Abstract
Objective:
Deficits in working memory and cognitive control in schizophrenia are associated with impairments in prefrontal cortical function, including altered gamma band oscillations. These abnormalities are thought to reflect a deficiency in the synchronization of pyramidal cell activity that is dependent, in part, on gamma-aminobutyric acid (GABA) neurotransmission through GABA type A (GABAA) receptors containing α2 subunits. The authors conducted a proof-of-concept clinical trial designed to test the hypothesis that a novel compound with relatively selective agonist activity at GABAA receptors containing α2 subunits would improve cognitive function and gamma band oscillations in individuals with schizophrenia.
Method:
Participants were male subjects (N=15) with chronic schizophrenia who were randomly assigned to receive 4 weeks of treatment with the study drug MK-0777, a benzodiazepine-like agent with selective activity at GABAA receptors containing α2 or α3 subunits, or a matched placebo in a double-blind fashion. Outcome measures were the Brief Psychiatric Rating Scale (BPRS), Repeatable Battery for the Assessment of Neuropsychological Status, three tests of working memory and/or cognitive control (N-back, AX Continuous Performance Test, and Preparing to Overcome Prepotency), and EEG measures of gamma band oscillations induced during the Preparing to Overcome Prepotency task.
Results:
Compared with placebo, the MK-0777 compound was associated with improved performance on the N-back, AX Continuous Performance Test, and Preparing to Overcome Prepotency tasks. The compound was also associated with increased frontal gamma band power during the Preparing to Overcome Prepotency task. No effects of the MK-0777 compound were detected in BPRS or Repeatable Battery for the Assessment of Neuropsychological Status scores, with the exception of improvement on the Repeatable Battery for the Assessment of Neuropsychological Status delayed memory index. The MK-0777 agent was well-tolerated.
Conclusions:
These findings provide preliminary support for the hypothesis that enhanced GABA activity at α2 subunit containing GABAA receptors improves behavioral and electrophysiological measures of prefrontal function in individuals with schizophrenia.
In individuals with schizophrenia, a characteristic pattern of cognitive deficits occurs with high frequency, is relatively stable over time, is independent of psychosis, and is the best predictor of long-term functional outcome (1-3). Thus, the development of effective treatments for cognitive deficits remains a major goal of schizophrenia research (4).
Substantial research has focused on impairments in working memory and cognitive control that are accompanied by altered activation of the dorsolateral prefrontal cortex (5-7). Studies examining neural activity using functional magnetic resonance imaging (fMRI) have indicated that individuals with schizophrenia exhibit an altered relationship with working memory load, behavioral performance, and dorsolateral prefrontal cortex activation (8).
In nonhuman primates, activity of gamma-aminobutyric acid (GABA) neurons in the dorsolateral prefrontal cortex is essential for normal working memory function (9), which suggests that altered GABA neurotransmission in the dorsolateral prefrontal cortex could contribute to the cognitive impairments in schizophrenia patients. Consistent with this hypothesis, postmortem studies have repeatedly found reduced levels of mRNA for the 67 kilodalton isoform of glutamic acid decarboxylase (GAD67)—the major determinant of GABA levels—in the dorsolateral pre-frontal cortex of individuals with schizophrenia (10-14). The affected GABA neurons include those that express the calcium binding protein parvalbumin. In contrast, the estimated 50% of GABA neurons that express the calcium binding protein calretinin appear to be unaffected (15). Parvalbumin-positive neurons include the chandelier subclass; the axon terminals of these neurons synapse exclusively on the axon initial segments of pyramidal neurons. In the dorsolateral prefrontal cortex of individuals with schizophrenia, GABA plasma membrane transporter-1 (GAT-1) immunoreactivity is reduced in chandelier axon terminals (16), whereas in the postsynaptic axon initial segments of pyramidal neurons immunoreactivity for the α2 subunit of the GABA type A (GABAA) receptor is markedly increased (17). Together, these findings suggest that reduced expression of GAD67 mRNA in protein parvalbumin-positive chandelier neurons results in decreased GABA synthesis and release. These changes appear to be accompanied by compensatory, but insufficient, responses that include decreased presynaptic GABA reuptake and upregulated postsynaptic GABAA receptors (18).
Reduced GABA signaling from chandelier cells to pyramidal neurons could contribute to working memory dysfunction via a pathophysiological mechanism involving protein parvalbumin-positive GABA neurons. Parvalbumin-positive GABA neurons regulate the synchronized oscillatory activity of cortical pyramidal neurons in the gamma band range (30–80 Hz) (19). Gamma band oscillations in the human dorsolateral prefrontal cortex increase in proportion to working memory load (20), and frontal lobe gamma band oscillations were reduced during a delay-dependent cognitive control task among individuals with schizophrenia (21). Consequently, a deficit in the synchronization of pyramidal cell activity, resulting from altered regulation by parvalbumin-positive GABA neurons, is hypothesized to contribute to both reduced gamma band oscillations and impaired dorsolateral prefrontal cortex-dependent cognitive performance in schizophrenia patients (18).
This hypothesis suggests that a positive allosteric modulator (i.e., a drug that increases chloride ion flow through GABAA receptors only when GABA is bound to the receptor) with selective activity at GABAA receptors containing the α2 subunit might have beneficial effects in schizophrenia patients. By augmenting GABA neurotransmission from chandelier neurons, such a medication is predicted to enhance the synchronization of pyramidal neuron activity at gamma band frequencies and thereby improve the function of dorsolateral prefrontal cortex circuitry in individuals with schizophrenia (22). In contrast, the adverse cognitive effects and sedation associated with the benzodiazepines currently available (which are attributable to their activity at GABAA receptors containing α1 and α5 subunits) are likely to mask the hypothesized cognitive benefits associated with α2 selectivity (23). As a proof-of-concept test of this hypothesis, we conducted a randomized double-blind, placebo-controlled trial to determine whether the Merck compound MK-0777, a benzodiazepine-like agent with selective activity at GABAA receptors containing α2 or α3 subunits, would improve prefrontal-mediated cognitive functions and gamma oscillations in schizophrenia patients.
Method
Subjects
Male outpatients, ages 18–50 years, who were enrolled in mental health programs affiliated with the University of Pittsburgh Medical Center and had a clinical diagnosis of schizophrenia, were invited to participate in the study. Of the 29 patients who gave written informed consent, 16 met the study inclusion criteria after formal screening. All enrolled subjects 1) met DSM-IV criteria for schizophrenia or schizoaffective disorder based on Structured Clinical Interview for DSM-IV and consensus diagnosis, 2) had been clinically stable on fixed doses of antipsychotics ≥3 months, 3) were unemployed, 4) had an IQ ≥80, and 5) had a baseline score <90 on the Repeatable Battery for the Assessment of Neuropsychological Status (24). The remaining 13 subjects were not eligible to participate in the study as a result of one or more of the following prespecified exclusion criteria: 1) current use of a benzodiazepine; 2) positive urine screen for substances of abuse; 3) history of psychoactive substance dependence within the past 6 months or substance abuse within the past month; 4) history of head trauma, seizure disorder, or ECT within the past year; and 5) use of medications that might be adversely affected by the MK-0777 compound (e.g., clinically relevant inhibitors or inducers of CYP3A4). Fifteen subjects comprised the final study sample (Table 1), since one of the 16 subjects who met inclusion criteria discontinued the study medication after 11 days, reporting that he had developed feelings of depression, irritability, and decreased appetite over the preceding 4 days. The subject was assigned to receive the MK-0777 compound. Per the study protocol, the blind was maintained and randomization of subsequent subjects continued.
TABLE 1.
Demographic and Clinical Characteristics for Schizophrenia Patients Randomly Assigned to MK-0777 or Placebo
| Treatment Group |
Age (years) |
Education (years) |
Marital Status |
Age at Onset of Illness (years) |
Duration of Illness (years)a |
Antipsychotic Treatment |
|---|---|---|---|---|---|---|
| Placebo | 31 | 12 | Single | 19 | 12 | Clozapine (325 mg/day) |
| MK-0777 | 46 | 12 | Married | 43 | 3 | Risperidone (4 mg/night) |
| MK-0777 | 23 | 10 | Separated | 20 | 3 | Risperidone (1 mg/night and 25 mg [intramuscular]/2 weeks) |
| Placebo | 34 | 14 | Single | 20 | 14 | Clozapine (100 mg/morning and 300 mg/night); Risperidone (2 mg/night) |
| MK-0777 | 49 | 13 | Single | 21 | 28 | Clozapine (375 mg/night) |
| MK-0777 | 46 | 12 | Single | 21 | 25 | Risperidone (3 mg/night) |
| Placebo | 38 | 13 | Divorced | 15 | 24 | Aripiprazole (30 mg/day) |
| Placebo | 48 | 10 | Single | 17 | 31 | Aripiprazole (30 mg/day); Haloperidol (6 mg/night) |
| MK-0777 | 50 | 9 | Single | 21 | 29 | Risperidone (2 mg/day); Haloperidol decanoate (25 mg/month) |
| MK-0777 | 45 | 16 | Single | 28 | 18 | Olanzapine (20 mg/night) |
| MK-0777 | 40 | 9 | Single | 17 | 24 | Olanzapine (7.5 mg/night); Fluphenazine decanoate (50 mg/2 weeks) |
| Placebo | 34 | 7 | Single | 25 | 9 | Risperidone (3 mg/night and 37.5 mg [intramuscular]/2 weeks); Ziprasidone (80 mg b.i.d.) |
| Placebo | 29 | 8 | Single | 29 | 1 | Quetiapine (800 mg/night) |
| MK-0777 | 31 | 10 | Single | 19 | 12 | Olanzapine (10 mg/night) |
| MK-0777 | 24 | 13 | Single | 18 | 6 | Risperidone (3 mg/night) |
Duration of illness was measured from the onset of the first psychotic symptoms to the date of consent to participate in the study.
Study Design
This double-blind, placebo-controlled clinical trial used a randomization procedure established by a statistician unaffiliated with the study. Nine subjects received the MK-0777 compound, a selective GABAA receptor α2,3 subunit partial agonist. Since MK-0777 lacks agonist activity at GABAA receptors containing α1 subunits, it does not produce the sedative effects of benzodiazepines (25). The initial dose of MK-0777 was 3.0 mg b.i.d. The dosage increased to 5.0 mg b.i.d. at the end of week 1 and 8.0 mg b.i.d at the end of week 2, which was continued for the remaining 2 weeks of the trial. Six subjects received matching placebo tablets. Medications were dispensed weekly in blister packs by the hospital pharmacy.
Following the baseline screening, subjects were assessed with the Brief Psychiatric Rating Scale (BPRS) at each of five weekly visits. A 10-point Likert scale (1=minimal to 10=severe) was used to assess the presence and severity of any adverse effects at each visit. Potential withdrawal symptoms were assessed 1 week after discontinuation of the study medication. An ECG, complete ophthalmology exam, and laboratory exam (i.e., blood count and liver and renal function tests) were obtained at the beginning and end of the trial. Neuropsychological assessments were conducted at baseline and after 2 and 4 weeks of drug administration. The 2-week test point was included in order to maximize the availability of data for each subject in the event that a subject withdrew from the trial as a result of side effects from the 8.0 mg dose of MK-0777. Since all subjects who completed the assessments at week 2 also completed the assessments at week 4, only the latter data are reported.
Neuropsychological Assessments
Repeatable Battery for the Assessment of Neuropsychological Status
The Repeatable Battery for the Assessment of Neuropsychological Status (24, 26) consists of subtests combined to form five index scale scores (immediate memory, language, visuospatial, attention, and delayed memory) and a total score. Index and total scores are expressed as standardized scores normalized to a population mean of 100, with a standard deviation of 15. All subjects received the “A” form at baseline and at the week-4 visit and the “B” form at the week-2 visit.
An increase of 10 points for the Repeatable Battery for the Assessment of Neuropsychological Status total score may be considered a strong signal of response, since 1) 10 points is equivalent to two-thirds of the difference in mean total scores between employed and unemployed schizophrenia subjects (24), and 2) scores change minimally after 12 weeks of treatment with placebo (27). As a result of the “experimental medicine probe” design and limited statistical power of the present study, a simple decision rule was developed based on the number of patients in both the placebo and active-treatment groups who showed improvement ≥10 points. Specifically, an improvement ≥10 points in the total score at the end of week 4 for ≥6 MK-0777-treated subjects and for ≤1 subject in the placebo group was determined, a priori, to constitute evidence of a positive clinical outcome.
Cognitive Tasks
For each of three cognitive tasks, subjects sat approximately 75 cm from a 15-in cathode ray tube monitor. Stimuli were generated and responses recorded using E-Prime (Psychology Software Tools, Pittsburgh). Responses were recorded using a button box or computer keyboard (see the data supplement accompanying the online version of this article).
The N-back task is a sequential-letter memory task for which working memory load is varied (28). Subjects with schizophrenia exhibit substantially impaired performance and decreased functional activation of the dorsolateral prefrontal cortex relative to matched comparison subjects for the 2-back load condition (29).
For the AX Continuous Performance Test, subjects are required to maintain an attentional set across a delay interval in order to overcome a prepotent response tendency (30). Under certain task conditions, subjects with schizophrenia perform worse than comparison subjects, and their impaired performance is associated with decreased dorsolateral prefrontal cortex activity (31).
The Preparing to Overcome Prepotency task is a cued stimulus-response reversal paradigm that, similar to the AX Continuous Performance Test, requires increases in cognitive control through the maintenance and use of context information to overcome prepotent response tendencies (21).
Electrophysiology
During the Preparing to Overcome Prepotency task, EEG data were acquired via a method previously described (21) (see the data supplement accompanying the online version of this article). Time-frequency analyses were conducted using Brain Vision Analyzer (Brain Products GmbH, Munich, Germany). For analysis of gamma band oscillations, we examined 40 Hz induced activity (i.e., not time locked to stimulus), which has been shown to be reduced in frontal brain areas in schizophrenia patients relative to healthy subjects while performing the Preparing to Overcome Prepotency task (21). Data were referenced to a −200 to −50 msec precue baseline interval.
Data Analysis
Data for BPRS and Repeatable Battery for the Assessment of Neuropsychological Status were assessed using analysis of variance (ANOVA). Behavioral analyses for the cognitive tasks were conducted separately, with reaction time and error rates as dependent measures. Given the modest sample size of the present study, multivariate analyses of variance (MANOVAs) were conducted in order to pool effects across tasks. Nine MK-0777-treated subjects and five subjects in the placebo group had sufficient data for both N-back and Preparing to Overcome Prepotency tasks, and these data were included in the MANOVAs. Since four subjects did not complete a sufficient number of trials for the AX Continuous Performance Test task at both testing periods, data from this task were analyzed separately in an ANOVA for seven MK-0777-treated subjects and four subjects in the placebo group. For MANOVAs, the dependent measure was a change from baseline measures (i.e., performance differences between the week-4 and baseline time points). Thus, group (MK-0777-treated group versus placebo group) was the only factor. For ANOVA, the model was a repeated-measures design with group as a between-subjects factor and time (baseline versus week 4) as a within-subjects factor.
For MANOVAs, the dependent measure for the N-back task was performance in the 2-back condition, which provides the best index of performance and dorsolateral prefrontal cortex disturbances in subjects with schizophrenia (29). The dependent measure for the Preparing to Overcome Prepotency task was the performance difference between the high versus low control conditions, which indexes cognitive control impairments in schizophrenia patients (21). For AX Continuous Performance Test data, the dependent measure was d′ at the long delay (calculated as AX hits minus BX false alarms [see the data supplement accompanying the online version of this article]), which is particularly sensitive to context processing impairments in individuals with schizophrenia (32).
EEG analysis involved t tests for treatment group differences in induced gamma power across high versus low control conditions across time (baseline versus week 4). The focus of this analysis was the delay period (500–1,500 msec relative to cue onset) during which schizophrenia subjects exhibit disturbances in the ability to appropriately modulate induced frontal gamma activity in accordance with cognitive control demands (21). Since many samples were acquired during the delay period (250 samples over 1,000 msec), data for this interval were divided into 250-msec nonover-lapping bins in order to reduce the number of comparisons.
Results
Clinical Measures
As detailed in Table 1, placebo subjects and subjects in the MK-0777-treated group did not differ in mean age (placebo group: 35.7 years [SD=6.8]; MK-0777-treated group: 39.3 years [SD=10.6]; t=−0.746, df=13, p=0.47) and education (placebo group: 10.7 years [SD=2.8]; MK-0777-treated group: 11.6 years [SD=2.3]; t=-0.673, df=13, p=0.5). In each group, 67% of the subjects were African American. Two subjects in each group were Caucasian, and one MK-0777-treated subject was Asian American. Only three subjects in the entire study sample had ever been married. Single subjects were 78% of the MK-0777-treated group and 83% of the placebo group. The mean age at onset of schizophrenia (t=−1.087, df=13, p=0.3) did not differ between placebo subjects (20.8 years [SD=5.2]) and MK-0777-treated subjects (23.1 years [SD=8.1]). One subject in the MK-0777-treated group had a relatively late onset of illness at age 43. The duration of illness (approximately 16 years) was very similar for both groups (t=0.016, df=13, p=0.99).
At baseline, mean total BPRS scores (t=3.23, df=13, p=0.02) were significantly higher for the placebo group (34.2 [SD=7.1]) relative to the MK-0777-treated group (24.3 [SD=2.6]). The between-group differences were primarily a result of subjects in the placebo group reporting greater positive (t=2.30, df=13, p=0.07) and negative (t=2.46, df=13, p=0.03) symptoms relative to subjects in the MK-0777-treated group. At week 4, mean BPRS total scores were nearly the same for the placebo group (27.0 [SD=3.0]) and MK-0777-treated group (26.3 [SD=5.3]) as a result of a significant reduction in positive symptoms reported for the placebo group (F=9.12, df=1, 13, p=0.01). For the MK-0777-treated group, neither total BPRS scores nor any of the factor scores changed over the course of the trial.
Few of the 15 subjects who completed the trial reported any side effects, and these side effects were generally mild in nature. During the final week of drug administration, three subjects in the MK-0777-treated group reported one adverse effect, and one subject reported two adverse effects (Table 2). No withdrawal symptoms were reported by any subject 1 week after drug discontinuation.
TABLE 2.
Reported Side Effects for Schizophrenia Patients Randomly Assigned to MK-0777 or Placebo
| Placebo Group (N=6) |
MK-0777 Group (N=9) |
|||||
|---|---|---|---|---|---|---|
| Side Effect | Pre-Trial Baseline |
Week 4 | Post-Trial Week 1 |
Pre-Trial Baseline |
Week 4 | Post-Trial Week 1 |
| Dizziness | 0 | 0 | 0 | 1 (1)a | 2 (4, 4)a | 0 |
| Somnolence | 0 | 0 | 0 | 0 | 2 (2, 6)a | 0 |
| Insomnia | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 |
| Dry mouth | 0 | 0 | 0 | 0 | 0 | 0 |
| Appetite increase | 0 | 0 | 0 | 0 | 1 (7)a | 0 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 |
| Impaired coordination | 0 | 0 | 0 | 0 | 0 | 0 |
Severity ratings for reported symptoms are in parentheses (1=minimal to 10=severe).
Neuropsychological Assessments
For all subjects, the mean Repeatable Battery for the Assessment of Neuropsychological Status total score at baseline was 68.5, which is more than two standard deviations below that previously reported for healthy comparison subjects (24). Both the placebo group and MK-0777-treated group had higher total Repeatable Battery for the Assessment of Neuropsychological Status scores at week 4, and the magnitude of improvement was rather similar for both groups (Table 3). Only one MK-0777-treated subject and two placebo subjects showed a ≥10-point increase in total Repeatable Battery for the Assessment of Neuropsychological Status scores. Thus, criteria for the prespecified decision rule for a positive clinical outcome were not met. However, of the five subindices, scores for the mean delayed memory index showed significant improvement (F=5.47, df=1, 13, p=0.04) for MK-0777-treated subjects from baseline (76.8 [SD=15.5]) to week 4 (85.3 [SD=15.7]) relative to changes in the placebo group (baseline score=60.8 [SD=16.1]; week 4 score=63.8 [SD=17.6]). The between-group effect size for this measure was 0.53.
TABLE 3.
Repeatable Battery for the Assessment of Neuropsychological Status Scores for Schizophrenia Patients Randomly Assigned to MK-0777 or Placebo
| Repeatable Battery for the Assessment of Neuropsychological Status Score |
Placebo Group (N=6) |
MK-0777 Group (N=9) |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Week 4 |
Baseline |
Week 4 |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Total | 63.5 | 7.1 | 70.8 | 12.4 | 71.8 | 10.6 | 76.7 | 13.5 |
| Immediate memory | 61.2 | 10.7 | 66.8 | 22.7 | 71.0 | 17.3 | 76.4 | 21.0 |
| Visual spatial/constructional | 71.8 | 13.9 | 83.0 | 11.2 | 73.3 | 9.8 | 77.8 | 15.0 |
| Language | 81.7 | 11.1 | 83.0 | 8.7 | 84.4 | 12.1 | 83.0 | 11.6 |
| Attention | 78.5 | 7.3 | 90.2 | 17.8 | 82.8 | 12.1 | 84.2 | 16.5 |
| Delayed memory | 60.8 | 16.1 | 63.8 | 17.6 | 76.6 | 15.5 | 85.3* | 15.7 |
p<0.05.
Cognitive Tasks
A MANOVA conducted for reaction time measures for the N-back and Preparing to Overcome Prepotency tasks revealed a significant group effect (F=5.93, df=2, 11, p<0.05). Examination of performance on each task using ANOVAs indicated that the MK-0777-treated group showed significant improvements from baseline, compared with the placebo group, on the Preparing to Overcome Prepotency task (F=4.87, df=1, 13, p=0.05) and a tendency toward a similar result for the N-back task (F=2.28, df=1, 13, p=0.16). To determine whether group baseline differences (Table 4) represented a confound (e.g., mediated by regression to the mean), a follow-up MANCOVA was conducted, with baseline values as a covariate, and revealed that the group effect remained significant (F=4.26, df=2, 9, p=0.05). The MANOVA for error rate measures yielded no significant group effect (F=1.36, df=2, 11, p=0.3). A MANCOVA conducted with baseline as a covariate did not appreciably change the results (F=2.43, df=2, 9, p=0.14).
TABLE 4.
Behavioral and Cognitive Performance Tasks for Schizophrenia Patients Randomly Assigned to MK-0777 or Placebo
| Performance Task | Placebo Group |
MK-0777 Group |
Between- Group Effect Sizea |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Week 4 |
Baseline |
Week 4 |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| AX Continuous Performance Test task b | |||||||||
| d′ | 0.7 | 0.9 | 0.7 | 0.9 | 0.9 | 0.7 | 1.9 | 0.9 | 1.07 |
| N-back task c | |||||||||
| Reaction time | 669 | 158 | 684 | 101 | 874 | 238 | 786 | 231 | 0.84 |
| Error rate | 0.250 | 0.028 | 0.297 | 0.075 | 0.247 | 0.102 | 0.239 | 0.062 | 0.83 |
| Preparing to Overcome Prepotency task d | |||||||||
| Reaction time | 36 | 61 | 66 | 55 | 66 | 48 | 57 | 66 | 1.74 |
| Error rate | 0.064 | 0.068 | 0.031 | 0.074 | 0.034 | 0.050 | 0.042 | 0.042 | −0.35 |
Positive effect sizes indicate that the MK-0777-treated group showed the hypothesized performance improvements (relative to the placebo group). Only the Preparing to Overcome Prepotency task error rates showed effects in the opposite direction.
Data for the d′ long-delay condition are reported.
Data for the 2-back condition are reported.
Data for performance differences between high and low control conditions are reported.
The ANOVA conducted for the long-delay d′ measure of the AX Continuous Performance Test task was not significant but was suggestive of a time-by-group interaction (F=2.92, df=1, 9, p=0.12). Post hoc within-group t tests showed that improvements (at week 4 relative to baseline long-delay d′) in the MK-0777-treated group approached significance (t=2.23, df=6, p=0.07), whereas changes in the placebo group did not (t=-0.36, df=3, p=0.74).
Since the study design was underpowered for statistical measures, we also calculated effect sizes (Table 4). For all tasks, positive effect sizes (0.83–1.74) indicated that the MK-077-treated group showed the hypothesized performance improvements relative to the placebo group. Only the error rate for the Preparing to Overcome Prepotency task showed effects of the MK-0777 compound in the opposite direction.
Electrophysiology
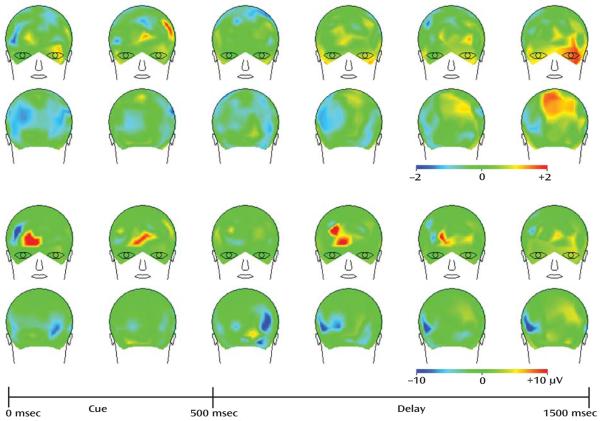
The t tests comparing MK-0777-treated subjects with the placebo group on changes in induced gamma over the 4-week treatment period yielded no significant results at the α=0.05 (t=2.2) level. However, the statistical map of t values (Figure 1) revealed that t values were in the hypothesized direction, particularly during the late-delay period in the left frontal and central parietal regions. These findings are consistent with the hypothesis that frontal gamma disturbances in schizophrenia (21) may be partially ameliorated by short-term treatment with the MK-0777 compound. The mean power values showing increased frontal area gamma band power in the MK-0777-treated subjects relative to placebo subjects across the delay period are illustrated in Figure 1.
FIGURE 1. Mean Power Values for MK-0777-Treated Group Versus Placebo Group Between Baseline and Week 4 for the High Minus Low Control Condition for Each Epoch of the Preparing to Overcome Prepotency Taska.
a The images illustrate EEG findings expressed as the MK-0777 minus placebo group difference between baseline and week 4 for the high minus low control condition for each 250-msec epoch of the Preparing to Overcome Prepotency Task. The top panel illustrates the statistical map of t values. Although statistical significance was not attained, the directionality of the t values are consistent with the hypothesis (that enhancing GABA signaling through GABAA receptors containing an α2 subunit improves the cognitive impairments associated with dorsolateral prefrontal cortex dysfunction in individuals with schizophrenia), particularly during the late-delay period in the left frontal and central parietal regions. The bottom panel illustrates the mean gamma band power values for the same comparison. Frontal area differences across the delay period are shown. Although similar in appearance in some portions of the spatiotemporal layout, the top and bottom panels are not closely matched, since the t values incorporate both mean and variance data, and the power values incorporate only mean values.
Discussion
The present study was designed as an initial test of the hypothesis that enhancing GABA signaling through GABAA receptors containing an α2 subunit improves the cognitive impairments associated with dorsolateral prefrontal cortex dysfunction in individuals with schizophrenia. Although the study drug was well-tolerated, delayed memory was the only domain of neuropsychological function that showed improvement on the Repeatable Battery for the Assessment of Neuropsychological Status. However, evidence of improvement in the MK-0777-treated group was found on each of three cognitive tests that tap cognitive processes mediated by the circuitry of the dorso-lateral prefrontal cortex and that are known to be impaired in schizophrenia patients. Furthermore, this improvement was accompanied by increased frontal gamma band power. Thus, the results of this preliminary study provide suggestive evidence supporting the hypotheses that 1) reduced GABA neurotransmission through GABAA receptors containing an α2 subunit is associated with impaired frontal gamma oscillations and cognitive performance (18), and 2) selective positive modulation of α2 containing GABAA receptors can lead to improvement in these measures (22).
However, there are several limitations to the study. The “experimental medicine probe” design was known at the outset to be limited in statistical power as a result of the small sample size. Perhaps of equal importance to the sample size limitation is the question of whether the Repeatable Battery for the Assessment of Neuropsychological Status has the appropriate sensitivity for a short-term study designed to test the ability of a drug to normalize the pathophysiology linking the neurobiological and cognitive alterations in schizophrenia. Neuropsychological test batteries, such as the Repeatable Battery for the Assessment of Neuropsychological Status and NIMH's MATRICS initiative (i.e., Measurement and Treatment Research to Improve Cognition in Schizophrenia), are advantageous for larger clinical trials because their psychometric properties, such as test-retest reliability and floor and ceiling effects, are thoroughly characterized. However, practice effects have been observed with such batteries (33). Indeed, the total and subtest scores for the Repeatable Battery for the Assessment of Neuropsychological Status improved in both the placebo and active-treatment groups in the present study, which might have obscured a real drug effect. Perhaps more importantly, a global summary score from these test batteries measures the engagement of multiple complex cognitive systems. If a drug has its effect on one specific cognitive system (e.g., prefrontal-dependent cognitive control functions regulating working memory and response inhibition), then more fine-grained cognitive measures that target those specific functions (e.g., the N-back task and AX Continuous Performance Test) may provide a more sensitive approach to measuring drug effects (34). The moderate to large effect sizes seen with these tasks in our data suggest that this might be the case for the MK-0777 compound. Furthermore, the improvement in delayed memory in the MK-0777-treated subjects supports the hypothesis that the information value of early-phase clinical trials is likely to be improved by the inclusion of selective probes that tap specific cognitive processes.
The randomized assignment of subjects resulted in between-group differences in baseline measures of clinical symptoms (BPRS) and neuropsychological function (Repeatable Battery for the Assessment of Neuropsychological Status). Whether these differences blunted or heightened the magnitude of the effect of MK-0777 can only be resolved by larger trials in which the randomization is more likely to result in subject groups with equivalent baseline performance levels. It is also important to note that the inclusion criterion of a baseline Repeatable Battery for the Assessment of Neuropsychological Status score <90 excluded subjects with more modest cognitive impairments who might be more likely to benefit from pharmacological interventions (35).
In addition to the target and compound, this treatment approach is novel in that the therapeutic intervention was designed to augment a compensatory response (i.e., increased density of α2 containing GABAA receptors) as opposed to correcting a detrimental molecular abnormality. That is, the reduced expression of GAD67 mRNA in protein parvalbumin-positive chandelier neurons has been interpreted to result in decreased GABA synthesis and release and to induce compensatory responses (18). For example, the reduced levels of GAT-1 in chandelier axon terminals in schizophrenia (16) are predicted to enhance GABA neurotransmission, since the blockade of GABA reuptake prolongs the duration of inhibitory postsynaptic currents when synapses located close to each other are activated synchronously (36). The prolongation of inhibitory postsynaptic currents increases the probability of inhibitory postsynaptic current summation, which increases the efficacy of inhibitory postsynaptic current trains. Similarly, the upregulation of the postsynaptic GABAA receptors containing α2 subunits in schizophrenia is predicted to increase the efficacy of the GABA that is released from chandelier neurons. However, the presence of cognitive impairments in individuals with schizophrenia indicates that these neuroplastic changes are insufficient as compensatory responses. Thus, as suggested by the results of the present study, pharmacological augmentation of these compensatory responses might be of therapeutic value.
Synchronization of cortical neuronal activity at gamma frequencies appears to be important for a number of perceptual and higher cognitive processes, such as working memory (20, 37). The precise mechanisms by which gamma oscillations support perception and cognition have been the subject of much theorizing, but both empirical (38, 39) and theoretical (40, 41) evidence suggest that synchronous oscillations serve as a mechanism by which cortical circuits can coordinate activity to support cognitive functions. For example, appropriate subsets of neurons can engage in such oscillations to represent specific information, such as location in a spatial working memory task (38, 39), and intracranial recordings in the human dorsolateral prefrontal cortex have demonstrated that gamma band power increases in proportion to working memory load (20). We previously reported that a cognitive control task was associated with increased induced gamma activity over prefrontal cortical areas in healthy comparison subjects but not in schizophrenia patients (21). The disturbances in gamma activity in patients also correlated with disorganization symptoms, similar to previous findings demonstrating that the degree of impaired prefrontal activation (as assessed by fMRI) in a similar cognitive control task predicted clinical ratings of disorganization (29). These findings indicate that disturbances in induced gamma oscillations might underlie the dorsolateral prefrontal cortex-mediated cognitive deficits in schizophrenia. Although the sample size was small and clearly requires replication and extension, the present findings, which suggest that there is increased gamma band power and improved cognitive performance following MK-0777 treatment, support this interpretation.
The potential clinical value of augmenting GABA neurotransmission via α2 containing receptors awaits the results of larger trials of the MK-0777 compound as well as future trials of other compounds currently in development that possess greater potency and selectivity for α2 containing GABAA receptors. In addition to an adjuvant treatment for cognitively impaired individuals with chronic schizophrenia, such compounds offer potential promise for early intervention for high-risk individuals. The presence of cognitive impairments before the onset of psychosis in such individuals suggests that the target clinical features and underlying molecular circuitry alterations are present. Furthermore, the potential for anxiolytic effects with positive modulation at α2 containing GABAA receptors (42) raises the possibility that such agents could also attenuate the increased stress responsivity that frequently precedes the onset of psychosis.
Acknowledgments
Dr. Lewis currently receives research support from the Bristol-Myers Squibb Foundation, Merck, and Pfizer; he has served as a consultant for Bristol-Myers Squibb, Lilly, Merck, Neurogen, Pfizer, Hoffman-Roche, Sepracor, and Wyeth. Dr. Carter has served as a consultant for Eli Lilly, Pfizer, and Hoffman-Roche. Drs. Cho, Kelly, and Montrose and Mr. Eklund and Ms. Forster report no competing interests.
Supported by Merck and NIH grants MH-043784 and MH-045156.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIMH or NIH.
The authors thank Dr. Allan Sampson for constructing the randomization procedure and the staff of the Western Psychiatric Institute and Clinic pharmacy for their assistance.
Footnotes
ClinicalTrials.gov Identifier: NCT00129441.
References
- 1.Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, Hoblyn J, Davis KL. Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. Am J Psychiatry. 1998;155:1080–1086. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- 4.Hyman SE, Fenton WS. Medicine: What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 6.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 7.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 8.Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- 9.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of pre-frontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 11.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor Trkb to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo T-U, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volk DW, Pierri JN, Fritschy J-M, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 18.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 19.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 21.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis DA, Volk DW, Hashimoto T. Selective alterations in pre-frontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 23.de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoe-maker RC, Rijnbeek B, Cohen AF, Vega JM, Agrawal NG, Goel TV, Simpson RC, Pearson LK, Li S, Hesney M, Murphy MG, van Gerven JM. Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2, 3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J Psychopharmacol. 2007;21:374–383. doi: 10.1177/0269881106072343. [DOI] [PubMed] [Google Scholar]
- 24.Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable Battery for the Assessment of Neuropsychological Status as a screening test in schizophrenia, I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156:1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- 25.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KM, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 26.Wilk CM, Gold JM, Bartko JJ, Dickerson F, Fenton WS, Knable M, Randolph C, Buchanan RW. Test-retest stability of the Repeatable Battery for the Assessment of Neuropsychological Status in schizophrenia. Am J Psychiatry. 2002;159:838–844. doi: 10.1176/appi.ajp.159.5.838. [DOI] [PubMed] [Google Scholar]
- 27.Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158:2071–2074. doi: 10.1176/appi.ajp.158.12.2071. [DOI] [PubMed] [Google Scholar]
- 28.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 29.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 31.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 32.Barch DM, Carter CS, MacDonald AW, III, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- 33.Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: Is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 34.Carter CS. Applying new approaches from cognitive neuro-science to enhance drug development for the treatment of impaired cognition in schizophrenia. Schizophr Bull. 2005;31:810–815. doi: 10.1093/schbul/sbi046. [DOI] [PubMed] [Google Scholar]
- 35.Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large-scale, double-blind, randomized study. Psychopharmacology (Berl) 2003;169:404–411. doi: 10.1007/s00213-002-1342-5. [DOI] [PubMed] [Google Scholar]
- 36.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 39.Camperi M, Wang XJ. A model of visuospatial working memory in prefrontal cortex: recurrent network and cellular bistability. J Comput Neurosci. 1998;5:383–405. doi: 10.1023/a:1008837311948. [DOI] [PubMed] [Google Scholar]
- 40.Traub RD, Bibbig A, LeBeau FE, Cunningham MO, Whittington MA. Persistent gamma oscillations in superficial layers of rat auditory neocortex: experiment and model. J Physiol. 2005;562(pt 1):3–8. doi: 10.1113/jphysiol.2004.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy J-M, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]