;97:309–320. [Google Scholar]
- Khodorova NV, Koteyeva NK, Miroslavov EA. Ultrastructural changes of tuber phloem parenchyma cells of Corydalis bracteata (Fumariaceae) growing outdoors and indoors. Botanicheskii Zhurnal. 2007;92:1011–1023. [in Russian] [Google Scholar]
- Lambrechts H, Rook F, Kollöfel C. Carbohydrate status of tulip bulbs during cold-induced flower stalk elongation and flowering. Plant Physiology. 1994;104:515–520. doi: 10.1104/pp.104.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Nard M, De Hertogh AA. Bulb growth and development and flowering. In: De Hertogh AA, Le Nard M, editors. The physiology of flower bulbs. Amsterdam: Elsevier Science Publishers; 1993. pp. 29–45. [Google Scholar]
- Lennartsson M, Ögren E. Predicting the cold hardiness of willow stems using visible and near-infrared spectra and sugar concentrations. Trees – Structure and Function. 2003;17:463–470. [Google Scholar]
- Levitt J. Response of plants to environmental stresses. Vol. I. Chilling, freezing and high temperature stresses. New York: Academic Press; 1980. [Google Scholar]
- Mäkelä P, McLaughlin JE, Boyer JS. Imaging and quantifying carbohydrate transport to the developing ovaries of maize. Annals of Botany. 2005;96:939–949. doi: 10.1093/aob/mci246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelis LFM, Heuvelink E, Baan Hofman-Eijer LR, Den Bakker J, Xue LB. Flower and fruit abortion in sweet pepper in relation to source and sink strength. Journal of Experimental Botany. 2004;55:2261–2268. doi: 10.1093/jxb/erh245. [DOI] [PubMed] [Google Scholar]
- Margesin R, Neuner G, Storey KB. Cold-loving microbes, plants, and animals – fundamental and applied aspects. Naturwissenschaften. 2007;94:77–99. doi: 10.1007/s00114-006-0162-6. [DOI] [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. The Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquat C, Vandamme M, Gendraud M, Pétel G. Dormancy in vegetative buds of peach. Relation between carbohydrate absorption potentials and carbohydrate specific content in the bud during dormancy and its release. Scientia Horticulturae. 1999;79:151–162. [Google Scholar]
- McLaughlin JE, Boyer JS. Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Annals of Botany. 2004;94:75–86. doi: 10.1093/aob/mch123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros PM, Simoneit BRT. Analysis of sugars in environmental samples by gas chromatography-mass spectrometry. Journal of Chromatography A. 2007;1141:271–278. doi: 10.1016/j.chroma.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Miller WB. A review of carbohydrate metabolism in geophytes. Acta Horticulturae. 1992;325:239–246. [Google Scholar]
- Ntsika G, Delrot S. Changes in apoplastic and intracellular leaf sugars induced by the blocking of export in Vicia faba. Physiologia Plantarum. 1986;68:145–153. [Google Scholar]
- Pollock CJ, Kingston-Smith AH. The vacuole and carbohydrate metabolism. Advances in Botanical Research. 1997;25:145–215. [Google Scholar]
- Rebers M, Vermeer E, Knegt E, Shelton CJ, Plas LHW. Gibberellin levels and cold-induced floral stalk elongation in tulip. Physiologia Plantarum. 1995;94:687–691. [Google Scholar]
- Rietveld PL, Wilkinson C, Franssen HM, et al. Low temperature sensing in tulip (Tulipa gesneriana L.) is mediated through an increased response to auxin. Journal of Experimental Botany. 2000;51:587–594. doi: 10.1093/jexbot/51.344.587. [DOI] [PubMed] [Google Scholar]
- Risser PG, Cottam G. Carbohydrate cycles in the bulbs of some spring ephemerals. Bulletin of the Torrey Botanical Club. 1968;95:359–369. [Google Scholar]
- Ryberg M. A morphological study of Corydalis nobilis, Corydalis cava, Corydalis solida and some allied species, with special reference to their underground organs. Acta Horti Bergiani. 1959;19:15–119. [Google Scholar]
- Steer M. Understanding cell structure. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Stitt M, Vaughan H. A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology. 2002;5:199–206. doi: 10.1016/s1369-5266(02)00258-3. [DOI] [PubMed] [Google Scholar]
- Taeb AG, Alderson PG. Effect of low temperature and sucrose on bulb development and on the carbohydrate status of bulbing shoots of tulip in vitro. Journal of Horticultural Science. 1990;65:193–197. [Google Scholar]
- Turgeon R. Phloem loading and plasmodesmata. Trends in Plant Science. 1996;1:418–423. [Google Scholar]
- Turgeon R, Medville R. Phloem loading. A reevaluation of the relationship between plasmodesmatal frequencies and loading strategies. Plant Physiology. 2004;136:3795–3803. doi: 10.1104/pp.104.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Toorn A, Zemah H, Van As H, Bendel P, Kamenetsky R. Developmental changes and water status in tulip bulbs during storage: visualization by NMR imaging. Journal of Experimental Botany. 2000;51:1277–1287. doi: 10.1093/jexbot/51.348.1277. [DOI] [PubMed] [Google Scholar]
- Van Kilsdonk MG, Nicolay MG, Franssen JM, Kolloffel C. Bud abortion in tulip bulbs studied by magnetic resonance imaging. Journal of Experimental Botany. 2002;53:1603–1611. doi: 10.1093/jxb/erf002. [DOI] [PubMed] [Google Scholar]
- Vanacker H, Harbinson J, Ruisch J, Carver TLW, Foyer CH. Antioxidant defences of the apoplast. Protoplasma. 1998;205:29–140. [Google Scholar]
- Vishnevetsky J, Zamskia E, Ziv M. Carbohydrate metabolism in Nerine sarniensis bulbs developing in liquid culture. Physiologia Plantarum. 2000;108:361–369. [Google Scholar]
- Voitsekhovskaja OV, Pakhomova MV, Syutkina AV, Gamalei YV, Heber U. Compartmentation of assimilate fluxes in leaves. II. Apoplastic sugar levels in leaves of plants with different companion cell types. Plant Biology. 2000;2:107–112. [Google Scholar]
- Weimar M, Rothe G. Preparation of extracts from mature spruce needles for enzymatic analysis. Physiologia Plantarum. 1986;69:692–698. [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Critical Reviews in Plant Sciences. 2000;19:31–67. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Zemah H, Bendel P, Rabinowitch HD, Kamenetsky R. Visualization of morphological structure and water status during storage of Allium aflatunense bulbs by NMR imaging. Plant Science. 1999;147:65–73. [Google Scholar]