Abstract
The origin of image artifacts in an off-resonance spin-locking experiment is shown to be imperfections in the excitation flip angle. A pulse sequence for off-resonance spin locking is implemented that compensates for imperfections in the excitation flip angle through an off-resonance rotary echo. The off-resonance rotary echo alternates the frequency offset and phase of the RF transmitter during two spin-locking pulses of equal duration. The underlying theory is detailed, and MR images demonstrate the effectiveness of the technique in agarose gel phantoms and in in vivo human brain at 3T.
Keywords: off-resonance T1ρ, spin locking, T1ρ-weighted imaging, rotary echo, T1ρ relaxation
The different magnetic relaxation properties of tissues make magnetic resonance imaging (MRI) one of the most clinically useful imaging modalities. Relaxation-dependent contrast is inherent to conventional T1- and T2-weighted images as well as the more recent steady-state free precession (SSFP) T1/T2-weighted images. Since relaxation times vary among healthy and diseased tissues, these techniques can be used to distinguish the diseased tissues in MR images. In addition to the widely employed T1 and T2 relaxation contrast techniques, a small but increasing number of studies are devoted to examining T1ρ relaxation contrast, in which tissue magnetization is “locked” by an on-resonance RF field. Redfield (1) showed that the T1ρ relaxation time characterizes the spin-lattice relaxation in the rotating frame. In most cases, as the amplitude of the locking RF field approaches zero, T1ρ- weighted imaging is functionally equivalent to T2- weighted imaging, since both generate tissue contrast based on the disappearance of transverse magnetization.
In practice, T1ρ-weighted imaging offers several advantages over T1- and T2-weighted imaging. For example, T1ρ-weighted imaging is sensitive to the slow motional processes in the 0.5–3 kHz range, such as proton exchange with amides and hydroxyl groups in proteins and residual dipole–dipole interactions (2,3). To mention a few applications, T1ρ-weighted imaging has shown improved contrast between healthy brain or breast tissues and tumors in mice (4) and humans (5,6), tracked the degeneration of the patellar cartilage matrix (7,8), is sensitive to posttraumatic cartilage injury (9) and has shown sensitivity to degradation of the nucleus pulposus in studies of degenerative intervertebral disc disease (10,11) and enabled the indirect detection of metabolic in vivo (12,13). This approach presents some difficulties compared to traditional relaxation contrast techniques, however. Long-duration RF pulses may cause coil damage, the specific absorption rate (SAR) delivered to tissues during a T1ρ sequence is high, and both B0 and B1 imperfections are significant sources of artifacts.
To overcome three of these difficulties (coil damage, high SAR, and B1 imperfections), we developed a T1ρoff pulse sequence that implements an off-resonance “rotary echo” during spin locking. An off-resonance spin lock reduces the spin-lock RF field strength ω1 by increasing the off-resonance component Δω0 to achieve the same effective field strength, and thus reduces the SAR and ω1 power demands on the RF coil. A rotary echo reduces artifacts due to B1 inhomogeneity. T1ρoff is an intrinsically different relaxation time from T1ρ, and instead combines both T1ρ- and T1-type contrast. In particular, when an off-resonance RF pulse is delivered far from resonance (Δω0 ≫ ω1), T1ρoff approaches T1 and on-resonance T1ρoff becomes T1ρ. Here we generalize the rotary echo to the off-resonance case using product operator theory and show that it reduces imperfect B1 MR image artifacts during off-resonance spin locking. In previous studies, off-resonance spin locking showed sensitivity to acute rat cerebral ischemia for ΔωRF < 2.5 kHz (14), and was used to obtain single-shot measurements of T1ρoff in the human breast (15). Despite the decrease in sensitivity to tissue pathology that occurs with decreasing ω1/ωΔRF (16), off-resonance spin locking may be useful for situations in which Δω0 inhomogeneity artifacts prohibit an on-resonance spin lock, particularly at higher B0 field strengths.
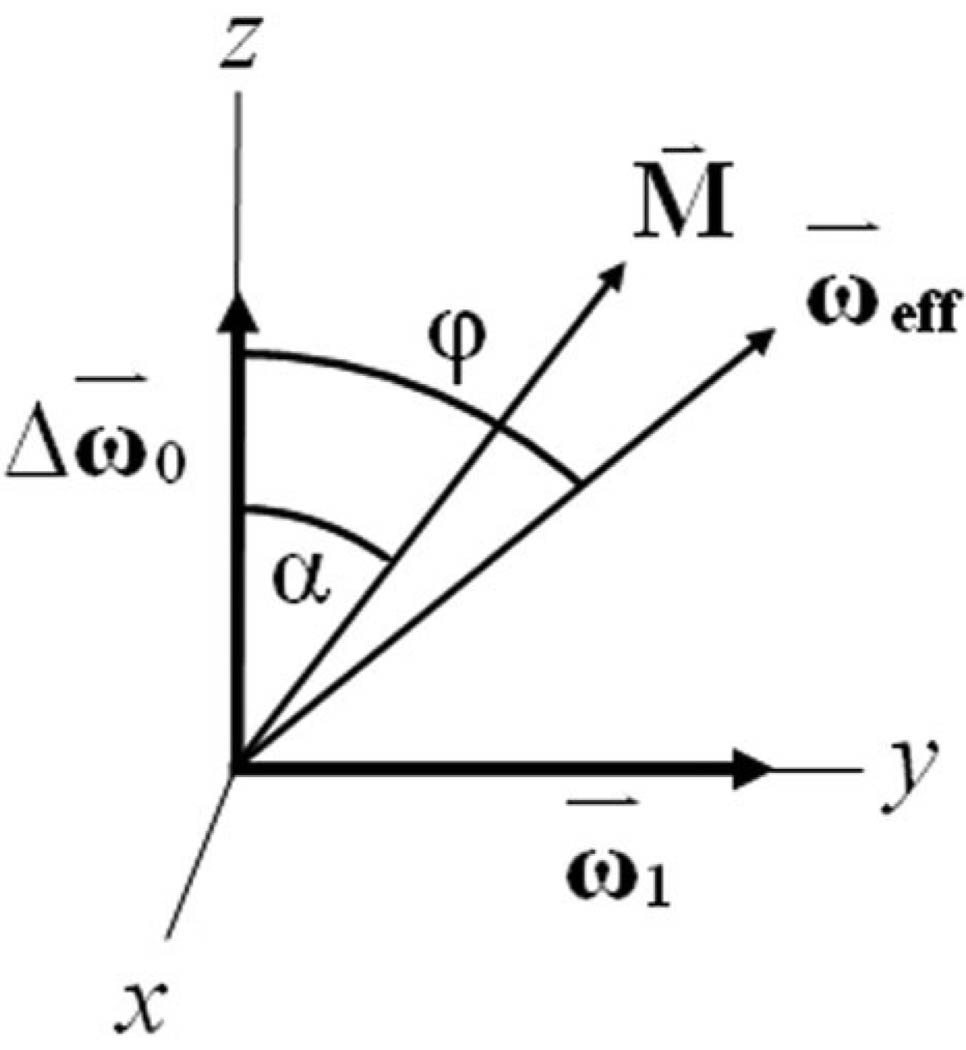
The geometry of the system during an off-resonance spin lock in the rotating frame is shown in Fig. 1. Magnetization is flipped parallel to the effective field, such that the flip angle α = ϕ, where the effective field ωeff makes an angle with the z-axis:
| [1] |
In the on-resonance case, ωeff = ω1 and ϕ = 90°.
FIG. 1.
Off-resonance spin-locking geometry. The effective field ωeff makes an angle φ = tan−1(ω1/Δω) with the z-axis. To perform off-resonance spin locking, the magnetization must be flipped parallel to the effective field such that the flip angle α = ϕ; however, B1 imperfections cause deviations in the expected flip angle α = ϕ − Δϕ. In on-resonance spin-locking experiments, ωeff = ω1 and ϕ = 90°.
Solomon (17) first introduced the rotary echo to correct for imperfections in the RF field. On-resonance, spins accumulate a phase ω1τ because of nutation due to the RF field. In particular, an inhomogeneous B1 field will cause the spin phase to vary throughout the sample and cause a decay of the net magnetization throughout the sample. Solomon (17) realized that if a second B1 pulse that is 180° out of phase with the first is applied for the same duration, the spins will accumulate the exact opposite phase ω1τ and an echo will be formed at 2τ. Sears (18,19) extended the rotary echo to off-resonance spins to remove the dipolar broadening in solid CFCl3. Further, Rhim et al. (20) used an off-resonance rotary echo to compensate for inhomogeneity in the RF field in time reversal experiments on dipolar coupled spins. Notably, the current implementation is used not only to compensate for the loss of coherence during the RF pulse, but also to correct for nutations about the effective field as a result of an imperfect excitation. Indeed, as we will show in Eq. [13], the origin of spin-lock artifacts is nutation about the effective field rather than the loss of phase coherence, which may shorten only the apparent relaxation time during the applied RF pulse.
More recently, several T1ρ-weighted pulse sequences with artifact correction have been implemented in MRI (see Table 1). Charagundla et al. (21) eliminated artifacts from both B1 imperfections and flip angles α ≠ 90° (usually also the result of an imperfect B1) in T1ρ-weighted imaging. By reducing these artifacts, it became possible to measure the spatial distribution of T1ρ relaxation times accurately (22). A complementary technique uses adiabatic excitation to correct for an imperfect flip angle (16). We suggest that an off-resonance rotary echo is useful for spin-locking off-resonance, corrects for both B1 imperfections and flip angles α ≠ ϕ, and can also complement an adiabatic excitation.
Table 1.
Sources of Artifacts in T1ρoff-Weighted Imaging and Their Pulse Sequence Correction Schemes.
| Reference | B1 imperfections (on-resonance) |
B1 imperfections (off-resonance) |
Flip angle α ≠ φ(φ = 90° on-resonance) |
|
|---|---|---|---|---|
| Conventionala | ||||
| Rotary echo | Wheaton et al. (8) | X | X | |
| Adiabatic excitation | Santyr et al. (16) | X | ||
| Off-resonance rotary echo | This work | X | X | X |
A conventional spin-lock is an on-resonance spin lock without a rotary echo and is sensitive to all three sources of artifacts in T1ρ-weighted imaging.
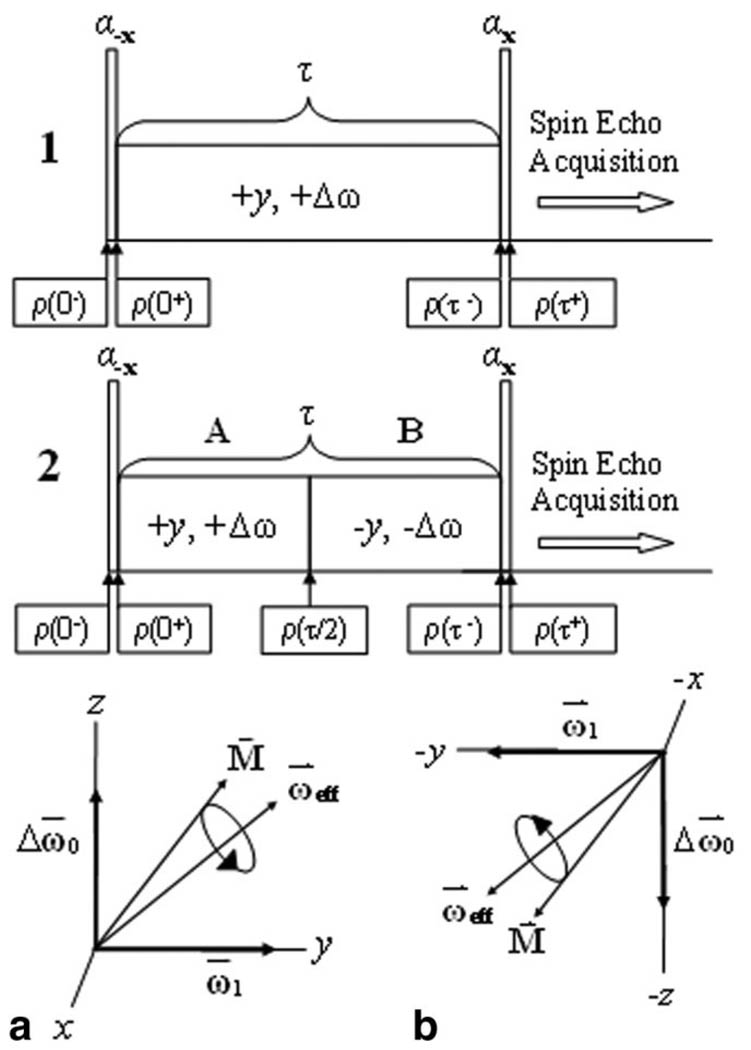
A traditional off-resonance, T1ρoff-weighted imaging sequence is shown in Fig. 2 (sequence 1). Magnetization is T1ρoff-weighted during the spin-locking cluster and spatially encoded in two or three dimensions by a spin-echo imaging sequence. If the flip angle α ≠ ϕ, images will have banding artifacts, as shown in Fig. 3.
FIG. 2.
Two preparatory pulse clusters for T1ρoff-weighted imaging. a: In sequence 1, if the excitation flip angle α is not the same as that of the effective field ϕ, the magnetization nutates about ωeff and produces imaging artifacts. b: In sequence 2, the magnetization is refocused by a frequency- and phase-inverted spin-locking pulse after τ/2.
FIG. 3.
Compensation for B1 inhomogeneity during on-resonance (ΔωRF = 0) and off-resonance (ΔωRF = 400 Hz) spin locking in 3% agarose phantoms (α = 65°, ω1 = 400 Hz). Magnetization nutates about the effective field ωeff, and without both phase and frequency inversion, banding artifacts are severe.
THEORY
The origin of the banding artifacts can be shown with the use of product operator theory. Although the on-resonance spin-lock theory of the rotary echo is well described by quantum mechanical (20) or Bloch (21) treatments, the off-resonance case has not been examined. Following the notation of Levitt (23), the density matrix at equilibrium is given by
| [2] |
where we omit the unity operator 1 and the Boltzmann factor for compactness. Ideally, a uniform pulse of flip angle α = ϕ nutates the magnetization parallel to the effective magnetic field ωeff so that immediately after the pulse
| [3] |
where the pulse nutation is assumed to be instantaneous, and thus RX(−φ) = e−iφIX. The density matrix evolves under the influence of the effective field ωeff, so
| [4] |
where the effective field in the rotating frame is , the evolution propagator is RZ′(ωeffτ) = e−iωeffIZ′τ and z′ denotes the axis of the effective field. After T1ρoff-weighting, the magnetization is stored along the z-axis with a −ϕ pulse:
| [5] |
To examine the density matrix evolution during the spin-locking pulse, we transform the density matrix to the tilted rotating frame using
| [6] |
Combining Eqs. [5] and [6] yields
| [7] |
where R(inv) denotes the inverse operators applied to the right of the density matrix at ρ(0−). Now, RX(φ)RX(φ) = 1. Since at τ = 0− the density matrix is proportional to IZ, the commutator [RZ(ωeffτ),IZ] = 0. Consequently, the density matrix in Eq. [7] is reduced to
| [8] |
in addition to the usual T1ρoff relaxation.
As Soloman (17) showed empirically, Eq. [8] is not correct in the presence of an imperfect B1 field, but can be corrected with a rotary echo. In addition, as the result of an imperfect spin flip where α ≠ ϕ, the magnetization now makes a small angle Δφ to ωeff:
| [9] |
Since the magnetization is no longer parallel to ωeff, Eq. [7] no longer simply reproduces ρ(τ+) = ρ(0−) in Eq. [8]. As a result, ωeff modulates the density matrix by RZ(ωeffτ), and signal oscillations proportional to both ωeff and τ are observed. Figure 3 shows the artifacts produced because of this oscillation.
Suppose now that ω1 is broken into two pulses of equal duration, as shown in Fig. 2 (sequence 2). During the first period τ/2 (Fig. 2a), ωeff modulates the density operator as in Eq. [6]. During the second period τ/2 (Fig. 2b), the phase of ωeff is rotated 180°. The transformation to the tilted rotating frame during this period may be written as
| [10] |
Since [RX(π), RX(−φ)] = 0, the π rotation inverts the direction of nutation:
| [11] |
As before, the density matrix at time τ/2 is
| [12] |
The density matrix now evolves according to Eq. [11], so
| [13] |
Finally, Eq. [13] reduces to
| [14] |
The effects of the nutation RZ(ωeffτ) in Eq. [13] are completely eliminated in Eq. [14] by RX(π). Of course, the form of RX(π) in Eq. [10] depends on whether spin locking is performed on-resonance (ωeff = ω1) or off-resonance (ωeff ≠ ω1). We examine both of these cases below.
RESULTS
Phase inversion of ω1 (±180°) meets the condition of RX(π) in Eq. [10] on-resonance, and is shown in Fig. 3 to reduce artifacts in 3% agarose phantoms. For example, if ω1 is parallel to the y-axis during the first spin-locking period, then ω1 must be parallel to the −y-axis during the second spin-locking period. Physically, when the flip angle α ≠ ϕ, the magnetization makes an angle Δϕ with the xy-plane. During the first spin-locking period, magnetization nutates about the spin-locking axis y for a time τ/2. In the second spin-locking period, the sense of precession is reversed (ω1 → −ω1) and the magnetization returns to its orientation prior to spin locking.
Combined inversion of ω1 and inversion of Δω, the transmitter offset frequency, satisfies RX(π) in Eq. [10] and is shown experimentally in Fig. 3. Once again, if α ≠ ϕ, the magnetization makes an angle Δϕ with ωeff. During the first spin-locking period, magnetization nutates about ωeff. Without inversion of the ωeff, the signal oscillates. If during the second spin-locking period, ω1 → −ω1 and Δω → −Δω, the axis of ωeff will be inverted and the sense of precession about ωeff will be reversed. This condition is called phase and frequency inversion.
The pulse sequence used to reduce off-resonance spin-locking artifacts is shown in Fig. 2, sequence 2, and is contrasted with the conventional off-resonance spin lock shown in Fig. 2, sequence 1. Magnetization is excited along the direction of the effective field with a hard pulse RX(−φ), where it is spin-locked by two phase and frequency symmetric spin-locking pulses (+y, +Δω and −y, −Δω). Consequently, the magnetization is stored along the z-axis with another hard pulse. During spin locking, the magnetization nutates first around the effective field (Fig. 2a) and is refocused during the second τ/2 period by nutation in the opposite direction (Fig. 2b).
DISCUSSION
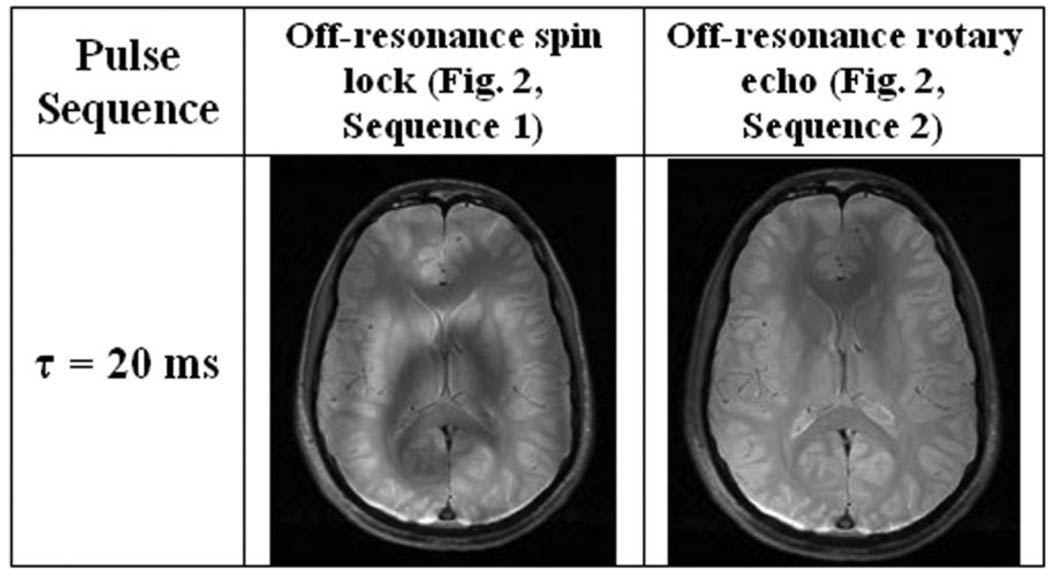
While it may be useful for single-spin systems, the off-resonance rotary echo has limited applicability to NMR spectroscopy because it cannot simultaneously rephase spins precessing at different frequencies. For example, suppose two spins are spin-locked off-resonance, where the frequencies of the two precessing spins are Δω1 and Δω2 in the rotating frame. Frequency inversion of the first spin (Δω1 → −Δω1) cannot simultaneously bring the second spin back into phase with the first at the end of the spin-locking sequence (t = τ). Despite this limitation, the off-resonance rotary echo pulse sequence in Fig. 2, sequence 2, is still useful for MRI because most imaging systems have a single resonant frequency. An additional complication is the presence of any appreciable B0 inhomogeneity, which may cause local spin density to precess off-resonance; however, the condition for an effective off-resonance spin lock is in fact an easier requirement to fulfill compared to the on-resonance condition ω1 ≫ Δω0. We demonstrate the utility of the off-resonance spin lock for imaging the human brain at 3T in Fig. 4 and call attention to the severe deviation of the initial excitation flip angle (α = 65°) from the nominal flip angle (α = 45°) for ω1 = ΔωRF = 400 Hz. For this reason, surface coil T1ρoff-weighted imaging in particular benefits from the compensated rotary echo.
FIG. 4.
T1ρoff-weighted brain images obtained in a healthy volunteer at 3T (α = 65°, ω1 = 400 Hz, ΔωRF = 400 Hz). Phase and frequency alternation of the spin-lock pulse compensates for imperfect flip artifacts; however, there is signal loss in regions of Δω0 inhomogeneity. The flip angle α = 65° deviates from the nominal tan−1(ω1/Δω) = 45° flip necessary for an off-resonance spin lock, and was chosen to amplify the image artifacts.
It is possible to estimate the reduction in SAR during the off-resonance spin lock using a model for SAR deposited in the human head by a quadrature birdcage coil developed by Collins et al. (24). It was shown that
| [15] |
where the SAR is a function of the shape factor f(= 1 for a rectangular pulse), flip angle α, pulse duration τ, and coefficient SAR90°,3 ms = 1.46 W/kg for a 3-ms, 90° rectangular pulse at 1.5T. Considering a single 500 Hz spin-locking pulse delivered for 100 ms, the average SAR delivered with TR = 3 s is approximately 2 W/kg, which is well under the 8 W/kg FDA mandated restriction in the head. For pulse sequences with shorter TR, such as a T1ρ-weighted 3D gradient-echo (GRE) or T1ρ-weighted balanced-SSFP sequence, the average SAR can often surpass FDA limitations. For example, the average SAR delivered during a T1ρ-weighted 3D GRE sequence with TR = 300 ms is nearly 18 W/kg, but can be reduced to less than 8 W/kg by implementing a T1ρoff sequence with ωeff = 500 Hz, ω1 = 325 Hz and Δω0 = 380 Hz. Even if assumptions about the filling factor or SAR90,3ms are incorrect, Eq. [15] predicts that the reduction in SAR will be (B1,off/B1,on)2 and the SAR will be reduced to a fraction of that obtained in an on-resonance T1ρ-weighted experiment. In addition, one can reduce the total scan time by lowering the minimum TR necessary to maintain FDA guidelines.
One unusual consequence may emerge from samples with an asymmetric z-spectrum, where the magnetization transfer (MT) effect may vary between the two spin-locking pulses. For example, suppose an off-resonance spin-locking or saturation experiment is performed in the presence of an asymmetric z-spectrum. The signal intensity might be expected to vary with the spin-locking length and the asymmetry in the z-spectrum. Also, in the conventional off-resonance spin lock, as τ → ∞ the magnetization approaches a steady state Meff along the direction of the effective field ωeff. During a rotary echo, Meff is expected to change halfway through the spin lock and consequently change the resultant image contrast. There are no obvious differences in contrast for τ < 100 ms, although they certainly may exist. ΔωRF inversion may even null tissue magnetization if it is timed appropriately.
CONCLUSIONS
We have introduced a spin-locking sequence that reduces B1 artifacts by means of a frequency- and phase-inverted spin-locking pulse cluster. In agreement with theory, we demonstrated that B1 nutation can be reversed by a 180° inversion of the effective field on- or off-resonance, and showed that B1 artifacts were reduced in experiments on agarose phantoms. We expect that this off-resonance rotary echo will be useful for off-resonance spin locking, since the technique removes complicating image artifacts and preserves exponential T1ρoff decay of the signal while it reduces the required spin-locking RF amplitude.
ACKNOWLEDGMENTS
The authors thank Luke Bloy, Jeremy Wellen, and Susanta Sarkar for stimulating discussions and technical expertise. This study was performed at the Metabolic Magnetic Resonance Research and Computing Center, an NIH-supported resource center (NIH RR02305).
Grant sponsor: National Institutes of Health (NIH); Grant numbers: R01AR045404; R01AR051041.
REFERENCES
- 1.Redfield AG. Nuclear magnetic resonance saturation and rotary saturation in solids. Phys Rev. 1955;98:1787–1809. [Google Scholar]
- 2.Duvvuri U, Goldberg AD, Kranz JK, Hoang L, Reddy R, Wehrli FW, Wand AJ, Englander SW, Leigh JS. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci USA. 2001;98:12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akella SV, Regatte RR, Wheaton AJ, Borthakur A, Reddy R. Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med. 2004;52:1103–1109. doi: 10.1002/mrm.20241. [DOI] [PubMed] [Google Scholar]
- 4.Poptani H, Duvvuri U, Miller CG, Mancuso A, Charagundla S, Fraser NW, Glickson JD, Leigh JS, Reddy R. T1rho imaging of murine brain tumors at 4 T. Acad Radiol. 2001;8:42–47. doi: 10.1016/S1076-6332(03)80742-0. [DOI] [PubMed] [Google Scholar]
- 5.Aronen HJ, Ramadan UA, Peltonen TK, Markkola AT, Tanttu JI, Jaaskelainen J, Hakkinen AM, Sepponen R. 3D spin-lock imaging of human gliomas. Magn Reson Imaging. 1999;17:1001–1010. doi: 10.1016/s0730-725x(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 6.Santyr GE, Henkelman RM, Bronskill MJ. Spin locking for magnetic resonance imaging with application to human breast. Magn Reson Med. 1989;12:25–37. doi: 10.1002/mrm.1910120104. [DOI] [PubMed] [Google Scholar]
- 7.Wheaton AJ, Casey FL, Gougoutas AJ, Dodge GR, Borthakur A, Lonner JH, Schumacher HR, Reddy R. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20:519–525. doi: 10.1002/jmri.20148. [DOI] [PubMed] [Google Scholar]
- 8.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozano J, Li X, Link TM, Safran M, Majumdar S, Ma CB. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349–1352. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 10.Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, Reddy R, Elliott DM. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auerbach JD, Johannessen W, Borthakur A, Wheaton AJ, Dolinskas CA, Balderston RA, Reddy R, Elliott DM. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15:S338–S344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tailor DR, Poptani H, Glickson JD, Leigh JS, Reddy R. High-resolution assessment of blood flow in murine RIF-1 tumors by monitoring uptake of H217O with proton T1rho-weighted imaging. Magn Reson Med. 2003;49:1–6. doi: 10.1002/mrm.10375. [DOI] [PubMed] [Google Scholar]
- 13.Tailor DR, Roy A, Regatte RR, Charagundla SR, McLaughlin AC, Leigh JS, Reddy R. Indirect 17O-magnetic resonance imaging of cerebral blood flow in the rat. Magn Reson Med. 2003;49:479–487. doi: 10.1002/mrm.10403. [DOI] [PubMed] [Google Scholar]
- 14.Grohn OH, Makela HI, Lukkarinen JA, DelaBarre L, Lin J, Garwood M, Kauppinen RA. On- and off-resonance T1rho MRI in acute cerebral ischemia of the rat. Magn Reson Med. 2003;49:172–176. doi: 10.1002/mrm.10356. [DOI] [PubMed] [Google Scholar]
- 15.Fairbanks EJ, Santyr GE, Sorenson JA. One-shot measurement of spin-lattice relaxation-times in the off-resonance rotating-frame using MR-imaging, with application to breast. J Magn Reson Ser B. 1995;106:279–283. doi: 10.1006/jmrb.1995.1044. [DOI] [PubMed] [Google Scholar]
- 16.Santyr GE, Fairbanks EJ, Kelcz F, Sorenson JA. Off-resonance spin locking for MR imaging. Magn Reson Med. 1994;32:43–51. doi: 10.1002/mrm.1910320107. [DOI] [PubMed] [Google Scholar]
- 17.Soloman I. Rotary spin echoes. Phys Rev Lett. 1959;2:301–302. [Google Scholar]
- 18.Sears REJ. Off-resonance rotary spin echoes in dipolar broadened solids. Bull Am Phys Soc. 1970;15:275. [Google Scholar]
- 19.Sears REJ. F-19 anistropic chemical-shift in solid CFCL3. Bull Am Phys Soc. 1972;17:573. [Google Scholar]
- 20.Rhim WK, Pines A, Waugh JS. Time-reversal experiments in dipolar-coupled spin systems. Phys Rev B. 1971;3:684–696. [Google Scholar]
- 21.Charagundla SR, Borthakur A, Leigh JS, Reddy R. Artifacts in T1ρ- weighted imaging: correction with a self-compensating spin-locking pulse. J Magn Reson. 2003;162:113–121. doi: 10.1016/s1090-7807(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 22.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229:269–274. doi: 10.1148/radiol.2291021041. [DOI] [PubMed] [Google Scholar]
- 23.Levitt MH. Spin dynamics: basics of nuclear magnetic resonance. New York: John Wiley & Sons, Ltd.; 2001. [Google Scholar]
- 24.Collins CM, Li S, Smith MB. SAR and B1 field distributions in a heterogeneous human head model within a birdcage coil. Magn Reson Med. 1998;40:847–856. doi: 10.1002/mrm.1910400610. [DOI] [PubMed] [Google Scholar]