Abstract
Although inflammation and altered barrier functions of the vasculature, due predominantly to the infection of endothelial cell lining of small and medium-sized blood vessels, represent salient pathological features of human rickettsioses, the interactions between pathogenic rickettsiae and microvascular endothelial cells remain poorly understood. We have investigated the activation of nuclear transcription factor-kappa B (NF-κB) and p38 mitogen-activated protein (MAP) kinase, expression of heme oxygenase 1 (HO-1) and cyclooxygenase 2 (COX-2), and secretion of chemokines and prostaglandins after Rickettsia rickettsii infection of human cerebral, dermal, and pulmonary microvascular endothelial cells in comparison with pulmonary artery cells of macrovascular origin. NF-κB and p38 kinase activation and increased HO-1 mRNA expression were clearly evident in all cell types, along with relatively similar susceptibility to R. rickettsii infection in vitro but considerable variations in the intensities/kinetics of the aforementioned host responses. As expected, the overall activation profiles of macrovascular endothelial cells derived from human pulmonary artery and umbilical vein were nearly identical. Interestingly, cerebral endothelial cells displayed a marked refractoriness in chemokine production and secretion, while all other cell types secreted various levels of interleukin-8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1) in response to infection. A unique feature of all microvascular endothelial cells was the lack of induced COX-2 expression and resultant inability to secrete prostaglandin E2 after R. rickettsii infection. Comparative evaluation thus yields the first experimental evidence for the activation of both common and unique cell type-specific host response mechanisms in macrovascular and microvascular endothelial cells infected with R. rickettsii, a prototypical species known to cause Rocky Mountain spotted fever in humans.
The obligately intracellular Alphaproteobacteria belonging to genus Rickettsia are now classified into four subgroups, which include an ancestral group and a transitional group in addition to two major antigenically defined groups comprised of spotted fever and typhus species (12). Pathogenic rickettsiae display tropism for vascular endothelial cell lining of small and medium-sized blood vessels in their mammalian hosts. As a consequence, the predominant pathological features of resultant clinical syndromes—for example, Rocky Mountain spotted fever due to Rickettsia rickettsii—are generally attributed to altered vascular functions due to preferential infection of the endothelium (27, 36). However, despite critical contributions of endothelial injury, inflammation, and dysfunction to the pathogenesis of vasculotropic rickettsioses, the biological basis of rickettsial interactions with microvascular endothelium of vertebrate hosts still remains poorly understood.
An initial breakthrough in the laboratory investigations of the interplay between host cells and pathogenic rickettsiae was the description of an in vitro model of infection utilizing infection of human umbilical vein-derived endothelial cells (HUVECs) with R. rickettsii. These studies exploited the contemporary development of procedures to establish primary in vitro cultures of vascular endothelium from human umbilical cords and documented that R. rickettsii replicates in both the nucleus and cytoplasm of infected endothelial cells and displays early intercellular spread without detectable injury to the host cells (31). Widespread dilatation of intracellular membranes, predominantly of rough endoplasmic reticulum, occurs with the progress of infection and later manifests as the loss of integrity and host cell lysis (30). Importantly, cultured HUVECs used in these studies were found to retain many characteristic features of capillary vascular endothelium such as the presence of Weibel-Palade bodies and positive staining for factor VIII antigen on the cell surface (30, 31). Subsequent experimental findings using this in vitro model of infection have since established that endothelial cells infected with R. rickettsii launch an antioxidant response via increased expression of heme oxygenase 1 (HO-1) to combat the oxidative stress due to increased accumulation of intracellular peroxides and oxygen free radicals (11, 22, 28). Endothelial cell activation in response to R. rickettsii and Rickettsia conorii involves the activation of nuclear factor kappa B (NF-κB) and p38 mitogen-activated protein (MAP) kinase signaling and is characterized by altered expression/secretion profile of various cytokines and chemokines including interleukin-1α (IL-1α), IL-8, monocyte chemoattractant protein 1 (MCP-1), Mig, and IP-10 (7, 16, 23, 33, 35).
The recently emerging concept of “endothelial cell heterogeneity” dictates that, depending on their location and physiological functions, vascular endothelium of different organ systems displays significant differences in its biological properties and activation patterns in response to known stressors (4, 17). Although brain microvascular endothelial cells (ECs) express surface adhesion molecules to regulate extravasation of leukocytes similar to other ECs, some unique features which distinguish them from other peripheral ECs include numerous intercellular “tight junctions” that display high transendothelial resistance and retard paracellular flux (10), absence of fenestrae, and a reduced level of fluid-phase endocytosis (21), and asymmetrically localized enzymes and carrier-mediated transport systems that engender a truly “polarized” phenotype (38). In addition, the pulmonary microvascular ECs are among the most important targets of reactive oxygen species (ROS) in lung injury (5). In this regard, an important consideration is that prominent pathological features directly relevant to rickettsial diseases include noncardiogenic pulmonary edema and cerebral edema (13, 35, 36, 40). Thus, keeping in mind that microvascular ECs of different origin may exhibit marked differences in their sensitivity and/or responses to infection, we have now conducted comparative studies using microvessel ECs derived from human dermis (HMEC-1), lungs (HPMEC), and brain (SV-HCEC) and macrovascular cell types, namely, human pulmonary artery endothelial cells (HPAECs) and HUVECs.
MATERIALS AND METHODS
Cell culture.
HUVECs from umbilical cords collected within 48 h of delivery were cultured as previously described (33). Cells were plated to achieve 80 to 90% confluence after 5 to 7 days in culture and were routinely used at passage 2. Human dermal microvascular ECs (HMEC-1) were obtained from the Centers for Disease Control and Prevention (Atlanta, GA). Human cerebral microvascular ECs (SV-HCECs) were kindly provided by R. K. Yu and S. S. Dasgupta, Institute of Molecular Medicine and Genetics, Medical College of Georgia, Augusta. These immortalized cell lines, which reportedly retain morphological, phenotypic, and functional characteristics of normal human endothelium, were cultivated as recommended (2, 19). Primary human pulmonary microvascular ECs (HPMECs) and primary human pulmonary artery ECs (HPAECs) were purchased from ScienCell Research Laboratories (San Diego, CA) and Lonza, Inc. (Walkersville, MD), respectively, and maintained in culture according to the manufacturers’ instructions.
Infection.
The Sheila Smith strain of R. rickettsii was cultivated in Vero cells to prepare seed stocks for use in infection experiments (26, 33). The titers of viable rickettsiae in such preparations were determined by plaque formation assay and calculated as the number of PFU per ml. The results of the plaque assay were then confirmed by a TaqMan quantitative PCR (qPCR) procedure using primer pair Rp877p-Rp1258n for the citrate synthase (gltA) gene and an internal probe with 100% homology to Rickettsia species (25). ECs were infected with approximately 6 × 104 to 1 × 105 PFU of viable rickettsiae for every cm2 of culture area. This dose yields a relatively synchronous infection with ≥80% of cells infected with an average of 4 rickettsiae per cell at 6 to 8 h (25, 33).
Indirect immunofluorescent staining.
ECs infected on gelatin-coated plastic coverslips were infected as described above. At 6 h, the cells were gently washed with phosphate-buffered saline and fixed with 3.7% formaldehyde solution. Immunofluorescent staining and enumeration of the number of infected cells and average number of rickettsiae in each infected cell were carried out employing an established procedure (29).
Northern blot analysis.
Equal amounts of RNA isolated from infected ECs and corresponding uninfected cells (controls) using TRI-reagent protocol were subjected to Northern blot analysis using radioactively labeled cDNA probes designed for specific detection of cyclooxygenase 2 (COX-2) (Cayman Chemical) and HO-1 transcripts (22, 24). The differences in sample loading on different lanes were corrected by stripping of the signals and reprobing for the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene.
Western blot analysis.
Equal amounts of protein from experimental cellular lysates were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and probed with either a phospho-specific p38 antibody (Cell Signaling Technology, Inc., Beverly, MA) or a monoclonal antibody capable of detecting human COX-2 (Cayman Chemical, Ann Arbor, MI). Blots were probed using compatible horseradish peroxidase (HRP)-conjugated secondary antibodies and SuperSignal chemiluminescence protocol, followed by exposure to an ECL Hyperfilm. The identity of the proteins on the blot was ascertained using electrophoresis standards of various molecular weights, biotinylated molecular weight markers detected with an antibiotin antibody, and the appropriate positive controls. The equality of protein loading for different samples was ascertained by stripping of the blots and reprobing with a monoclonal antibody against α-tubulin (Accurate Chemicals, Westbury, NY).
Preparation of nuclear extracts and EMSA.
For the electrophoretic mobility shift assay (EMSA), cell extracts enriched in nuclear proteins were isolated from R. rickettsii-infected cells and corresponding uninfected controls using a previously established method (26, 33). The protein concentration in these preparations was determined by Bradford assay reagent using bovine serum albumin (BSA) as the standard (Bio-Rad Laboratories, Inc., Hercules, CA). DNA-protein binding assays were performed using a gel shift assay system (Promega). For each gel shift reaction, 2 μg of nuclear proteins was incubated with a consensus NF-κB oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′), which was end labeled using T4 polynucleotide kinase and [γ-32P]ATP (3,000 Ci/mmol, 10 mCi/ml; Dupont/NEN) according to the manufacturer's instructions. Specificity of DNA-protein binding was simultaneously confirmed by addition of a 10-fold molar excess of unlabeled oligonucleotide to the gel shift reaction mixture prior to the addition of labeled probe. The DNA-protein complexes were then resolved on a 4% nondenaturing low-ionic-strength polyacrylamide gel prepared in 0.5× Tris-borate-EDTA (TBE). Gels were electrophoresed at 100 V for 2 h, vacuum dried, and subjected to radiographic exposure for different periods of time.
Measurement of chemokine and PG secretion.
Culture supernatants from R. rickettsii-infected and simultaneously processed uninfected cells were cleared by centrifugation at 10,000 × g for 5 min. The amounts of IL-8, MCP-1, and prostaglandin E2 (PGE2) were measured by enzyme-linked immunosorbent assay (ELISA) kits following manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN). The minimum detectable doses of IL-8, MCP-1, and PGE2 for the assay systems used were 1.5 to 7.5 pg/ml, <5.0 pg/ml, and 18.2 to 36.8 pg/ml, respectively. At least two dilutions of each sample were assayed in duplicate, and the optical density (OD) values corresponding to the linear range of the standard curve were used for the determination of chemokine and prostaglandin concentrations.
Densitometric and statistical analysis.
The radiograms with optimum exposure were scanned in the grayscale mode using an HP ScanJet 6300C scanner at a resolution of 600 dpi for quantitative analysis of data from blotting experiments and EMSAs. Volume analysis was performed using ImageQuant software, version 3.3, and band intensities were determined as densitometric units. For comparison, the normalized band intensity for uninfected control in each experiment was assigned a value of 1. Data were calculated as the mean ± standard error for at least three independent experiments, and comparisons between the study and control groups were performed using Student's t test. P values of ≤0.05 were considered statistically significant.
RESULTS
R. rickettsii infection of different types of endothelial cells in vitro.
The tropism for vascular endothelium lining small and medium-sized vessels in vivo is rather intriguing considering that pathogenic rickettsiae are capable of infecting a wide range of cultured cell types in vitro. To test the infectivity of ECs of different tissues, cells were infected with identical doses of R. rickettsii followed by determination of the extent of infection by indirect immunofluorescent staining. As established earlier, infection efficiency of human umbilical vein endothelial cells (HUVECs) was included as a control in these experiments (29, 33). All types of ECs, including both primary cells and cell lines, were efficiently infected by R. rickettsii. Also, relative efficiencies for different ECs were strikingly similar, with more than 80% of cells infected with an average of 4 to 5 rickettsiae per infected cell, with the exception of HPAECs carrying 7 to 8 organisms per cell (Table 1). Thus, all human ECs tested were susceptible to R. ricekttsii infection, yielding levels of infection very similar to HUVECs (29).
TABLE 1.
Infection of different types of endothelial cells with Rickettsia rickettsiia
| Cell type | % of infected cells | Avg no. of rickettsiae/infected cell |
|---|---|---|
| HMECs | 88 | 4.7 |
| HPAECs | 91 | 7.5 |
| HPMECs | 86 | 4.3 |
| HUVECs | 86 | 5.4 |
| SV-HCECs | 90 | 4.5 |
Endothelial cells of different origin were cultured on sterile plastic coverslips to 80 to 90% confluence and infected with approximately 6 × 104 PFU of R. rickettsii in antibiotic-free culture medium. At 6 h, cells were gently washed, fixed in a 3.7% formaldehyde solution in PBS and stained by fluorescence with anti-R. rickettsii antiserum. For each condition, a minimum of 100 cells in randomly selected fields were scored for the number of R. rickettsii organisms per cell (29).
Effect of R. rickettsii infection on NF-κB activation in different types of host endothelial cells.
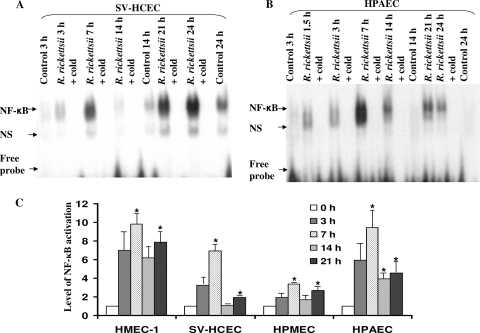
Employing infection of cultured HUVECs to understand host-pathogen interactions, our laboratory has demonstrated that R. rickettsii inhibits the apoptosis of the infected host cell early during the infection through the activation of NF-κB, suggesting an important role for this mechanism in the host cell survival and sustained infection (7, 15, 27). With an aim to define potential similarities as well as differences in the intensities and kinetics of host responses of different types of endothelial cells to R. rickettsii, we initially investigated the activation pattern of NF-κB during in vitro infection of human microvascular endothelial cells originating from the dermis (HMECs), brain (SV-HCECs), and lungs (HPMECs). The infection-induced activation profile in these cell types was compared to that seen in human pulmonary artery endothelial cells (HPAECs) of macrovascular origin. ECs were infected for various lengths of time ranging from 1.5 to 24 h for subsequent analysis of nuclear translocation of NF-κB by electrophoretic mobility shift assay (Fig. 1A and B). The results suggest that R. rickettsii infection triggers NF-κB activation in all studied EC types, with a prominent early increase in DNA binding activity at 7 h postinfection (p.i.). The highest intensity of this initial, transient phase of NF-κB activation was seen in infected HMECs and HPAECs, followed by a comparatively less intense response in SV-HCECs, and with the lowest level in HPMECs. Furthermore, there was an appreciable decrease at 14 h followed by a trend toward another increase in DNA binding activity of NF-κB later at 21 h. Thus, different types of ECs used in this comparative analysis consistently demonstrated a significant increase over the basal level of NF-κB activation at 7 h and 21 h (Fig. 1C). Clearly, while the intensity of NF-κB activation exhibited considerable variations among the studied EC types, the overall pattern of the activation kinetics was similar to that reported earlier for HUVECs (33).
FIG. 1.
Effect of R. rickettsii infection on NF-κB activation in micro- and macrovascular human endothelial cells. ECs were either infected with R. rickettsii or incubated with the antibiotic-free culture medium alone (Control). Gel-shift analysis was carried out to measure the DNA-binding activity using nuclear extracts (2 μg protein) and a consensus NF-κB sequence. Specificity of DNA-protein binding was ascertained by inclusion of approximately 50-fold molar excess of unlabeled probe in the assay (+cold). The autoradiographic exposures from a typical experiment with human microvascular cerebral ECs (SV-HCECs [A]) and pulmonary artery ECs (HPAECs [B]) are shown. The arrows indicate relative positions of the NF-κB complex, a nonspecific band (NS), and unbound radioactivity in the reaction mixture (Free probe) on the gel. Panel C represents quantitative densitometric analysis of NF-κB activation in organ-specific ECs after R. rickettsii infection in relation to the uninfected controls (0 h), which were assigned a value of 1. The data are presented as the mean ± standard error of the mean (SEM) from a minimum of three independent experiments (n ≥ 3). The asterisks indicate statistically significant differences (P ≤ 0.05) in comparison to the corresponding uninfected controls.
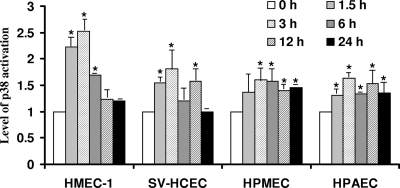
Activation of p38 MAP kinase in cerebral, dermal, and pulmonary microvascular endothelial cells following R. rickettsii infection.
p38 is an important stress-activated MAP kinase, the inhibitors of which have promising efficacy in several models of disease, including inflammatory disorders, septic shock, and other diseases (9). Our previous studies have revealed the evidence for increased phosphorylation (a marker of activation) and enzymatic activity of p38 MAP kinase in HUVECs infected with R. rickettsii and a role for p38-mediated signaling in rickettsial invasion into host cells and consequent chemokine response (23). Because all MAP kinases are activated following dual phosphorylation of the threonine-X-tyrosine motif, where X is glycine in p38 kinases, we investigated the activation of p38 in ECs from different organs after R. rickettsii infection by determining its phosphorylation status. As reported previously, HUVECs infected with R. rickettsii displayed p38 activation as early as 1.5 h postinfection with about a 2.8-fold increase in the phospho-p38 level in comparison to the corresponding uninfected control. All microvascular and pulmonary artery ECs infected with R. rickettsii for different times ranging from 30 min to 24 h also had increased steady-state levels of phospho-p38 starting at 1.5 h postinfection and displayed maximum p38 activation at 3 h, followed by a decline toward the baseline at later times (Fig. 2). Among the EC types used for comparative analysis, HMEC-1 demonstrated maximal p38 activation of 2.5-fold over the basal level in uninfected cells at 3 h, whereas HPMECs had an approximately 1.8-fold increase in the steady-state level of phospho-p38 (Fig. 2). Thus, the intensities and dynamics of p38 activation were apparently similar among different cell types and resembled the kinetics of p38 activation in HUVECs (23).
FIG. 2.
Activation of p38 MAP kinase in different types of ECs following R. rickettsii infection for various lengths of time. Total protein lysates from ECs infected with R. rickettsii for 1.5 to 24 h or incubated with the culture medium alone (Control) were subjected to Western blot analysis to determine the steady-state levels of phosphorylated p38 kinase. Blots were probed with a phosphorylation-state-specific (Thr180/Tyr182) p38 antibody, followed by an anti-α-tubulin antibody to normalize for any variations in protein loading among different lanes. Shown are the changes in phospho-p38 levels in different types of ECs infected with R. rickettsii, as determined by quantitative densitometric analysis. For comparison, the basal level of p38 phosphorylation in each cell type was assigned a value of 1. The data are presented as the mean ± standard error from a minimum of three independent observations (n ≥ 3). The asterisks indicate statistically significant increase in cellular phospho-p38 levels in comparison to the corresponding uninfected controls.
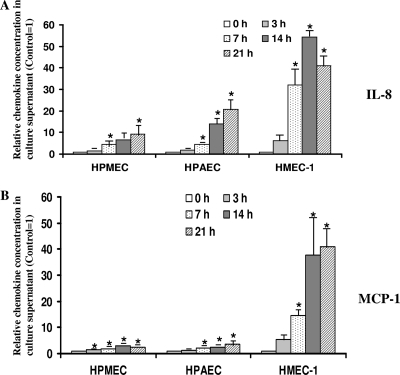
Increased production and secretion of IL-8 and MCP-1 during R. rickettsii infection of different types of ECs.
Chemokines IL-8 and MCP-1 play an active role in activating and recruiting neutrophils and monocytes to the sites of inflammation. We have earlier reported on enhanced secretion of IL-8 and MCP-1 by R. rickettsii-infected HUVECs in an NF-κB- and p38 MAP kinase-dependent manner (8, 23). To test whether R. rickettsii infection results in increased secretion of these important inflammatory chemokines in other types of endothelial cells, we measured the production of IL-8 and MCP-1 in culture supernatants of infected and uninfected ECs by ELISA. With the notable exception of SV-HCEC, infection with R. rickettsii triggered increased secretion of IL-8 and MCP-1 by all other endothelial cells in a time-dependent manner (Fig. 3). The levels of both IL-8 and MCP-1 in culture supernatants from SV-HCECs were significantly lower than those for all other endothelial cells and below the limit of detection for the assay. In comparison to the basal level of secretion from uninfected cells, the secretion of IL-8 and MCP-1 was significantly increased in culture medium samples collected from human dermal microvascular, pulmonary microvascular, and pulmonary artery ECs infected with R. rickettsii. These results also reveal that HPMECs exhibit minimal activation in terms of chemokine secretion with increases of 1.4- and 10.5-fold for IL-8 (Fig. 3A) and 1.4- and 2.5-fold at 3 and 21 h postinfection, respectively, for MCP-1 (Fig. 3B). Conversely, HMECs displayed the most prominent chemokine secretion response, as evidenced by an average of 6.3-fold and 5.2-fold increases for IL-8 and MCP-1 at 3 h with even more robust increases of 42.3- and 41.0-fold in the accumulation of these chemokines at 21 h, respectively (Fig. 3A and B).
FIG. 3.
IL-8 and MCP-1 production by R. rickettsii-infected ECs of different origin. Culture supernatants from cells infected with R. rickettsii for 3, 7, 14, and 21 h and corresponding uninfected controls were collected and assessed for IL-8 and MCP-1 levels by ELISA. At least two dilutions of each sample were assayed in duplicate, and OD values corresponding to the linear range of the standard curve were used for the determination of chemokine concentrations. Results are expressed as mean fold induction of IL-8 (A) and MCP-1 (B) over the basal levels of secretion by uninfected cells (Control), which were assigned a value of 1 for the ease of comparison. The data sets are presented as the mean ± standard error (SE) of at least three independent experiments (n ≥ 3). Statistically significant differences in comparison to the basal IL-8/MCP-1 secretion by uninfected cells are indicated by the asterisks.
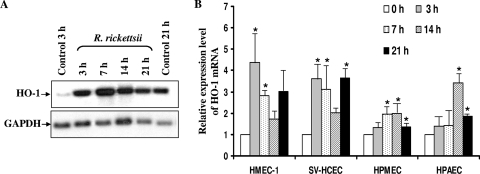
Induced mRNA expression of heme oxygenase 1 after R. rickettsii infection.
It is now well established that oxidative stress-mediated injury plays an important role in the pathogenesis of spotted fever group (SFG) rickettisioses. Our laboratory identified induced expression of heme oxygenase 1, an activatable isoform of the first regulatory enzyme in the pathway for heme catabolism, as one of the potential antioxidant mechanisms to counter infection-induced oxidative stress. In the present study, comparative analysis of HO-1 expression in both microvascular and macrovascular ECs from the lungs, dermal microvascular ECs, and cerebral ECs further revealed increased expression of HO-1 at the level of transcription after R. rickettsii infection of all of these cell types. In addition, the kinetics and intensities of induced HO-1 mRNA expression activation during 24 h of infection were almost identical among these specialized endothelial cell types (Fig. 4A and B) and quite similar to those reported earlier for infected HUVECs (22). All ECs had a peak of HO-1 mRNA expression, predominantly at 3 to 7 h pi, with the exception of HPAECs, where this response was delayed to 14 h. Intriguingly, HMECs and SV-HCECs demonstrated the highest level of HO-1 mRNA expression of about 4.5- and 3.8-fold in comparison with uninfected control, whereas HPMECs were least responsive, with about 2.2-fold induction (Fig. 4B).
FIG. 4.
Dynamics of HO-1 mRNA expression in organ-specific micro- and macrovascular ECs infected with R. rickettsii. Total RNA (8 μg) from uninfected ECs (Control) or those infected with R. rickettsii for 3 to 21 h were processed for Northern blot analysis to determine the level of HO-1 transcriptional activation. Blots were first probed with a cDNA probe specific for human HO-1, followed by a GAPDH probe to normalize for potential variations in RNA loading among different samples. Results of a representative blot obtained from infected HPMECs are shown in panel A. The detailed densitometric analysis of changes in steady-state levels of HO-1 transcript due to R. rickettsii infection of different types of ECs is presented as the mean fold induction over the basal level of 1 (B). The data are presented as the mean ± standard error of three independent experiments (n ≥ 3). The asterisks indicate statistically significant differences in HO-1 mRNA expression relative to the corresponding uninfected controls.
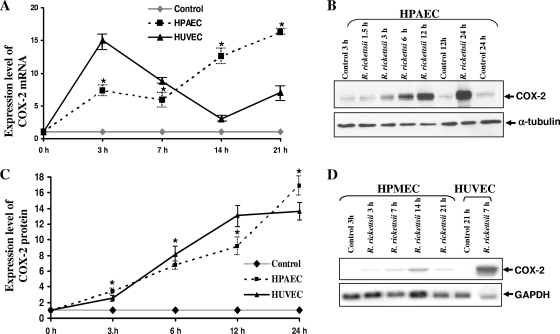
Differential expression of cyclooxygenase 2 and secretion of prostaglandins by macro- versus microvascular endothelial cells following R. rickettsii infection.
Composed of a housekeeping form and an inducible isoform, the cyclooxygenase family of isozymes plays a critical role in the regulation of vascular functions by generating a number of vasoactive substances, including prostaglandins (PGs), prostacyclin, and thromboxanes (32). In addition, experimental evidence further implicates an intimate interrelationship between HO and COX enzyme systems in the vasculature. Because infection with SFG rickettsiae stimulates both mRNA and protein expression of COX-2 in HUVECs, leading to enhanced secretion of 6-keto PGF1α and PGE2 (24), we have further compared the dynamics of COX-2 activation in EC types of different origin. The steady-state levels of COX-2 mRNA were significantly increased in infected pulmonary artery ECs at 3, 7, 14, and 21 h. Both HPAECs and HUVECs displayed two peaks of mRNA expression (Fig. 5A) and corresponding increases in the levels of steady-state levels of cellular COX-2 protein (Fig. 5B and C). Importantly, the protein levels of COX-2 in infected HPAECs demonstrated statistically significant differences in comparison to uninfected controls (P ≤ 0.05) at all times postinfection. In contrast, all of the microvascular EC types (i.e., HMECs, SV-HCECs, and HPMECs) demonstrated either negligible or very minimal levels of COX-2 expression in uninfected controls and after infection with R. rickettsii, at the levels of transcription (Fig. 5D) and translation (not shown). In subsequent experiments, induced COX-2 mRNA expression in infected HPAECs and HUVECs, but not in microvascular HMECs, SV-HCECs, and HPMECs, was further confirmed by a qPCR-based gene expression assay (assay ID Hs01573471_m1 from Applied Biosystems) (data not shown). As expected, there was an apparent correlation between the intensity of COX-2 response and prostaglandin release by HPAECs and HUVECs. Specifically, a time-dependent increase in PGE2 release into the culture medium was evident during R. rickettsii infection of HPAECs, with significant differences in its level in the culture supernatants starting at 7 h postinfection and a noticeable increase from 0.02 pg/ml at 3 h p.i. to 6.2 pg/ml at 24 h p.i. These results corroborate our earlier findings of increased PGE2 release by HUVECs during R. rickettsii infection (24). On the other hand, analysis of culture supernatants from uninfected and R. rickettsii-infected HMEC-1, SV-HCECs, and HPMECs revealed either very low or barely detectable PGE2 in culture supernatants of uninfected cells as well as those infected with R. rickettsii for various lengths of time. Collectively, these results demonstrate a differential pattern of COX-2 expression by macro- and microvascular ECs in response to R. rickettsii infection: i.e., cells derived from major vessels (HUVECs and HPAECs) demonstrate substantial increase in COX-2 activity following R. rickettsii infection, whereas ECs derived from microvessels of different origin (HMEC-1, SV-HCECs, and HPMECs) had almost negligible level of COX-2 activation and consequently, no detectable release of PGE2.
FIG. 5.
Increased expression of COX-2 mRNA and protein synthesis in macrovascular ECs infected with R. rickettsii. Panel A depicts densitometric analysis of changes in steady-state levels of COX-2 transcriptional activation in HPAECs and HUVECs after R. rickettsii infection. The results are presented as the mean fold induction over the basal level ± SE (n ≥ 3). The asterisks indicate statistically significant differences in COX-2 mRNA expression relative to the baseline in uninfected controls. Panel B shows the results of a typical Western blot with uninfected (Control) and R. rickettsii-infected HPAECs. Total protein lysates of HPAECs infected for 1.5 to 24 h or incubated with medium alone (Control) were analyzed by Western blotting to determine the steady-state levels of cellular COX-2 protein. Blots were probed in succession with anti-human COX-2-specific antibody and an anti-α-tubulin antibody. Densitometric analysis of changes in COX-2 protein levels in HPAECs and HUVECs infected with R. rickettsii was then performed (C). The data are presented as the mean fold induction ± standard error over the baseline value of 1 (n ≥ 3). Panel D shows a representative Northern blot for the detection of basal and R. rickettsii-induced COX-2 expression in HPMECs infected for different lengths of time. HUVECs infected with R. rickettsii for 7 h were included as a positive control in this experiment.
DISCUSSION
Both during the natural course of arthropod vector-mediated transmission to humans and in established laboratory models mimicking major characteristics of human rickettsial diseases, vascular endothelial cells lining the small- and medium-sized blood vessels are the focal point of infection by pathogenic Rickettsia species. Invasion of vascular endothelium by these intracellular bacteria relying on the host cell's nutrient-rich “intracytoplasmic niche” for their metabolic requirements, replication, and intercellular spread not only contributes to the establishment and progression of infection, but also triggers a number of host defense mechanisms and deleterious effects on the barrier functions of the endothelium, leading to rickettsial vasculitis characterized by vascular inflammation and compromised permeability properties. Accordingly, salient pathological features of human rickettsioses include fluid imbalance in vital organ systems, which likely represents a major underlying cause of pulmonary and cerebral edema associated with severe rickettsial infections (13, 27, 36, 39, 40). Supported by the evidence from clinical cases and experimental models of infection, these findings imply considerable accumulation of rickettsiae in the lungs and their ability to cross the blood-brain barrier into the host's central nervous system and target the brain's vasculature. However, despite their critical importance in determining the course and outcome of disease, the interactions between pathogenic rickettsiae and organ-specific microvascular endothelial cells remain poorly understood. Such a critical gap in the knowledge of host-pathogen interactions acquires additional physiological significance in the context of emerging concept of endothelial cell heterogeneity, which essentially dictates that there exist significant variations in the behavioral and biological response patterns of different types of endothelium, depending on its spatial location, functions, extracellular environment, and epigenetic modifications in different vascular beds and organ systems (3, 4). In this regard, the present study provides first evidence for relatively similar levels of infectivity and activation of both NF-κB and p38 MAP kinase as common signaling mechanisms and HO-1 mRNA expression in different types of macro- and microvascular endothelial cells infected with R. rickettsii. Interestingly, the data also reveal refractoriness of brain-derived microvascular endothelium to secrete chemokines in response to infection and reduced ability of microvascular endothelial cells to induce COX-2 expression and consequently, attenuated secretion of PGE2 in response to R. rickettsii infection.
Although systematic comparative analysis of mechanisms underlying host cell transcriptional activation illustrates activation of NF-κB and p38 MAP kinase in all microvascular cell types tested, i.e., human brain, dermal, and pulmonary endothelium, the most striking changes in terms of the intensity of R. rickettsii-induced responses were observed in dermal HMECs. Correspondingly, infected HMECs were also found to secrete the largest amounts of IL-8 and MCP-1. These results are in agreement with our earlier findings from in vitro infection of primary cultures of HUVECs (8, 23) and further implicate an important role for signaling through NF-κB and p38 MAP kinase pathways in inflammatory responses to R. rickettsii. Because HMECs, known to display nearly all important functional, phenotypic, and morphological characteristics of human microvasculature (2, 19), respond most efficiently to in vitro infection, they likely represent a relevant and well-suited culture model system to define rickettsial interactions with their preferred target cell type during human disease.
Lining the intraluminal surface of brain capillaries and responsible for the formation of blood-brain barrier, cerebral endothelial cells display lower levels of endocytosis and transcytosis than their peripheral counterparts and form tight intercellular junctions conferring the restraining nature necessary to maintain and protect brain microenvironment. In addition, these cells possess specialized transport mechanisms and enzyme systems such as monoamine oxidase (1). Although primary cultures of endothelial cells isolated from brain microvessels constitutively express, produce, and secrete IL-8 and MCP-1 under resting conditions (6, 20) and in response to ischemic stress (34), the basal secretion by SV-HCECs was undetectable by ELISA with the sensitivity limits of 3.5 and less than 5 pg/ml, respectively, for IL-8 and MCP-1. Interestingly, response to R. rickettsii infection was characterized by their inability to secrete either of the chemokines despite evidence for the activation of NF-κB and p38 upstream signaling mechanisms. Infection of SV-HCECs by R. rickettsii causes a dose-dependent decrease in transendothelial electrical resistance reflecting compromised permeability, a response exacerbated by exogenous inflammatory stimuli (40). The relative inertness of SV-HCECs to secrete chemotactic activities in response to R. rickettsii infection is intriguing, because published evidence suggests augmented secretion of IL-8 and macrophage inflammatory protein 1α by cerebral endothelial cells infected with Anaplasma phagocytophilum and enhancement of this effect by coinfection with Borrelia burgdorferi (14). Interestingly, the human brain microvascular EC line in this study and SV-HCECs used in our experiments are both derived by immortalization of primary HCECs via stable transfection of pSV plasmid encoding the simian virus 40 large T antigen and maintain the typical cobblestone morphology and major phenotypic properties of endothelial cells (2, 14, 19). Despite these important interpretive considerations, our data do not completely rule out the possibility of differential regulation of the chemokine secretion response in primary versus immortalized cerebral endothelium and dictate the need for further “side-by-side” investigations of primary and immortalized cerebral microvascular endothelium in response to infection.
R. rickettsii infection of large vessel endothelial cells of different origins (i.e., those isolated from human pulmonary artery [HPAECs] and umbilical vein [HUVECs]) triggered an almost identical pattern of host cell activation as defined by translocation of active NF-κB to the nucleus, enhanced phosphorylation of p38 and secretion of IL-8 and MCP-1, and induced expression of HO-1 and COX-2. Another notable similarity was the presence of significant amounts of PGE2 into the culture supernatants of infected HPAECs, which may be attributable to the increased activity of COX-2. These similarities in the behavior of ECs isolated from the anatomically distinct large vessels within the same host species thus suggest the likely involvement of identical mechanisms of generation of reactive oxygen species, protective antioxidant enzyme systems, and activation of upstream cellular signaling machinery leading to recruitment of inflammatory cells.
An intriguing observation from our studies was that increased expression of COX-2 was only evident in macrovascular endothelial cells, whereas microvascular cells from the brain, dermis, or lungs did not display increased COX-2 expression, rendering them unable to secrete detectable levels of prostaglandins following R. rickettsii infection. Although prostaglandins are best known for modifying the inflammatory response affecting symptoms, including hyperthermia, pain, and swelling, PGE2 also has anti-inflammatory functions such as suppression of lymphocyte proliferation and inhibition of cytokine and interleukin production. Also, there is compelling evidence to implicate COX-2 and prostaglandins in increased permeability of brain microvasculature due to tumor necrosis factor alpha (TNF-α) (18). In this regard, the absence of changes in COX-2 activity during R. rickettsii infection suggests that alterations in vascular permeability of cerebral endothelial cells apparently involve PG-independent mechanisms. Another potent vasodilator, prostacyclin PGI2 is also important in maintaining vascular homeostasis and mediates vascular pathologies manifesting as atherogenesis and thrombosis. Consistent with previous findings that prostacyclin is the main product of macrovascular endothelium (37), our results also demonstrate COX-2 expression and PG secretion only by infected macrovascular endothelial cells. Taken together, these findings uncover activation of common as well as unique macro- versus microvascular endothelial activation mechanisms during Rickettsia infection and identify physiologically important differences in the consequences of disseminated infection of site- and vessel size-specific endothelium.
Acknowledgments
We thank Julia Ablaeva and Semion Kiriakidi for assistance with primary cell cultures and data analysis throughout the course of this study, Punsiri M. Colonne for assistance with quantitative PCR assays, and Andrew Buckley for his contributions while rotating through our laboratory.
This research was supported in part by USPHS grants AI040689 and AI067613 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda, MD.
In accordance with the HHS Select Agent requirements, the corresponding author's laboratory is registered with the Centers for Disease Control and Prevention and has obtained approval for the possession, use, and transfer of Rickettsia rickettsii for biomedical research (registration no. C20090827-0899; expiration date, 20 August 2012). The procedures and policies for inventory, storage, and usage of R. rickettsii in a biosafety level 3 containment laboratory, as recommended by the latest edition of “Biosafety in Microbiological and Biomedical Laboratories” (BMBL) and approved by the Institutional Biosafety Committee (IBC), are followed for all experiments employing viable organisms.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 12 April 2010.
REFERENCES
- 1.Abbott, N. J. 2002. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 200:629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 3.Aird, W. C. 2004. Endothelial cell heterogeneity: a case for nature and nurture. Blood 103:3994-3995. [Google Scholar]
- 4.Aird, W. C. 2008. Endothelium in health and disease. Pharmacol. Rep. 60:139-143. [PubMed] [Google Scholar]
- 5.Brigham, K. L. 1990. Oxidant stress and adult respiratory distress syndrome. Eur. Respir. J. 11:S482-S484. [PubMed] [Google Scholar]
- 6.Charalambous, C., L. B. Pen, Y. S. Su, J. Milan, T. C. Chen, and F. M. Hofman. 2005. Interleukin-8 differentially regulates migration of tumor-associated and normal human brain endothelial cells. Cancer Res. 65:10347-10354. [DOI] [PubMed] [Google Scholar]
- 7.Clifton, D. R., R. A. Goss, S. K. Sahni, D. van Antwerp, R. B. Baggs, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc. Natl. Acad. Sci. U. S. A. 95:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifton, D. R., E. Rydkina, H. Huyck, G. Pryhuber, R. S. Freeman, D. J. Silverman, and S. K. Sahni. 2005. Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: regulation by nuclear transcription factor NF-κB. Int. J. Med. Microbiol. 295:267-278. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, P. 2009. Targeting protein kinases for the development of anti-inflammatory drugs. Curr. Opin. Cell Biol. 21:317-324. [DOI] [PubMed] [Google Scholar]
- 10.Crone, C., and S. P. Oleson. 1992. Electrical resistance of brain microvessel endothelium. Brain Res. 241:49-55. [DOI] [PubMed] [Google Scholar]
- 11.Eremeeva, M. E., and D. J. Silverman. 1998. Effects of the antioxidant α-lipoic acid on human umbilical vein endothelial cells infected with Rickettsia rickettsii. Infect. Immun. 66:2290-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillespie, J. J., M. S. Beier, M. S. Rahman, N. C. Ammerman, J. K. Shallom, A. Purkayastha, B. S. Sobral, and A. F. Azad. 2007. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS One 7:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonçalves da Costa, P. S., M. E. Brigatte, E. Pereira de Almeida, and L. M. de Carvalho Valle. 2002. Atypical fulminant Rickettsia rickettsii infection (Brazilian spotted fever) presenting as septic shock and adult respiratory distress syndrome. Braz. J. Infect. Dis. 6:91-96. [DOI] [PubMed] [Google Scholar]
- 14.Grab, D. J., E. Nyarko, N. C. Barat, O. V. Nikolskaia, and J. S. Dumler. 2007. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin. Vaccine Immunol. 14:1420-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi, S. G., C. W. Francis, D. J. Silverman, and S. K. Sahni. 2003. Nuclear factor-kappa B protects against host cell apoptosis during Rickettsia rickettsii infection by inhibiting activation of apical and effector caspases and maintaining mitochondrial integrity. Infect. Immun. 71:4127-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplanski, G., N. Teysseire, C. Farnarier, S. Kaplanski, J. C. Lissitzky, J. M. Durand, J. Soubeyrand, C. A. Dinarello, and P. Bongrand. 1995. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1α-dependent pathway. J. Clin. Invest. 96:2839-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little, M. A., and C. O. Savage. 2008. The role of the endothelium in systemic small vessel vasculitis. Clin. Exp. Rheumatol. 26:S135-S140. [PubMed] [Google Scholar]
- 18.Mark, K. S., W. J. Trickler, and D. W. Miller. 2001. Tumor necrosis factor-α induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 297:1051-1058. [PubMed] [Google Scholar]
- 19.Muruganandam, A., L. M. Herx, R. Monette, J. P. Durkin, and D. B. Stanimirovic. 1997. Development of immortalized human cerebromicrovascular endothelial cell line as an in vitro model of the human blood-brain barrier. FASEB J. 11:1187-1197. [DOI] [PubMed] [Google Scholar]
- 20.Prat, A., K. Biernacki, J. F. Lavoie, J. Poirier, P. Duquette, and J. P. Antel. 2002. Migration of multiple sclerosis lymphocytes through brain endothelium. Arch. Neurol. 59:391-397. [DOI] [PubMed] [Google Scholar]
- 21.Reese, T. S., and M. J. Karnovsky. 1967. Fine structural localization of blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rydkina, E., A. Sahni, D. J. Silverman, and S. K. Sahni. 2002. Rickettsia rickettsii infection of cultured human endothelial cells induces heme oxygenase-1 expression. Infect. Immun. 70:4045-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rydkina, E., D. J. Silverman, and S. K. Sahni. 2005. Activation of p38 stress-activated protein kinase during Rickettsia rickettsii infection of human endothelial cells: role in the induction of chemokine response. Cell. Microbiol. 7:1519-1530. [DOI] [PubMed] [Google Scholar]
- 24.Rydkina, E., A. Sahni, R. B. Baggs, D. J. Silverman, and S. K. Sahni. 2006. Infection of human endothelial cells with spotted fever group rickettsiae stimulates cyclooxygenase-2 expression and release of vasoactive prostaglandins. Infect. Immun. 74:5067-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rydkina, E., A. Sahni, D. J. Silverman, and S. K. Sahni. 2007. Comparative analysis of host-cell signaling mechanisms activated in response to infection with Rickettsia conorii and Rickettsia typhi. J. Med. Microbiol. 56:896-906. [DOI] [PubMed] [Google Scholar]
- 26.Sahni, S. K., D. J. Van Antwerp, M. E. Eremeeva, D. J. Silverman, V. J. Marder, and L. A. Sporn. 1998. Proteasome-independent activation of nuclear factor-κB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii. Infect. Immun. 66:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahni, S. K., and E. Rydkina. 2009. Host-cell interactions with pathogenic Rickettsia species. Future Microbiol. 4:323-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santucci, L. A., P. L. Gutierrez, and D. J. Silverman. 1992. Rickettsia rickettsii induces superoxide radical and superoxide dismutase in human endothelial cells. Infect. Immun. 60:5113-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi, R. J., P. J. Simpson-Haidaris, N. B. Lerner, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. Transcriptional regulation of endothelial cell tissue factor expression during Rickettsia rickettsii infection: involvement of the transcription factor NF-κB. Infect. Immun. 66:1070-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman, D. J. 1984. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect. Immun. 44:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman, D. J., and S. B. Bond. 1984. Infection of human vascular endothelial cells by Rickettsia rickettsii. J. Infect. Dis. 149:201-206. [DOI] [PubMed] [Google Scholar]
- 32.Simmons, D. L., R. M. Botting, and T. Hla. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56:387-437. [DOI] [PubMed] [Google Scholar]
- 33.Sporn, L. A., S. K. Sahni, N. B. Lerner, V. J. Marder, D. J. Silverman, L. C. Turpin, and A. L. Schwab. 1997. Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect. Immun. 65:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanimirovic, D. B., and K. Satoh. 2000. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 10:113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valbuena, G., W. Bradford, and D. H. Walker. 2003. Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group. Am. J. Pathol. 163:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valbuena, G., and D. H. Walker. 2006. The endothelium as a target for infections. Annu. Rev. Pathol. 1:171-198. [DOI] [PubMed] [Google Scholar]
- 37.Venugopal, S. K., S. Devaraj, and I. Jialal. 2003. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation 108:1676-1678. [DOI] [PubMed] [Google Scholar]
- 38.Vorbrodt, A. W. 1988. Ultrastructural cytochemistry of blood-brain barrier endothelia. Prog. Histochem. Cytochem. 18:1-99. [DOI] [PubMed] [Google Scholar]
- 39.Walker, D. H., and J. H. S. Gear. 1985. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am. J. Trop. Med. Hyg. 34:361-371. [DOI] [PubMed] [Google Scholar]
- 40.Woods, M. E., and J. P. Olano. 2008. Host defenses to Rickettsia rickettsii infection contribute to increased microvascular permeability in human cerebral endothelial cells. J. Clin. Immunol. 28:174-185. [DOI] [PMC free article] [PubMed] [Google Scholar]