Summary
The phosphoinositide 3-kinase (PI3K) signaling pathway plays a critical role in the development, activation, and homeostasis of T cells by modulating the expression of survival and mitogenic factors in response to a variety of stimuli. Ligation of the antigen receptor, costimulatory molecules, and cytokine receptors activate PI3K, resulting in the production of the lipid second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3). A number of molecules help to regulate the activity of this pathway, including the lipid phosphatase PTEN (phosphatase and tensin homolog deleted on chromosome 10). By limiting the amount of PIP3 available within the cell, PTEN directly opposes PI3K activity and influences the selection of developing thymocytes as well as the activation requirements of mature T cells. T cells with unchecked PI3K activity, as a result of PTEN deficiency, contribute to the development of both autoimmune disease and lymphoma. This review dissects our current understanding of PI3K and PTEN and discusses why appropriate balance of these molecules is necessary to maintain normal T-cell responses.
Keywords: Tcells, cell activation, costimulation
Introduction
T-cell fate is dictated by the integration of signals delivered through a variety of receptors expressed at the cell surface. Receptors including the T-cell antigen receptor (TCR), costimulatory molecules, and cytokine receptors ultimately control T-cell development, tolerance induction, homeostasis, activation, differentiation, and death. A surprisingly few number of downstream signaling pathways can individually and collectively mediate this broad array of outcomes. Appropriate balance and regulation of these pathways is therefore essential to prevent deleterious T-cell responses.
Ligation of TCR, CD28, or common γ chain (γc) cytokine receptors all result in the recruitment and activation of, among other pathways, phosphoinositide-3 kinase (PI3K) (1). Signaling via PI3K is well documented to promote T-cell survival, proliferation, and motility. Unchecked activation of PI3K, therefore, has the potential to drive harmful responses such as autoimmunity and tumor formation. The lipid phosphatase PTEN (phosphatase and tensin homolog deleted on chromosome 10) provides one level of regulation of PI3K signaling. This review focuses on how regulation of this signaling pathway, most prominently via PTEN, is critical for the physiologic control of the immune response.
PI3K
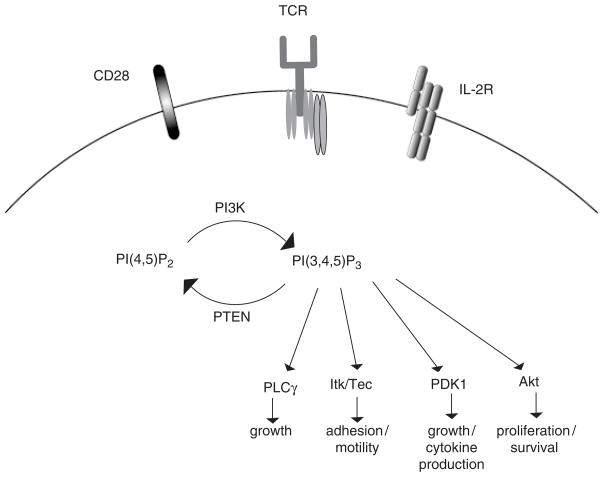
PI3K enzymes are lipid kinases that phosphorylate the 3′ position of the inositol ring of phosphoinositides, resulting in the production of phosphatidylinositol(3)-monophosphate (PIP), phosphatidylinositol(3,4)-biphosphate (PIP2), and phosphatidylinositol(3,4,5)-triphosphate (PIP3) (2). Accumulation of PIP3 within the cell leads to the recruitment and activation of a number of pleckstrin homology (PH) domain-containing proteins (3), including Akt, Itk [interleukin-2 (IL-2)-induced kinase]/Tec, PLCγ (phospholipase Cγ), Vav, and phosphoinositide-dependent kinase-1 (PDK1) (Fig. 1). The function of each of these molecules and its contribution to PI3K signaling has been reviewed extensively (4–6). Ultimately, activation of these targets in response to PI3K activation promotes a wide range of cellular responses such as cell cycle progression, growth, prevention of apoptosis, cell migration, and differentiation.
Fig. 1. PI3K signaling in T cells.
Ligation of a number of receptors expressed on the surface of T cells (shown: TCR, CD28, γc cytokine receptors) results in the activation of PI3K. Once activated, PI3K phosphorylates PI(4,5)P2 to generate the lipid product PI(3,4,5)P3. PH domain-containing proteins bind to and are activated downstream of PI(3,4,5)P3. Ultimately, these proteins promote T-cell growth, motility, cytokine production, proliferation, and survival. Accumulated PI(3,4,5)P3 is hydrolyzed by the phosphatase PTEN.
Structural studies have revealed that PI3K is a heterodimeric protein that consists of a catalytic (p110) and a regulatory (p85) subunit. Since the identification of PI3K in 1988 (7), eight known catalytic isoforms have been identified in mammals that have been divided into class IA, IB, II, and III. Only the class IA and IB isoforms are able to generate the potent second messenger PIP3 and are the most thoroughly studied isoforms in immune cells (8, 9). T cells express three class IA catalytic isoforms (p110α, p110β, and p110δ), which are constitutively bound to one of five Src-homology 2 (SH2) domain-containing regulatory subunits (p85α, p55α p50α, p85β, or p55γ; often referred to as ‘p85s’). Class IA isoforms can be activated by TCR, CD28 family costimulatory receptors, and cytokine receptors. T cells also express a single class IB PI3K. It consists of the p110γ catalytic subunit complexed to the p101 regulatory subunit, signals downstream of G-protein-coupled receptors (GPCRs), and is activated mainly by chemokines and by adenosine (10–12). As the class II and III PI3Ks have not been well examined in immune signaling, they are not discussed in this review.
Multiple ways to activate PI3K
Recent microscopy studies demonstrated that T-cell activation is accompanied by rapid and sustained production of PIP3, which is concentrated at the immunological synapse (13, 14). The exact mechanism by which TCR engagement leads to PI3K activation remains uncertain. However, data suggest that PI3K may be coupled to TCR signals through p85 interactions with adapter molecules either containing canonical YxxM motifs, such as T-cell receptor interaction molecule (TRIM) (15), or with signaling molecules containing non-canonical tyrosine-based motifs such as ζ-associated protein of 70 kDa (ZAP-70) or SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) (16, 17).
Our understanding of PI3K activation downstream of costimulatory molecules is more clear. The cytoplasmic tail of CD28 and ICOS (inducible costimulator) each encode a YxxM motif. Upon T-cell activation, CD28 is tyrosine phosphorylated by Lck (18), providing a docking site for the SH2 domains of the p85 regulatory subunit of class IA PI3K, which in turn recruit the catalytic p110 subunits of PI3K to the membrane, leading to PI3K enzymatic activity (19, 20).
Additionally, class IA PI3K has been linked to costimulation by mitogenic signals generated by γc cytokines (IL-2, IL-4, IL-7, and IL-15)(21–24). While some cytokine receptors have direct binding sites for p85, others interact with PI3K through adapter molecules. Activation of PI3K downstream of IL-2 receptor (IL-2R), for example, involves the successive recruitment of the adapter proteins Shc, Grb2, and Gab2, and p85 is recruited to the cell membrane via interactions with phosphorylated Gab2 (25, 26).
Role of PI3K in T cells
p110
To evaluate the role of different class I PI3K isoforms in transmitting signals in vivo, several laboratories have generated PI3K mutant or knockout mice. Mice deficient in p110α or p110β are embryonic lethal, precluding an analysis of the role of these isoforms in T-cell function. Mice expressing a catalytically inactive form of p110δ (p110δD910A/D910A), the principal isoform involved in antigen receptor signaling, are viable. These mice exhibited impaired calcium flux in response to TCR crosslinking and were defective in proliferating and producing IL-2 in response to peptide stimulation (27). p110δ has also been implicated in CD28-mediated clonal expansion and T-helper (Th) cell differentiation (28). More recently it has become evident that p110δ plays a critical role in regulating the activity of regulatory T cells (Tregs). In addition to having a decreased proportion of Tregs in the spleen and lymph nodes, Tregs from p110δD910A/D910A mice are defective in IL-10 production and have a diminished suppressive capacity both in vitro and in vivo (29).
Like p110δ, p110γ also plays a role in signaling downstream of the TCR. Impaired proliferation of both CD4+ and CD8+ T cells from p110γ−/− mice correlated with diminished tyrosine kinase, Akt, and mitogen-activated protein kinase (MAPK) activation as well as a reduction in F-actin polymerization and conjugate formation (30).
In addition to peripheral signaling events, analysis of p110γ−/− mice provided early evidence for a role for PI3K in T-cell development. While the effect of p110γ deficiency was somewhat modest, there was a reduction in the total number of thymocytes in these mice, which became more apparent as the mice aged. This decreased number was attributed to a decreased survival capacity in the absence of p110γ (11). More recent studies shed additional light on these findings and suggest the moderate defect in p110γ knockout mice was due to redundancy between PI3K isoforms, as mice lacking both p110γ and p110δ have more severe T-cell developmental defects (31). More specifically, in the absence of both p110γ and p110δ, overall thymic cellularity was reduced by > 50%, with profound reductions in the number of double negative 4 (DN4), double positive (DP), and mature single positive (SP) cells. Defects in both the proliferative burst following β-selection as well as reduced survival within the DP population were proposed to account for the overall reduction in cell numbers. These data cumulatively suggest an important thymic role for PI3K activation downstream of the pre-TCR. Complementary studies show that overexpression of Akt in thymocytes confers increased viability and resistance to apoptosis that is associated with increased expression of Bcl–xL (32).
p85
T cells from mice deficient in p85α or p85β develop normally and proliferate in response to various mitogens (33–35). This observation suggested that class IA regulatory subunits have either limited or redundant functions. Indeed, characterization of mice lacking multiple regulatory subunits (p85α/p55α/p50α/p85β knockout) revealed a critical role for PI3K in response to TCR crosslinking. T cells from combined knockout mice have impaired calcium flux, diminished proliferation and cytokine production, and are unable to induce expression of Bcl–xL in response to antigen receptor stimulation (36). These defects in TCR signaling are partially rescued by exogenous IL-2 or by addition of CD28 costimulation. Although more work needs to be done to clarify the unique and overlapping roles of class IA and IB PI3K isoforms in T-cell activation, these genetic studies underline the pivotal role of PI3K signaling during development and in peripheral tissues is response to a variety of cellular cues.
PI3K and costimulation
In addition to its role downstream of the antigen receptor, it is also clear that PI3K is activated in response to ligation of CD28. However, the precise role for PI3K signals downstream of CD28 remains controversial. Conflicting results have been generated by groups studying CD28 knockout mice reconstituted with a tyrosine-to-phenylalanine (Y170F) mutant of CD28 that cannot bind PI3K. Early indications were that CD28-mediated PI3K signals were critical for driving both the survival and proliferation of CD4+T cells (37). However, more recent studies indicate that PI3K signaling downstream of CD28 is only required for the upregulation of the pro-survival factor BcL–xL (38).
γc chain cytokines provide an additional means of activating PI3K, with IL-2 signals being the best characterized in T cells. It has long been appreciated that signaling through the IL-2R promotes both T-cell survival and proliferation by modulating the expression of Bcl-2 family members and cell cycle regulators, respectively. These outcomes are largely dependant on PI3K activity, as deletion within the S-domain of the IL-2Rβ, which prevents activation of PI3K, reduces IL-2-mediated proliferation (39). Further, addition of chemical inhibitors to block PI3K activity prevents IL-2-mediated induction of Bcl–xL and the cell cycle regulator E2F (40–42). The proliferative and anti-apoptotic signals delivered by the IL-2R are mediated in large part by the PI3K pathway member Akt. IL-2 signaling induces phosphorylation of Akt and also promotes its translocation to the cell membrane (43). Constitutive activation of either PI3K or Akt induces c-Myc expression and prevents cell cycle arrest following IL-2 withdrawal (43, 44). These data suggest that PI3K is critical for mediating the mitogenic effects of IL-2 signaling. In addition to a direct role for PI3K signaling downstream of the IL-2R, PI3K may also promote cell survival and division by potentiating IL-2-induced signal transducer and activator of transcription 5 (STAT5) signals (45).
PI3K regulation
Controlling kinase activity
PI3K can be regulated by different means, which include control of PI3K activity and catabolism of PIP3. This first level of regulation, terminating the activity of the kinase itself, is mediated by several molecules, including the tyrosine phosphatase SHP-1. It has been demonstrated that SHP-1 can dephosphorylate tyrosine residues that serve as binding sites for p85, resulting in relocation of p85 away from the membrane and into the cytosol (46). Additionally, SHP-1 may inactivate PI3K by directly dephosphorylating Tyr688 of p85. Another potential mechanism of PI3K inactivation involves the E3 ubiquitin ligase Cbl-b, which can associate with and ubiquitinate p85 (47). While ubiquitination does not induce degradation of p85, it does prevent its recruitment to the TCR and CD28 (48). Interestingly, the T-cell defects associated with Cbl-b deficiency, including increased cytokine production and proliferation in response to TCR signals, appear to be largely due to dysregulated PI3K signaling. Blockade of PI3K using chemical inhibitors restores a wildtype (WT) phenotype to Cbl-b−/− CD4+ T cells. In addition to SH2 domain-containing phosphatase-1 (SHP-1) and Cbl-b, cytotoxic T-lymphocyte antigen-4 (CTLA-4) may also play a role in inactivating PI3K signals. The intracellular domain of CTLA-4 contains a YxxM motif that binds p85. While the significance of this interaction remains unclear, its possible that CTLA-4 also serves as a sink, preventing p85 from associating with activating receptors such as the TCR and CD28 (49).
In addition to terminating the activity of PI3K, a second mode of regulation of this pathway involves hydrolysis of the lipid second messenger PIP3. This level of regulation is provided by two inositol phosphatases, SHIP (Src-homology 2 containing phosphatase) and PTEN.
SHIP
Upon TCR/CD28 engagement, SHIP is recruited to the plasma membrane (50). Once recruited, SHIP hydrolyzes PIP3 at the 5′ position to generate phosphatidylinositol (3,4)-biphosphate [PI(3,4)P2]. Hydrolysis of PIP3 by SHIP prevents the membrane localization of certain PH-domain-containing proteins such as Akt. Interestingly, some proteins, such as TAPP1 and TAPP2, actually have a higher affinity for PI(3,4)P2 than for PIP3 (51). These data suggest that SHIP does not just quantitatively diminish the PI3K signal but also qualitatively modulates the signaling pathway. The importance of SHIP in regulating lymphocyte responses has been demonstrated by the production of SHIP knockout mice, which have a shortened life span and develop both splenomegaly and autoimmunity (52, 53). More recent studies in which SHIP was specifically deleted within the T-cell compartment suggest that SHIP plays a role in regulating the Th1/Th2 lineage decision and also limits CD8+ T-cell cytotoxicity (54).
PTEN
PTEN (also called MMAC or TEP1) is a tumor suppressor gene that was cloned by three groups in 1997 (55–57). Deletions or mutations in the PTEN gene have been identified in up to 50% of tumors, making it one of the most commonly mutated genes in human cancer. PTEN is thought to act predominantly as a lipid phosphatase, dephosphorylating PIP3 at the 3′ position to generate PI(4,5)P2 (58) (Fig. 1). By preventing the accumulation of PIP3 within the cell, PTEN limits the activation of Akt/PKB and therefore blunts the survival and proliferative signal delivered by PI3K. It is through its regulation of this pathway that PTEN is thought to exert its tumor suppressor activity.
While PIP3 is thought to be its primary substrate, PTEN also has documented protein phosphatase activity. PTEN can directly bind and dephosphorylate focal adhesion kinase (FAK) (59, 60). Mutants of PTEN that lack lipid phosphatase activity retain this capacity to dephosphorylate FAK, the result of which is decreased cell adhesion and migration (61).
While still little is known about how PTEN is regulated, structural studies have provided insights into several different potential mechanisms (62). PTEN is a 54 kDa protein that contains an N-terminal phosphatase domain, two PEST sequences, a PDZ-binding domain, and a number of phosphorylation sites at the C-terminus. PTEN can be phosphorylated on Ser380, Thr382, and Thr383. Mutation studies demonstrate that phosphorylation at these sites stabilizes PTEN but also reduces its catalytic activity (63). Additional work has identified kinases, such as the protein kinase CK2, that can phosphorylate PTEN in vitro, but it still remains unclear which kinases are responsible for this event in vivo (64).
A second possible mechanism of regulation of PTEN is mediated by protein–protein interactions. PTEN binds to the scaffolding molecule MAGI-2 through its PDZ-binding domain. This interaction prevents PTEN degradation and also promotes its activity. Thus, binding of PTEN to MAGI-2 may improve the efficiency of PTEN activity by stabilizing it as part of a multi-protein complex at the cell membrane (65).
More recent studies have identified a third potential mechanism of PTEN regulation, as the two PEST motifs of PTEN are targets of ubiquitination (66–68). Wang et al. (66) demonstrated that poly-ubiquitination of PTEN leads to its degradation and that this event can be mediated by the E3 ubiquitin ligase NEDD4-1. Interestingly, mono-ubiquitination of PTEN does not promote its degradation but rather results in increased nuclear import of PTEN. While it has long been recognized that PTEN is expressed in both the cytoplasm and nucleus of many tissues, the role of nuclear PTEN had not been appreciated. These studies shed light on this observation and suggest a phosphatase-independent role for PTEN in maintaining chromosomal stability (68).
Given PTEN’s role in regulating cellular responses such as survival, proliferation, and transformation, many groups have focused on characterizing both cell lines and mouse models with defects in PTEN. Several laboratories attempted to generate PTEN null mutant mice. However, these mice die early during embryogenesis (69). Subsequent characterization of PTEN heterozygous mice revealed a high tumor incidence in various tissues, including some spontaneous tumors of T-cell origin (70–72). Additionally, T cells from PTEN+/− mice were hyperproliferative and less sensitive to activation-induced cell death. With age PTEN heterozygous mice develop classic signs of autoimmunity including lymphadenopathy, kidney disease, and elevated serum autoantibody levels (70). Together, these observations suggest a potential role for PTEN in regulating T-cell proliferation and survival.
To better study the role of PTEN in T cells, mice with a T-cell-specific deficiency in PTEN were generated using the Cre-loxP conditional gene targeting system (73). The results of these studies are the focus of the following sections. In particular, we describe our current understanding of PTEN’s involvement in regulating the responses of both developing thymocytes as well as mature T-cell subsets.
Role of PTEN in T cells: development
In the last few years, it has become increasingly evident that PTEN-mediated regulation of PI3K signaling in the thymus plays a critical role in T-cell development. The first evidence for this was provided by Suzuki et al. (73), who generated mice with a T-cell-specific deficiency in PTEN by crossing Lck-Cre transgenic animals with mice in which one allele of Pten was deleted and the other floxed (LckCre-PTENflox/−). Deletion of PTEN early in T-cell development has a profound impact on both the size and composition of the thymus. By 6–8 weeks of age, there was an approximate twofold increase in total thymic cellularity of LckCre-PTENflox/− mice. This change was associated with increased cell numbers in all thymic populations as well as an increase in the percentage of cells with an activated phenotype. Breeding these mice onto an H–Y TCR transgenic background revealed that PTEN deficiency altered both positive and negative selection in the thymus. With age, these mice displayed hallmarks of autoimmunity, developed T-cell-derived lymphomas, and died by 17 weeks after birth.
These results provided strong evidence that PTEN is a critical regulator of T-cell development. However, the disadvantage of the model by Suzuki et al. (LckCre-PTENflox/−) was that expression of PTEN was reduced not just in T cells but in all cellular compartments, which could complicate the interpretation of these results, as PTEN+/− mice develop autoimmune disorders and lymphoid hyperplasia. To address similar questions in a model where the PTEN levels were modulated only within the T-cell compartment, Hagenbeek et al. (74)studied T-cell development in LckCre-PTENflox/flox mice. By crossing these mice with recombination activating gene 2 (Rag2)- and γc-deficient mice, the authors showed that PTEN deficiency can substitute for both IL-7R and pre-TCR signals in the thymus. Higher basal PI3K signals maintained in the absence of PTEN likely promote survival and proliferation of developing thymocytes, even in the absence of normally required environmental and cellular cues.
In addition to a role for PTEN in the development of conventional T cells, recent work suggests that PTEN may also regulate the development of invariant natural killer T cells (iNKT) (75). In the absence of PTEN, there is a 4–5-fold reduction in the number of NKT cells in the thymus as well as a reduction in peripheral tissues. This reduction is associated with a block in development between stage 2 (CD44high NK1.1 −) and the final stage of NKT cell maturation (CD44high NK1.1+). The authors went on to show that reducing the number of functionally mature iNKT cells circulating in the mouse results in diminished NKT cell-mediated tumor surveillance, as protection against the metastasis of melanoma cells to the lung was impaired in the absence of PTEN. By promoting iNKT cell development, these data highlight a secondary mechanism by which PTEN acts to suppress tumor formation.
These data suggest that PTEN is critical for regulating T-cell selection signals in the thymus. Expression of PTEN imposes a requirement for growth and activating signals at various stages of development and may ultimately play a role in regulating lineage fate decisions. The contribution of defective T-cell development in the absence of PTEN to the aberrant T-cell activation and lymphoma formation that is observed is still not clear. Given the broad array of functions that PI3K signaling mediates, additional experiments will be required to determine which dysregulated signals contribute to disease progression in this mouse model.
Role of PTEN in T cells: effector CD4+ T cells
Our laboratory has characterized the effect of PTEN deficiency on peripheral CD25−CD4+ effector T cells. For these purposes, we have crossed CD4-Cre transgenic animals with PTENflox/flox mice (progeny referred to as PTEN-ΔT) and have used young animals (~2.5–4.5 weeks of age) to examine the activation requirements of naive CD4+ T cells deficient in PTEN, as the splenic and lymphoid compartments in young PTEN-ΔT mice are grossly normal, containing appropriate numbers and ratios of CD4 and CD8 cells. Additionally, CD4+ T cells isolated from young PTEN-ΔT mice are phenotypically and functionally naive. Interestingly, CD4+ T cells deficient in PTEN are hyperresponsive to sub-optimal stimulation through the TCR (76). There is enhanced phosphorylation of molecules downstream of PI3K such as Akt, GSK3β, and p70 S6 kinase when CD4+ T cells deficient in PTEN receive low doses of isolated TCR signals. Enhanced activation of the PI3K pathway by PTEN-ΔT cells corresponds with augmented IL-2 production and enhanced proliferation.
As discussed previously, T-cell development is altered in the absence of PTEN. To determine if the phenotype of mature PTEN-ΔT CD4+ T cells was independent of developmental defects, floxed PTEN mice were bred with mice expressing an inducible Cre recombinase (ERCre-PTENflox/flox), so that PTEN could be deleted in vivo in mature, post-thymic T cells. Importantly, peripheral deletion of PTEN within mature CD4+ T cells recapitulates the hyperresponsive phenotype of PTEN-ΔT cells, suggesting the augmented response to TCR signals in the absence of PTEN is not entirely due to defects arising in the thymus.
The two signal hypothesis proposes that naive T cells require antigen receptor signaling (signal 1) in the context of costimulation (signal 2) for optimal activation. CD28 is the best characterized T-cell costimulatory pathway, and activation of PI3K downstream of CD28 is thought to be a critical step in its effects. The observation that PTEN-deficient CD4+ T cells generate augmented PI3K activity in response to isolated TCR signals raised the possibility that these cells may no longer have a stringent requirement for costimulation to become activated. Indeed, in vivo proliferative responses that require CD28 costimulation under WT conditions are intact in the absence of CD28 when T cells are deficient in PTEN. Consistent with this observation, CD4+ T cells from PTEN-ΔT mice are refractory to anergy induction both in vitro and in vivo following TCR stimulation in the absence of CD28 costimulation (76). Cumulatively, these data suggest that by negatively regulating PI3K in response to sub-optimal TCR signals, PTEN imposes a requirement for costimulation that may in turn play a role in maintaining immune homeostasis and self-tolerance.
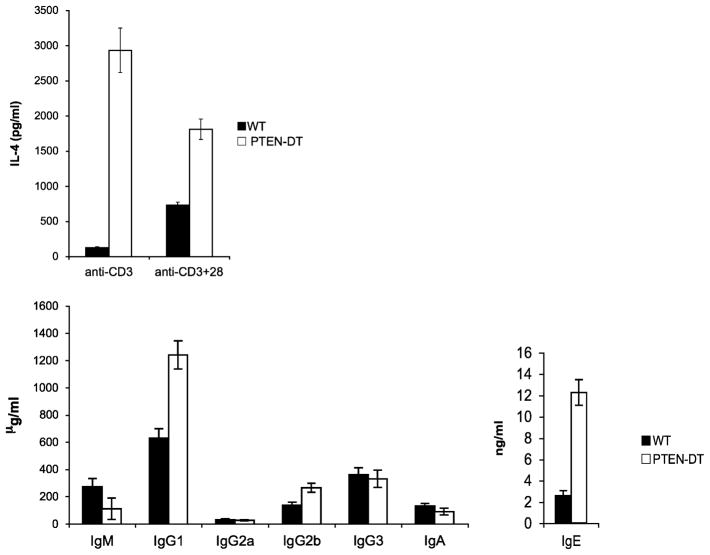
In addition to promoting T-cell activation, CD28 costimulatory signals are critical for driving Th2 responses. Consistent with the hypothesis that PTEN-deficient cells have a diminished requirement for costimulation, CD4+ T cells that do not express PTEN produce elevated levels of classic Th2 cytokines even in the absence of CD28 signals (Fig. 2). In response to stimulation through the TCR alone or in combination with CD28, PTEN-ΔT CD4+T cells produce approximately 5–10 fold more IL-4 and IL-10 than WT T cells. Differences in IFN-γ and IL-17 production are much more modest (authors’ unpublished data). This exaggerated Th2 response in vitro is complemented by the observation that there are greatly increased levels of immunoglobulin G1 (IgG1) and IgE in the serum of PTEN-ΔT mice (Fig. 2). These data provide preliminary evidence that PTEN-deficient T cells may have a Th2 bias and that appropriate regulation of PI3K signaling is important for balancing effector cell differentiation in the periphery.
Fig. 2. PTEN-ΔT CD4+cells have a Th2 bias in vitro and in vivo.
(Top) CD4+T cells MACS purified from the spleen and lymph nodes of three-week-old WT and PTEN-ΔT mice were stimulated for 96 h with anti-CD3 (1 μg/ml) alone or in combination with anti-CD28 (5 μg/ml). Supernatants were harvested, and IL-4 production was measured by enzyme-linked immunosorbent assay (ELISA). (Bottom) Representative serum Ig ELISA analysis from eight-week-old WT and PTEN-ΔT mice.
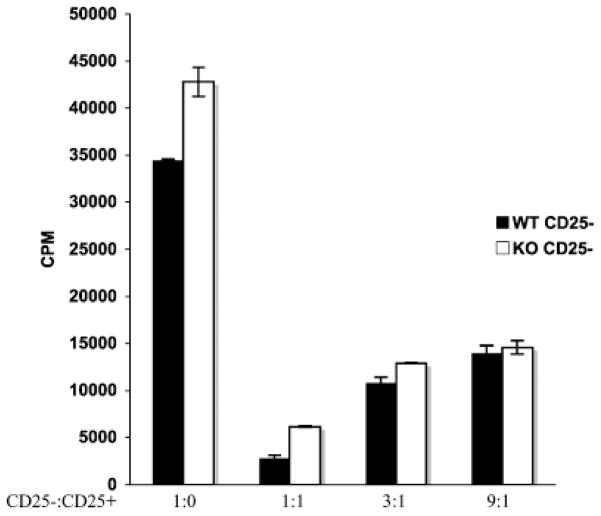
The phenotype of PTEN-deficient T cells is similar in many ways to other models where key negative regulatory elements, such as Cbl-b or tumor necrosis factor (TNF) receptor-associated factor-6 (TRAF-6), have been mutated (77–82). Reports characterizing the defects in Cbl-b and TRAF-6 mutant mice show that deficiency of either of these molecules generates a population of CD4+ T cells that are hyperresponsive to TCR signals, have a Th2 bias, and cannot be properly anergized. In addition, Cbl-b or TRAF6 mutant CD4+ T cells are not susceptible to the suppressive effects of Tregs. Interestingly, despite enhanced signaling, PTEN-deficient CD4+ T cells remain responsive to Treg-mediated suppression (Fig. 3). Dissecting the different targets regulated by these various molecules may help shed light on the mechanism by which effector T cells are held in check by Tregs.
Fig. 3. PTEN-ΔT CD4+CD25− T cells are susceptible to suppression by Tregs.
CD4+ CD25+ and CD4+ CD25 − cells were FACS purified from the spleen and lymph node of three-week-old WT and PTEN-ΔT mice. 1.0 × 105 CD4+CD25 − cells were stimulated for 72 h with antigen-presenting cells and soluble anti-CD3 (1 μg/ml) in the presence or absence of various ratios of CD4+CD25+ cells (as shown). Wells were pulsed with tritiated thymidine for the final 16 h of the culture.
Role of PTEN in T cells: Tregs
Naturally occurring Tregs are generated in the thymus, express the transcription factor forkhead box protein 3 (Foxp3), and play a critical role in preventing the activation of autoreactive T cells. Given the evidence that PTEN has a profound impact on many aspects of T-cell development and that deletion of PTEN is associated with autoimmunity, one might predict that the Treg population is altered in this model. Interestingly, Tregs appear to develop normally in PTEN-ΔT mice (83). In young mice, before the onset of disease, there is a normal number and percentage of Foxp3+cells in both the thymus and in the periphery. Additionally, mixed bone marrow chimeras suggest that PTEN deficiency does not provide a competitive advantage or disadvantage to developing Tregs, as both WTand PTEN-ΔT bone marrow contributed equally to the Foxp3+ population. In addition to normal numbers in PTEN-ΔT mice, PTEN-deficient Tregs maintain their ability to suppress the proliferation of effector T cells in vitro.
PTEN deficiency, however, does alter the proliferative capacity of Tregs. WT Tregs are hypoproliferative to IL-2R signaling due to an inability to activate the PI3K pathway (84). This inability to activate PI3K targets is likely due to negative regulation of this pathway by PTEN, as deletion of PTEN generates Tregs capable of proliferating in response to IL-2 alone (83). PTEN-ΔT Tregs expand 15–25-fold in culture with recombinant IL-2, in contrast to WT Tregs which fail to accumulate under these conditions. Importantly, expansion of PTEN-deficient Tregs with IL-2 does not alter their suppressive capacity, as they retain their ability to prevent colitis in an in vivo model of inflammatory bowel disease. Knockdown of PTEN, therefore, may be a useful tool for expansion of this potentially therapeutic cell population.
These data strongly support the idea that PTEN plays a critical role in regulating the response of mature CD4+ T cells in the periphery. Little work has been done characterizing the role that PTEN-mediated regulation of PI3K plays in the context of other T-cell populations such as mature CD8+ T cells and effector versus central memory cells.
Concluding remarks
The common theme among all these results is that PTEN appears to set a high threshold for signaling. Expression of PTEN in T cells tunes the strength of signal necessary to mediate a response. We propose that a primary function of PTEN is to prevent aberrant T-cell survival and activation, and it accomplishes this by imposing a stringent requirement for appropriate environmental signals at various stages during the life of the T cell. T cells activated in the absence of appropriate cues can contribute to deleterious fates, such as malignancies and autoimmune disorders.
There is increasing evidence that a common link exists between autoimmunity and cancer (85). PTEN is one of many different regulatory elements, including BIM, Fas, and CTLA4, that serve as checkpoints to prevent prolonged signaling and aberrant lymphocyte activation, and mutations in each of these molecules are associated with lymphoid hyperplasia, autoimmune disease, and malignancy (86–88). Studying models in which cancer and autoimmunity are coincident will allow us to better understand if common or divergent signaling events lead to the onset of autoimmunity and the initiation of transformation.
While much work has been done in recent years to elucidate the role that PTEN plays in regulating T-cell responses, many questions remain. Importantly, we still have a limited understanding of how PTEN expression and function are regulated in T cells. While it has been shown that TCR signals can result in PTEN degradation, little is known about how other cell signaling events, such as costimulatory molecule and cytokine signaling, influence PTEN function. Additionally, other T-cell subsets, such as CD8+ T cells and memory T cells, also express PTEN. Yet, there is no work describing a role for PTEN in these populations. Finally, it would be of great interest to know if modulation of PTEN within various T-cell subsets could be beneficial as a therapeutic tool. Downmodulation of PTEN might, for example, improve the anti-tumor response of effector CD4+ T cells. Tumor cells are known to have decreased expression of costimulatory molecules, preventing robust effector T-cell activation. PTEN deficiency, however, relieves T cells of the requirement for costimulation, suggesting they may be less susceptible to tumor tolerizing mechanisms. Additionally, downmodulation may be a practical approach for effectively expanding regulatory T cells ex vivo, a process that could prove useful in the setting of transplantation.
References
- 1.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins PT, Jackson TR, Stephens LR. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- 3.Fruman DA, Rameh LE, Cantley LC. Phosphoinositide binding domains: embracing 3-phosphate. Cell. 1999;97:817–820. doi: 10.1016/s0092-8674(00)80792-8. [DOI] [PubMed] [Google Scholar]
- 4.Seminario MC, Wange RL. Lipid phosphatases in the regulation of T cell activation: living up to their PTEN-tial. Immunol Rev. 2003;192:80–97. doi: 10.1034/j.1600-065x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Ward SG, Cantrell DA. Phosphoinositide 3-kinases in T lymphocyte activation. Curr Opin Immunol. 2001;13:332–338. doi: 10.1016/s0952-7915(00)00223-5. [DOI] [PubMed] [Google Scholar]
- 6.Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr Opin Immunol. 2004;16:314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 8.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 9.Vanhaesebroeck B, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 10.Laffargue M, et al. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki T, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 12.Ward SG. T lymphocytes on the move: chemokines, PI 3-kinase and beyond. Trends Immunol. 2006;27:80–87. doi: 10.1016/j.it.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- 14.Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Im-munol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- 15.Bruyns E, et al. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR-CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 17.Shim EK, Moon CS, Lee GY, Ha YJ, Chae SK, Lee JR. Association of the Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) with the p85 subunit of phosphoinositide 3-kinase. FEBS Lett. 2004;575:35–40. doi: 10.1016/j.febslet.2004.07.090. [DOI] [PubMed] [Google Scholar]
- 18.Raab M, Cai YC, Bunnell SC, Heyeck SD, Berg LJ, Rudd CE. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc Natl Acad Sci USA. 1995;92:8891–8895. doi: 10.1073/pnas.92.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad KV, et al. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci USA. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truitt KE, Hicks CM, Imboden JB. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat T cells. J Exp Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remillard B, et al. Interleukin-2 receptor regulates activation of phosphatidylinositol 3-kinase. J Biol Chem. 1991;266:14167–14170. [PubMed] [Google Scholar]
- 22.Xiao H, et al. Specificity of interleukin-2 receptor gamma chain superfamily cytokines is mediated by insulin receptor substrate-dependent pathway. J Biol Chem. 2002;277:8091–8098. doi: 10.1074/jbc.M106650200. [DOI] [PubMed] [Google Scholar]
- 23.Dadi HK, Roifman CM. Activation of phosphatidylinositol-3 kinase by ligation of the interleukin-7 receptor on human thymocytes. J Clin Invest. 1993;92:1559–1563. doi: 10.1172/JCI116736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambricki E, et al. Signaling T-cell survival and death by IL-2 and IL-15. Am J Transplant. 2005;5:2623–2631. doi: 10.1111/j.1600-6143.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 27.Okkenhaug K, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 28.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 29.Patton DT, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+ Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 30.Alcazar I, et al. Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J Exp Med. 2007;204:2977–2987. doi: 10.1084/jem.20070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 32.Jones RG, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fruman DA, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, et al. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 35.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol. 2004;172:6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 36.Deane JA, et al. T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109:2894–2902. doi: 10.1182/blood-2006-07-038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada Y, et al. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 38.Okkenhaug K, et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 39.Merida I, Williamson P, Kuziel WA, Greene WC, Gaulton GN. The serine-rich cytoplasmic domain of the interleukin-2 receptor beta chain is essential for interleukin-2-dependent tyrosine protein kinase and phosphatidylinositol-3-kinase activation. J Biol Chem. 1993;268:6765–6770. [PubMed] [Google Scholar]
- 40.Gonzalez-Garcia A, Merida I, Martinez AC, Carrera AC. Intermediate affinity interleukin-2 receptor mediates survival via a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 1997;272:10220–10226. doi: 10.1074/jbc.272.15.10220. [DOI] [PubMed] [Google Scholar]
- 41.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 42.Brennan P, Babbage JW, Thomas G, Cantrell D. p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol Cell Biol. 1999;19:4729–4738. doi: 10.1128/mcb.19.7.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reif K, Burgering BM, Cantrell DA. Phosphatidylinositol 3-kinase links the interleukin-2 receptor to protein kinase B and p70 S6 kinase. J Biol Chem. 1997;272:14426–14433. doi: 10.1074/jbc.272.22.14426. [DOI] [PubMed] [Google Scholar]
- 45.Moon JJ, Nelson BH. Phosphatidylinositol 3-kinase potentiates, but does not trigger, T cell proliferation mediated by the IL-2 receptor. J Immunol. 2001;167:2714–2723. doi: 10.4049/jimmunol.167.5.2714. [DOI] [PubMed] [Google Scholar]
- 46.Cuevas B, et al. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J Biol Chem. 1999;274:27583–27589. doi: 10.1074/jbc.274.39.27583. [DOI] [PubMed] [Google Scholar]
- 47.Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem. 2001;276:4872–4878. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- 48.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 49.Schneider H, Prasad KV, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edmunds C, Parry RV, Burgess SJ, Reaves B, Ward SG. CD28 stimulates tyrosine phosphorylation, cellular redistribution and catalytic activity of the inositol lipid 5-phosphatase SHIP. Eur J Immunol. 1999;29:3507–3515. doi: 10.1002/(SICI)1521-4141(199911)29:11<3507::AID-IMMU3507>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Dowler S, et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helgason CD, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helgason CD, et al. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of b lymphocytes in ship −/−mice. J Exp Med. 2000;191:781–794. doi: 10.1084/jem.191.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S. T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci USA. 2007;104:11382–11387. doi: 10.1073/pnas.0704853104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 56.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 57.Steck PA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 58.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 59.Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 60.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 61.Gu J, et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146:389–403. doi: 10.1083/jcb.146.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JO, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 63.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 65.Wu X, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA. 2000;97:4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trotman LC, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen WH, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 69.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 70.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki A, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 72.Podsypanina K, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki A, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 74.Hagenbeek TJ, et al. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kishimoto H, et al. The Pten/PI3K pathway governs the homeostasis of Valpha14iNKT cells. Blood. 2007;109:3316–3324. doi: 10.1182/blood-2006-07-038059. [DOI] [PubMed] [Google Scholar]
- 76.Buckler JL, Walsh PT, Porrett PM, Choi Y, Turka LA. Cutting edge: T cell requirement for CD28 costimulation is due to negative regulation of TCR signals by PTEN. J Immunol. 2006;177:4262–4266. doi: 10.4049/jimmunol.177.7.4262. [DOI] [PubMed] [Google Scholar]
- 77.King CG, et al. Cutting edge: requirement for TRAF6 in the induction of T cell anergy. J Immunol. 2008;180:34–38. doi: 10.4049/jimmunol.180.1.34. [DOI] [PubMed] [Google Scholar]
- 78.King CG, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 79.Jeon MS, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 80.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 81.Chiang YJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 82.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 83.Walsh PT, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bensinger SJ, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 86.Rieux-Laucat F, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 87.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 88.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]