Abstract
The rising number of antibiotic-resistant bacterial strains represents an emerging health problem that has motivated efforts to develop new antibacterial agents. Endogenous cationic antibacterial peptides (CAPs) that are produced in tissues exposed to the external environment are one model for the design of novel antibacterial compounds. Here, we report evidence that disubstituted dexamethasone-spermine (D2S), a cationic corticosteroid derivative initially identified as a by-product of synthesis of dexamethasone-spermine (DS) for the purpose of improving cellular gene delivery, functions as an antibacterial peptide-mimicking molecule. This moiety exhibits bacterial killing activity against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa present in cystic fibrosis (CF) sputa, and Pseudomonas aeruginosa biofilm. Although compromised in the presence of plasma, D2S antibacterial activity resists the proteolytic activity of pepsin and is maintained in ascites, cerebrospinal fluid, saliva, and bronchoalveolar lavage (BAL) fluid. D2S also enhances S. aureus susceptibility to antibiotics, such as amoxicillin (AMC), tetracycline (T), and amikacin (AN). Inhibition of interleukin-6 (IL-6) and IL-8 release from lipopolysaccharide (LPS)- or lipoteichoic acid (LTA)-treated neutrophils in the presence of D2S suggests that this molecule might also prevent systemic inflammation caused by bacterial wall products. D2S-mediated translocation of green fluorescent protein (GFP)-labeled glucocorticoid receptor (GR) in bovine aorta endothelial cells (BAECs) suggests that some of its anti-inflammatory activities involve engagement of glucocorticoid receptors. The combined antibacterial and anti-inflammatory activities of D2S suggest its potential as an alternative to natural CAPs in the prevention and treatment of some bacterial infections.
The soluble factors expressed by most organisms to kill bacteria are sufficiently general to prevent not only bacterial growth but also bacterial resistance. These factors, known in mammals as cationic antimicrobial peptides (CAPs), are small peptides derived by posttranslational and often extracellular cleavage of precursor proteins that can have functions independent of the antibacterial activity of their cleavage products (3, 7, 28). The relative lack of specificity of CAPs is essential to their general effectiveness and possible lack of bacterial resistance (24). The exact downstream action of CAPs is not yet known, but their initial bacterial targets are well defined. These targets include the outer wall constituents, lipopolysaccharides (LPS) in Gram-negative bacteria, and lipoteichoic acids (LTA) in Gram-positive bacteria (7, 9, 28). Interaction between CAPs and bacterial wall LPS or LTA precedes the insertion of CAPs into the bacterial membrane that results in membrane permeabilization and/or disruption. This effect leads to changes in second messenger systems that further augment the abnormal electrical activity and disrupt signal transduction, causing bacterial death (30). CAPs can also influence both the quality and effectiveness of the immune response (52). Therefore, much of the antibacterial function within the body is mediated by cationic peptides, and the primary factor determining their initial interaction with bacteria is electrostatic. This fundamental electrostatic action of CAPs can be exploited for the development of novel antimicrobial agents. Covalent linkage of the polycation spermine (SP) to a specific site on dexamethasone (DX) allows it to carry DNA across the cell membrane and, by exploiting binding of dexamethasone to the glucocorticoid receptor (GR), delivers it to the nucleus (37). Dexamethasone-spermine (DS) is pharmacologically active: in addition to its ability to induce nuclear localization of the glucocorticoid receptor, it also reduces inflammation in vivo (22). At least partial activity described for DS may be expected from disubstituted DS (D2S). DS and D2S (20, 22, 37) share some structural and functional properties with squalamine, a membrane-active cationic steroid antibiotic (CSA) (34), and some previously described cholic acid antimicrobial derivatives (23, 42). Squalamine, first isolated from tissues of the dogfish shark, and its analogues with different polyamines added to the 3-keto group by reductive amination (29) effectively kill different strains of bacteria, but mammals appear not to produce cationic steroids as antimicrobial factors (17). Synthetic cationic steroid antibiotics were developed with the intent of mimicking the antibacterial activities of polymyxin B (18). The bactericidal properties of these compounds are due to membrane disruption, and they display a moderate degree of selectivity for prokaryotic over eukaryotic membranes (40). Many cholic acid derivates, such as l-lysine, lysyl-lysine (4), cholic acid with basic amino acids (5, 32), guanidine (41), and spermidine (13), were previously characterized as potent antimicrobial agents that kill a wide range of strains, including multidrug-resistant bacteria (42) and fungi (26). In addition to previously described steroidal conjugates (40), we found that D2S displays strong antibacterial activity with low lytic effect on eukaryotic plasma membranes (20) and inhibits the inflammatory response induced by bacterial wall products in vitro.
MATERIALS AND METHODS
Materials.
Tryptic soy broth (TSB), Mueller-Hinton agar (MHA), and Pseudomonas isolation agar were purchased from Difco (Sparks, MD). Brain heart infusion (BHI) agar was from Emapol (Gdańsk, Poland). Mannitol salt agar (MSA) and an ID 32 Staph kit to identify staphylococcal isolates were from bioMérieux, (La Balme Les Grottes, France). Etests to determine susceptibility to methicillin were obtained from AB Biodisk (Solna, Sweden). The beta-lactamase (cefinase) test was from Becton Dickinson (San Jose, CA). LPS (Escherichia coli, serotype O26:B6), fluorescein isothiocyanate (FITC), and dexamethasone were purchased from Sigma (St. Louis, MO). Pseudomonas aeruginosa Xen 5 (a mucoid clinical strain isolated from human septicemia) that was engineered through conjugation and transposition of a plasmid carrying transposon Tn5 luxCDABE was purchased from Caliper Life Science, Inc. (CA). LL-37 and HB-71 peptides were purchased from Bachem (King of Prussia, PA). Human interleukin-6 (IL-6) and IL-8 enzyme-linked immunosorbent assay (ELISA) kits were obtained from Thermo Fisher Scientific, Inc. (Rockwood, TN). Purified LTA (Staphylococcus aureus) was a kind gift from T. Hartung (University of Konstanz, Germany). A solution of human albumin was from Baxter Healthcare Corporation (Glendale, CA). DS, D2S, and CSA-13 were synthesized as described previously (16, 20). Cystic fibrosis (CF) sputum samples were collected by spontaneous expectoration from patients attending the University of Pennsylvania Health System Adult Cystic Fibrosis Center at Presbyterian Hospital (PA; IRB-803255).
Bacterial killing assay.
The bactericidal activities of spermine, dexamethasone, DS, D2S, and LL-37 against kanamycin-resistant Pseudomonas aeruginosa PAO1 were determined as described previously (8). P. aeruginosa PAO1 was grown to mid-log phase at 37°C (controlled by the evaluation of optical density at 600 nm) and resuspended in phosphate-buffered saline (PBS). The bacterial suspensions were then diluted in solutions containing antibacterial agents by themselves or with CaCl2. When required, D2S and LL-37 were preincubated (3 h, 37°C) in solution at low pH (0.01 M HCl, 150 mM NaCl, pH ∼1.5) with and without addition of pepsin (0.5 mg/ml). After 1 h of incubation with bacteria at 37°C, the suspensions were placed on ice and diluted 10- to 1,000-fold. Ten-microliter aliquots of each dilution were spotted on Pseudomonas isolation agar containing 25 μg/ml of kanamycin for overnight culture at 37°C. The number of colonies at each dilution was counted the following morning. The CFU (per ml) of the individual samples were determined from the dilution factor.
Antimicrobial activity against clinical bacterial strains.
Mannitol salt agar was used to isolate bacteria from clinical specimens. Most Staphylococcus aureus strains were from pus samples (infected surgical wounds, diabetic foot, or furunculus). S. aureus identification was performed with an ID 32 Staph kit, and the results were read using the ATB system (bioMérieux, La Balme Les Grottes, France) according to the manufacturer's instructions. The presence of beta-lactamase and susceptibility to methicillin and vancomycin were determined using cefinase and Etest, respectively. For these assays, aerobic bacterial growth was conducted in BHI agar according to provider recommendations. S. aureus susceptibility to macrolides, lincosamides, and streptogramins B was evaluated using diffusion methods on MHA, with a bacterial inoculum at a density of 0.5 (McFarland scale) (15, 21, 31). The D2S MIC and minimal bactericidal concentrations (MBCs) against different strains of Staphylococcus aureus (8 × 105 CFU/ml) were determined using Mueller-Hinton broth and MHA, respectively. In a different set of experiments, the antibacterial activity of sublethal concentrations of D2S in combination with sublethal concentrations of amoxicillin and clavulanic acid (AMC), tetracycline (T), erythromycin (E), and amikacin (AN) was evaluated against six selected strains of S. aureus with MIC measurements. The bactericidal activities of D2S, HB-71, and LL-37 (Fig. 1) against Pseudomonas aeruginosa in sputum samples collected from CF patients with chronic lung infection were measured as previously described (10). The CF sputum specimens were diluted 10 times, and 100-μl samples were treated with antibacterial agents (10 to 200 μM). After a 1-h incubation at 37°C, the suspensions were placed on ice and diluted 10- to 1,000-fold. Ten-microliter aliquots of each dilution were spotted on Pseudomonas isolation agar plates for overnight culture at 37°C. The number of colonies at each dilution was counted the following morning. The CFU (per ml) of the individual samples were determined from the dilution factor and were used to calculate the percentage of bacterial outgrowth.
FIG. 1.
Structure of LL-37 and HB-71 peptides and D2S molecules. For amino acids, the one-letter code is used. MW, molecular weight.
Biofilm assay.
Starting from an overnight culture of Pseudomonas aeruginosa Xen 5, grown in TSB to late stationary phase, a dilution containing ∼108 CFU/ml was made. For each experiment, bacterial suspensions were placed in 24-well polystyrene plates and a biofilm was allowed to form for 24 h. Bacteria adherent to the plate were considered a biofilm, and cells not adherent to the surface of the plate were considered planktonic and were washed out before D2S addition to individual wells. Biofilm density after D2S treatment was evaluated using crystal violet (CV) staining (0.1%) as described previously (35). The mutagen plasmid (Xen5) gives the P. aeruginosa bacteria a measurable chemiluminescence, which was also quantified using a Fuji Film LAS-300 system before and after D2S treatment as a measure of biofilm viability. Chemiluminescence (densitometry analysis) was performed using Image Gauge (version 4.22) software (Fuji Photo Film Co).
Evaluation of D2S antibacterial activity in different body fluids.
Natural CAPs as a part of the host defense system can kill bacteria on mucosal or skin surfaces, but their antibacterial activity is inhibited in circulation due to interaction with blood lipoproteins. Accordingly, ApoA-1 was found to specifically inhibit the bactericidal ability of cathelicidin LL-37 (47). Many other factors present at infection sites, such as mucins, negatively charged DNA, and F-actin can compromise CAP bactericidal activity as well (8, 27, 48). To assess the D2S potential to kill bacteria in different environments, using a Fuji Film LAS-300 system, we evaluated changes of P. aeruginosa Xen5 luminescence (∼108 CFU/ml) in PBS or PBS mixed with 50% of plasma, ascites, cerebrospinal fluid, saliva, or bronchoalveolar lavage (BAL) fluid at different time points, up to 6 h after D2S addition (10 and 30 μM).
Determination of IL-6 and IL-8 concentrations.
Neutrophils were isolated from human blood using the endotoxin-free lympholyte-poly kit (Cedarlane, Ontario, Canada). At the end of the isolation process, neutrophils were suspended (∼7 × 106 cells/ml) in RPMI buffer containing 2% human albumin and activated with LPS from Escherichia coli (50 ng/ml) or LTA from Staphylococcus aureus (10 μg/ml). When required, SP, DX, DS, or D2S was added to neutrophil samples ∼1 min before LPS or LTA addition. Cell-free neutrophil supernatants were collected 24 h after addition of bacterial wall products by centrifugation (5,000 × g, 5 min) and stored at −80°C until cytokine determination. IL-6 and IL-8 were measured using a sandwich ELISA according to the manufacturer's instructions. The detection limit was 30 pg/ml. Viability of neutrophils under SP, DX, or D2S treatment was evaluated using a manual trypan blue exclusion method.
Cultivation of Saccharomyces cerevisiae and labeling with fluorescein.
Yeast cells were grown in media containing 1% yeast extract, 2% peptone, and 2% glucose at 30°C for 24 h. The cells were then washed twice in distilled water and heat killed in boiling water for 30 min. After one rinse, cells were resuspended in 0.9% NaCl and stored at −20°C. To evaluate phagocytic activity, heat-inactivated cells were labeled with fluorescein isothiocyanate (100 μg/ml) in buffer (0.5 M carbonate, pH 9.5) containing 108 yeast cells. The mixture was incubated for 30 min at 37°C and washed four times in PBS.
Microscopic evaluation of neutrophil morphology and viability.
Neutrophil activation is associated with marked changes in cellular morphology. Typically, shortly after activation by bacteria, fungi, or their products, neutrophils adopt an elongated shape with a rough surface and form protrusions and aggregates. Neutrophils freshly prepared from human blood were subjected to activation with heat-inactivated yeast, and microscopic evaluation at different time points (1 to 12 h) was performed using a Leica microscope (Bannockburn, IL) with a 40× objective. When required, before yeast addition, neutrophils were treated with D2S (10 to 20 μM).
Evaluation of RAW264.7 murine macrophage phagocytic activity.
RAW264.7 murine macrophage cells were seeded at 0.5 × 105 cells/ml in 24-well plates. Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. FITC-labeled yeast were opsonized during 30-min incubation with mouse serum before their addition to cultured RAW264.7 cells. To test whether D2S affected phagocytosis, RAW264.7 cells were treated with D2S (1 to 10 μM) immediately before addition of yeast. Two steps of phagocytosis, engulfment and digestion, were evaluated by fluorescence microscopy. The presence of fluorescent yeast cells or their fragments in the cytoplasm of RAW264.7 cells 24 h and 7 days after treatment was quantified as a measure of engulfment and digestion, respectively. About 100 cells were analyzed per condition.
GFP-GR translocation study.
Bovine aorta endothelial cells (BAECs) were seeded at 1 × 105 cells/ml in 24-well plates and allowed to spread overnight. Cells were transfected with green fluorescent protein (GFP)-GR chimeric protein by adding 450 ng of DNA complexed with 2 μl of Lipofectamine in a 200-μl volume of Optimem growth media to each well of a 24-well plate for ∼1.5 h. The DNA-Lipofectamine complex was then removed, and cells were placed in DMEM supplemented with 0.5% calf serum. This reduced level of calf serum was used to prevent nonspecific translocation of GFP-GR receptors in response to hormones in the serum. After 24 h, GFP-GR expression was observed with ∼70% of cells, and they were then treated with dexamethasone, DS, D2S, CSA-13, and LL-37 (0.01 to 1 μM) for 1 h and imaged. Analysis of GFP-GR translocation was conducted by counting the number of cells in each field of view which had GFP-GR nuclear localization and comparing that with the total number of cells. About 40 cells were analyzed per condition.
RESULTS
D2S bactericidal activity against P. aeruginosa PAO1.
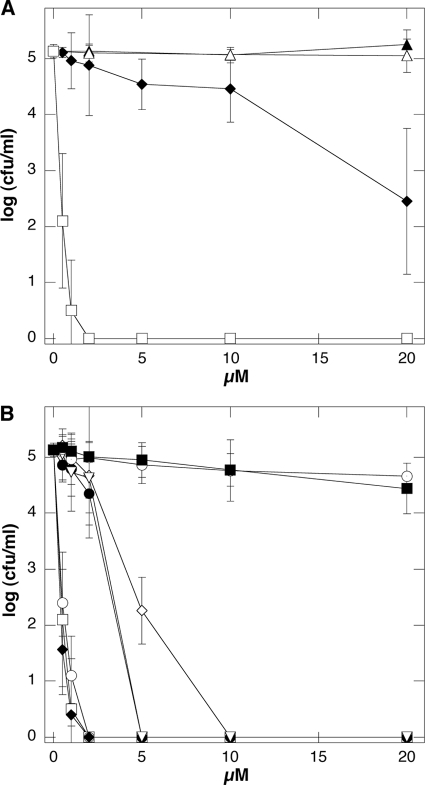
In PBS, DS and D2S exhibit bactericidal activity against P. aeruginosa PAO1, but D2S bactericidal activity is much higher than that of DS (Fig. 2A). In our experimental setting, after 1 h of incubation, SP and DX have not shown any antibacterial activity against P. aeruginosa PAO1 up to 50 μM (Fig. 2A and data not shown). This observation is consistent with previous studies that indicated a lack of or limited antibacterial activity of SP and DX, which was observed only after a long period of incubation (19, 39, 45). D2S bactericidal activity against P. aeruginosa PAO1 was also stronger compared to LL-37 peptide activity and resistant to the proteolytic activity of pepsin. Similar to that of LL-37, D2S activity was found to decrease in the presence of calcium ions (Fig. 2B).
FIG. 2.
(A) Bactericidal activity of dexamethasone (▴), spermine (▵), dexamethasone-spermine (♦), and D2S (□) against P. aeruginosa PAO1. (B) Bactericidal activity of D2S (□) and LL-37 (•) against P. aeruginosa PAO1 and after 3 h of incubation in solution at pH 1.5 (○ and ▿ for D2S and LL-37, respectively), after 3 h of incubation in solution at pH 1.5 containing 0.5 mg/ml of porcine pepsin (♦ and ⊙ for D2S and LL-37, respectively), and in solution containing 2 mM CaCl2 (⋄ and ▪ for D2S and LL-37, respectively). Error bars represent standard deviations from four measurements.
D2S bactericidal activity against clinical strains of S. aureus and P. aeruginosa.
Susceptibility testing of the clinical isolates of S. aureus demonstrates that the D2S MICs and MBCs range between 1.56 and 3.125 μM and 1.56 and 12.5 μM, respectively (Table 1). The observed concentrations that effectively eradicated each of 16 tested clinical strains of S. aureus bacteria were similar to those observed for CSA-13 (data not shown) as well as to previously reported CSA-13 values, when its activity was evaluated against clinical isolates of vancomycin-intermediate S. aureus (VISA), heterogeneous VISA (hVISA), and vancomycin-resistant S. aureus (VRSA) (14). We have not observed any difference in D2S MIC values obtained from four S. aureus groups that we defined based on bacterial susceptibility to methicillin, streptogramins B, and the presence of beta-lactamase (Table 1).
TABLE 1.
MIC and MBC of D2S against different clinical isolates of S. aureus
| Group or strain/descriptiona | Strain | D2S (μM) |
|
|---|---|---|---|
| MIC | MBC | ||
| 1/MRSA MLSB+, β-lactamase+ | 1 | 1.56 | 3.125 |
| 2 | 1.56 | 3.125 | |
| 3 | 3.125 | 6.25 | |
| 4 | 3.125 | 6.25 | |
| 2/MSSA MLSB+, β-lactamase+ | 1 | 1.56 | 3.125 |
| 2 | 1.56 | 6.25 | |
| 3 | 3.125 | 12.5 | |
| 4 | 3.125 | 6.25 | |
| 3/MSSA MLSB−, β-lactamase+ | 1 | 3.125 | 6.25 |
| 2 | 3.125 | 6.25 | |
| 3 | 1.56 | 3.125 | |
| 4 | 1.56 | 3.125 | |
| 4/MSSA MLSB−, β-lactamase− | 1 | 3.125 | 6.25 |
| 2 | 3.125 | 6.25 | |
| 3 | 1.56 | 6.25 | |
| 4 | 1.56 | 6.25 | |
| S. aureus ATCC 29213 | 1.56 | 3.125 | |
Groups 1 to 4 represent data from 4 strains with similar characteristics. MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
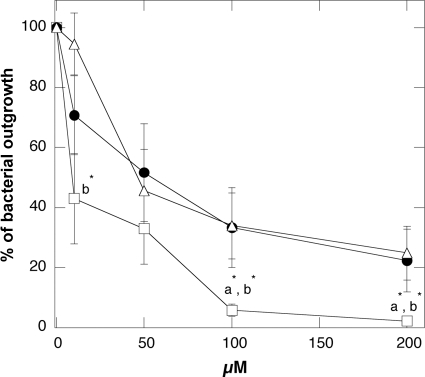
In addition to its intrinsic antibacterial activity, sublethal concentrations of D2S were found to enhance different S. aureus strains' susceptibilities to antibiotics (Table 2). Table 3 shows the reduction in the MICs of amoxicillin and clavulanic acid (AMC), amikacin (AN), and tetracycline (T) when these drugs were used in combination with sublethal concentrations of D2S. This finding suggests possible synergy between generally applied antibiotics (via oral administration or injection) and topical/local administration of D2S. In another set of experiments, we compared the antibacterial activity of D2S against clinical strains of P. aeruginosa to the antibacterial activity of the naturally occurring antibacterial agent LL-37 peptide and a synthetic HB-71 peptide. This comparison, performed in diluted CF sputum samples, reveals lower bacterial outgrowth after D2S addition than after LL-37 or HB-71 addition (Fig. 3).
TABLE 2.
Antibacterial activity of AMC, T, E, AN, and D2S against six different strains of S. aureus
| Bacterial strain/description (strain no.) | MIC (μg/ml)a |
MIC/MBC D2S (μM) | |||
|---|---|---|---|---|---|
| AMC | T | E | AN | ||
| S. aureus ATCC 29213/MSSA, MLSB−, β-lactamase+ (1) | 0.4 (S) | 1.25 (S) | 0.4 (S) | 1.6 (S) | 1.56/3.125 |
| S. aureus ATCC 43300/MRSA, MLSB+, β-lactamase+ (2) | 12.5 (R) | 0.625 (S) | 400 (R) | 25 (I) | 3.125/3.125 |
| S. aureus (clinical strain)/MSSA, MLSB−, β-lactamase+ (3) | 0.2 (S) | 1.25 (S) | 0.2 (S) | 1.6 (S) | 3.125/3.125 |
| S. aureus (clinical strain)/MSSA, MLSB−, β-lactamase+ (4) | 1.6 (S) | 1.25 (S) | 0.4 (S) | 3.2 (S) | 1.56/1.56 |
| S. aureus (clinical strain)/MSSA, MLSB−, β-lactamase+ (5) | 3.2 (S) | 40 (R) | 0.2 (S) | 1.6 (S) | 3.125/3.125 |
| S. aureus (clinical strain)/MRSA, MLSB+, β-lactamase+ (6) | 12.5 (R) | 20 (R) | 400 (R) | 25 (I) | 3.125/3.125 |
S, sensitive; R, resistant; I, intermediate; AMC, amoxicillin and clavulanic acid (S ≤ 4μg/ml; R ≥ 8μg/ml); T, tetracycline (S ≤ 4μg/ml; I = 8μg/ml; R ≥ 16μg/ml); E, erythromycin (S ≤ 0.5 μg/ml; I = 1 to 4μg/ml; R ≥ 8μg/ml); AN, amikacin (S ≤ 16μg/ml; I = 32μg/ml; R ≥ 64μg/ml).
TABLE 3.
Antibacterial activity (MIC) of AMC, T, E, and AN against six selected strains of S. aureus in combination with D2Sa
| Strain no.b | Antibiotic | MIC of antibiotic (μg/ml) | MIC (μg/ml) of antibiotic with D2S at concentration (μM) of: |
||||
|---|---|---|---|---|---|---|---|
| 0.4 | 0.8 | 1.56 | 3.125 | 6.25 | |||
| 1 (3.125) | AMC | 0.4 | ≤0.05 | ||||
| T | 1.25 | 0.625 | 0.321 | 0.321 | ≤0.16 | ||
| E | 0.4 | >0.4 | 0.2 | 0.1 | ≤0.05 | ||
| AN | 1.6 | 0.4 | 0.4 | ≤0.2 | |||
| 2 (3.125) | AMC | 12.5 | 6.25 | 6.25 | 3.125 | ≤1.6 | |
| T | 0.625 | 0.312 | 0.16 | ≤0.08 | |||
| E | 400 | >400 | 400 | 200 | 100 | ≤50 | |
| AN | 25 | 12.5 | 12.5 | ≤6.2 | |||
| 3 (3.125) | AMC | 0.2 | 0.1 | ≤0.05 | |||
| T | 1.25 | 1.25 | 0.625 | 0.321 | ≤0.16 | ||
| E | 0.2 | >0.2 | 0.2 | ≤0.025 | |||
| AN | 1.6 | 0.8 | 0.4 | ≤0.2 | |||
| 4 (1.56) | AMC | 1.6 | ≤0.2 | ||||
| T | 1.25 | 0.321 | ≤0.16 | ||||
| E | 0.4 | >0.4 | 0.2 | ≤0.05 | |||
| AN | 3.2 | 0.8 | ≤0.4 | ||||
| 5 (3.125) | AMC | 3.125 | 0.8 | ≤0.4 | |||
| T | 40 | >40 | 20 | 10 | ≤5 | ||
| E | 0.2 | 0.2 | 0.2 | ≤0.025 | |||
| AN | 1.6 | 0.8 | ≤0.2 | ||||
| 6 (3.125) | AMC | 12.5 | ≤3.125 | ||||
| T | 20 | 20 | 10 | 5 | 5 | ≤2.5 | |
| E | 400 | >400 | >400 | 200 | 200 | ≤50 | |
| AN | 25 | 12.5 | 6.25 | ≤3.125 | |||
AMC, amoxicillin and clavulanic acid; T, tetracycline; E, erythromycin; AN, amikacin. Please see Table 2 for strain characteristics. Numerical values represent antibiotic concentrations (μg/ml).
MBCs (μM) of D2S are indicated by the bold type in parentheses. For each bacterial strain, a positive control of bacterial growth without an antibacterial agent was performed.
FIG. 3.
P. aeruginosa outgrowth from cystic fibrosis sputum samples after 1-h treatment with D2S (□), LL-37 (•), and HB-71 (▵) peptides. Error bars represent standard deviations from eight measurements. *, significantly lower in comparison to LL-37 (a) and HB-71 (b) peptides.
D2S activity against bacteria embedded in biofilm.
Pulmonary infections caused by biofilm-forming P. aeruginosa are major risk factors for high morbidity and mortality in patients with cystic fibrosis. In a biofilm state, bacteria are highly resistant to antibiotics and the host immune system. Figure 4 shows D2S activity in a preformed P. aeruginosa Xen 5 biofilm environment, evaluated by quantifying biofilm mass with CV staining and luminescence, indicative of bacterial viability. Because cells (both living and dead), as well as the matrix, are indistinguishably stained by CV (36), a decrease in CV staining over time after D2S addition indicates mostly a decrease of biofilm formation. However, D2S treatment may also cause the disruption of preformed biofilm. With D2S concentrations above 25 μM, the biofilm mass decreased almost 50%, which is above the estimated biofilm growth. The observed decrease in the luminescence of P. aeruginosa biofilm after D2S treatment indicates a strong ability of these molecules to reach and affect bacterial cells residing in the complex structure of a biofilm mass.
FIG. 4.
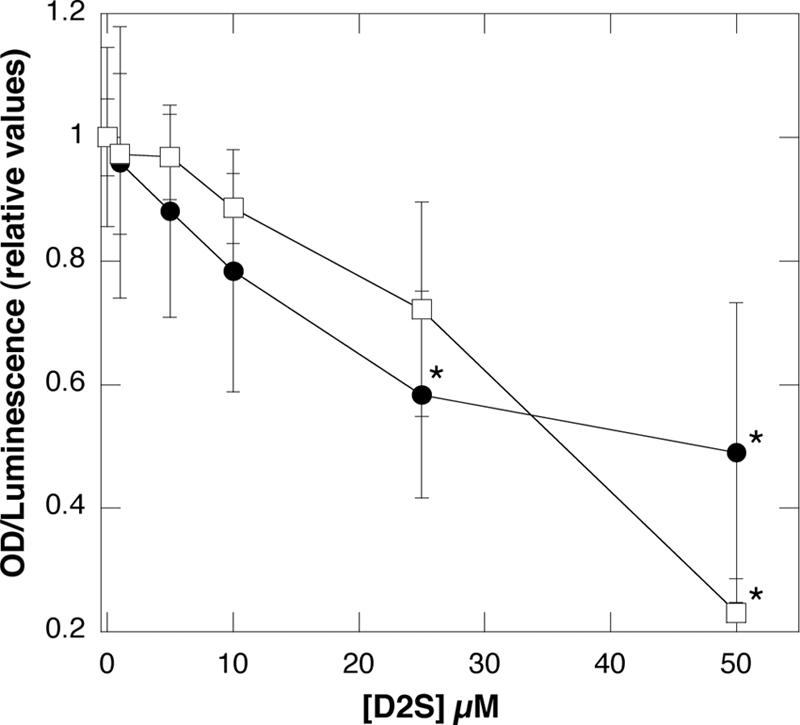
Antibacterial activity of D2S against biofilm of a mucoidal strain of P. aeruginosa Xen 5, evaluated 4 h after D2S administration. Slopes represent relative values of crystal violet absorbance (•) and luminescence (□) that progressively decrease with addition of D2S. Error bars represent standard deviations from three to five measurements. *, significantly different from samples without D2S addition; OD, optical density.
D2S activity in different body fluids.
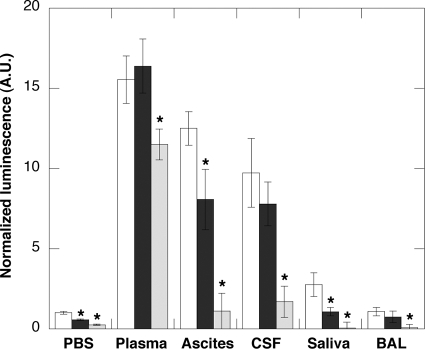
Bacterial infection takes place in various areas of the body which may have variable antibacterial activity depending on the local environment. To assess the potential of D2S to kill bacteria in different body compartments, we evaluated the luminescence of P. aeruginosa Xen 5 bacteria in PBS suspensions containing 50% of different body fluids, including plasma, ascites, cerebrospinal fluid, saliva, and BAL fluid (Fig. 5). Human body fluid specimen collection was performed in accordance with an approved protocol by the Medical University of Białystok Ethics Committee for Research on Humans and Animals (written consent was obtained from all subjects). In this set of experiments, D2S ability to decrease P. aeruginosa Xen 5 luminescence was used as a measure of antibacterial activity. The increased bacterial luminescence that was observed 6 h after bacterial growth in the presence of different body fluids, compared to luminescence in PBS, indicates differences in bacterial growth that likely reflect the availability of nutrition and the variable presence of endogenous host immune defense molecules. Under 10 μM D2S treatment, a decrease of bacterial luminescence was observed with all evaluated body fluids, except for blood plasma specimens. With an increase of D2S concentration from 10 to 30 μM, luminescence decreased significantly for blood plasma as well as the other body fluids. More precisely, luminescence decrease in plasma samples was 26% and 91, 88, 96, and 97% in ascites, cerebrospinal fluid, saliva, and BAL fluid samples, respectively. These data suggest that the antibacterial activity of D2S is compromised in blood plasma, which might limit D2S use for systemic application. However, D2S has potential for local/apical applications, such as in the oral cavity, airways, or skin. The chemical basis whereby D2S antibacterial activity is compromised in plasma is not yet known, but understanding this basis may help to overcome this limitation, with synthetic alteration of D2S structure.
FIG. 5.
Antibacterial activity of D2S against P. aeruginosa Xen 5 in different body fluids was determined based on luminescence reading 3 h after addition of equal amounts of bacteria to equal volumes of PBS or PBS supplemented with 50% of human plasma, ascites, cerebrospinal fluid (CSF), saliva, or bronchoalveolar lavage (BAL) specimens. In each condition, the white column indicates the luminescent signal in control samples. Black and gray columns indicate the luminescent signal in the presence of 10 and 30 μM D2S, respectively. Data from one experiment performed in triplicate are shown. Two other experiments with samples obtained from different subjects show similar trends. *, significantly different from control sample.
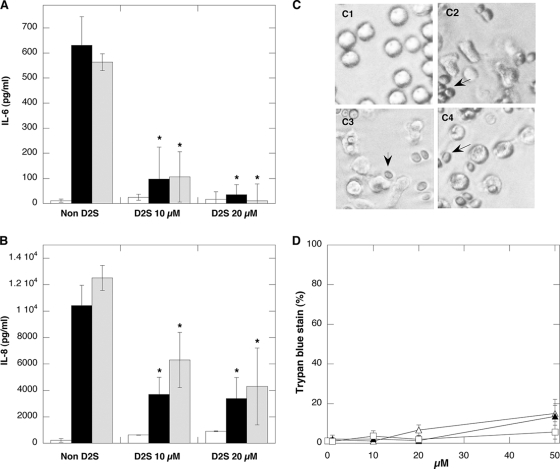
D2S prevents LPS- and LTA-induced release of interleukins from human neutrophils.
IL release in response to bacterial products during infection represents an important step toward development of systemic inflammation. Therefore, developing anti-LPS/LTA molecules might aid in preventing the negative effects of bacterially derived molecules released at infection sites. In human neutrophils, bacterial wall products, such as LPS and LTA, govern IL production via Toll-like receptor (TLR)/NF-kB pathways which trigger the transcription of proinflammatory cytokine genes (43). Accordingly, as shown in Fig. 6, the total IL-6 and IL-8 amounts detected in neutrophil supernatant 24 h after their activation were significantly higher than the amounts detected in nonactivated samples. D2S treatment (10 to 20 μM) results in a significant and concentration-dependent decrease in IL-6 and IL-8 release from neutrophils (Fig. 6). These data are consistent with the hypothesis that D2S binds to LPS and LTA, and this binding results in attenuation of these bacterial wall products' ability to reach and activate TLR. Decreased IL-8 release was also observed in LPS-activated neutrophils in the presence of spermine, dexamethasone, and DS (10 to 20 μM each; data not shown). These observations agree with previous studies reporting downregulation of cytokine synthesis in human mononuclear cells after SP (51, 53) or DX (6) treatment, indicating that the ability of D2S to prevent neutrophil cytokine release is based partially on posttranscriptional mechanisms.
FIG. 6.
D2S inhibits IL-6 (A) and IL-8 (B) release from neutrophils treated with LPS (0.05 μg/ml) from E. coli (black column) or purified LTA (10 μg/ml) from S. aureus (gray column). White columns represent values obtained in control (nontreated samples or neutrophils treated with indicated D2S amount). Error bars represent standard deviations from three measurements performed in duplicate. *, significantly different from neutrophil samples treated with LPS or LTA. Morphology of human neutrophils (C) in resting stage (C1) or with addition of yeast indicated by arrows (C2) in the presence of 10 μM (C3) and 20 μM (C4) D2S. Viability of neutrophils (D) after 1 h of incubation of their suspension with dexamethasone (▴), spermine (▵), and D2S (□). Error bars represent standard deviations from three measurements.
Neutrophil morphology and viability.
Neutrophil activation is associated with significant changes in cellular morphology. As shown in Fig. 6C, neutrophils prepared in sterile conditions maintain a round shape indicative of their resting state. After addition of heat-inactivated yeast (that were opsonized by incubation with human serum for 10 min at 37°C), neutrophils adopt an elongated shape with a rough surface and form protrusions. Those changes were observed within 1 h after yeast addition. Treatment of neutrophils with D2S did not eliminate neutrophil response to the presence of antigen. Additionally, neutrophil incubation with SP, DX, and D2S (up to 20 μM) for 12 h did not decrease their viability (Fig. 6D).
D2S has little effect on intracellular digestion of yeast cells in RAW264.7 murine macrophage.
Detection, engulfment, and killing of pathogens or cancerous cells represent different functions of circulating macrophages. After incubation of RAW264.7 macrophages with FITC-labeled yeast, we observed fluorescence of whole-yeast cells in the cytosol of the macrophages (engulfment) accompanied by multiple smaller fluorescent yeast fragments, indicating their digestion (data not shown). Quantitative analysis of RAW264.7 macrophages containing intact yeast cells compared to macrophages with both intact cells and smaller fragments reveals a slightly reduced intracellular digestion (∼15%) of yeast cells after D2S treatment (data not shown).
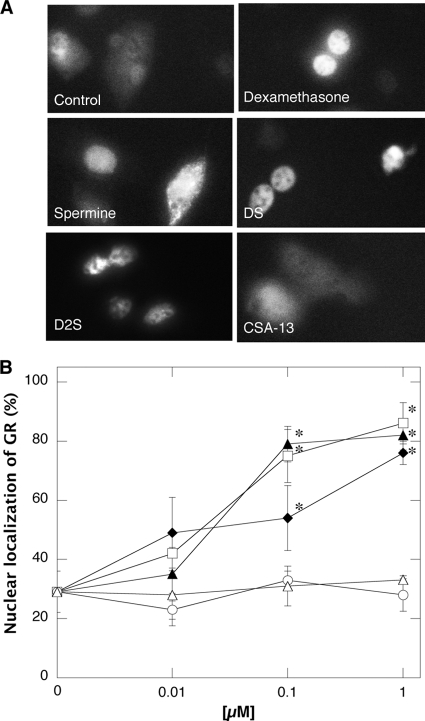
D2S glucocorticoid activity.
Nuclear localization of GR is one test of glucocorticoid activity that was previously used to study DS pharmacological effects (22). As shown in Fig. 7A, efficient transfection of BAECs results in a fluorescent signal that can be detected and analyzed with microscopy. A 60-min treatment of BAECs expressing GFP-GR protein with DX, DS, or D2S caused nuclear localization of GFP-GR at doses from 0.01 to 1 μM. However, CFP-GR translocation was not observed with SP, CSA-13 (Fig. 7), or LL-37 treatment (data not shown) at similar tested concentrations. This finding indicates that D2S has strong (comparable to DS and DX) glucocorticoid activity, which should enlarge the spectrum of its anti-inflammatory actions.
FIG. 7.
D2S induced GFP-GR translocation from cytosol to nucleoplasm in BAECs. (A) GFP-GR nuclear localization. In this experiment BAECs were transfected with GFP-GR plasmid using Lipofectamine as a gene delivery strategy. (B) Quantitative evaluation of BAECs with nuclear localization of GFP-GR. After transfection, cells were grown for 24 h and treated with spermine (▵), dexamethasone (▴), dexamethasone-spermine (♦), disubstituted dexamethasone-spermine conjugate (□), and ceragenin CSA-13 (○). *, significantly different from control sample.
DISCUSSION
Novel membrane-active cationic antibacterial peptides and their mimics, such as cationic lipids, offer alternatives to antibacterial agents that can be used to combat bacterial infections, especially those caused by drug-resistant strains. Host inflammatory response to infection can strongly aggravate its consequences, and, in many cases, the current therapy for infection involves antibacterial and anti-inflammatory drug administration. Indeed, combined antibacterial and anti-inflammatory treatment with dexamethasone was found beneficial in some ocular (33, 46), airway (44), skin (25), and central nervous system infections (12). Corticosteroids are also used early in CF lung disease to prevent and delay inflammation leading to lung damage and respiratory failure. On the other hand, application of glucocorticoids in sepsis or severe infection is still disputed as an appropriate clinical treatment, and the benefits of glucocorticoid administration should always be weighed against occurrence of adverse events, especially development of cataracts and effects on linear growth (50). However, the adverse effects are more likely to occur with long-term exposure, while antibiotic therapy is usually brief. The strong antibacterial and anti-inflammatory activity of D2S described in this study, which is at least partly mediated through GR activation, provides a new therapeutic solution with potential for the treatment of certain apical infections. Similar MIC values recorded for 16 different S. aureus isolates that include bacteria with different mechanisms of antibiotic resistance and observed activities against P. aeruginosa provide strong experimental evidence to support this possibility.
Another potential benefit of D2S use in infection treatment is suggested by the ability of D2S to increase S. aureus susceptibility to several antibiotics currently used in treatment of infections caused by this strain of bacteria. These data also suggest that the antibacterial mechanism of D2S to kill bacteria is likely universal and will not be compromised by bacterial ability to resist the activity of currently used antibiotics. However, our data do not provide a mechanism for D2S antibacterial action. Possible modes, such as competitive binding to receptors on the membrane surface, complex formation with DNA or proteins in the bacterial cytoplasm, and channel/pore formation in the plasma membrane that involve interaction with LPS or LTA molecules should be considered (13). One possible mechanism for D2S bacterial killing is due to its membrane activity, and if this activity is not specific toward bacteria, the potential toxicity toward host cells needs to be addressed. A simple measure of membrane selectivity can be determined by dividing the value of minimal concentration required to lyse red blood cells (RBCs) (minimal hemolytic concentration) by the value of the MIC, which was determined in our study for different S. aureus strains to be in the range of 1.6 to 3.2 μM. Previous observations show that D2S is not lytic to RBCs at concentrations of ∼50 μM (D. Fein, unpublished data). These data show the degree of selectivity of D2S for prokaryotic membranes. Results of in vitro evaluations indicate that toxicity of D2S at its antibacterial concentration is very unlikely to occur. We have not observed significant changes in neutrophil response to antigen after D2S treatment, nor was the viability of neutrophils and yeast engulfment by macrophages compromised after incubation with D2S.
In addition to its antibacterial activity, our data indicate a strong ability of D2S to inactivate bacterial wall products, such as LPS and LTA, as indicated by the loss of their ability to stimulate IL-6 and IL-8 release from human neutrophils. Similar to that of D2S, lipopolysaccharide sequestering ability was described for mono- and bis-acyl polyamines with various acyl chain lengths (2), CSA-13 (11), and other steroid conjugates (38, 40), with chemical characteristics similar to those of D2S. Neutralization of bacterial wall molecules by cationic lipids can occur directly, based on molecular binding or by interference with LPS/LTA signaling. It was recently shown that LPS treatment induced activation of TLR4 via its translocation into membrane lipid raft microdomains, and this translocation was inhibited by incubation of the cells with the surfactant lipid. Lipid-mediated inhibition of TLR4 membrane repartition can downregulate the synthesis of IL-8 by an alveolar epithelial cell line in response to LPS (1, 49). This recent finding gives rise to the hypothesis that D2S membrane activity may result in rearrangement of lipid packing after D2S membrane insertion, which will interfere with TLR membrane repartition. The effect of D2S on eukaryotic plasma membrane lipid organization deserves future investigation.
In conclusion, we show that D2S, like the synthetic ceragenin CSA-13 or natural LL-37 peptide, has a large spectrum of antibacterial activity that is active in different body fluids and is able to prevent inflammatory response through interaction with LPS and LTA that interferes with TLR signaling pathways. Additionally, D2S ability to induce GR nuclear localization suggests that it might prevent inflammation through engagement of GR pathways. Given that D2S is an experimental antibacterial molecule, future safety, efficacy, and pharmacokinetic studies in vivo should be performed to confirm our in vitro findings.
Acknowledgments
This work was supported by NIH grant HL67286, the Cystic Fibrosis Foundation, and Medical University of Bialystok grants 3-22695L and 3-22477F.
We gratefully acknowledge the assistance of M. Ferrin and patients of the Adult Cystic Fibrosis Center of the University of Pennsylvania for providing sputum samples.
P. B. Savage is a paid consultant for Ceragenix Pharmaceuticals, Innate Immune, Inc., and WittyCell. R. Bucki and P. A. Janmey began a sponsored research agreement with Critical Biologics, Inc., in a project directed at evaluating the potential clinical use of gelsolin but not otherwise related to the present study. None of the research reported in this paper was supported by Ceragenix Pharmaceuticals or by any other corporate entity. The other authors have no conflicts of interest to declare.
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Abate, W., A. A. Alghaithy, J. Parton, K. P. Jones, and S. K. Jackson. 2009. Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J. Lipid Res. 51:334-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakrishna, R., S. J. Wood, T. B. Nguyen, K. A. Miller, E. V. Suresh Kumar, A. Datta, and S. A. David. 2006. Structural correlates of antibacterial and membrane-permeabilizing activities in acylpolyamines. Antimicrob. Agents Chemother. 50:852-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beisswenger, C., and R. Bals. 2005. Functions of antimicrobial peptides in host defense and immunity. Curr. Protein Pept. Sci. 6:255-264. [DOI] [PubMed] [Google Scholar]
- 4.Bellini, A. M., G. Cavazzini, and G. Vertuani. 1976. Antibacterial activity of the derivatives of cholic acid. Ann. Sclavo 18:461-468. [PubMed] [Google Scholar]
- 5.Bellini, A. M., G. Vertuani, M. P. Quaglio, and G. Cavazzini. 1979. Bile acid derivatives with antimicrobial activity. Farmaco Sci. 34:967-978. [PubMed] [Google Scholar]
- 6.Bhavsar, P., N. Khorasani, M. Hew, M. Johnson, and K. F. Chung. 2010. Effect of p38 MAPK inhibition on corticosteroid suppression of cytokine release in severe asthma. Eur. Respir. J. 35:750-756. [DOI] [PubMed] [Google Scholar]
- 7.Boman, H. G. 2003. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 254:197-215. [DOI] [PubMed] [Google Scholar]
- 8.Bucki, R., F. J. Byfield, and P. A. Janmey. 2007. Release of the antimicrobial peptide LL-37 from DNA/F-actin bundles in cystic fibrosis sputum. Eur. Respir. J. 29:624-632. [DOI] [PubMed] [Google Scholar]
- 9.Bucki, R., and P. A. Janmey. 2006. Interaction of the gelsolin-derived antibacterial PBP10 peptide with lipid bilayers and cell membranes. Antimicrob. Agents Chemother. 50:2932-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucki, R., J. J. Pastore, P. Randhawa, R. Vegners, D. J. Weiner, and P. A. Janmey. 2004. Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob. Agents Chemother. 48:1526-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucki, R., A. G. Sostarecz, F. J. Byfield, P. B. Savage, and P. A. Janmey. 2007. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J. Antimicrob. Chemother. 60:535-545. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri, A., P. Martinez-Martin, P. G. Kennedy, R. Andrew Seaton, P. Portegies, M. Bojar, and I. Steiner. 2008. EFNS guideline on the management of community-acquired bacterial meningitis: report of an EFNS Task Force on acute bacterial meningitis in older children and adults. Eur. J. Neurol. 15:649-659. [DOI] [PubMed] [Google Scholar]
- 13.Chen, W. H., X. B. Shao, R. Moellering, C. Wennersten, and S. L. Regen. 2006. A bioconjugate approach toward squalamine mimics: insight into the mechanism of biological action. Bioconjug. Chem. 17:1582-1591. [DOI] [PubMed] [Google Scholar]
- 14.Chin, J. N., M. J. Rybak, C. M. Cheung, and P. B. Savage. 2007. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 51:1268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement, vol. 29. M-100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 16.Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. [DOI] [PubMed] [Google Scholar]
- 17.Ding, B., U. Taotofa, T. Orsak, M. Chadwell, and P. B. Savage. 2004. Synthesis and characterization of peptide-cationic steroid antibiotic conjugates. Org. Lett. 6:3433-3436. [DOI] [PubMed] [Google Scholar]
- 18.Ding, B., N. Yin, Y. Liu, J. Cardenas-Garcia, R. Evanson, T. Orsak, M. Fan, G. Turin, and P. B. Savage. 2004. Origins of cell selectivity of cationic steroid antibiotics. J. Am. Chem. Soc. 126:13642-13648. [DOI] [PubMed] [Google Scholar]
- 19.Fair, W. R., and N. Wehner. 1971. Antibacterial action of spermine: effect on urinary tract pathogens. Appl. Microbiol. 21:6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fein, D. E., M. P. Limberis, S. F. Maloney, J. M. Heath, J. M. Wilson, and S. L. Diamond. 2009. Cationic lipid formulations alter the in vivo tropism of AAV2/9 vector in lung. Mol. Ther. 12:2078-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiebelkorn, K. R., S. A. Crawford, M. L. McElmeel, and J. H. Jorgensen. 2003. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J. Clin. Microbiol. 41:4740-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruneich, J. A., A. Price, J. Zhu, and S. L. Diamond. 2004. Cationic corticosteroid for nonviral gene delivery. Gene Ther. 11:668-674. [DOI] [PubMed] [Google Scholar]
- 23.Guan, Q., C. Li, E. J. Schmidt, J. S. Boswell, J. P. Walsh, G. W. Allman, and P. B. Savage. 2000. Preparation and characterization of cholic acid-derived antimicrobial agents with controlled stabilities. Org. Lett. 2:2837-2840. [DOI] [PubMed] [Google Scholar]
- 24.Hancock, R. E., and A. Patrzykat. 2002. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Targets Infect. Disord. 2:79-83. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto, Y., Y. Kaneda, N. Takahashi, S. Akashi, I. Arai, and S. Nakaike. 2003. Clarithromycin inhibits the development of dermatitis in NC/Nga mice. Chemotherapy 49:222-228. [DOI] [PubMed] [Google Scholar]
- 26.Hazra, B., V. Pore, S. Dey, S. Datta, M. Darokar, D. Saikia, S. P. Khanuja, and A. Thakur. 2004. Bile acid amides derived from chiral amino alcohols: novel antimicrobials and antifungals. Bioorg. Med. Chem. Lett. 14:773-777. [DOI] [PubMed] [Google Scholar]
- 27.Herasimenka, Y., M. Benincasa, M. Mattiuzzo, P. Cescutti, R. Gennaro, and R. Rizzo. 2005. Interaction of antimicrobial peptides with bacterial polysaccharides from lung pathogens. Peptides 26:1127-1132. [DOI] [PubMed] [Google Scholar]
- 28.Jenssen, H., P. Hamill, and R. E. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones, S. R., W. A. Kinney, X. Zhang, L. M. Jones, and B. S. Selinsky. 1996. The synthesis and characterization of analogs of the antimicrobial compound squalamine: 6 beta-hydroxy-3-aminosterols synthesized from hyodeoxycholic acid. Steroids 61:565-571. [DOI] [PubMed] [Google Scholar]
- 30.Kourie, J. I., and A. A. Shorthouse. 2000. Properties of cytotoxic peptide-formed ion channels. Am. J. Physiol. Cell Physiol. 278:C1063-C1087. [DOI] [PubMed] [Google Scholar]
- 31.Leclercq, R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34:482-492. [DOI] [PubMed] [Google Scholar]
- 32.Li, C., M. R. Lewis, A. B. Gilbert, M. D. Noel, D. H. Scoville, G. W. Allman, and P. B. Savage. 1999. Antimicrobial activities of amine- and guanidine-functionalized cholic acid derivatives. Antimicrob. Agents Chemother. 43:1347-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, F., A. K. Kwok, and B. M. Cheung. 2008. The efficacy of intravitreal vancomycin and dexamethasone in the treatment of experimental bacillus cereus endophthalmitis. Curr. Eye Res. 33:761-768. [DOI] [PubMed] [Google Scholar]
- 34.Moore, K. S., S. Wehrli, H. Roder, M. Rogers, J. N. Forrest, Jr., D. McCrimmon, and M. Zasloff. 1993. Squalamine: an aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. U. S. A. 90:1354-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peeters, E., H. J. Nelis, and T. Coenye. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72:157-165. [DOI] [PubMed] [Google Scholar]
- 36.Pitts, B., M. A. Hamilton, N. Zelver, and P. S. Stewart. 2003. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 54:269-276. [DOI] [PubMed] [Google Scholar]
- 37.Price, A., M. Limberis, J. A. Gruneich, J. M. Wilson, and S. L. Diamond. 2005. Targeting viral-mediated transduction to the lung airway epithelium with the anti-inflammatory cationic lipid dexamethasone-spermine. Mol. Ther. 12:502-509. [DOI] [PubMed] [Google Scholar]
- 38.Randazzo, R. A., R. Bucki, P. A. Janmey, and S. L. Diamond. 2009. A series of cationic sterol lipids with gene transfer and bactericidal activity. Bioorg. Med. Chem. 17:3257-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rozansky, R., U. Bacharach, and N. Grossowicz. 1954. Studies on antibacterial action of spermine. J. Gen. Microbiol. 10:11-16. [DOI] [PubMed] [Google Scholar]
- 40.Salunke, D. B., B. G. Hazra, and V. S. Pore. 2006. Steroidal conjugates and their pharmacological applications. Curr. Med. Chem. 13:813-847. [DOI] [PubMed] [Google Scholar]
- 41.Savage, P. B., and C. Li. 2000. Cholic acid derivatives: novel antimicrobials. Expert Opin. Investig. Drugs 9:263-272. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, E. J., J. S. Boswell, J. P. Walsh, M. M. Schellenberg, T. W. Winter, C. Li, G. W. Allman, and P. B. Savage. 2001. Activities of cholic acid-derived antimicrobial agents against multidrug-resistant bacteria. J. Antimicrob. Chemother. 47:671-674. [DOI] [PubMed] [Google Scholar]
- 43.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 44.Tagliabue, C., C. M. Salvatore, C. Techasaensiri, A. Mejias, J. P. Torres, K. Katz, A. M. Gomez, S. Esposito, N. Principi, and R. D. Hardy. 2008. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J. Infect. Dis. 198:1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaara, M., and T. Vaara. 1983. Polycations sensitize enteric bacteria to antibiotics. Antimicrob. Agents Chemother. 24:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Endt, J. J., H. G. Veraart, R. Kramer, A. G. Janssen, and P. Sunder Raj. 1997. A comparison of two ophthalmic steroid-antibiotic combinations after cataract surgery. Eur. J. Ophthalmol. 7:144-148. [DOI] [PubMed] [Google Scholar]
- 47.Wang, Y., B. Agerberth, A. Lothgren, A. Almstedt, and J. Johansson. 1998. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J. Biol. Chem. 273:33115-33118. [DOI] [PubMed] [Google Scholar]
- 48.Weiner, D. J., R. Bucki, and P. A. Janmey. 2003. The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am. J. Respir. Cell Mol. Biol. 28:738-745. [DOI] [PubMed] [Google Scholar]
- 49.Wong, S. W., M. J. Kwon, A. M. Choi, H. P. Kim, K. Nakahira, and D. H. Hwang. 2009. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 284:27384-27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, Z., J. P. Ouyang, and Y. P. Li. 2009. Dexamethasone attenuated endotoxin-induced acute lung injury through inhibiting expression of inducible nitric oxide synthase. Clin. Hemorheol. Microcirc. 41:117-125. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, M., T. Caragine, H. Wang, P. S. Cohen, G. Botchkina, K. Soda, M. Bianchi, P. Ulrich, A. Cerami, B. Sherry, and K. J. Tracey. 1997. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J. Exp. Med. 185:1759-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao, H., and P. K. Kinnunen. 2003. Modulation of the activity of secretory phospholipase A2 by antimicrobial peptides. Antimicrob. Agents Chemother. 47:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, S., M. Ashok, J. Li, W. Li, H. Yang, P. Wang, K. J. Tracey, A. E. Sama, and H. Wang. 2009. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol. Med. 15:275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]