Abstract
Weight loss involving diet modification improves urinary incontinence (UI) in women, but little is known about dietary correlates of UI. The authors examined intakes of total energy, carbohydrate, protein, and fats in relation to UI in a cross-sectional sample of 2,060 women in the population-based Boston Area Community Health Survey (2002–2005). Data were collected from in-person home interviews and food frequency questionnaires. Logistic regression was used to calculate odds ratios and 95% confidence intervals for the presence of moderate-to-severe UI; a severity index was analyzed in secondary analysis of 597 women with urine leakage. Greater total energy intake was associated with UI (Ptrend = 0.0001; highest quintile vs. lowest: adjusted odds ratio = 2.86, 95% confidence interval: 1.56, 5.23) and increased severity. No associations were observed with intake of carbohydrates, protein, or total fat. However, the ratio of saturated fat intake to polyunsaturated fat intake was positively associated with UI (highest quintile vs. lowest: adjusted odds ratio = 2.48, 95% confidence interval: 1.22, 5.06) and was strongly associated with severity (Ptrend < 0.0001). Results suggest that dietary changes, particularly decreasing saturated fat relative to polyunsaturated fat and decreasing total calories, could independently account for some of the benefits of weight loss in women with UI.
Keywords: diet, dietary fats, energy intake, fatty acids, nutritional status, urinary incontinence, women
Urinary incontinence diminishes the quality of life for millions of women on a daily basis and has been associated with an estimated $20 billion in annual direct health-care costs (1–4). Recent population-based estimates from the United States show that approximately 16% of women report moderate-to-severe urinary incontinence (2, 5). Pathophysiologic mechanisms of urinary incontinence may have various origins and generally have been theorized to be related to urothelium-based, myogenic, and/or neurogenic changes (6–8). In epidemiologic studies, urinary incontinence in women has been associated with increased age, white/Caucasian race/ethnicity, central obesity, vaginal child delivery, hysterectomy, heart disease, asthma, and arthritis/rheumatism (4, 9–11).
Research priorities in urinary incontinence include identifying modifiable lifestyle factors (2, 12). Recently, a randomized clinical trial of overweight women confirmed that weight loss significantly reduces the frequency of urinary incontinence episodes (13). The authors noted that 74% of the reduction in incontinence frequency was statistically explained by the weight change (14), proposing the mechanism that weight loss reduces intraabdominal pressure, thereby decreasing pressures on the bladder and pelvic floor. However, the authors acknowledged the possibility that changes in diet or physical activity may have accounted for some of the intervention effect. Women in the intervention group were instructed to reduce their total daily energy intake to 1,200–1,500 kcal/day, limit their fat intake, and consume a low-fat beverage daily as a meal replacement for part of the trial duration, along with increasing their physical activity to 200 minutes per week or more. Physical activity has been found to be inversely associated with urinary incontinence in women, although the relation is not observed across all studies and may be partly explained by weight maintenance (4, 15, 16). Considering that the sympathetic and parasympathetic nervous systems control micturition and that inflammation and endothelial dysfunction may be involved in urologic symptoms (6–8, 17–20), it is plausible that changes in energy intake and specific macronutrients have direct effects on urinary incontinence, independently of weight loss or physical activity. In support of this hypothesis, investigators in the Leicestershire MRC Incontinence Study reported a positive association between total and saturated fat intake and onset of stress incontinence (21). A better understanding of the contribution of diet—specifically, the dietary changes that are often made for weight loss (i.e., changes in total energy intake and specific macronutrients such as fat)—would be helpful both for understanding pathophysiologic mechanisms and for clinical practice in treatment of urinary incontinence.
To test the hypothesis that increased total energy and macronutrient intakes are positively associated with urinary incontinence in women, we analyzed these dietary factors in relation to urinary incontinence symptoms in a population-based, racially/ethnically diverse cross-sectional study, the Boston Area Community Health (BACH) Survey. We focused our analysis on dietary factors, rather than physical activity, partly because in a previous report from the BACH Survey, Tennstedt et al. (4) analyzed the relative contribution of physical activity and found that it was not associated with urinary incontinence in this sample of women.
MATERIALS AND METHODS
Participants and data collection
The BACH Survey.
The BACH Survey is a community-based study of urologic symptoms and risk factors in Boston, Massachusetts. From 2002 to 2005, BACH investigators used multistage stratified random sampling to recruit 3,202 women aged 30–79 years from 3 racial/ethnic groups into the study. Anthropometric measurements (including height, weight, and waist circumference) were taken, and information about urologic symptoms, comorbid conditions, and lifestyle was obtained at an in-person home interview. All participants were mailed an English or Spanish version of the Block food frequency questionnaire (FFQ) (22). Details on the methods used in the BACH Survey have been published elsewhere (23). The study was approved by the institutional review board of the New England Research Institutes, and all participants provided written informed consent.
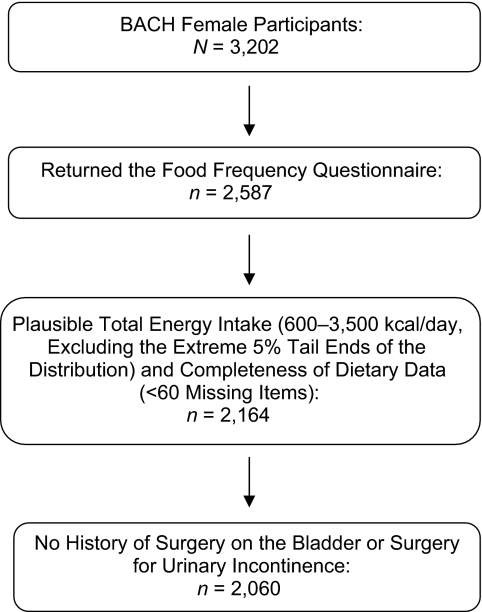
The final sample size for this analysis was 2,060 women. Exclusions from the original sample of 3,202 female BACH participants were applied as noted in Figure 1. Standard dietary data criteria were used to obtain acceptable data quality and led to most of the exclusions (24). Compared with the larger BACH sample, the resulting analytic sample had fewer Hispanics (26.7% vs. 34.7%) and more whites (39.7% vs. 32.0%), but there were no differences in age, physical activity, body mass index (weight (kg)/height (m)2), waist circumference, alcohol consumption, or prevalence of urinary incontinence or overall lower urinary tract symptoms.
Figure 1.
Inclusion criteria used for an analysis of dietary macronutrient and energy intake and urinary incontinence among women in the Boston Area Community Health (BACH) Survey, 2002–2005.
Assessment of diet.
Participants completed the Study of Women's Health Across the Nation (SWAN) 2001/2002 version of the 1995 Block FFQ (22). Both English and Spanish versions of the SWAN/Block FFQ have been validated in various settings, with moderate-to-high validity and reliability (22, 25, 26). In comparison with 4 24-hour dietary recalls, deattenuated energy-adjusted correlation coefficients obtained in women were 0.67 for fat, 0.66 for carbohydrates, and 0.53 for protein (27). The SWAN FFQ performed similarly well in Hispanics: Deattenuated correlation coefficients were 0.78 for fat, 0.61 for carbohydrates, and 0.61 for protein (25).
Assessment and operational definition of urinary incontinence.
The presence of urinary incontinence was assessed during the in-home interview using questions from validated and reliable scales, in accordance with the classification of urinary incontinence in the 2003 report of the standardization subcommittee of the International Continence Society (28). Women who reported experiencing any urinary incontinence in the past 12 months completed the validated Sandvik severity index (29), which assesses frequency (less than once per month, 1 or more times per month, 1 or more times per week, or every day) and amount (drops, small splashes, or more) of urine leakage, resulting in a composite score ranging from 1 to 12. For our primary analysis, incontinence case status was defined by a severity score of 3 or more, indicating moderate-to-severe urinary incontinence (5, 11, 30). This definition corresponds to at-least-weekly leakage or monthly leakage of volumes greater than a few drops in the past 12 months. To confirm that this common dichotomization of the severity index was appropriate for our nutritional analyses, we conducted 2 additional analyses. First, in preliminary analyses, we tested a 4-level multinomial outcome of no urinary incontinence, low severity (score 1 or 2), moderate severity (score 3 or 4), or high severity (score 5–12) and found no appreciable differences between the no-incontinence and low-severity groups with regard to variables of interest. Second, in sensitivity analyses, we used an outcome definition based on frequency of urinary incontinence regardless of severity, defined as weekly urinary incontinence (n = 208), and excluded women with less frequent urine leakage from the reference group (389 were excluded, leaving 1,671 women in the analysis). Results were similar to those of the main analysis and thus are not presented.
Type of leakage, defined as either stress, urge, or mixed urinary incontinence, was assessed by responses to questions on leakage during physical activity (including coughing, sneezing, lifting, or exercising) and during times when there was a strong urge to empty the bladder. As in previous reports on BACH data (4, 11), most women with urinary incontinence had mixed incontinence (n = 121), and the low prevalence of stress-only (n = 38) or urge-only (n = 30) incontinence precluded separate statistical analyses.
Data analysis
Nutrient intakes were adjusted for total energy intake using residuals (31). Participants were grouped into quintiles of daily intake of each nutrient and the ratio of intake of saturated fatty acids (SFA) to intake of polyunsaturated fatty acids (PUFA). To minimize the influence of outliers, we conducted linear tests for trend using the median values of deciles of intake to represent the exposure of all participants in the same decile.
We used logistic regression to calculate odds ratios and 95% confidence intervals for the associations between nutrient intakes and the primary outcome of urinary incontinence among all 2,060 women. In secondary analyses, we examined the association between nutrient intakes and the continuous severity score among 597 women with any urinary incontinence in the past year, using linear regression to obtain β estimates and standard errors. We examined smoothing (LOESS) plots to confirm linearity of the regression models in preliminary model-building procedures. Initial models were adjusted for age and total energy intake. In full multivariate models, we additionally adjusted for factors that were established risk factors from prior studies (4, 9, 10), were associated with both diet and urinary incontinence, and changed the estimate of the dietary association more than 10%: race/ethnicity, waist circumference, ever giving birth vaginally, menopause/hormone-use status, use of antispasmodic or anticholinergic medication, total energy intake, cardiac disease, diabetes, asthma, and arthritis/rheumatism; in models for type of fat, we additionally adjusted for the other types of fat. We also considered the following factors in the multivariate models but did not include them because they did not affect the final results: physical activity, body mass index, cigarette smoking, alcohol consumption, average daily fluid intake, sodium intake, socioeconomic status, cancer, depression symptoms, Parkinson's disease, multiple sclerosis, stroke, use of a bladder catheter, and use of diuretics, α-blockers, or tricyclic antidepressants. Effect modification of total energy intake by waist circumference or physical activity was examined by means of stratified analyses and statistical tests for interaction.
The sampling design of the BACH Survey requires weighting observations inversely proportional to their probability of selection in order for the results to be generalizable to the larger population. Weights were poststratified to the 2000 US Census population of Boston. All statistical tests were 2-sided, performed at α = 0.05, and conducted in SUDAAN, version 10.0 (Research Triangle Institute, Research Triangle Park, North Carolina).
RESULTS
Of the 2,060 women included in this analysis, 257 (12.5%) had a severity score of 3 or more and were therefore considered to have moderate-to-severe urinary incontinence. The average severity score among the cases was 4.8 (standard error, 0.2), with 63.1% being moderate cases and 36.9% being severe cases. Case women most commonly (43.9%) had mixed urinary incontinence, although 22.1% reported only stress incontinence, 7.6% only urge incontinence, and 4.9% only nonspecific incontinence. Among the 1,803 women who were not classified as incontinence cases, 79.5% reported never leaking urine in the past 12 months and 20.5% reported infrequent leakage of low severity (mean severity score = 1.4).
Weighted mean values and prevalences of characteristics that may be associated with urinary incontinence are shown in Table 1, overall and by case status. Women with urinary incontinence were less likely to be physically active and more likely to be overweight, to be surgically postmenopausal, to have depression symptoms, to have delivered a child vaginally, and to have had a urinary tract infection, diabetes, cardiac disease, arthritis/rheumatism, or asthma. Pearson correlation coefficients for correlations between dietary factors and physical activity, body mass index, or waist circumference were low (r < 0.10). The factors that influenced estimates of diet–urinary incontinence associations most in subsequent multivariate models were waist circumference, diabetes, and asthma.
Table 1.
Weighted Characteristics of 2,060 Women in the Boston Area Community Health Survey, Overall and by Urinary Incontinence Statusa, 2002–2005
| Total (N = 2,060 ) |
UI (n = 257) |
No/Low UI (n = 1,803) |
||||
| Mean (SE) | % | Mean (SE) | % | Mean (SE) | % | |
| Age, years | 48.9 (0.6) | 52.3 (1.5) | 48.4 (0.6) | |||
| Race/ethnicity | ||||||
| Black | 30.2 | 31.0 | 30.1 | |||
| Hispanic | 13.0 | 9.1 | 13.6 | |||
| White | 56.8 | 59.9 | 56.3 | |||
| Cigarette smoking status | ||||||
| Never smoker | 50.6 | 45.3 | 51.4 | |||
| Former smoker | 26.5 | 26.2 | 26.5 | |||
| Current smoker | 22.9 | 28.5 | 22.2 | |||
| Alcohol intake, g/day | 5.7 (0.4) | 4.8 (0.7) | 5.9 (0.5) | |||
| Body mass indexb | 28.9 (0.3) | 31.4 (0.7) | 28.6 (0.3) | |||
| Waist circumference, cm | 89.6 (0.6) | 95.1 (1.7) | 88.8 (0.6) | |||
| Physical activity levelc | ||||||
| Low | 26.6 | 35.0 | 25.4 | |||
| Medium | 53.4 | 54.1 | 53.3 | |||
| High | 19.9 | 10.9 | 21.2 | |||
| Medical history | ||||||
| Diabetes mellitus | 8.2 | 13.7 | 7.4 | |||
| Cardiac disease | 7.3 | 12.7 | 6.6 | |||
| Cancer | 9.2 | 12.6 | 8.7 | |||
| Arthritis or rheumatism | 28.3 | 50.9 | 25.0 | |||
| Asthma | 18.2 | 33.2 | 16.0 | |||
| Depression symptoms | 18.4 | 34.5 | 16.1 | |||
| Urinary tract infection | 46.1 | 57.8 | 44.4 | |||
| Use of diuretic agents | 13.0 | 18.9 | 12.1 | |||
| Use of antispasmodic or anticholinergic agents | 1.4 | 5.7 | 0.8 | |||
| Menopausal/hormone-use status | ||||||
| Premenopausal | 24.1 | 10.5 | 26.1 | |||
| Perimenopausal | 21.1 | 25.9 | 20.4 | |||
| Naturally postmenopausal | 22.4 | 23.0 | 22.3 | |||
| Surgically postmenopausal | 14.5 | 22.6 | 13.3 | |||
| Hormone use | 15.5 | 14.5 | 15.6 | |||
| Undetermined | 2.5 | 3.5 | 2.3 | |||
| Ever delivering a child vaginally | 61.0 | 73.2 | 59.3 | |||
| Total energy intaked, kcal/day | 1,589 (19) | 1,803 (74) | 1,558 (19) | |||
| Energy-adjusted dietary intake | ||||||
| Protein, g/day | 82.7 (0.6) | 82.3 (1.5) | 82.8 (0.7) | |||
| Carbohydrates, g/day | 239 (1.8) | 242 (4.0) | 239 (1.9) | |||
| Saturated fat, g/day | 25.0 (0.2) | 25.9 (0.7) | 24.9 (0.2) | |||
| Monounsaturated fat, g/day | 30.1 (0.3) | 29.8 (0.6) | 30.1 (0.3) | |||
| Polyunsaturated fat, g/day | 13.8 (0.2) | 13.0 (0.4) | 14.0 (0.2) | |||
| Saturated fat:polyunsaturated fat ratio | 2.02 (0.03) | 2.22 (0.11) | 1.99 (0.03) | |||
| Sodium, g/day | 2,621 (23) | 2,580 (54) | 2,627 (25) | |||
| Cholesterol, mg/day | 297 (5.5) | 305 (11.5) | 296 (6.1) | |||
Abbreviations: SE, standard error; UI, urinary incontinence.
UI was defined as moderate-to-severe urine leakage using the Sandvik severity scale (29), which considers frequency and amount of urine leakage in the past 12 months. Moderate-to-severe UI was compared with no/nonsevere UI.
Weight (kg)/height (m)2.
Physical activity was measured by means of the Physical Activity Scale for the Elderly (51); scores were classified as follows: <100 = low, 100–250 = medium, ≥250 = high.
Total energy intake does not include energy derived from alcohol consumption.
Results of the multivariate analyses are presented in Table 2. In both age-adjusted and multivariate-adjusted models, greater total energy intake was significantly associated with higher odds of urinary incontinence (Ptrend = 0.0001). Women in the highest total energy intake group were almost 3 times as likely to report urinary incontinence as women in the lowest group (odds ratio = 2.86, 95% confidence interval: 1.56, 5.23; P = 0.0007). Although it was not quite as strong, a linear association with the continuous severity score was also apparent among women who had had any urinary incontinence in the past 12 months (Ptrend = 0.03).
Table 2.
Odds Ratios for Urinary Incontinence and Statistical Significance of Trend Tests for Severity Among 2,060 Women, by Macronutrient Intake, Boston Area Community Health Survey, 2002–2005a
| Macronutrient | Quintile of Dietary Intake (257 Cases, 1,803 Noncases) |
P for Trend | P for Trend in Severity Score (n = 597) | ||||||||
| 1 (Referent) | 2 |
3 |
4 |
5 |
|||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| TEI | |||||||||||
| Median value, kcal/day | 840 | 1,171 | 1,494 | 1,860 | 2,453 | ||||||
| Adjusted for age | 1.00 | 1.02 | 1.27 | 1.26 | 2.62** | 1.39, 4.92 | 0.0002 | ||||
| Multivariateb | 1.00 | 1.28 | 0.67, 2.44 | 1.40 | 0.65, 3.03 | 1.30 | 0.65, 2.63 | 2.86** | 1.56, 5.23 | 0.0001 | 0.03 |
| Carbohydrates | |||||||||||
| Median value, g/day | 188 | 221 | 244 | 267 | 305 | ||||||
| Adjusted for age and TEI | 1.00 | 0.89 | 0.81 | 1.04 | 1.11 | 0.57, 2.15 | 0.78 | ||||
| Multivariateb | 1.00 | 0.86 | 0.45, 1.61 | 0.86 | 0.41, 1.81 | 1.08 | 0.51, 2.31 | 1.24 | 0.62, 2.46 | 0.53 | 0.26 |
| Protein | |||||||||||
| Median value, g/day | 57 | 71 | 81 | 91 | 110 | ||||||
| Adjusted for age and TEI | 1.00 | 1.41 | 1.79 | 1.07 | 1.03 | 0.54, 1.96 | 0.78 | ||||
| Multivariateb | 1.00 | 1.60 | 0.79, 3.25 | 1.96 | 0.89, 4.33 | 1.07 | 0.51, 2.27 | 1.04 | 0.52, 2.08 | 0.67 | 0.71 |
| Total fat | |||||||||||
| Median value, g/day | 55 | 67 | 76 | 84 | 97 | ||||||
| Adjusted for age and TEI | 1.00 | 1.01 | 1.03 | 0.91 | 1.09 | 0.57, 2.09 | 0.95 | ||||
| Multivariateb | 1.00 | 0.91 | 0.45, 1.86 | 0.87 | 0.43, 1.74 | 0.71 | 0.39, 1.29 | 0.86 | 0.46, 1.64 | 0.44 | 0.14 |
| SFA:PUFA ratio | |||||||||||
| Median value (range)c | 1.1 (0.5–1.4) | 1.6 (1.4–1.7) | 1.9 (1.7–2.0) | 2.3 (2.0–2.6) | 3.0 (2.6–7.5) | ||||||
| Adjusted for age and TEI | 1.00 | 1.92 | 2.01 | 2.20** | 2.46** | 1.37, 4.42 | 0.005 | ||||
| Multivariateb | 1.00 | 1.84 | 0.89, 3.80 | 2.13 | 0.97, 4.68 | 1.93* | 0.99, 3.76 | 2.48** | 1.22, 5.06 | 0.02 | <0.0001 |
| Saturated fat | |||||||||||
| Median value, g/day | 16.5 | 21.2 | 24.6 | 27.8 | 33.0 | ||||||
| Adjusted for age and TEI | 1.00 | 0.56 | 0.84 | 0.88 | 1.46 | 0.80, 2.66 | 0.03 | ||||
| Multivariateb | 1.00 | 0.60 | 0.29, 1.22 | 0.96 | 0.37, 2.49 | 0.90 | 0.37, 2.20 | 1.63 | 0.65, 4.09 | 0.04 | 0.0005 |
| Monounsaturated fat | |||||||||||
| Median value, g/day | 20.3 | 25.6 | 29.6 | 33.5 | 39.8 | ||||||
| Adjusted for age and TEI | 1.00 | 0.82 | 0.81 | 1.00 | 0.85 | 0.43, 1.65 | 0.86 | ||||
| Multivariateb | 1.00 | 0.73 | 0.33, 1.62 | 0.70 | 0.27, 1.77 | 0.63 | 0.23, 1.72 | 0.61 | 0.20, 1.82 | 0.18 | 0.01 |
| Polyunsaturated fat | |||||||||||
| Median value, g/day | 7.7 | 10.6 | 12.8 | 15.3 | 19.8 | ||||||
| Adjusted for age and TEI | 1.00 | 0.56 | 0.67 | 0.72 | 0.58 | 0.31, 1.06 | 0.24 | ||||
| Multivariateb | 1.00 | 0.58 | 0.30, 1.10 | 0.68 | 0.35, 1.34 | 0.66 | 0.34, 1.34 | 0.57 | 0.27, 1.17 | 0.22 | 0.005 |
Abbreviations: CI, confidence interval; OR, odds ratio; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TEI, total energy intake.
* P = 0.05; **P ≤ 0.01.
For improved readability, some 95% confidence intervals are not shown. Data are available from the authors upon request.
The multivariate model included age (5-year categories), race/ethnicity (black, Hispanic, or white), waist circumference (cm; quintiles), ever giving birth vaginally (yes/no), menopausal/hormone-use status (premenopausal, perimenopausal, postmenopausal, hysterectomy without hormones, hysterectomy with hormones, hormone replacement therapy, birth control, use of raloxifene hydrochloric acid, use of progesterone only, or undetermined), use of antispasmodic or anticholinergic medication, total energy intake (kcal/day; quintiles), and history of cardiac disease, diabetes, asthma, urinary tract infection, or arthritis/rheumatism. Models for saturated fat, monounsaturated fat, and polyunsaturated fat additionally adjusted for the other types of fat (g/day; quintiles), and models for SFA:PUFA ratio additionally adjusted for monounsaturated fat (g/day; quintiles).
Cutpoints overlap because of rounding.
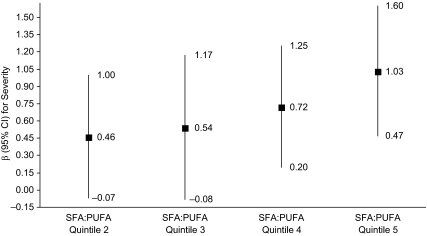
No consistent or statistically significant associations between intake of carbohydrates, protein, or total fat and urinary incontinence were observed in any of the analyses. However, SFA:PUFA ratio was significantly associated with urinary incontinence. Women with an SFA:PUFA ratio greater than 2:1 had over twice the odds of urinary incontinence (quartile 5 vs. quartile 1: odds ratio = 2.48, 95% confidence interval: 1.22, 5.06; P = 0.01). In secondary analyses of severity of urinary incontinence, the role of this ratio was highly statistically significant (Ptrend < 0.0001; Figure 2); women in the highest quintile of SFA:PUFA ratio (consumption of approximately 3–7 times’ more saturated fat than polyunsaturated fat) had, on average, a severity score 1 point higher than women in the lowest quintile (SFA:PUFA ratio closer to 1:1; P = 0.0004). A similar but slightly weaker association was observed for the ratio of saturated fat to the sum of polyunsaturated fat plus monounsaturated fat (Ptrend < 0.0001; data not shown). When the different types of fat were examined individually, higher intake of saturated fat had a positive trend towards increased severity of urinary incontinence, which was attenuated after adjustment for other factors (primarily waist circumference and arthritis/rheumatism) but remained statistically significant (Ptrend = 0.0005). Meanwhile, severity of urinary incontinence was inversely related to intake of monounsaturated fat (Ptrend = 0.01) or polyunsaturated fat (Ptrend = 0.005). To determine whether SFA:PUFA ratio or the absolute level of intake of each type of fat was more relevant to urinary incontinence or its severity, we mutually adjusted for each variable in multivariate models. Results showed that the ratio measure was a more robust predictor of urinary incontinence or severity of incontinence than was absolute intake of either saturated fat or polyunsaturated fat (data not shown).
Figure 2.
Multivariate linear regression results for the association between the ratio of dietary intakes of saturated fatty acids (SFA) to polyunsaturated fatty acids (PUFA) and continuous urinary incontinence severity score among 597 women with any urine leakage in the past 12 months, Boston Area Community Health Survey, 2002–2005 (Ptrend < 0.0001). The β estimates (squares) represent the adjusted mean difference in severity score, comparing a given intake quintile to the reference (lowest) intake quintile, with adjustment for the factors listed in footnote “b” of Table 2. Severity score was calculated from the validated Sandvik severity index (29), which considers frequency and amount of urine leakage to obtain a score ranging from 1 to 12. The mean severity score among the 597 women with any urinary incontinence was 2.80 (standard error, 0.12). Bars, 95% confidence interval (CI).
Results from analysis of total energy intake stratified by waist circumference or physical activity are shown in Table 3. Across all categories of waist circumference, greater total energy intake was significantly associated with greater odds of urinary incontinence. The association was stronger among women with low-to-moderate waist circumference, but there was no statistically significant interaction. No significant differences were found for the positive relation between total energy intake and urinary incontinence across levels of physical activity.
Table 3.
Associationa Between a 500-kcal/Day Increase in Total Energy Intake and Urinary Incontinence in 2,060 Women, by Waist Circumference Category and Physical Activity Level, Boston Area Community Health Survey, 2002–2005
| Total No. of Women | No. of Cases | No. of Noncases | Multivariate Odds Ratio | 95% Confidence Interval | P Value | |
| Waist circumferenceb,c | ||||||
| Low–moderate (≤89 cm) | 979 | 91 | 888 | 1.41 | 1.09, 1.84 | 0.01 |
| High (90–109 cm) | 751 | 94 | 657 | 1.34 | 1.01, 1.77 | 0.04 |
| Very high (≥110 cm) | 330 | 72 | 258 | 1.38 | 1.01, 1.87 | 0.04 |
| Physical activityd,e | ||||||
| Low | 723 | 114 | 609 | 1.38 | 1.10, 1.75 | 0.006 |
| Medium | 1,014 | 114 | 900 | 1.33 | 1.04, 1.69 | 0.02 |
| High | 323 | 29 | 294 | 1.68 | 0.99, 2.85 | 0.055 |
Results from multivariate models including age (5-year categories), race/ethnicity (black, Hispanic, or white), menopausal/hormone-use status (premenopausal, perimenopausal, naturally postmenopausal, surgically postmenopausal, hormone use, or undetermined), urinary tract infection, vaginal child delivery, cardiac disease, asthma, arthritis/rheumatism, and use of antispasmodic or anticholinergic medication. Models for very high waist circumference and high physical activity controlled for age in 10-year categories because of the smaller sample size.
Waist circumference categories were adapted from recommendations regarding obesity-related health risk (50).
Interaction term: Wald P = 0.94. Likelihood ratio test comparing models with and without the interaction term: P = 0.85.
Physical activity was measured by means of the Physical Activity Scale for the Elderly (51); scores were classified as follows: <100 = low, 100–250 = medium, ≥250 = high.
Interaction term (quintile of total energy intake × physical activity level): Wald P = 0.98. Likelihood ratio test comparing models with and without the interaction term: P = 0.97.
DISCUSSION
In this population-based cross-sectional study, women who had a higher SFA:PUFA ratio or higher total daily calorie intake had significantly greater odds of urinary incontinence. No associations were observed for total fat, carbohydrate, or protein intake. These results were robust to adjustment for interviewer-measured obesity, physical activity, comorbid conditions, and relevant pharmaceutical usage as determined by a home inventory. Results were consistent, and stronger for SFA:PUFA ratio, when we examined the severity of urinary incontinence among women with any reported urine leakage in the past year, indicating that the relative intake of certain types of fat may be important for the progression of urinary incontinence as well as its initial development.
Behavioral and lifestyle modifications, including weight loss, remain the preferred first line of treatment for most urinary incontinence patients, yet there have been few studies of dietary correlates of urinary incontinence. The most comparable research to date was carried out in the Leicestershire MRC Incontinence Study, which examined diet in relation to incidence of stress incontinence after 1 year of follow-up (21). Similarly to us, those investigators found a positive association between saturated fat intake and urinary incontinence. However, they also reported a positive association with total fat, whereas our results for total fat were null and most likely reflected the opposing associations held for saturated fats versus unsaturated fats.
Indeed, a novel finding from our analysis was that SFA:PUFA ratio was more important than the absolute amount of each type of fat consumed. Specific pathophysiologic mechanisms that may underlie an association between relative intake of saturated and polyunsaturated fats and urinary incontinence plausibly involve inflammation or vascular changes that result in endothelial dysfunction relevant to the bladder, the detrusor, and urologic symptoms (6–8, 19). The theory that inflammatory processes may be involved in the etiology of urologic symptoms is supported by separate analyses of the BACH Survey in which Kupelian et al. (20) reported that serum C-reactive protein measures were positively associated with lower urinary tract symptoms in women. Additionally, the findings of 2 longitudinal cohort studies of men also support this theory: St. Sauver et al. (32) reported that C-reactive protein was associated with rapid onset of storage symptoms, and Schenk et al. (33) reported that inflammatory markers were associated with incidence of high-moderate to severe overall lower urinary tract symptoms. Meanwhile, there is consistent evidence that the relative intakes of saturated and unsaturated fatty acids and postprandial lipoproteins have effects on vascular inflammation, as measured by significant changes in serum C-reactive protein and the proinflammatory interleukins 6, 7, and 18, as well as endothelial dysfunction (34–39). Studies suggest that for heart disease prevention, an SFA:PUFA ratio of 1:1 and less than 1.5:1 is most beneficial (38, 40, 41). In our study, women in the reference quintile of lowest intake consumed just this amount, averaging a 1:1 ratio and at most a 1.5:1 ratio, whereas women in the highest quintile of intake had an SFA:PUFA ratio closer to 3:1 and upwards of 8:1, as is possible with the Western dietary pattern.
An additional novel finding of our analysis was a positive association between total energy intake and urinary incontinence. A common concern with analyses of total energy intake is that it is closely related to body size and physical activity, and results can be difficult to interpret. For example, in our analysis, the positive association was primarily carried by women in the highest quintile of caloric intake, despite the statistically significant linear trend across intake groups. Still, the findings were robust and were not appreciably affected by adjustment for fluid intake, fruit and vegetable intake (as a marker for foods with high water content), or physical activity. Our results are strengthened by the fact that the positive association was observed across all strata of body mass index, waist circumference, and physical activity and was strongest among women in the lowest body-size group. The possibility that an effect of total energy intake is stronger in leaner women is plausible, in that leaner women may have fewer competing causes of urinary incontinence than overweight women, whose urinary incontinence may be more likely to be related to intraabdominal pressure (13) or effects of the proinflammatory state of obesity (39, 42). A possible pathophysiologic mechanism explaining our observed associations is that intake of total energy, including saturated fat, increases autonomic nervous system activity, which is associated with an overactive bladder and lower urinary tract symptoms (8, 17, 18, 43–45).
Intervention studies show that weight loss reduces the frequency of urinary incontinence in overweight women (13, 46). It is noteworthy that weight loss was achieved by instructing women to reduce their total energy intake to 1,200–1,500 kcal/day and limit saturated fat intake. Our results suggest that these dietary changes could directly improve urinary incontinence, in addition to any indirect effects mediated by weight loss. That is, part of the observed effects of weight loss on frequency of urinary incontinence episodes may be due to decreased caloric and SFA intakes. To further explore the importance of both diet and body size in urinary incontinence using our data, we conducted additional analyses (not shown) which found that 70% of the cases of urinary incontinence in the BACH Survey women might have been preventable solely through management of macronutrient intake and weight, by following these evidence-based guidelines for a healthy lifestyle: SFA:PUFA ratio ≤ 1.5:1, total energy intake ≤ 1,800 kcal/day, and body mass index ≤ 25 (“normal”).
Among the limitations of our study is the fact that the observational nature of this analysis precluded us from making definitive causal inferences. The pathophysiologic mechanisms we have discussed are plausible but remain speculative until definitive experimental research is conducted. Although we found no associations between physical activity and urinary incontinence, a specific measure of exercises that strengthen core pelvic area muscles may have been more informative. To explore this possibility, we analyzed data (not shown) on pelvic floor exercise, available only in the subset of women who had sought health care for urinary incontinence (n = 176; 39.4% responded positively to pelvic floor exercise); results showed that pelvic floor exercise was not associated with dietary intakes and did not alter our findings. Regarding dietary intakes, we did not have information on type of polyunsaturated fat. There may be important differences, for example, between cis- and trans-fats, which should be examined in future studies.
Another concern may be that 16% of the returned FFQs were excluded for questionable dietary data quality. This concern is alleviated by the fact that the resulting analytic sample was similar to the original BACH sample in outcome measures as well as in relevant demographic and lifestyle factors. Furthermore, for a diverse population-based sample, the initial response rate of 81% to the mailed self-administered FFQ actually surpassed expectations and was similar to the 80% response rate for the Block FFQ in the National Cancer Institute's Eating at America's Table Study (27).
A noteworthy limitation is that we were unable to examine dietary associations separately for stress incontinence and urge incontinence, because most women reported mixed incontinence. Prior studies have indicated that many predictors of urinary incontinence may differ for stress and urge types (13, 46, 47). A detailed examination of the association of total energy intake and macronutrients with each type of urinary incontinence would help elucidate pathophysiologic mechanisms in our results. At the same time, urodynamic studies show that self-reports on type of urinary incontinence are prone to error, particularly error in discriminating between mixed and stress incontinence (48). Thus, our analysis of total urinary incontinence had the advantage of avoiding misclassification by type of urinary incontinence, since urodynamic measures were unavailable.
A major strength of this study was its community-based, random sample of women of various ages and racial/ethnic groups. We assessed symptoms of urinary incontinence in the entire population sample, which is a distinct epidemiologic advantage compared with studying women clinically diagnosed with urinary incontinence, because it expanded our case group beyond only those women who had the knowledge to seek health care and/or had access to health care. Thus, diagnostic bias was avoided, and our results are applicable to various socioeconomic groups. In our sample, the weighted energy-adjusted mean dietary intakes of various nutrients were similar to results obtained from the 1999–2000 National Health and Nutrition Examination Survey (49), and participants consumed close to the Recommended Dietary Intakes for most nutrients (data not shown). Therefore, the BACH sample is likely to be representative of the general US population in terms of dietary consumption patterns.
In summary, in this study, total energy intake and SFA:PUFA ratio were each independently positively associated with urinary incontinence in women. These associations were not accounted for by body size or physical activity and in fact were strong among leaner women. Additional research should be pursued not only to confirm these findings but also to experimentally test hypothesized pathophysiologic mechanisms involving inflammation, endothelial dysfunction, and autonomic nervous system activity.
Acknowledgments
Author affiliations: Department of Epidemiology, New England Research Institutes, Watertown, Massachusetts (Nancy N. Maserejian, John B. McKinlay); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Edward L. Giovannucci); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Edward L. Giovannucci); Department of Medicine, Channing Laboratory, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Edward L. Giovannucci); Department of Urology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Kevin T. McVary); and Department of Health Sciences, School of Medicine, University of Leicester, Leicester, United Kingdom (Catherine McGrother).
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant R21DK081844).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Dr. Kevin T. McVary reports having received speaking fees from GlaxoSmithKline, Eli Lilly & Company, Sanofi-Aventis, and Advanced Health Media; having received grant-research support from GlaxoSmithKline, Eli Lilly & Company, and Allergan; and having provided consulting services for Pfizer, Eli Lilly & Company, and Allergan. The other authors report no disclosures.
Glossary
Abbreviations
- BACH
Boston Area Community Health
- FFQ
food frequency questionnaire
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
- SWAN
Study of Women's Health Across the Nation
References
- 1.Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63(3):461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Nygaard I, Thom DH, Calhoun EA. Urinary incontinence in women. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2007. pp. 157–192. (NIH publication no. 07-5512) [Google Scholar]
- 3.Tennstedt SL, Chiu GR, Link CL, et al. The effects of severity of urine leakage on quality of life in Hispanic, white, and black men and women: the Boston Area Community Health Survey. Urology. 2010;75(1):27–33. doi: 10.1016/j.urology.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tennstedt SL, Link CL, Steers WD, et al. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) Survey. Am J Epidemiol. 2008;167(4):390–399. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002;4(suppl 4):S7–S18. [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology. 2003;62(5 suppl 2):3–10. doi: 10.1016/j.urology.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Roosen A, Chapple CR, Dmochowski RR, et al. A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.07.044. Aug 4 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194(2):339–345. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrother CW, Donaldson MM, Hayward T, et al. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing. 2006;35(1):16–24. doi: 10.1093/ageing/afi205. [DOI] [PubMed] [Google Scholar]
- 11.Connolly TJ, Litman HJ, Tennstedt SL, et al. The effect of mode of delivery, parity, and birth weight on risk of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(9):1033–1042. doi: 10.1007/s00192-006-0286-4. [DOI] [PubMed] [Google Scholar]
- 12.Herbison P, Hay-Smith J, Paterson H, et al. Research priorities in urinary incontinence: results from citizens’ juries. BJOG. 2009;116(5):713–718. doi: 10.1111/j.1471-0528.2008.02093.x. [DOI] [PubMed] [Google Scholar]
- 13.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subak LL, Wing R, Vittinghoff E. The authors reply: weight loss to treat urinary incontinence in overweight and obese women [letter] N Engl J Med. 2009;360(21):2257. [Google Scholar]
- 15.Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109(3):721–727. doi: 10.1097/01.AOG.0000255973.92450.24. [DOI] [PubMed] [Google Scholar]
- 16.Townsend MK, Danforth KN, Rosner B, et al. Physical activity and incident urinary incontinence in middle-aged women. J Urol. 2008;179(3):1012–1016. doi: 10.1016/j.juro.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4):1327–1433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 18.Hubeaux K, Deffieux X, Ismael SS, et al. Autonomic nervous system activity during bladder filling assessed by heart rate variability analysis in women with idiopathic overactive bladder syndrome or stress urinary incontinence. J Urol. 2007;178(6):2483–2487. doi: 10.1016/j.juro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Dupont MC, Spitsbergen JM, Kim KB, et al. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166(3):1111–1118. [PubMed] [Google Scholar]
- 20.Kupelian V, McVary KT, Barry MJ, et al. Association of C-reactive protein and lower urinary tract symptoms in men and women: results from Boston Area Community Health Survey. Urology. 2009;73(5):950–957. doi: 10.1016/j.urology.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallosso H, Matthews R, McGrother C, et al. Diet as a risk factor for the development of stress urinary incontinence: a longitudinal study in women. Eur J Clin Nutr. 2004;58(6):920–926. doi: 10.1038/sj.ejcn.1601913. [DOI] [PubMed] [Google Scholar]
- 22.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 23.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52(2):389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett WC. Issues in analysis and presentation of dietary data. In: Willett WC, editor. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. pp. 321–346. [Google Scholar]
- 25.Block G, Wakimoto P, Jensen C, et al. Validation of a food frequency questionnaire for Hispanics [electronic article] Prev Chronic Dis. 2006;3(3):A77. [PMC free article] [PubMed] [Google Scholar]
- 26.Boucher B, Cotterchio M, Kreiger N, et al. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 27.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 28.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 29.Sandvik H, Seim A, Vanvik A, et al. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19(2):137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):520–524. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 32.St Sauver JL, Sarma AV, Jacobson DJ, et al. Associations between C-reactive protein and benign prostatic hyperplasia/lower urinary tract symptom outcomes in a population-based cohort. Am J Epidemiol. 2009;169(11):1281–1290. doi: 10.1093/aje/kwp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schenk JM, Kristal AR, Neuhouser ML, et al. Biomarkers of systemic inflammation and risk of incident, symptomatic benign prostatic hyperplasia: results from the Prostate Cancer Prevention Trial. Am J Epidemiol. 2010;171(5):571–582. doi: 10.1093/aje/kwp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22(1):18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 35.Kris-Etherton P, Daniels SR, Eckel RH, et al. AHA scientific statement: summary of the Scientific Conference on Dietary Fatty Acids and Cardiovascular Health. Conference summary from the Nutrition Committee of the American Heart Association. J Nutr. 2001;131(4):1322–1326. doi: 10.1093/jn/131.4.1322. [DOI] [PubMed] [Google Scholar]
- 36.Alipour A, Elte JW, van Zaanen HC, et al. Postprandial inflammation and endothelial dysfunction. Biochem Soc Trans. 2007;35(3):466–469. doi: 10.1042/BST0350466. [DOI] [PubMed] [Google Scholar]
- 37.Berry SE, Tucker S, Banerji R, et al. Impaired postprandial endothelial function depends on the type of fat consumed by healthy men. J Nutr. 2008;138(10):1910–1914. doi: 10.1093/jn/138.10.1910. [DOI] [PubMed] [Google Scholar]
- 38.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk SJ, Feskens EJ, Bos MB, et al. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90(6):1656–1664. doi: 10.3945/ajcn.2009.27792. [DOI] [PubMed] [Google Scholar]
- 40.Kang MJ, Shin MS, Park JN, et al. The effects of polyunsaturated:saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br J Nutr. 2005;94(4):526–532. doi: 10.1079/bjn20051523. [DOI] [PubMed] [Google Scholar]
- 41.Grundy SM. What is the desirable ratio of saturated, polyunsaturated, and monounsaturated fatty acids in the diet? Am J Clin Nutr. 1997;66(4 suppl):988S–990S. doi: 10.1093/ajcn/66.4.988S. [DOI] [PubMed] [Google Scholar]
- 42.Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 43.Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clin Sci (Lond) 1994;86(2):159–167. doi: 10.1042/cs0860159. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz JH, Young JB, Landsberg L. Effect of dietary fat on sympathetic nervous system activity in the rat. J Clin Invest. 1983;72(1):361–370. doi: 10.1172/JCI110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman NU, Phonsombat S, Bochinski D, et al. An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int. 2007;100(3):658–663. doi: 10.1111/j.1464-410X.2007.07069.x. [DOI] [PubMed] [Google Scholar]
- 46.Brown JS, Wing R, Barrett-Connor E, et al. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care. 2006;29(2):385–390. doi: 10.2337/diacare.29.02.06.dc05-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Townsend MK, Curhan GC, Resnick NM, et al. BMI, waist circumference, and incident urinary incontinence in older women. Obesity (Silver Spring) 2008;16(4):881–886. doi: 10.1038/oby.2008.14. [DOI] [PubMed] [Google Scholar]
- 48.Sandvik H, Hunskaar S, Vanvik A, et al. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol. 1995;48(3):339–343. doi: 10.1016/0895-4356(94)00147-i. [DOI] [PubMed] [Google Scholar]
- 49.Wright JD, Wang CY, Kennedy-Stephenson J, Ervin RB. Dietary intake of ten key nutrients for public health, United States: 1999–2000. Adv Data. 2003 (334):1–4. [PubMed] [Google Scholar]
- 50.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 51.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]