Abstract
Microglia provide immune surveillance for the brain through both the removal of cellular debris and protection against infection by microorganisms and “foreign” molecules. Upon activation, microglia display an altered morphology and increased expression of proinflammatory molecules. Increased numbers of activated microglia have been identified in a number of neurodegenerative diseases including Parkinson's disease (PD). What remains to be determined is whether activated microglia result from ongoing cell death or are involved in disease initiation and progression. To address this question we utilized a transgenic mouse model that expresses a mutated form of a key protein involved in Parkinson's disease, α-synuclein. Herein, we report an increase in activated microglia and proinflammatory molecules in 1-month-old transgenic mice well before cell death occurs in this model. Frank microglial activation is resolved by 6 months of age while a subset of proinflammatory molecules remain elevated for 12 months. Both tyrosine hydroxylase mRNA expression and α-synuclein protein are decreased in the striatum of older animals evidence of dystrophic neuritic projections. To determine whether mutated α-synuclein could directly activate microglia primary microglia-enriched cell cultures were treated with exogenous mutated α-synuclein. The data reveal an increase in activated microglia and proinflammatory molecules due to direct interaction with mutated α-synuclein. Together, these data demonstrate that mutated α-synuclein mediates a proinflammatory response in microglia and this activity may participate in PD pathogenesis.
Keywords: Dopaminergic neurons, Parkinson's disease, Neurodegeneration, Proinflammatory molecules
Introduction
α-Synuclein gene (SNCA) mutations were the first underlying genetic defects linked to early-onset familial Parkinson's disease (PD). Three missense mutations in SNCA, A53T, A30P, and E46K, as well as gene duplications and triplications have been identified in familial PD (Polymeropoulos et al. 1997; Kruger et al. 1998; Singleton et al. 2003; Zarranz et al. 2004; Nishioka et al. 2006). Although the majority of PD cases are sporadic and arise from as yet unknown etiology they are posited to be caused by an interplay between genetic vulnerability and environmental toxins (Maguire-Zeiss and Federoff 2003; Migliore and Coppede 2008). In fact, a small number of sporadic PD patients carrying SNCA duplications have recently been identified confirming that α-synuclein protein levels are important in both familial and sporadic PD (Ahn et al. 2008). Even prior to the identification of SNCA duplication in these sporadic PD cases, α-synuclein had been associated with PD because (1) the hallmark pathologic feature, the Lewy body is replete with α-synuclein (Spillantini et al. 1997), (2) α-Synuclein is enriched in dopaminergic presynaptic terminals which degenerate in PD, and (3) animal and cell culture α-synuclein overexpression, as well as neurotoxicant models display some of the hallmark pathological features of PD (reviewed in Emborg 2007; Chesselet 2008; Kahle 2008; Meredith et al. 2008; Terzioglu and Galter 2008). What remains to be determined is the exact role that α-synuclein plays in the pathogenesis of PD.
α-Synuclein, a 16–22 kDa protein with widespread nervous system distribution, was first isolated from the Torpedo cholinergic synaptic vesicles and later identified as the non-amyloid component of amyloid in Alzheimer's disease patients (Maroteaux et al. 1988; Ueda et al. 1993). In a separate study, immunochemical confocal analysis localized α-synuclein to synaptophysin-immunoreactive presynaptic terminals and synaptic vesicles (Iwai et al. 1995). Synelfin, a homolog of human α-synuclein, is upregulated during zebra finch song learning suggesting a role for α-synuclein protein in neural plasticity (George et al. 1995). Furthermore, α-synuclein is able to rescue a mouse model deficient in cysteine-string protein α (CSPα), a chaperone protein required for refolding of SNARE (soluble NSF attachment protein receptor) complexes and critical for synaptic vesicle docking (Bonini and Giasson 2005; Chandra et al. 2005). Another chaperone function ascribed to α-synuclein is the trafficking of the dopamine transporter (DAT) to the presynaptic terminal which when disrupted leads to dopamine dysregulation and neurotoxicity (Sidhu et al. 2004a, b). α-Synuclein's role in the maintenance of the synapse is also supported by the observation that α-synuclein knockout mice have altered fatty acid metabolism and altered dopamine release following paired stimuli (Abeliovich et al. 2000; Golovko et al. 2005; Barceló-Coblijn et al. 2007; Golovko et al. 2007). Furthermore, α-synuclein is capable of regulating tyrosine hydroxylase (TH) activity, the rate-limiting step in the synthesis of dopamine (Perez et al. 2002). Together this evidence suggests an important role for α-synuclein in the maintenance of synaptic integrity.
Wild-type human α-synuclein, as well as mutant forms of α-synuclein exist as natively unfolded monomers and possess the proclivity to misfold into oligomeric protofibrils and fibrils (Conway et al. 2000; Fink 2006). Several lines of evidence suggest that the pathologic role of α-synuclein is connected to its ability to self-aggregate. First, pathogenic mutations in α-synuclein have increased rates of self-assembly and fibrillization (Conway et al. 2000; Serpell et al. 2000; Greenbaum et al. 2005). Second, fibrillar α-synuclein protein is a major component of the intracytoplasmic protein-containing inclusions, Lewy Bodies, which are a pathological hallmark of PD (Spillantini et al. 1997). Third, studies using α-synuclein transgenic models suggest that formation of fibrillar α-synuclein is associated with neuronal dysfunction/degeneration (Dawson et al. 2002; Moussa et al. 2004). Taken together, α-synuclein misfolding is an apparent critical step in the pathogenesis of PD and α-synuclein mutations are implicated in accelerating the progression of disease. Studies on the pathological role of mutant α-synuclein have mainly focused on its actions within neurons where it is highly expressed and associated with neuronal death (Chung et al. 2001; Outeiro and Lindquist 2003; Wood-Kaczmar et al. 2006). However, recent studies provide evidence that α-synuclein may exist extracellularly where it is available to interact with surrounding glia and neurons (El-Agnaf et al. 2003; Lee et al. 2005). As a result, the study of the interaction of extracellular α-synuclein with its surrounding cellular environment in the central nervous system, especially with the environment-surveilling microglia may provide a new perspective on the neurotoxic role of α-synuclein in PD.
Increasing evidence points to an inflammatory process characterized by the activation of microglia in parallel with PD progression (McGeer and McGeer 2004; McGeer et al. 2005; Block et al. 2007). In the present study, we address whether microglia are activated in a mutant human α-synuclein-expressing transgenic mouse model (SYNDM+/+) where expression is under the direction of the tyrosine hydroxylase promoter. Our laboratory generated transgenic mice that overexpress two of the human α-synuclein mutations first identified in PD (A53T and A30P) within a single transgene sequence (hm2a-SYN; (Richfield et al. 2002)). These hm2a-SYN mice display more severe PD-related pathology compared to transgenic mice that overexpress wild-type human α-synuclein (hwa-SYN; (Richfield et al. 2002)). Observed pathology in these transgenic mice include age-related loss of dopaminergic substantia nigral neurons, dystrophic neurites, increased locomotor response following amphetamine treatment, and decreased dopamine and dopamine metabolite levels with aging (Richfield et al. 2002; Thiruchelvam et al. 2004). We have since homozygoused the α-synuclein locus for this model (SYNDM+/+) and employ these animals in the present studies.
Herein, we present data demonstrating increased microglial activation, which is accompanied by prominent inflammation in young SYNDM+/+ mice. While the frank microglial activation is resolved by 6 months of age, several proinflammatory molecules remain up-regulated compared to non-transgenic animals. Age-related decreases in both tyrosine hydroxylase mRNA expression and double-mutant human α-synuclein (DM SYN) protein are evident in the striatum of these animals supporting a role for mutated α-synuclein in the early initiation of neuroinflammation and as a contributing factor in the nigrostriatal pathology of PD. Furthermore, we demonstrate that DM SYN directly mediates microglial activation and that this activation is partially inhibited in microglia derived from CD36-deficient mice. Taken together, this work implicates microglia as an important early mediator of PD pathogenesis and accordingly represents a productive target for early therapeutic interventions.
Materials and Methods
Animals
The transgenic mouse model overexpressing human DM SYN overexpression under the control of the 9-kb rat tyrosine hydroxylase promoter was previously developed in our laboratory (Richfield et al. 2002). We have since homozygoused the α-synuclein locus for this model (SYNDM+/+). Age-matched male C57BL/6 mice were used as non-transgenic controls (NTG) in all experiments. CD36−/− mice were a gift of Drs. Andrew D. Luster and Kathryn Moore (El Khoury et al. 2003). All animal housing and procedures were performed in compliance with guidelines established by the University Committee of Animal Resources at the University of Rochester.
Bacterial Expression and Purification of α-synuclein
The bacterial expression vector pRK172 containing double-mutant human α-synuclein (dm syn) cDNA was constructed in our laboratory. DM SYN was expressed in Escherichia coli BL21 (DE3) and purified as previously described (Giasson et al. 1999; Maguire-Zeiss et al. 2006; Su et al. 2008). DM SYN was stored in TE buffer (10 mM Tris-HCl, pH 7.5, and 1 mM EDTA) containing 20 mM sodium chloride (NaCl) at 4°C. The contamination of lipopolysaccharide (LPS) in the purified DM SYN was assessed using E-TOXATE test kits (Sigma, Saint Louis, MI). The detection limit of the kit was 0.06 Endotoxin Units.
Primary Microglial Cells
Cerebral cortices of neonatal mice (1-day old; C57BL/6 or CD36−/−) were stripped of meninges and minced in Hepes balanced salt solution (HBSS; Mediatech Inc., Herndon, VA). Cells were dissociated in minimum essential media (MEM; Invitrogen, Frederick, MD) containing Earle's salts, L-glutamine, 0.01% pyruvate, 0.6% glucose, 4% fetal bovine serum, and 6% horse serum (complete medium), centrifuged, resuspended, and plated into flasks containing 10 ml complete medium at a density of one brain per T75 flask. Cultures were grown at 37°C under 5% CO2. After 1 day, the flasks were tapped gently to remove cell debris, media removed and replaced with fresh complete media. Cultures were grown as above for approximately 12 days at which time the microglia were harvested by tapping the flasks and collecting the microglia-enriched containing medium. Microglia were pelleted by centrifugation (1,000 rpm, 5 min), resuspended in MEM containing 0.01% pyruvate, 0.6% glucose, and 5% fetal bovine serum and enumerated. Primary microglial cells were plated in 24-well plates on cover glass (Carolina Biological Supply Company, Burlington, NC) at a density of 4 × 104 cells per well. Cells were incubated for 24 h followed by the treatment with 2.5, 5, or 10 nM DM SYN. LPS (0.007 ng) and buffer treatment served as negative activation controls. All treatments were in triplicate. Following DM SYN treatment, cells were incubated for 24 h under culture conditions and analyzed by immunocytochemistry for rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1; 1:750; Wako, Richmond, VA), a protein specifically expressed in microglia and macrophages, followed by nuclear staining (4′,6-diamidino-2-phenylindole; DAPI, Invitrogen). Approximately 95% of the DAPI fluorescent cells co-stained with Iba1.
Antibodies
Antibodies were used at the following dilutions: rabbit anti-phospho-ERK (1:1000; Cell Signaling Technology, Danvers, MA), rabbit anti-ERK (1:1000; Cell Signaling Technology, Danvers, MA), rabbit anti-β-actin (1:20000; Sigma, Saint Louis, MI), rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1; 1:5000 for immunohistochemistry & 1:750 for immunocytochemistry; Wako, Richmond, VA), rabbit anti-human synuclein (SYN) (1:5000; Biomol, Plymouth Meeting, PA), rabbit IgG horseradish peroxidase linked whole antibody (from sheep or donkey; 1:2000; GE Healthcare, Little Chalfont Buckinghamshire, UK), goat anti-rabbit IgG 594 (1:500, Invitrogen, Carlsbad, CA), biotinylated goat anti-rabbit immunoglobulin (1:1000, Vector Laboratories, Burlingame, CA).
Immunohistochemistry
Animals
One-, 6-, and 12-month-old SYNDM+/+ and non-transgenic mice (n = 4/genotype) were sacrificed, perfused with 4% (w/v) paraformaldehyde (PFA), brains removed and post-fixed in 4% PFA overnight at 4°C. Fixed brains were then transferred to 20% (w/v) sucrose overnight and stored in 30% (w/v) sucrose at 4°C until sectioning. Brain sections (30 μm) were cut on a sliding microtome and stored in cryoprotectant (300 g/l sucrose and 30% ethylene glycol) at −20°C until immunohistochemical analyses (IHC). For IHC, sections were washed 3 times in 0.1 M phosphate buffer solution (PBS; 80 g/l NaCl, 2 g/l KCl, 11.5 g/l Na2HPO4H2O, 2 g/l KH2PO4) for 10 min each followed by an overnight incubation to remove the cryoprotectant. Sections were treated with 3% H2O2 (v/v) in PBS for 20 min at room temperature (RT; 22°C) followed by permeabilization in PBS containing 0.1% Triton X-100 (v/v; PBS/0.1% TX-100) for 5 min. Sections were incubated in blocking solution containing PBS/10% filtered goat serum (v/v) for 1 h at RT followed by incubation with rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1) antibody (1:5000; Wako, Richmond, VA) or rabbit anti-human synuclein (SYN) (1:5000; Biomol, Plymouth Meeting, PA) for 2 days at 4°C. Following 3 rinses in PBS/0.1% TX-100/0.1% goat serum for 10 min each at RT, sections were incubated in PBS/0.1% TX-100/1% goat serum containing 2° antibody (biotinylated goat anti-rabbit immunoglobulin; 1:1000; Vector Laboratories, Burlingame, CA) for 2 h at RT followed by washes and colorimetric development (DAB; Vector Laboratories, Burlingame, CA). Controls included sections stained in the absence of 1° antibody. Iba1 specificity for activated and resting microglia was demonstrated following immunostaining of brain sections from MPTP-lesioned and untreated non-transgenic mice (MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; data not shown). MPTP-treatment has previously been demonstrated to activate microglia (Czlonkowska et al. 1996; Kohutnicka et al. 1998; Sawada et al. 2007). Immunostained sections were mounted on slides and sealed with cytoseal (Richard-Allan Scientific, Kalamazoo, MI). For image analysis, Iba1-positive cells were visualized using an Olympus AX-70 microscope equipped with a motorized stage (Olympus, Melville, NY) and the MCID 6.0 Elite Imaging Software (GE healthcare, Piscataway, NJ). Sections were tiled under 4× magnification. Four equal sections of substantia nigra (SN) or striatum (STR) from each mouse (n = 4 mice/genotype × 4 sections/mouse) were analyzed in a blinded fashion. Eighty percent for SN or fifty percent for STR of the defined region of interest was assessed under 40× magnification. Two groups of morphologically distinct Iba1? cells, resting (small cell bodies and thin processes) and activated (large cell bodies and thick processes) were counted (Su et al. 2008).
For densitometric analysis, the optical density of human α-synuclein-immunostained sections in the STR was estimated using an Olympus AX-70 microscope and a computer-assisted image analysis system as described above. The microscope captures images for striatal human α-synuclein-stained sections (8 sections per mouse; 4 mice/genotype). For each section, the STR was first manually delineated on the screen and the optical density was assessed using MCID 6.0 Elite Imaging Software (GE healthcare, Piscataway, NJ). To estimate the human α-synuclein-immunostaining density, the optical density readings were corrected for nonspecific background density, as measured from the white matter.
Cell Culture
Following treatment, cells were washed with 1 × PBS for 5 min, fixed in 4% PFA at room temperature for 20 min, permeabilized in 1 × PBS containing 0.1% Triton X-100 for 5 min and blocked for 1 h with 1 × PBS containing 10% goat serum. Cells were incubated overnight at 4°C with rabbit anti-Iba1 antibody (1:750; Wako, Richmond, VA) in blocking buffer, followed by a 1-h incubation with a fluorescent 2° antibody (goat anti-rabbit IgG 594; 1:500; Invitrogen, Carlsbad, CA). Unbound 2° antibody was removed by washing with 1 × PBS containing 0.1% Triton X-100. Nuclei were stained with DAPI (1:5000) in 1 × PBS for 5 min. Following two washes with 1 × PBS the cover glasses were mounted to slides using Mowiol solution (333 g/l glycerol, 133 g/l Mowiol, 0.133 M Tris-HCl, pH 8.5). For enumeration of total and activated microglia, six random 20× ICC images per sample were taken using an Olympus AX70 microscope (Olympus, Melville, NY). Two distinct groups of cells were counted based on cell area and morphology (circularity or elongation) using ImageJ software (NIH, Bethesda, MD). Cells were considered inactivated if the cell area was less than 1 μm2 (27 × 27 pixels) and/or had thin and elongated morphology. All others were counted as activated [Fig. 1b and (Su et al. 2008)].
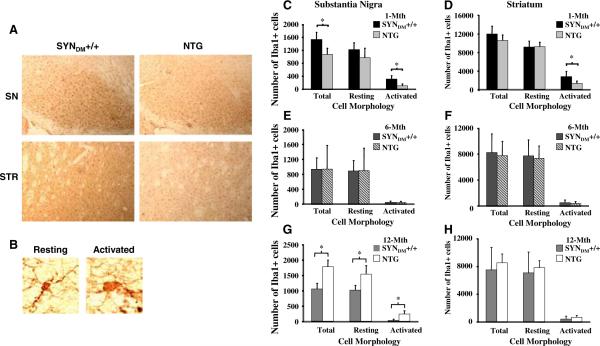
Fig. 1.
Dynamics of microglial activation in the SN and STR of SYNDM+/+ mice. Iba1+ cells were enumerated from 1-, 6-, and 12-month-old SYNDM+/+ and non-transgenic (C57/Bl6, NTG) mice following immunohistochemistry for the microglial marker, ionized calcium-binding adaptor molecule 1 (Iba1; n = 4 mice/genotype 9 × 4 sections/mouse). Two groups of morphologically distinct Iba1? cells, resting cells (small cell bodies and long processes; b), and activated cells (large cell bodies and thick processes; b) were counted in the substantia nigra (SN; c, e, and g) and the striatum (STR; d, f, and h). a Representative images of Iba1 immunohistochemistry in the SN and STR from 1-month-old SYNDM+/+ and NTG mice (109 magnification). b Image of resting and activated Iba1? cells showing the morphological criteria used for enumeration of microglia. We observed statistically significant increases in total and activated microglia in the SN and of activated microglia in the STR of 1-month-old SYNDM+/+ mice (c and d, respectively). No significant changes in either total or activated microglia were found in the SN (e) and STR (f) of 6-month-old SYNDM+/+ or in the STR of 12-month-old SYNDM+/+ (h). However, 12-month-old non-transgenic (NTG) mice displayed a significant increase in the numbers of total, resting and activated microglia in the SN (g) compared with SYNDM+/+ mice (* P < 0.05)
Quantitative Real-Time RT-PCR
Animals
RNA was isolated from microdissected SN and STR of 1-, 6-, and 12-month-old SYNDM+/+ and non-transgenic mice using TRIZOL (Invitrogen, Carlsbad, CA) (n = 4 mice/genotype). RNA was treated with RQ1 DNase (Promega, Madison, WI) followed by phenol:chloroform extraction and ethanol/lithium chloride precipitation. RNA integrity was determined using a bioanalyzer (Functional Genomic Center, University of Rochester, Rochester, NY). One microgram of total RNA was reverse transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The quality of cDNA was assessed by β-actin PCR. Ten microliters of total cDNA from each sample was used for PCR on Taqman Low Density Array Micro Fluidic Cards which were preloaded with probes and primers for 15 targets and one endogenous control 18S rRNA. The assay for each target was performed in triplicate. Real-time PCR reactions were conducted using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The results were analyzed using the relative quantification ΔΔCT method. First, the CT values of the target genes were normalized to the internal control gene 18S, referred to as ΔCT in order to correct for differences between samples. Then the relative gene expression was performed, which allowed for comparison of the gene expression in SYNDM+/+ samples relative to age-matched NTG. ΔΔCT was calculated by subtracting the ΔCT for transgenic samples from the ΔCT for NTG samples. Statistical analysis was performed by Student's t-test on ΔCT with the significance level set at P < 0.05.
To quantify the mRNA level for human double-mutant human α-synuclein in the SN, cDNA samples were analyzed by the 7300 real-time PCR system using quantitative real-time PCR with the specific primer and probe for human α-synuclein (Syntech). A calibration curve was generated using human α-synuclein cDNA. IL10 was quantified using real-time PCR Taqman Gene Expression Assays (IL10 [Mm00439616_m1]; Applied Biosystems, Foster City, CA). The quantification of target transcripts was based on a calibration curve (Su et al. 2008).
Cells
Primary microglia-enriched cells were plated in 12-well plates at a density of 2.6 × 105 per well for 24 h followed by incubation with 10 nM DM SYN or buffer alone for various times. Each time point was performed in triplicate. Total RNA was extracted from primary microglial cells using Trizol reagent according to the manufacturer's specifications (Invitrogen, Carlsbad, CA). Reverse transcription was conducted using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). cDNA samples were analyzed by the 7300 real-time PCR system using quantitative real-time PCR Taqman Gene Expression Assays with specific primers and probes for the following targets: TNFα (Mm00443258_m1), COX2 (Mm00478374_m1), IL1 β (Mm00434228_m1), IL6 (Mm00446190_m1), IL10 (Mm00439616_m1), NOS2 (Mm00440485_m1), NOX2 (Mm00432775_m1) (Applied Biosystems, Foster City, CA). The quantification of target transcripts was based on a calibration curve (Su et al. 2008).
Determination of TNFα and IL1β Levels
TNFα and IL1β secretion into microglia-conditioned media following DM SYN treatment (2.5, 5, or 10 nM DM SYN) was measured using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Fluorometric Assay of ROS Production
Primary microglia were plated in 24-well plates at the density of 1 × 105 cells per well for 24 h followed by treatment with 10 nM DM SYN or buffer (control) for various times. Cells were washed once with HBSS and incubated with 2′,7′-dichlorofluorescein diacetate (DCF-DA, Invitrogen, Carlsbad, CA) in HBSS at a concentration of 7.5 μg/ml for 1 h at 37°C in the dark. DCF-DA-treated cells were washed with HBSS, and incubated for an additional 40 min in HBSS. DCF fluorescence intensities were measured using a Bio-Rad FluoromakTM Plate Reader (excitation at 485 nm and emission at 538 nm; Gain 20; Hercules, CA).
Western Blot Analysis
Primary microglial cells were plated in 6-well plates at a density of 1 × 106 cells per well, incubated for 24 h prior to treatment with 10 nM DM SYN or buffer only for 5, 10, or 15 min. Cells were washed in ice-cold PBS 5 times and lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl) containing protease inhibitors (1 mM EDTA; 1 mM phenylmethylsulphonyl fluoride (PMSF); 1 μg/ml aprotinin; 1 μg/ml leupeptin; 1 μg/ml pepstatin) and phosphatase inhibitors (1 mM Na3VO4; 1 mM NaF). Cell lysates were sonicated 3 times with 5-s bursts followed by gentle rotation for 1 h at 4°C. Lysates were cleared by centrifugation at 13,000 rpm for 5 min. Thirty micrograms of protein was subjected to denaturing polyacrylamide gel electrophoresis [(Laemmli 1970); 10% PAG-SDS] and electrophoretically transferred to PVDF membrane (PerkinElmer, Wellesley, MA). Membranes were blocked for 1 h in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween) containing 5% (w/v) non-fat dry milk (NFDM), incubated with 1° antibody in TBST containing 5% NFDM overnight with gentle shaking at 4°C, followed by 4 washes in TBST, and incubation with the appropriate horse radish peroxidase-conjugated 2° antibody. After washing for 3 × 5 min in TBST and 1 × 5 min in TBS, antibody:antigen complexes were visualized using a Western Lightning Chemiluminescence Reagent kit (PerkinElmer, Wellesley, MA). Subsequently, blots were stripped and re-probed with different 1° antibodies. β-actin served as the loading control.
Results
To evaluate microglial activation in the SYNDM+/+ transgenic mouse model, 1-, 6-, and 12-month-old SYNDM+/+ mice were examined using Iba1 immunohistochemistry analysis followed by computer-assisted unbiased cell enumeration. We observed a significant increase in activated Iba1+ microglia as defined by morphological criteria (Fig. 1b) in the SN and STR of 1-month-old SYNDM+/+ compared to age-matched non-transgenic (NTG; C57/Bl6) mice (Fig. 1a, c, and d). Moreover, a significant increase in total microglia (sum of resting microglia and activated microglia) was appreciated in the SN of 1-month-old SYNDM+/+ (Fig. 1c). Frank microglial activation in SYNDM+/+ mice was resolved by 6 months of age (Fig. 1e–h). Paradoxically by 12 months of age, non-transgenic animals have an apparent increase in activated microglia in the SN compared with SYNDM+/+ mice (Fig. 1g).
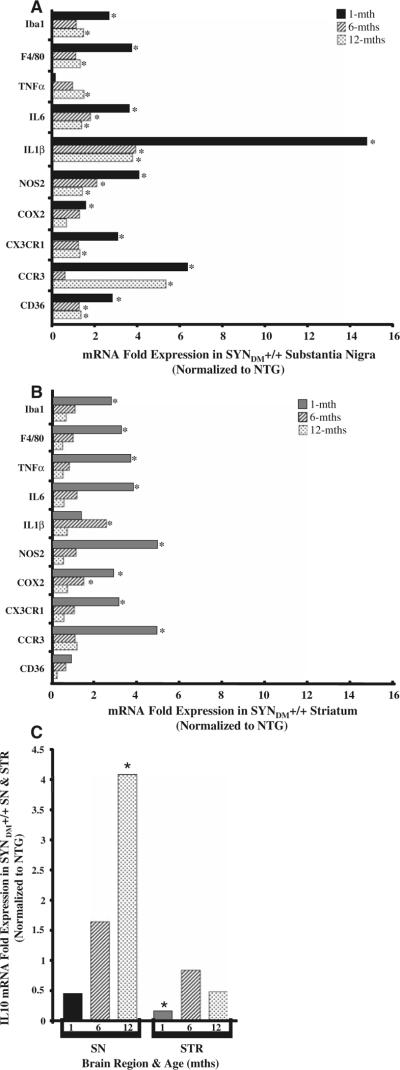
To identify the potential inflammatory mediators associated with microglial activation in the young SYNDM+/+ mice, the levels of target transcripts in the SN and STR of 1-, 6-, and 12-month-old SYNDM+/+ mice were determined by relative quantitative real-time PCR (qRT-PCR) analysis using TaqMan® Low Density Arrays (384-Well Micro Fluidic Cards) with customized probes and an endogenous control (18S ribosomal RNA). We demonstrated a prominent inflammatory process in young SYNDM+/+ mice accompanied with significant upregulation in the expression of proinflammatory mediators and downregulation of anti-inflammatory mediator gene expression (Fig. 2). Specifically, the common microglial cell markers Iba1 and F4/80 were over 2.5-fold increased at the transcript level in both the SN and STR of SYNDM+/+ mice, supporting an increase in total and/or activated microglia which comports with the histological cell count data. In addition, the mRNA expression of the class B scavenger receptor CD36 was increased to almost 3-fold in the SN. Increasing evidence suggests that CD36 is involved in the microglia-mediated cytotoxicity associated with inflammation (Febbraio et al. 2001; Coraci et al. 2002; Cho et al. 2005). The elevated CD36 mRNA expression suggests a role for this pattern recognition receptor in microglia-associated inflammation in young SYNDM+/+ mice. The mRNA expression levels of proinflammatory cytokines IL1β and IL6 and inflammation-inducible enzymes nitric oxide synthase iNOS (NOS2) and cyclooxygenase 2 (COX2) were also significantly increased in both the SN and STR of SYNDM+/+ mice. We observed that the increase in IL1β mRNA level within the SN was particularly remarkable, an over 15-fold increase relative to age-matched NTG mice. TNFα mRNA expression was also statistically upregulated in the STR of SYNDM+/+ mice (about 3.7-fold increase), although it did not reach significance in the SN. We further observed a significant increase in the transcript levels of the chemokine receptors CCR3 and CX3CR1. Finally, we demonstrated using a real-time PCR Taqman Gene Expression Assay that the anti-inflammatory cytokine IL10 was downregulated, perhaps facilitating an ongoing inflammation in SYNDM+/+ mice. Taken together, the early increased microglial activation accompanied with upregulation of proinflammatory mediators suggests that inflammation plays a role in initiating the pathology of SYNDM+/+.
Fig. 2.
Target mRNA expression in the SN and STR of 1-, 6-, and 12 month-old SYNDM+/+ mice. RNA extracted from the substantia nigra (SN; a) and striatum (STR; b) of SYNDM+/+ and nontransgenic (C57/Bl6, NTG; n = 4 mice/genotype) was examined using Taqman Low Density Array Micro Fluidic Card/qRT-PCR technology to determine changes in expression levels of the indicated target mRNA (* P < 0.05). c Results of real-time PCR Taqman Gene Expression Assay for IL10 in both the SN and STR of 1-, 6-, and 12-month-old SYNDM+/+ mice (* P < 0.05). We observed statistically significant changes in a subset of inflammatory markers at early (1 month) and later times (6 and 12 months) compared to similarly aged non-transgenic mice
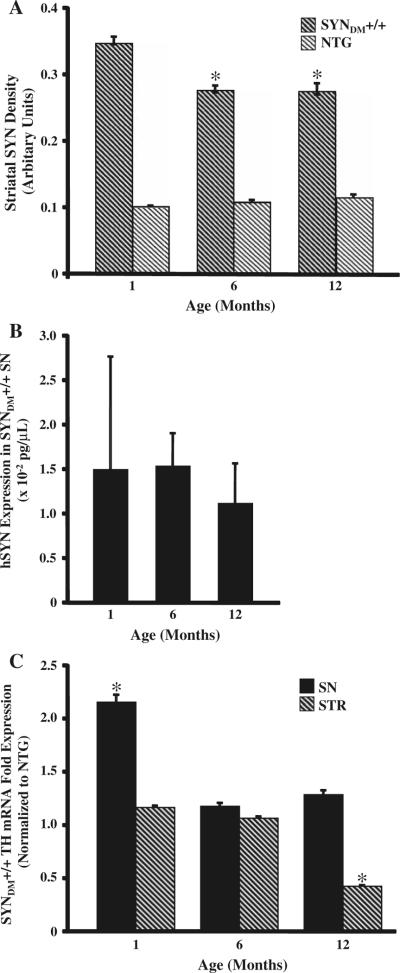
Although microglial activation as defined by morphologic criteria following Iba1 immunohistochemistry was resolved by 6 months of age in SYNDM+/+ mice, the expression of a subset of inflammatory target transcripts remained upregulated compared to non-transgenic animals (Fig. 2). Specifically, transcripts for Iba1, F4/80, TNFα, CCR3, and CXCR1 were increased in 12-month-old SYNDM+/+ SN compared to non-transgenic transcripts while CD36, IL1β, IL6, and NOS2 transcripts were increased at both 6 and 12 months of age in the same brain region. Most notably IL10 was upregulated in the SN of 12-month-old SYNDM+/+ mice suggesting an anti-inflammatory response at this later timepoint. In SYNDM+/+ STR upregulated transcripts were observed for IL1β and COX2 at 6 months of age only. To ascertain whether there were attendant age-related changes in nigrostriatal α-synuclein and/or tyrosine hydroxylase expression we interrogated both protein and mRNA expression levels (Fig. 3). Immunohistochemistry revealed that striatal α-synuclein was significantly decreased in 6- and 12-month-old SYNDM+/+ mice compared with 1-month-old SYNDM+/+ animals while the mRNA expression patterns were not significantly different (Fig. 3a and b). Furthermore, older SYNDM+/+ mice had a significant reduction in tyrosine hydroxylase mRNA expression in the STR compared to younger mice (Fig. 3c). Taken together, these data suggest that following early microglial and proinflammatory activation presynaptic striatal terminals become compromised as SYNDM+/+ mice age.
Fig. 3.
Progressive reduction in α-synuclein and tyrosine hydroxylase expression in the SYNDM+/+ striatum with age. Optical densities of human α-synuclein-immunostained striatal sections at three time points were analyzed using a computer-assisted image analysis system (8 sections/mouse; 4 mice/genotype; * P < 0.05). The densities of α-synuclein-positive striatal (STR) terminals were significantly decreased at 6 and 12 months of age compared to the 1-month time point (a). Quantitative real-time RT-PCR for human α-synuclein mRNA (hSYN) revealed no significant differences in hSYN expression with age (b). Real-time RT-PCR for tyrosine hydroxylase (TH) mRNA revealed a down-regulation of TH mRNA expression in the striatum of 12-month-old SYNDM+/+ compared with age-matched non-transgenic mice (c; * P < 0.05)
The mechanism(s) underlying the early microglial activation in SYNDM+/+ mice remains to be investigated. We recently demonstrated that wild-type human α-synuclein was capable of activating microglia directly in a cell culture system (Su et al. 2008). Previous studies have shown that microglia have greater response to aggregated wild-type human α-synuclein than monomeric wild-type human α-synuclein (Zhang et al. 2005; Thomas et al. 2007). Since the two point mutations, A30P and A53T, have been shown to accelerate α-synuclein aggregation in vitro (Narhi et al. 1999), we hypothesized that DM SYN would be a potent activator of microglia.
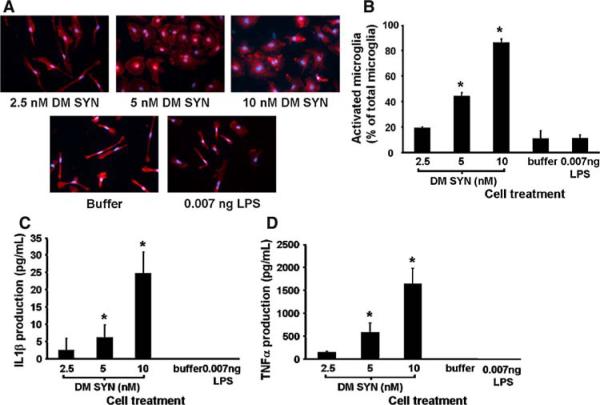
To examine the activating effect of DM SYN on microglia, primary microglial-enriched cultures from C57/BL6 (non-transgenic; NTG) mice were treated with various amounts of exogenous DM SYN. To eliminate the possibility that microglial activation might be triggered by contaminating lipopolysaccharide (LPS) that could co-purify with bacterially expressed and purified DM SYN, the amount of LPS contamination in the DM SYN sample (10 nM) was predetermined and added to parallel cultures of microglia as a control (0.007 ng LPS). As shown in Fig. 4 DM SYN activates microglia in a dose-dependent manner, as determined by a distinct change in cell size and morphology (Fig. 4a and b). The activated Iba1+ microglia possess large cell bodies with an amoeboid shape, while resting microglia display an elongated shape. As predicted DM SYN (10 nM) triggered activation, assessed using this morphologic assay, in approximately 90% of microglia. Neither buffer nor 0.007 ng LPS treatment caused significant microglial activation, confirming that activation was due to the presence of DM SYN.
Fig. 4.
Exogenously applied double-mutant α-synuclein activates cultured microglia. Primary microglia-enriched cultures were prepared from the cerebral cortices of neonatal mice (1-day-old C57BL/6). Cells were incubated with buffer, double-mutant α-synuclein (DM SYN; 2.5, 5, or 10 nM) or 0.007 ng LPS for 24 h (n = 3/condition). Activated Iba1+ microglia were enumerated following Iba1 immunocytochemistry with cell counting based on cell area and morphology (a; circularity or elongation) using ImageJ software (NIH, Bethesda, MD). Cells were considered inactivated if the cell area was less than 1 μm2(27 × 27 pixels) or displayed thin and elongated morphology. All other Iba1+ cells were considered “morphologically” activated. We observed a DM SYN dose-dependent activation response (b). Likewise, conditioned media from DM SYN-treated microglia demonstrated a dose-dependent increase in both IL1β (c) and TNFα (d) release, respectively. Buffer only and 0.007 ng LPS served as negative controls (* P < 0.05)
As noted above, we observed a prominent increase in the level of IL1β mRNA level in the SN and STR of 1-month-old SYNDM+/+ mice. In addition, TNFα gene expression was also significantly upregulated in the STR. Although the cellular localization of IL1β and TNFα requires further investigation, extensive evidence supports that microglia are the major source of these proinflammatory cytokines (Banati et al. 1993; Combs et al. 2001; Kim and Joh 2006). Consequently, we next examined the release of IL1β and TNFα in the microglia-enriched cultures treated with DM SYN. These results demonstrated that microglia produce IL1β and TNFα in a dose-dependent fashion in response to DM SYN and suggest an inflammatory process driven directly by DM SYN-mediated microglial activation (Fig. 4c and d).
Double-mutant human α-synuclein-stimulated microglial production of IL1β and TNFα suggested the possibility of an activation of signaling pathways triggering an inflammatory cascade. Therefore, we next explored the key components of the microglia-mediated inflammatory cascade. Previous work has demonstrated that upregulation of the proinflammatory cytokines TNFα, IL1β, IL6, COX2, and NOS2, reactive oxygen species-associated enzyme NADPH oxidase (NOX2), and the immunosuppressive cytokine IL10 paralleled microglial activation (Knott et al. 2000; McGeer et al. 2005; Ovanesov et al. 2006; Purisai et al. 2006; Su et al. 2008). To evaluate the mRNA expression of the above inflammatory mediators during the microglial activation process, we exposed microglial cultures to 10 nM DM SYN for various periods of time followed by qRT-PCR analysis of isolated RNA.
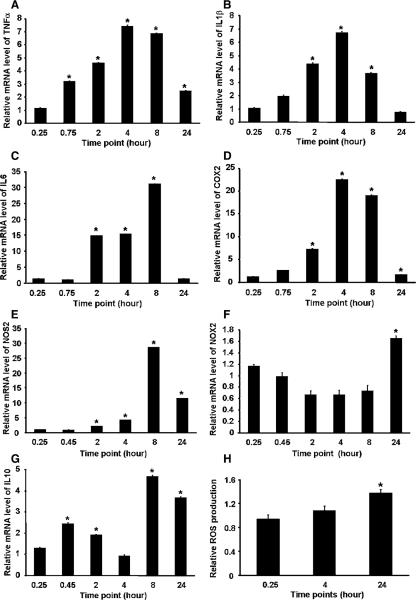
Quantitative RT-PCR results revealed time dependent changes in the patterns of TNFα, IL1β, IL6, COX2, NOS2, NOX2, and IL10 mRNA expression following DM SYN treatment (Fig. 5). In the control buffer-treated cultures, a low baseline mRNA level for all investigated mediators was observed. The kinetics of TNFα and IL1β mRNA expression showed a similar pattern (Fig. 5a and b) whereby expression of these cytokines rapidly increased, within 0.75 h, peaked at 4 h and then declined. At 24-h TNFα mRNA was still elevated compared to buffer-treated microglia while IL1β expression had returned to baseline. By contrast, IL6 mRNA expression displayed a delayed increase beginning at 2 h with a prominent peak at 8 h (approximately 31-fold over basal levels) before returning to baseline by 24 h (Fig. 5c). The temporal profile for COX2 mRNA followed a very similar pattern to TNFα and IL1β with a peak at 4 h (Fig. 5d) indicative of a primary COX2 response to DM SYN, rather than a secondary response triggered by TNFα and/or IL1β. Compared with COX2, NOS2 gene expression had a delayed pattern, with a moderate increase by 2 h followed by a peak at 8 h (Fig. 5e). Elevated NOS2 expression was still evident at 24 h. In contrast, the kinetics of NOX2 gene expression was maintained at basal levels until the 24-h time point when an increase was observed (Fig. 5f). Consistent with the qRT-PCR results, the production of cellular ROS in microglial cultures was not increased until 24 h after exposure to DM SYN (Fig. 5h). Finally, the immunosuppressive cytokine IL10 mRNA expression pattern was biphasic; initially increased at 0.75 h, followed by a decline at 4 h and a second increase at 8 h (Fig. 5g). IL10 mRNA levels remained upregulated compared to control treated cultures at 24 h.
Fig. 5.
Double-mutant α-synuclein-mediated microglial activation induces pro/anti-inflammatory molecular cascades. Kinetics of the TNFα (a), IL1β (b), IL6 (c), COX2 (d), NOS2 (e), NOX2 (f), IL10 (g) mRNA expression, and ROS production (h) were evaluated using primary C57BL/6 microglia-enriched cultures following treatment with 10 nM double-mutant α-synuclein (DM SYN) for 0.25, 0.75, 2, 4, 8, and 24 h. Values indicate relative expression normalized to the buffer control (n = 3; * P<0.05)
Double-mutant human α-synuclein-induced microglial production of proinflammatory cytokines and neurotoxic effectors, such as nitric oxide (NO) and reactive oxygen species (ROS), suggests that microglial activation may endanger neurons. We reasoned therefore that attenuation of double-mutant human α-synuclein-mediated microglial activation might be achieved by blocking the interaction between DM SYN and microglia, potentially a receptor-mediated process (Klegeris et al. 2008; Su et al. 2008). Multiple membrane receptors have been implicated in microglial activation and intracellular signal transduction pathways (Block et al. 2007). Among those, the class B scavenger receptor CD36 has been implicated in microglial phagocytosis of another misfolded protein, fibrillar amyloid β (Koenigsknecht and Landreth 2004). Moreover, we previously demonstrated that WT SYN-dependent microglial activation was attenuated in CD36 deficient cultures (Su et al. 2008). Since double-mutant human α-synuclein shares the self-aggregation propensity of amyloid β and wild-type human α-synuclein, we postulated that the scavenger receptor CD36 might be involved in double-mutant human α-syniclein-stimulated microglial activation as well.
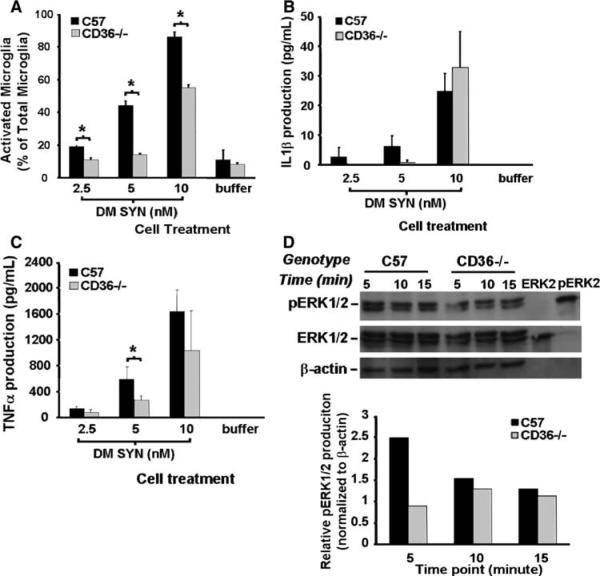
To investigate the role of CD36 in signaling microglial activation, primary CD36-deficient (CD36−/−) mouse microglia-enriched cultures were examined following treatment with exogenous double-mutant human α-syniclein (DM SYN). CD36−/− microglia were treated with 2.5, 5, or 10 nM DM SYN for 24 h followed by immunocytochemistry and Iba1+ cell enumeration categorized by microglial activation stages (activated or resting microglia). C57BL6 (C57) cultures were used for comparison. The results demonstrate that whereas CD36−/− and C57 microglia responded to DM SYN in a dose-dependent fashion, CD36−/− microglial activation was attenuated compared to C57 (Fig. 6a). We next examined whether CD36 status affected the amount of IL1β and TNFα released into the microglial-conditioned media. With a low dose of DM SYN (5 nM) the production of IL1β and TNFα was reduced in CD36−/− microglial cultures compared to C57-derived microglia (IL1β, P = 0.07; TNFα, P < 0.05) while at the highest dose of DM SYN (10 nM) IL1β and TNFα production was comparable between CD36−/− and C57 cultures (Fig. 6b and c). Utilizing these cultures we investigated the downstream signaling pathway normally activated in response to CD36 engagement. We hypothesized that the extracellular signal regulated kinase (ERK) signaling pathway might be interrupted in double-mutant human α-syniclein-treated CD36−/− microglia (DM SYN). Protein extracts from CD36−/− and C57 microglia following treatment with 10 nM DM SYN for 5, 10, or 15 min were subjected to western blot analysis for total ERK and phosphorylated ERK. As shown in Fig. 6d, the signal for phosphorylated ERK (pERK) was attenuated in CD36−/− microglia cultures compared to C57 cultures at the earliest time point (~2.5-fold reduction; density ratio of pERK to β-actin). Collectively, these results demonstrate that CD36 is involved in DM SYN-mediated microglial activation and suggest that blockade of CD36 may represent a potential therapeutic strategy to attenuate microglial activation.
Fig. 6.
Attenuation of α-synuclein-mediated microglial activation in CD36−/− derived cultures. Primary microglia-enriched cultures were prepared from the cerebral cortices of neonatal control and CD36-null mice (C57BL/6 and CD36−/−). Cells were incubated with double-mutant α-synuclein (DM SYN; 2.5, 5, or 10 nM) or buffer alone for 24 h followed by Iba1 immunocytochemistry and enumeration of labeled cells. We observed a reduction in activated microglia from CD36−/− cultures (a;* P<0.05). Likewise, the release of IL1β and TNFα protein from microglia-conditioned media was either delayed or reduced in the CD36−/− cultures (b and c; * P<0.05). To evaluate the effect of CD36 status on phospho-ERK1/2 (pERK1/2) production, primary C57B1/6 and CD36−/− microglia-enriched cells were incubated with 10 nM DM SYN for 5, 10, or 15 min and cell lysates prepared. Cell lysates (30 μg) as well as 10 μl of purified non-phospho-ERK2 and phospho-ERK2 were analyzed by western blot analysis using anti-phospho-ERK1/2, ERK1/2, and β-actin antibodies (d) followed by densitometric analysis. This analysis demonstrated that pERK1/2 production in CD36−/− microglia at the earliest time point was 2-fold less than in similarly treated C57 microglia (d). Taken together, these data demonstrate a reduced effect of DM SYN on CD36 knock-out microglia compared to C57BL/6 microglial suggesting this scavenger receptor is in part responsible for DM SYN-dependent microglial activation
Discussion
The goal of the present study was to investigate the effect of PD-related human α-syniclein genetic mutations on microglia. To address this, the activation status of microglia was examined in 1-, 6-, and 12-month-old transgenic mice expressing double-mutant human α-syniclein (a single transgene containing both the A53T and A30P mutations). The results presented herein demonstrate that young 1-month-old SYNDM+/+ mice develop a prominent microglia-mediated inflammation that resolves by 6 months of age. The expression of proinflammatory molecules increases at 1 month of age and for a subset of molecules remains upregulated relative to similarly aged non-transgenic mice (6- and 12-months old). Concomitant with changes in proinflammatory molecules aged SYNDM+/+ mice exhibit decreased double-mutant human α-syniclein density in the STR in the absence of significant alterations in the attendant mRNA expression and reduced tyrosine hydroxylase mRNA expression within the same region. To further dissect the mechanism(s) underlying this increased microglial activation in the SYNDM+/+ mice we used a primary microglia-enriched cell culture model and treated the cultures with exogenously expressed and purified double-mutant human α-syniclein (DM SYN). Similar to the results from our in vivo model, these data demonstrate that exogenously applied DM SYN had a greater potency to activate cultured microglia in comparison with wild-type human α-syniclein [10 nM DM SYN vs 250 nM WT SYN (Su et al. 2008)]. This activation process was partially mediated by CD36 and the downstream activation of the ERK signaling pathway. Together, these findings identify double-mutant human α-synuclein as a potent trigger of microglial activation and illustrate the contribution of neuroinflammation in the pathophysiological effects of mutant α-synuclein in PD.
The identification of early microglial activation accompanied by upregulation of proinflammatory mediators in SYNDM+/+ mice is of great interest. Our data indicate that an early event, vastly antedating any neuropathological changes, is the initiation of microglial activation and neuroinflammation in the affected brain regions (SN and STR) of a PD-related transgenic mouse model. Previously, the double-mutant human α-synuclein animal model demonstrated age-related changes which included dystrophic neurites and alterations in dopamine metabolism (Richfield et al. 2002) while dopaminergic cell death was not significant until the animals were greater than 15-months old (Thiruchelvam et al. 2004). For this reason we assume that very few if any dopaminergic neurons die in our SYNDM+/+ mice at the early time point (1 month) where we observed increased microglial activation. Therefore, in the SYNDM+/+ mice the trigger for early microglial activation is unlikely due to proinflammatory molecules released from dead neurons. More likely signals emanating from viable neurons are responsible for this activation. One candidate is double-mutant human α-synuclein itself since α-synuclein has been shown to be released from cells in culture following overexpression (Su et al. 2008). Although we have not detected double-mutant human α-synuclein in the parenchyma of SYNDM+/+ mice it is possible that DM SYN released from intact dopaminergic neurons and would be available as an initial trigger to activate microglia in the SYNDM+/+ mice. However, we cannot rule out the possibility that a cytokine or proinflammatory molecule released from dopaminergic neurons is responsible for the activation of microglia in vivo and that is an area of ongoing research.
We have established that exogenous double-mutant human α-synuclein is capable of direct microglial activation. Furthermore, in the present study, we verified a more pronounced proinflammatory response in SYNDM+/+ mice compared with age-matched SYNWT+/+ mice as measured by the temporal expression profiles of target mRNAs (Su et al. 2008). Moreover, our in vitro studies demonstrated that the concentration of human α-synuclein necessary to induce approximately 90% microglial activation was 10 nM for DM SYN, compared with 250 nM for WT SYN (Su et al. 2008). Our findings are consistent with a recent study that demonstrated differential abilities of human α-synuclein and mutant forms of α-synuclein to induce cytotoxicity (Klegeris et al. 2008). The difference between WT SYN- and mutant α-synuclein-directed pathology is purported to be due to their different propensities to self-aggregate. This ability to form misfolded/oligomeric forms may also be responsible for our reported differences between wild-type and DM SYN-directed microglial activation.
Increasing evidence suggests that overproduction of proinflammatory cytokines in the CNS contributes to neuronal cell death in a number of neurodegenerative diseases and that the major cellular source for these cytokines is activated microglia (Banati et al. 1993; Carson et al. 2004; Dheen et al. 2007). In the present study, we observed that mRNA expression for the proinflammatory cytokines IL1β and IL6, and the inflammation-inducible enzymes NOS2 and COX2 were significantly elevated in the SN and STR of 1-month-old SYNDM+/+ mice. Likewise, we demonstrated an inflammatory cascade in response to exogenous double-mutant human α-synuclein in primary microglia-enriched cultures. Together these data suggest that double-mutant human α-synuclein initiates a cascade of events leading to the induction of multiple cytokines and neurotoxic factors including prostaglandins (PGs), NO, and ROS.
In this inflammatory process, IL1β and/or TNFα may play a crucial role in setting this cascade in motion. The present in vitro study demonstrates the rapid up-regulation of mRNAs for IL1β and TNFα which precedes the induction of NOS2 and NOX2, suggesting IL1β and/or TNFα are prominent regulators that stimulate the subsequent production of free radicals, such as NO and ROS. Previous studies reported that IL1β and TNFα exert toxic effects by binding to their cognate receptors, IL1-R1 and TNFR1, respectively, triggering downstream signaling pathways that activate transcription factors, such as NFκB or AP1 which in turn lead to the production of NO and ROS (Bonizzi et al. 1999; Zhang et al. 2000; Li et al. 2006). IL1β-/TNFα-stimulated NO/ROS production has been suggested to be detrimental in several neurodegenerative disorders including AIDS dementia complex, multiple sclerosis, Alzheimer's disease, and PD (Brosnan et al. 1997; Akama and Van Eldik 2000; Gloire et al. 2006). Therefore, disruption of IL1β and TNFα signaling pathways by neutralizing these cytokines or blocking their receptors may provide potential therapeutic interventions for several neurodegenerative diseases.
Here we also demonstrated changes in CCR3 mRNA expression. The increased expression of CCR3 raises the question as to what role it may play in microglial activation and migration. CCR3 can serve as a receptor for several chemokines found in the central nervous system (CNS), including CCL2 (MCP1), CCL8 (MCP2), CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES) (Glabinski and Ransohoff 1999; Fife et al. 2000; Siebert et al. 2000; Gerard and Rollins 2001). In the CNS, CX3CR1 is only expressed by microglia (Harrison et al. 1998) and its exclusive ligand fractalkine (CX3CL1) is synthesized by neurons. It has been shown that neurons suppress microglial activation through the CX1CR3-fractalkine signaling pathway (Cardona et al. 2006). Therefore, the upregulation of CX1CR3 implies a neuron-mediated compensatory response to DM SYN-induced inflammation.
In the present study, the expression of IL10 mRNA increased rapidly and then dropped back to basal levels at 4 h in parallel with the peak expression of TNFα and IL1β. After 8 h, the expression of IL10 mRNA increased again to a much higher degree than the initial elevation. It has been shown that IL10 suppresses inflammation via deactivating monocytes/macrophages/microglia and inhibiting the production of proinflammatory cytokines (Kishore et al. 1999; Lee et al. 2002; Qian et al. 2006; Rajasingh et al. 2006). This early IL10 expression may be a compensatory response of the microglia to attenuate the inflammatory process. However, our data indicated that the production of IL10 at these early time points was insufficient to suppress proinflammatory cytokine production. The rebound of IL10 mRNA expression at the later time points may be attributable to a stronger autoregulatory mechanism which remains to be further investigated. Furthermore, in vivo SYNDM+/+ mice demonstrate a delayed upregulation of IL10 mRNA expression suggesting these animals are mounting a compensatory response to the early expression of proinflammatory molecules.
As professional phagocytes, microglia have evolved to express various surface receptors that engage a wide range of molecular determinants (Block et al. 2007). Scavenger receptor CD36 is a pattern recognition receptor and its expression on microglia in CNS has been previously established (Husemann et al. 2002). The major function of CD36 is to facilitate cell adhesion to various native and pathological substances with subsequent transduction of intracellular signals with relevance to inflammation, phagocytosis, and endocytosis (Husemann et al. 2002). Increasing evidence suggests that CD36 mediates adhesion of microglia to fibrillar amyloid β (Husemann et al. 2001; Coraci et al. 2002). Additionally, we previously demonstrated that CD36 plays a role in wild-type human α-synuclein-mediated microglial activation (Su et al. 2008). The present study showed that the expression of CD36 mRNA was significantly elevated in young SYNDM+/+ mice. Moreover, inflammatory responses to double-mutant human α-synuclein in CD36−/− microglial cell cultures were attenuated. Taken together, the results suggest a role of CD36 in double-mutant human α-synuclein-dependent microglial activation as well. It should be noted that microglial activation is not exclusively CD36-mediated since we observed pronounced microglial activation and proinflammatory cytokine production in CD36−/− cultures treated with higher concentrations of DM SYN. In addition, the activation of the downstream signaling molecule ERK1/2 was only partially inhibited in CD36−/− microglial cultures. In short, the results indicate that multiple receptors may be involved in facilitating microglial activation. A recent study demonstrated that microglial neurotoxicity secondary to mutant α-synuclein (A30P and A53T) treatment is primarily mediated by the macrophage antigen-1 (Mac-1) receptor, rather than by internalization of mutant α-synuclein by scavenger receptors (Zhang et al. 2007). It has also been shown that engagement of the Mac-1 receptor is linked to the activation of downstream NADPH oxidase and ERK1/2, leading to the release of ROS and inflammatory mediators such as prostaglandin E2 (PGE2) (Zouki et al. 2001; Zhang et al. 2003). Therefore, Mac-1 receptor maybe one compensatory mechanism utilized in our CD36 knockout culture system allowing for continued microglial activation in response to double-mutant human α-synuclein.
In conclusion, early microglia-mediated neuroinflammation was observed in vivo in a transgenic model of PD which expresses double-mutant human α-synuclein in dopaminergic neurons and in vitro in DM SYN-treated microglia-enriched cultures. The in vitro studies suggest double-mutant human α-synuclein as a putative direct trigger of microglial activation and attendant proinflammatory cascades. Present findings imply that mutant forms of α-synuclein not only contribute to neurodegeneration directly by interacting with neuronal intracellular machinery, but also indirectly by activating microglia extracellularly, leading to the production of proinflammatory substances. In our model there is an apparent loss of presynaptic tyrosine hydroxylase positive fibers in older SYNDM+/+ mice following the initial microglial activation. Together these studies suggest that PD therapeutic strategies directed at prevention of α-synuclein-directed microglial activation either by blocking direct interaction with surface receptors on microglia or by interrupting/regulating the microglial-mediated inflammation cascades may prevent downstream pathogenesis.
Acknowledgments
We thank Catherine Dunn and Qiong Liu for technical assistance with the synuclein subcloning and protein preparations and Landa Prifti for assistance with animal colony maintenance. This work was supported by DAMD17-03-1-0009 to H.J.F. and R01ES014470 to K.M.Z.
Abbreviations
- DAB
3,3′-Diaminobenzidine
- DM SYN
Double-mutant human α-synuclein protein
- ERK
Extracellular signal regulated kinase
- Iba1
Ionized calcium-binding adaptor molecule
- LBs
Lewy Bodies
- LPS
Lipopolysaccharide
- MEM
Minimum essential medium
- NaCl
Sodium chloride
- NTG
Non-transgenic
- PBS
Phosphate-buffered saline
- PD
Parkinson's disease
- SN
Substantia nigra
- STR
Striatum
- SYN
α-Synuclein
- SYNDM+/+
Homozygous double-mutant α-synuclein transgenic mice
- SYNWT+/+
Homozygous wild type α-synuclein transgenic mice
- WT SYN
Wild-type human α-synuclein protein
- TE buffer
Tris-EDTA buffer
References
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, Cho J, Jeon BS. Alpha-synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70(1):43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta-and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275(11):7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7(1):111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Barceló-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J Neurochem. 2007;101(1):132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Giasson BI. Snaring the function of alpha-synuclein. Cell. 2005;123(3):359–361. doi: 10.1016/j.cell.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19(3):1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Lee SC, Liu J. Regulation of inducible nitric oxide synthase expression in human glia: implications for inflammatory central nervous system diseases. Biochem Soc Trans. 1997;25(2):679–683. doi: 10.1042/bst0250679. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Lo D. Analysis of microglial gene expression: identifying targets for CNS neurodegenerative and autoimmune disease. Am J Pharmacogenomics. 2004;4(5):321–330. doi: 10.2165/00129785-200404050-00005. [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson's disease? Exp Neurol. 2008;209(1):22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25(10):2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7(10):1144–1150. doi: 10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. Beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21(4):1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry. 2000;39(10):2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160(1):101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Członkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Członkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson's disease mice model. Neurodegeneration. 1996;5(2):137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dawson T, Mandir A, Lee M. Animal models of PD: pieces of the same puzzle? Neuron. 2002;35(2):219–222. doi: 10.1016/s0896-6273(02)00780-8. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14(11):1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, Cookson MR, Hardy J, Allsop D. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17(13):1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- Emborg ME. Nonhuman primate models of Parkinson's disease. ILAR J. 2007;48(4):339–355. doi: 10.1093/ilar.48.4.339. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108(6):785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(6):899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39(9):628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15(2):361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2(2):108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274(12):7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Ransohoff RM. Chemokines and chemokine receptors in CNS pathology. J Neurovirol. 1999;5(1):3–12. doi: 10.3109/13550289909029740. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72(11):1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Faergeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 2005;44(23):8251–8259. doi: 10.1021/bi0502137. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Rosenberger TA, Feddersen S, Faergeman NJ, Murphy EJ. Alpha-synuclein gene ablation increases docosahexaenoic acid incorporation and turnover in brain phospholipids. J Neurochem. 2007;101(1):201–211. doi: 10.1111/j.1471-4159.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280(9):7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95(18):10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Kodama T, Silverstein SC. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar beta-amyloid. J Neuroimmunol. 2001;114(1–2):142–150. doi: 10.1016/s0165-5728(01)00239-9. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Kahle PJ. Alpha-synucleinopathy models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):87–95. doi: 10.1007/s00401-007-0302-x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38(4):333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Kishore R, Tebo JM, Kolosov M, Hamilton TA. Cutting edge: clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162(5):2457–2461. [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29(5):739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16(6):724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24(44):9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Członkowski A, Członkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39(3):167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee YB, Nagai A, Kim SU. Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res. 2002;69(1):94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25(25):6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26(1):140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire-Zeiss KA, Federoff HJ. Convergent pathobiologic model of Parkinson's disease. Ann N Y Acad Sci. 2003;991:152–166. doi: 10.1111/j.1749-6632.2003.tb07473.x. [DOI] [PubMed] [Google Scholar]
- Maguire-Zeiss KA, Wang CI, Yehling E, Sullivan MA, Short DW, Su X, Gouzer G, Henricksen LA, Wuertzer CA, Federoff HJ. Identification of human alpha-synuclein specific single chain antibodies. Biochem Biophys Res Commun. 2006;349(4):1198–1205. doi: 10.1016/j.bbrc.2006.08.127. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26(Suppl 1):94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson's disease progression. Acta Neuropathol. 2008;115(4):385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res: Fundam Mol Mech Mutagen. 2008 doi: 10.1016/j.mrfmmm.2008.10.011. doi:10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Moussa CE, Wersinger C, Tomita Y, Sidhu A. Differential cytotoxicity of human wild type and mutant alpha-synuclein in human neuroblastoma SH-SY5Y cells in the presence of dopamine. Biochemistry. 2004;43(18):5539–5550. doi: 10.1021/bi036114f. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274(14):9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol. 2006;59(2):298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302(5651):1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovanesov MV, Sauder C, Rubin SA, Richt J, Nath A, Carbone KM, Pletnikov MV. Activation of microglia by borna disease virus infection: in vitro study. J Virol. 2006;80(24):12141–12148. doi: 10.1128/JVI.01648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2006;25(2):392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Block ML, Wei SJ, Lin CF, Reece J, Pang H, Wilson B, Hong JS, Flood PM. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319(1):44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB J. 2006;20(12):2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175(1):35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Sawada H, Hishida R, Hirata Y, Ono K, Suzuki H, Muramatsu S, Nakano I, Nagatsu T, Sawada M. Activated microglia affect the nigro-striatal dopamine neurons differently in neonatal and aged mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci Res. 2007;85(8):1752–1761. doi: 10.1002/jnr.21241. [DOI] [PubMed] [Google Scholar]
- Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci USA. 2000;97(9):4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. Alpha-synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004a;565(1–3):1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004b;18(6):637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- Siebert H, Sachse A, Kuziel WA, Maeda N, Brück W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol. 2000;110(1–2):177–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29(11):1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzioglu M, Galter D. Parkinson's disease: genetic versus toxin-induced rodent models. FEBS J. 2008;275(7):1384–1391. doi: 10.1111/j.1742-4658.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J NeuroSci. 2004;19(4):845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Thomas MP, Chartrand K, Reynolds A, Vitvitsky V, Banerjee R, Gendelman HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson's disease. J Neurochem. 2007;100(2):503–519. doi: 10.1111/j.1471-4159.2006.04315.x. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90(23):11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson's disease. Trends Mol Med. 2006;12(11):521–528. doi: 10.1016/j.molmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12(3):301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278(31):28443–28454. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B, Miller DS, Chen B, Zhang W, McGeer PL, Hong JS, Zhang J. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55(11):1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- Zouki C, Zhang SL, Chan JS, Filep JG. Peroxynitrite induces integrin-dependent adhesion of human neutrophils to endothelial cells via activation of the Raf-1/MEK/Erk pathway. FASEB J. 2001;15(1):25–27. doi: 10.1096/fj.00-0521fje. [DOI] [PubMed] [Google Scholar]