Abstract
Rationale
Transient Receptor Potential Melastatin-3 (TRPM3) is a calcium-permeable ion channel activated by the neurosteroid pregnenolone sulphate and positively coupled to insulin secretion in β-cells. Although vascular TRPM3 mRNA has been reported, there is no knowledge of TRPM3 protein or its regulation and function in the cardiovascular system.
Objective
To determine the relevance and regulation of TRPM3 in vascular biology.
Methods and Results
TRPM3 expression was detected at mRNA and protein levels in contractile and proliferating vascular smooth muscle cells. Calcium entry evoked by pregnenolone sulphate or sphingosine was suppressed by TRPM3 blocking antibody or knock-down of TRPM3 by RNA interference. Low-level constitutive TRPM3 activity was also detected. In proliferating cells, channel activity was coupled negatively to interleukin-6 secretion via a calcium-dependent mechanism. In freshly-isolated aorta, TRPM3 positively modulated contractile responses independently of L-type calcium channels. Concentrations of pregnenolone sulphate required to evoke responses were higher than the known plasma concentrations of the steroids, leading to a screen for other stimulators. β-cyclodextrin was one of few stimulators of TRPM3, revealing the channels to be partially suppressed by endogenous cholesterol, the precursor of pregnenolone. Elevation of cholesterol further suppressed channel activity and loading with cholesterol to generate foam cells precluded observation of TRPM3 activity.
Conclusions
The data suggest functional relevance of TRPM3 in contractile and proliferating phenotypes of vascular smooth muscle cells, significance of constitutive channel activity, regulation by cholesterol, and potential value of pregnenolone sulphate in therapeutic vascular modulation.
Keywords: Calcium channel, Transient Receptor Potential, Vascular smooth muscle, Neurosteroid, Cholesterol, Interleukin
Introduction
Mammalian cells contain numerous types of trans-membrane ion channel that are permeable to Ca2+, including many of the 28 mammalian homologues of the Drosophila melanogaster Transient Receptor Potential (TRP) channel1-3. The channels are thought to have structural similarity to α-subunits of voltage-gated K+ channels, with intracellular amino and carboxy termini and four proteins required for coordination of a single ion pore. As with K+ channels, heteromultimerisation confers greater diversity. However, unlike voltage-gated K+ channels, membrane depolarization is not the primary trigger for channel activity. Instead, chemical factors are considered to be primary stimuli. Details of the chemical sensing properties are becoming apparent and hold promise for revealing further complexity and novelty. In addition, important roles of TRP channels have emerged, including in sensation and cell survival, but we are far from a full appreciation of the purposes of these channels and, in some cases, there is relatively little understanding of TRP family members – one example being TRPM3.
TRPM3 is a member of the M (melastatin) sub-type of TRP channel4-10. It is expressed most obviously in the brain and kidney but wider expression is also apparent. When exogenously over-expressed it forms Ca2+-permeable non-selective cationic channels (i.e. channels that are also permeable to Na+). Ion channels form without the need for co-expression with other TRP channels and so TRPM3 seems capable of function as a homomeric channel. Substantial and species-specific splice variation is evident, the biological relevance of which has largely to be determined – intriguingly, one splicing event confers change in ionic selectivity6,7. Activity of heterologously over-expressed TRPM3 channels has been observed to be enhanced by chemical factors including sphingosine, pregnenolone sulphate and dehydroepiandrosterone sulphate (DHEAS)5,9. In the mouse, endogenous TRPM3 is activated by pregnenolone sulphate and coupled to insulin secretion in pancreatic β-cells9. In humans the TRPM3 gene is on chromosome 9 (9q21.11-q21.12), relatively close to regions linked to coronary artery disease11 and tentatively linked to Kabuki syndrome12. However, no firm genetic linkage to disease has been established and there are no reports on the function or properties of vascular TRPM3 or endogenous human TRPM3.
Even before the discovery of TRP channels it was appreciated that smooth muscle cells contain voltage-gated Ca2+ channels and a variety of other Ca2+-permeable channels13. The smooth muscle cell has, therefore, been a focus for investigating the relevance of TRP channels in mammalian systems13,14. However, although mRNA analysis has indicated expression of the TRPM3 gene in vascular smooth muscle cells (VSMCs)14,15 there are no reports on the TRPM3 protein, its regulation or function in this context.
Smooth muscle cells have crucial roles in all organs of the body. In the physiological setting the cells are most-often in a stable (non-proliferating) contractile phenotype but in development, injury and disease there is modulation to a phenotype characterized by proliferation, motility, increased secretion, and absence of contractility16. The capacity for modulation is fundamentally important for adult life because it enables temporary physiological change, adaptation, and recovery from injury. It also plays pivotal roles in life-threatening vascular diseases. In some instances the remodeling generates potentially lethal neointimal formations following invasive procedures that include percutaneous transluminal angioplasty and coronary artery bypass graft surgery17-19; in the latter case, remodeling is prominent in the saphenous vein, a routine graft17,19,20. TRP channels appear to be particularly important in the remodeling situation21. Here we report on investigation of TRPM3 in proliferating and contractile VSMCs.
Methods
Human VSMCs and murine arteries
Human saphenous vein segments were obtained with ethical approval and proliferating VSMCs were prepared using an explant technique. For contraction studies, 8 week old mice were killed in accordance with the UK Animals Scientific Procedures Act and thoracic aorta was mounted for isometric tension recording in a myograph. For femoral artery studies, 12 week old mice underwent guide wire arterial injury conducted in accord with accepted standards of humane animal care under a UK Home Office Project License.
Cell-based assays
Intracellular Ca2+ was detected using fura-2 and measured on a 96-well fluorescence plate reader or a single-well microscope system. Membrane currents were recorded using a planar patch-clamp system in voltage-clamp mode. Secretions were quantified using ELISAs.
Molecular biology
Total RNA was extracted using a Tri-reagent protocol followed by DNase I treatment and reverse transcribed before PCR analysis. Human TRPM3 cDNA was expressed under tetracycline regulation in a HEK 293 cell-line2. For short interfering (si) RNA delivery, cells were suspended in Nucleofector solution, mixed with siRNA and transferred into a cuvette for electroporation.
Antibodies and labeling
Rabbit polyclonal anti-TRPM3 (TM3E3) and anti-TRPC1 (T1E3) antibodies have been described2,5. Antibody labeling of cells and intact vein sections was largely as described previously1. For calcium imaging, cells were pre-incubated with TM3E3 for 3.5 hours at 37 °C. For contraction studies mouse aorta was pre-incubated with TM3E3 in Hanks solution overnight at 4 °C.
Data analysis
Averaged data are presented as mean±s.e.mean. Data produced in pairs were compared using t tests, where statistical significance is indicated by * (P<0.05) and no significant difference by NS. Independent experiments were repeated on tissue/cells from at least 3 different patients.
For detailed methods, see Supplementary Information.
Results
Expression of TRPM3 in contractile and proliferating VSMCs
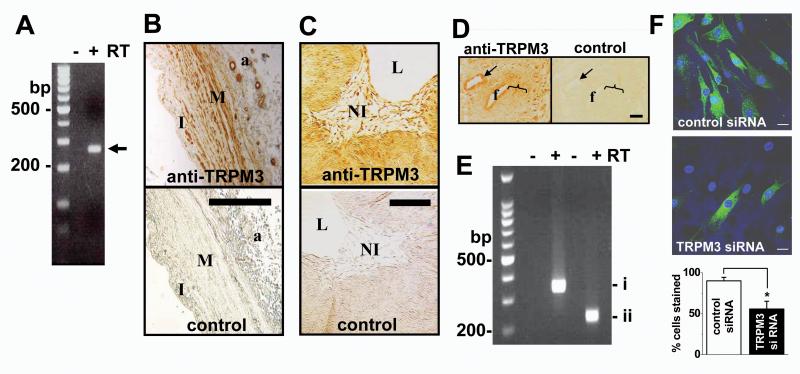
To investigate the relevance of TRPM3 to VSMCs we first focused on saphenous vein obtained from patients undergoing coronary artery bypass operations. TRPM3 mRNA was detected in samples of vein (Fig 1A). Anti-TRPM3 antibody labeled the medial layer, adventitial vessels, and intimal cells (Fig 1B), suggesting that TRPM3 occurs in contractile and pre-existing intimal VSMCs. To investigate the relevance to remodeling, veins were organ-cultured for 2 weeks to elicit neointimal hyperplasia20 (Fig 1C). TRPM3 was detected in the neointimal cells (Fig 1C), showing that TRPM3 is a feature also of human VSMCs proliferating in situ. Further evidence of a link to remodeling came from in vivo studies in the mouse where the femoral artery was injured with a guide wire to evoke VSMC remodeling: TRPM3 was detected in, but not restricted to, remodeling VSMCs and VSMCs of the uninjured (contractile) medial layer (Fig 1D). VSMCs proliferating in planar cell culture also contained TRPM3 mRNA (Fig 1E) and showed anti-TRPM3 antibody labeling (Fig 1F), which was confirmed to reflect TRPM3 protein by specific siRNA knock-down (Fig 1F, Supplementary Fig I). Endothelial TRPM3 was not obvious in the intact vessels labeled with anti-TRPM3 antibody (Fig 1B, C); consistent with this observation, cultured endothelial cells showed less TRPM3 mRNA compared with VSMCs (Supplementary Fig II). The data suggest that TRPM3 is expressed in contractile and proliferating VSMCs.
Figure 1.
Native TRPM3 expression in vascular smooth muscle cells (VSMCs). (A) RT-PCR analysis of mRNA in fresh human saphenous vein medial layer without (−) and with (+) prior reverse transcription (RT). DNA markers (M) are on the left. The arrow indicates the predicted size of the TRPM3 product (268 bp). (B, C) Cross-sections of intact saphenous vein without (B) and with (C) induction of neointima. (B) I, pre-existing intima; M, medial layer; a, adventitia containing vasa vasorum. (C) NI, neointima; L, lumen. Controls were primary antibody omitted (B) and antibody preadsorbed to antigenic peptide (C). (D) Tissue section containing in vivo injured mouse femoral artery (f) and an uninjured artery (arrow), with the bracket indicating the region of remodeling VSMCs; control staining of the adjacent tissue section was with antibody preadsorbed to antigenic peptide. (B-D) Labeling with anti-TRPM3 antibody is shown in brown colour (B, TM3N1 Ab8; C & D, TM3E3 Ab10); scale bars are 100 μm. (E, F) Data from human saphenous vein VSMCs in culture, representative of n=4 (E) and 3 (F). (E) RT-PCR analysis using two PCR primer sets, showing mRNA of TRPM31325 (i) without TRPM3f insert (ii) (see Supplementary Table I). (F) Immunofluorescence labeling with TM3E3 (green) after transfection with scrambled (control) or TRPM3 siRNA. Cell nuclei were stained with DAPI (blue). The scale bars are 20 μm. The bar chart is mean data for the type of experiments illustrated by the images (n/N = 4/81 for control, 4/100 for TRPM3 siRNA).
Pregnenolone sulphate responses in proliferating VSMCs
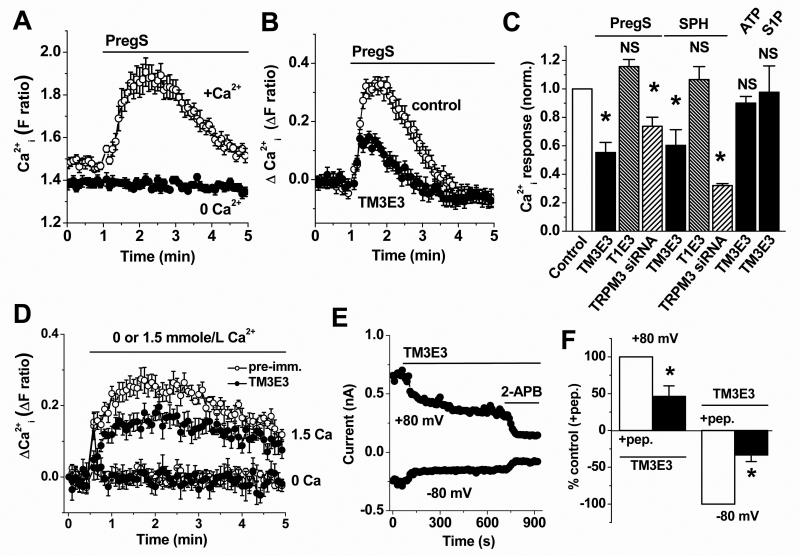
To investigate if TRPM3 is functional in VSMCs we made intracellular Ca2+ measurements because TRPM3 is Ca2+-permeable8,10. The TRPM3 agonist, pregnenolone sulphate, elicited reliable rises in intracellular Ca2+ that depended on extracellular Ca2+, consistent with activation of Ca2+-entry (Fig 2A). Non-specific TRPM3 inhibitors (gadolinium ions and 2-APB) inhibited the responses (Supplementary Fig III), as did anti-TRPM3 blocking antibody TM3E310 (Fig 2B, C). Responses to ATP and sphingosine-1-phosphate were unaffected by TM3E3 (Fig 2C, Supplementary Fig IV), consistent with these responses occurring via other mechanisms and confirming specificity of TM3E3. T1E3, a blocking antibody targeted to a different TRP channel (TRPC1)20, had no effect on pregnenolone sulphate responses (Fig 2C). Further support for the involvement of TRPM3 was the observation that transfection with TRPM3 siRNA inhibited pregnenolone sulphate responses (Fig 2C). Despite investigation of several TRPM3 siRNA molecules (e.g. Supplementary Fig I), only partial knock-down of the pregnenolone sulphate response could be achieved (Fig 2C). Whole-cell voltage-clamp recordings from VSMCs showed large membrane currents in response to pregnenolone sulphate. In 27 out of 37 recordings the evoked current-voltage relationships (I-Vs) were relatively linear and reversed polarity near 0 mV (Supplementary Fig V), similar to the currents reported for over-expressed human TRPM3 recorded under similar conditions10. However, in the remaining recordings the I-Vs were outwardly rectifying and did not reverse at 0 mV, suggesting activation of additional undefined ionic mechanisms (Supplementary Fig V). The data suggest that TRPM3 channels are functional in VSMCs.
Figure 2.
Functional activity of endogenous TRPM3 in proliferating VSMCs. Data are based on intracellular Ca2+ measurement (A-D) or whole-cell electrophysiology (E, F) applied to human saphenous vein VSMCs. (A) Responses to 25 μmole/L PregS in the presence (+Ca2+) or absence (0 Ca2+) of 1.5 mmole/L extracellular Ca2+ (representative of n/N = 5/30 for each). (B) Example responses to 25 μmole/L PregS after pretreatment with TM3E3 antiserum or its preimmune control. (C) Mean data summarizing effects on responses to 25 μmole/L PregS, 20 μmole/L sphingosine (SPH), 100 μmole/L ATP or 1 μmole/L S1P of pretreatments with TM3E3, anti-TRPC1 antiserum (T1E3) or their preimmune controls (n/N = 7/76 for TM3E3 and 6/47 for T1E3 tests and controls), or transfection with TRPM3 siRNA compared with scrambled siRNA (n/N = 3/18 for each test and control). Each test data set was normalized to its own control. (D) Effect of returning 1.5 mM Ca2+ to the extracellular solution following pre-incubation with TM3E3 or its pre-immune serum (N = 6 for each; representative of n=5). Data without Ca2+ add-back (0 Ca) are shown for comparison. (E) Example spontaneous current at the indicated voltages, showing effects of extracellular TM3E3 and then 75 μmole/L 2-aminoethoxydiphenylborate (2-APB). (F) Mean data for the type of experiment shown in (E) normalized to currents in paired controls (TM3E3 preadsorbed to its antigenic peptide (+pep.)).
Sphingosine responses in proliferating VSMCs
Sphingosine and dihydrosphingosine have also been suggested to be agonists at TRPM35. In VSMCs the compounds elicited Ca2+ elevations that were similar to those of HEK 293 cells over-expressing TRPM310 (Fig 2C, Supplementary Fig VI). TM3E3 or TRPM3 siRNA suppressed the VSMC sphingosine responses, again suggesting functional TRPM3 channels (Fig 2C, Supplementary Fig VI).
Constitutive TRPM3 activity in VSMCs
TRPM3 has also been suggested to exhibit constitutive activity8. To investigate this possibility we performed extracellular Ca2+ add-back experiments in the absence of an exogenous TRPM3 stimulator. The Ca2+-entry was attenuated by TM3E3 (Fig 2D). Furthermore, electrophysiology recordings revealed basal ionic current was inhibited by TM3E3 (Fig 2E, F); the I-Vs were mildly outwardly rectifying and reversed polarity near 0 mV (Supplementary Figure VII). The data suggest that endogenous TRPM3 channels of VSMCs have moderate constitutive activity.
Negative coupling to cytokine secretion
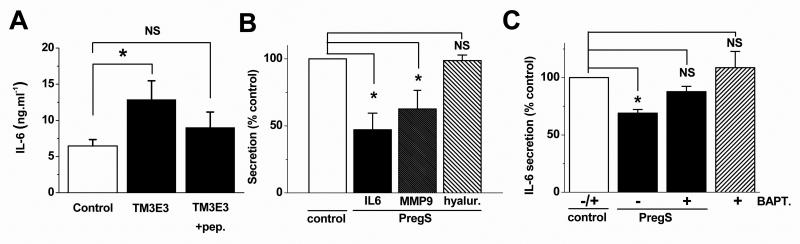
Based on prior experience with other TRP channels28 we hypothesized that TRPM3 might be relevant to VSMC secretion. Impact was found on secretion of the cytokine interleukin-6 (IL-6), which is generated during bypass surgery and stimulates neointimal formation and atherogenesis29-31. Averaged data from VSMCs of 9 patients revealed stimulation of IL-6 secretion following TRPM3 inhibition by TM3E3 (Fig 3A), which is consistent with constitutive TRPM3 activity having tonic inhibitory effect against IL-6. Pregnenolone sulphate suppressed secretion of IL-6 or the matrix metalloproteinase MMP-9 but lacked effect on hyaluronan (Fig 3B). To investigate if Ca2+ entry was the cause of the effect, cells were incubated in 1 μM BAPTA-AM to confer intracellular Ca2+ chelation via BAPTA free acid. BAPTA suppressed the inhibitory effect of pregnenolone sulphate and slightly (but not significantly) enhanced IL-6 secretion in the absence of pregnenolone sulphate (Fig 3C). Higher concentrations of BAPTA were not studied because of general suppressive effects on secretion (data not shown). The data suggest that TRPM3 activity is functionally relevant to secretion of factors including IL-6.
Figure 3.
Relationship to secretion from proliferating VSMCs. (A) Absolute interleukin-6 (IL-6) concentration in extracellular medium exposed to TM3E3 (n=9) or TM3E3 preadsorbed to its antigenic peptide (n=7). (B) Effects 10 μmole/L PregS on secretion of IL-6, MMP-9 and hyaluronan (n=8 patients for each condition). (C) Effects 1 μmole/L BAPTA-AM (BAPT.) on IL-6 secretion and its inhibition by 10 μmole/L PregS (n=7 patients for each condition). All data are from human saphenous vein VSMCs.
Positive coupling to contractile function
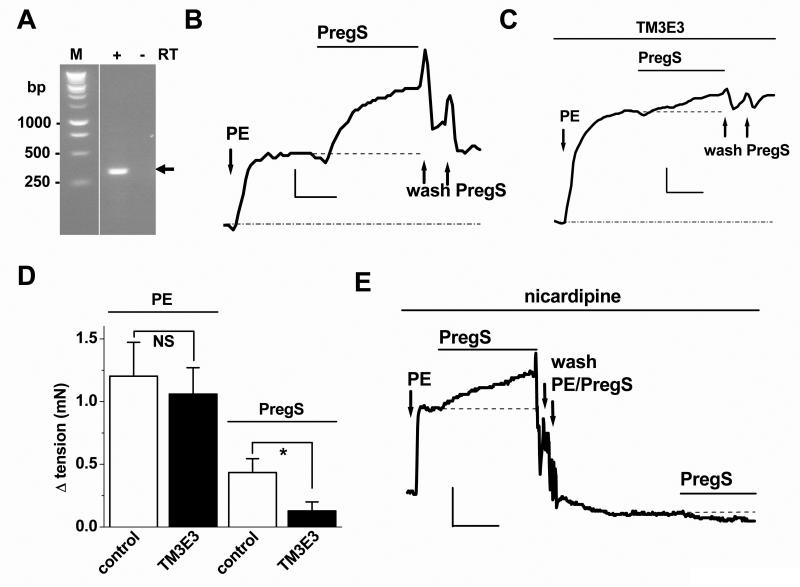
For these studies we focused on mouse aorta where physiological contractile responses could be reliably observed. Expression of TRPM3 was confirmed by RT-PCR (Fig 4A). Isometric tension recordings revealed contractile responses to pregnenolone sulphate (≥60 μmole/L) in vessels precontracted with a submaximal concentration of the α-adrenoceptor agonist, phenylephrine (e.g. Fig 4B). Pregnenolone sulphate responses were suppressed by TM3E3 (Fig 4B-D), which was validated as an inhibitor of mouse TRPM3 (Supplementary Fig VIII). Responses to phenylephrine were unaffected by TM3E3 (Fig 4D). Pregnenolone sulphate responses occurred despite the presence of 100 nM nicardipine (Fig 4E), which blocked voltage-gated Ca2+ channels as shown by suppression of 60 mM K+ responses (data not shown). Pregnenolone sulphate failed to evoke contraction in the absence of pre-tone (Fig 4E). Smooth muscle cells freshly-isolated from the aorta exhibited ionic current in response to pregnenolone sulphate that was similar to the current observed through exogenously-expressed mouse TRPM3 channels (Supplementary Fig IX). The data suggest the presence of functional native TRPM3 channels in contractile VSMCs which positively modulate contractile responses independently of voltage-gated Ca2+ channels.
Figure 4.
Relationship to contraction in mouse aorta. (A) PCR analysis of aorta mRNA using primers for TRPM3 (expected product size, 295 bp). PCR products are shown with (+) or without (−) reverse transcription (RT) and DNA markers (M) are on the left. (B-E) Isometric tension recordings. (B, C) Example paired recording showing responses to 10 nmole/L phenylephrine (PE) and 200 μmole/L PregS. Arrows mark PregS wash-out artefacts. Vessel segments were pre-incubated with dialysed and boiled TM3E3 (B, control) or dialysed TM3E3 (C, test ). The verticle scale bars are 0.5 and 0.2 mN (B, C) and the horizontal bars are 5 min. (D) Mean data for the type of experiment illustrated in (B, C) (n=9 pairs). (E) As for (B, C) but excluding TM3E3 and in the continuous presence of 100 nmole/L nicardipine (representative of n=4). Verticle scale bar, 0.5 mN; horizontal scale bar, 10 min.
Physiological relevance of steroid and sphingolipid stimulators
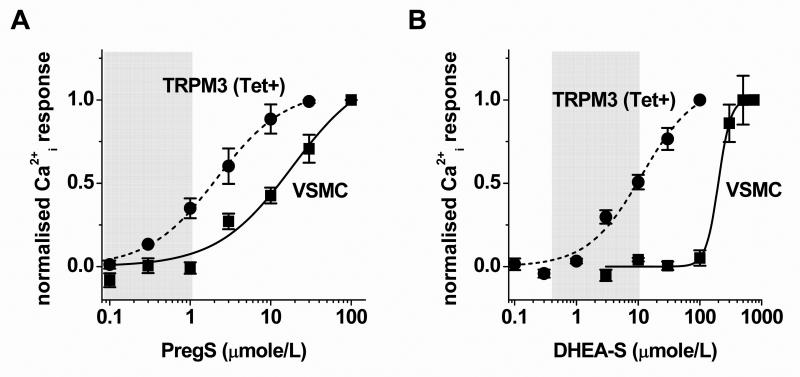
Concentration-response curves were generated to determine relevance to plasma concentrations of steroids (Fig 5). Although pregnenolone sulphate activated over-expressed TRPM3 at quite low concentrations, more than 1 μmole/L was required to evoke responses in saphenous vein VSMCs (Fig 5A). Similarly, higher concentrations of DHEAS were required for VSMC responses (Fig 5B, Supplementary Fig X). The differences may be accounted for by different channel expression levels, but they also raise the possibility that TRPM3 in VSMCs does not confer sensitivity to plasma concentrations of pregnenolone sulphate or DHEAS22. Sphingolipids are alternative stimulators but, despite the observed effects of sphingosine, it is difficult to generate a case that VSMCs express plasma membrane TRPM3 to sense extracellular sphingosine or related lipids including ceramide and sphingosine-1-phosphate, which do not activate TRPM35. Physiological functions of sphingosine have been suggested23 but serum concentrations of sphingosine24 do not reach the micromolar concentrations necessary to activate vascular TRPM3 (Supplementary Fig VI). Without excluding importance of local accumulation of factors, we embarked on a chemical screen to potentially identify other natural activators.
Figure 5.
Sensitivity to neurosteroids. (A, B) Concentration-dependence of responses to PregS (A) or dihydroepiandrosterone sulphate (DHEAS, B) in HEK 293 cells induced to express TRPM3 (Tet+: n/N=3/12, A; n/N=3/9, B) or VSMCs (n/N=4/12, A; n/N=4/16, B). Shaded areas indicate the physiological plasma concentrations22.
Chemical screening of TRPM3
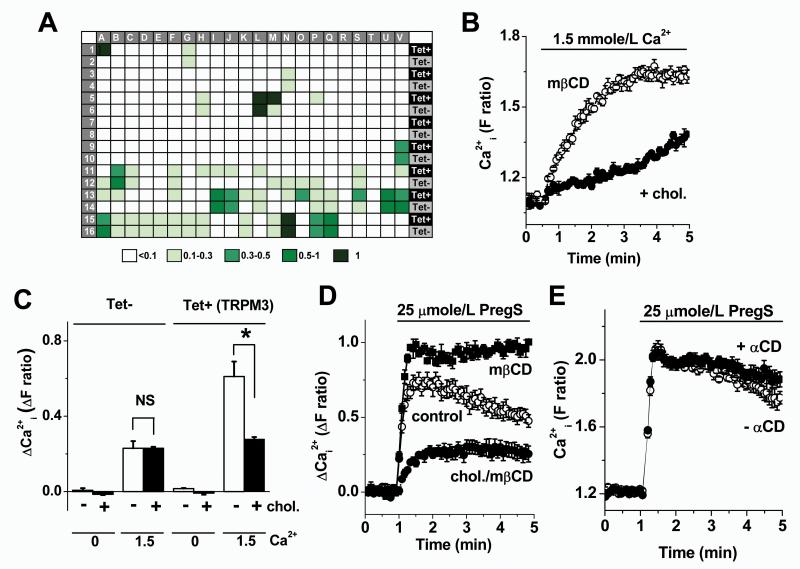
176 compounds were selected for screening against HEK 293 cells with (Tet+) and without (Tet−) tetracycline-induced expression of human TRPM3. The majority of compounds were inactive as TRPM3 stimulators, despite inclusion of a large range of endogenous biologically-active substances (Fig 6A, Supplementary File I). The only confirmed positive hit was β-cyclodextrin (Fig 6A, Supplementary File I).
Figure 6.
Chemical screen and identification of cholesterol as a TRPM3 inhibitor. Shown are data from intracellular Ca2+ responses in HEK 293 cells without (Tet− in A and C) or with induction of TRPM3 expression (Tet+ in A, and all data in B, D and E). (A) Summary of the outcomes of the chemical screen. Response amplitudes were graded according to the indicated colour scale. Squares A1-2 were for PregS and S13-14 for β-cyclodextrin (βCD). For full decoding of the plate see Supplementary File I. (B) Effect of returning 1.5 mmole/L Ca2+ to the extracellular solution following pre-incubation with and without 0.5 mmole/L cholesterol in the presence of 2.5 mmole/L mβCD (N = 4 for each condition). (C) As for (B) but mean data with and without addition of 1.5 mmole/L Ca2+, with and without addition of cholesterol to the mβCD, and with (Tet+) and without (Tet−) induction of TRPM3 expression (n/N = 3/12 for each). (D) Example comparison of responses to 25 μmole/L PregS with (+mβCD) or without pre-incubation with 2.78 mmole/L mβCD (control) or with preincubation with 1 mmole/L cholesterol (chol.) delivered on 5 mmole/L mβCD as the carrier. (E) Example responses to 25 μmole/L PregS with (+αCD) and without pre-incubation with 2.78 mmole/L α-cyclodextrin (αCD). (D, E) Each is representative of 3 independent experiments (N = 6 for each condition).
Suppression of constitutive TRPM3 by membrane cholesterol
The β-cyclodextrins are not endogenous substances but exogenous carriers or chelators of cholesterol25, the precursor of pregnenolone. It was striking that β-cyclodextrin stimulated TRPM3 in the absence of another exogenous activator, suggesting that constitutive activity of TRPM3 is normally partially suppressed by endogenous cholesterol of the membrane. In subsequent experiments methyl β-cyclodextrin (mβCD) was used because it has been most commonly used for extraction of endogenous cholesterol in the membrane. Cells were pretreated with mβCD alone or cholesterol delivered with mβCD as a carrier25; cholesterol and mβCD were washed out prior to measuring Ca2+ signals. To observe constitutive channel activity we measured Ca2+ entry in response to add-back of Ca2+ to Ca2+-free extracellular medium. The signal was inhibited by cholesterol in TRPM3-expressing HEK 293 cells (Fig 6B, C). Without TRPM3 expression there was significantly less Ca2+-entry and cholesterol had no effect (Fig 6C). The data support the hypothesis that TRPM3 has constitutive activity that is vulnerable to suppression by cholesterol.
Intermediate set-point and further suppression by cholesterol loading
We hypothesized that the channels exist at an intermediate point of modulation, such that elevated cholesterol, a well-established driver of vascular disease26,27, can act as a negative regulator of TRPM3. The hypothesis was investigated using pregnenolone sulphate as the TRPM3 stimulator to order to maximize clarity of the TRPM3 signal relative to background signals. Pretreatment with mβCD alone potentiated the effect of pregnenolone sulphate on TRPM3-expressing cells (Fig 6D) while having no effect on control cells (Supplementary Fig XI). Exogenous cholesterol suppressed the pregnenolone sulphate response (Fig 6D). Importantly, the control Ca2+ signal (no mβCD or exogenous cholesterol) was intermediate in amplitude, between the mβCD (cholesterol extraction) and mβCD plus exogenous cholesterol (cholesterol loading) signals (Fig 6D). α-Cyclodextrin, which has a binding cleft too small to accept cholesterol, was without effect (Fig 6E). The data suggest that TRPM3 channels normally exist at an intermediate point in relation to inhibition by cholesterol, with excess cholesterol driving further suppression of channel activity.
Relationship to cholesterol in VSMCs
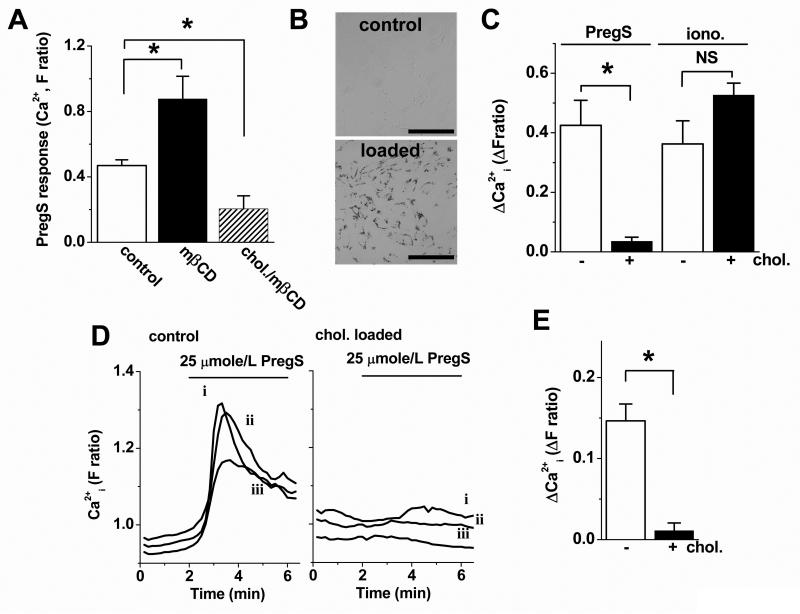
In VSMCs pregnenolone sulphate responses were enhanced by cholesterol depletion and suppressed below control amplitude by acute cholesterol loading (Fig 7A). Therefore, the endogenous channels are also at an intermediate set-point and vulnerable to suppression by excess cholesterol. To further investigate the hypothesis we mimicked the pathological cholesterol overload condition by loading VSMCs with cholesterol over 48 hr to generate foam cells (Fig 7B, Supplementary Figure XII). Pregnenolone sulphate responses were much-reduced in foam VSMCs (Fig 7C). Because the cell density was lower for these cells, we also used microscopy to observe responses; again the foam VSMCs had smaller responses to pregnenolone sulphate (Fig 7D, E). The data support the hypothesis that physiological cholesterol of the membrane partially suppresses TRPM3 activity and that excess cholesterol further suppresses the channel activity.
Figure 7.
Effects of cholesterol loading on responses in VSMCs. (A, C-E) Data from intracellular Ca2+ measurements in 96-well (A, C) or microscope recording systems (D, E). (A) Mean responses to 25 μmole/L PregS with (mβCD) or without (control) pre-incubation with 2.78 mmole/L mβCD or with pre-incubation with 1 mmole/L cholesterol (chol./mβCD) delivered on 5 mmole/L mβCD (n/N=3/18). (B) Bright-field images of Oil Red-O stained cells without and with cholesterol loading for 48 hr. Scale bar, 50 μm. (C) Mean responses to 25 μmole/L PregS or 1 μmole/L ionomycin in cells without (−) and with (+) prior cholesterol loading for 48 hr (n/N = 4/32 for PregS and 4/20 for ionomycin). (D) Example single cell effects of 25 μmole/L PregS without and with prior cholesterol loading for 48 hr (traces are shown for 3 cells: i, ii, iii). (E) Mean data for the type of experiment illustrated in (D) (n/N = 4/28 for control and 4/23 for +chol.). All data are from human saphenous vein VSMCs.
Discussion
Identification of functional TRPM3 in VSMCs adds to an expanding picture of TRP channels as complex and numerous players in VSMC function13,14. We observed TRPM3 mRNA and protein in contractile and proliferating VSMCs and showed functional activity and relevance to secretion and contraction. Much of the study focused on human cells, providing the first evidence for function of endogenous TRPM3 in this species. Through a chemical screen we found that TRPM3 is resistant to stimulation by many agents, including common vascular agonists, but we identified cholesterol as a previously unrecognized modulator. Constitutive cholesterol in the membrane conferred about 50 % inhibition of TRPM3 activity, allowing cholesterol loading to confer additional effect. Pregnenolone sulphate sensing by the channels may be useful for therapeutic cardiovascular modulation, but not in conditions of cholesterol loading.
The observed pregnenolone sulphate responses suggest a mechanistic foundation for considering pregnenolone sulphate as a naturally-occurring substance for use therapeutically to suppress unwanted vascular inflammation without the adverse effects of glucocorticoids. Effects on proliferating VSMCs occurred at concentrations that have been readily achieved in individuals after oral administration of pregnenolone, which is sulphated in vivo. Other TRPM3-mediated benefits of pregnenolone sulphate would be expected, including enhanced insulin secretion in hyperglycaemia9. Pregnenolone has been described as a fountain of youth, but rigorous clinical trials are needed to determine its true efficacy and safety. We observed contractile effects of pregnenolone sulphate in the aorta but at relatively high concentrations, such that risk of increased vascular tone may only be a potential concern at high doses. Recent studies have suggested that DHEA/DHEAS could be useful to counter unwanted vascular remodeling32,33 but our data do not suggest that such effects relate to vascular TRPM3 channels.
Pregnenolone sulphate was not observed to activate other TRP channels9 but it is not specific for TRPM3; there is also modulation of NMDA and GABA-A receptors, for example. NMDA and GABA-A receptors are not known features of VSMCs but we did make observations that were difficult to explain by TRPM3 alone. Divergence of the I-V shape from that of over-expressed TRPM3 could be accounted for by expression of multiple TRPM3 splice variants or heteromultimerisation with other TRP channels, and the transient nature of the pregnenolone sulphate response in VSMCs could be explained by an inactivation mechanism that is lacking in HEK 293 cells. However, observation of outward ionic current at negative voltages (Supplementary Fig V) could not be explained by a cation-selective channel such as TRPM3.
TRPM3 appears not to be receptor-activated because a large number of G protein-coupled receptor agonists were tested in our chemical screen and none activated TRPM3. Constitutive activity could, however, be critical in TRPM3 and other TRP channels8,28, enabling substances such as cholesterol to act in the absence of another agent. There are few reports of effects of cholesterol on TRP channels but an enhancing effect was observed on endogenous channels involving TRPC134, a TRP channel that facilitates neointimal hyperplasia in human saphenous vein20. Putatively, elevated cholesterol associated with disease and high-fat diets may suppress anti-secretory TRPM3 and enhance pro-proliferative TRPC1. We emphasize, nevertheless, that cholesterol has wide-ranging effects on membranes and the associated proteins and so effects on TRP channels should be viewed in this broader context25,26.
In summary, the study reveals functional relevance of TRPM3 and pregnenolone sulphate to secretion in proliferating VSMCs and contraction in contractile VSMCs. The pregnenolone precursor cholesterol has been identified as a previously unrecognized TRPM3 modulator, adding to an emerging picture of TRP channel modulation by this key factor and strengthening the case for functional relevance of constitutive TRP channel activity. The data suggest that pregnenolone sulphate should be explored further as a vascular modulator for use therapeutically, particularly in combination with cholesterol-lowering strategies.
Novelty and Significance.
What Is Known?
To-date 28 mammalian homologues of the fruit fly’s Transient Receptor Potential (TRP) ion channel have been described. The function of most of these channels is t to link various slow chemical and physical signals to intracellular calcium pathways
TRPM3 is one of the mammalian TRP channels. Its mRNA has been detected in blood vessels, but not much else is known about this channel
TRPM3 is activated by the so-called ‘fountain of youth’ neurosteroids, which decline in abundance with age and correlate with coronary artery disease
What New Information Does This Article Contribute?
This works shows that TRPM3 protein exists in smooth muscle cells of human and mouse blood vessels and its function is relevantto contraction and cytokine secretion
Neurosteroid concentrations required to evoke activity in smooth muscle cells are relevant to plasma concentrations achieved by dietary supplementation
TRPM3 has constitutive activity. Itis suppressed by cholesterol, the neurosteroid precursor and driver of atherosclerosis
In this study we found that TRPM3 protein is present in native contractile and proliferating human and mouse vascular smooth muscle cells. We provide the first evidence for constitutive activity of endogenous TRPM3 channels, suggesting the possibility of regulation by inhibitors without the need for an activator. Signals were, however, also evoked by known TRPM3 stimulators and were accounted for partly by TRPM3. TRPM3 channel was found to play a role in aortic contraction and the suppression of the secreted pro-inflammatory interleukin-6. However, concentration-response curves suggested pharmacological rather than physiological relevance of neurosteroid effects. From screening 176 compounds we found that cholesterol is, negative, modulator of TRPM3. The study shows that TRPM3 is a new ion channel type of the cardiovascular system, which protects against cytokine secretion. Our observations provide mechanistic insights that should encourage consideration of neurosteroids as therapeutic vascular modulators when combined with cholesterol-lowering strategies.
Supplementary Material
Acknowledgments
Sources of Funding The work was supported by Wellcome Trust and British Heart Foundation grants to DB. JN was a BBSRC Collaborative PhD Student with AstraZeneca, PS was an Overseas and University of Leeds Research Scholar, and YM was a University of Leeds Scholar.
Non-Standard Abbreviations and Acronyms
- TRP
transient receptor potential
- VSMC
vascular smooth muscle cell
- HEK
human embryonic kidney
- PregS
pregnenolone sulphate
- DHEAS
dehydroepiandrosterone sulphate
- IL-6
interleukin-6
- TM3E3
functional antibody targeted to the third extracellular loop of TRPM3
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flockerzi V. An introduction on TRP channels. Handb Exp Pharmacol. 2007;179:1–19. doi: 10.1007/978-3-540-34891-7_1. [DOI] [PubMed] [Google Scholar]
- 2.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, Huang M, Lin JH, Feder JN, Janovitz EB, Levesque PC, Blanar MA. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3) J Biol Chem. 2003;278:20890–20897. doi: 10.1074/jbc.M211232200. [DOI] [PubMed] [Google Scholar]
- 5.Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol Pharmacol. 2005;67:798–805. doi: 10.1124/mol.104.006734. [DOI] [PubMed] [Google Scholar]
- 6.Oberwinkler J, Lis A, Giehl KM, Flockerzi V, Philipp SE. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem. 2005;280:22540–22548. doi: 10.1074/jbc.M503092200. [DOI] [PubMed] [Google Scholar]
- 7.Oberwinkler J, Phillipp SE. Trpm3. Handb Exp Pharmacol. 2007;179:253–267. doi: 10.1007/978-3-540-34891-7_15. [DOI] [PubMed] [Google Scholar]
- 8.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- 9.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- 10.Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ. Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol. 2008;155:567–573. doi: 10.1038/bjp.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuniba H, Yoshiura KI, Kondoh T, Ohashi H, Kurosawa K, Tonoki H, Nagai T, Okamoto N, Kato M, Fukushima Y, Kaname T, Naritomi K, Matsumoto T, Moriuchi H, Kishino T, Kinoshita A, Miyake N, Matsumoto N, Niikawa N. Molecular karyotyping in 17 patients and mutation screening in 41 patients with Kabuki syndrome. J Hum Genet. 2009;54:304–309. doi: 10.1038/jhg.2009.30. [DOI] [PubMed] [Google Scholar]
- 13.Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2005;32:597–603. doi: 10.1111/j.1440-1681.2005.04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 15.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 16.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 17.Angelini GD, Jeremy JY. Towards the treatment of saphenous vein bypass graft failure--a perspective of the Bristol Heart Institute. Biorheology. 2002;39:491–499. [PubMed] [Google Scholar]
- 18.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaki CK. Cytokines and the early vein graft: strategies to enhance durability. J Vasc Surg. 2007;45:A92–98. doi: 10.1016/j.jvs.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beech DJ. Ion channel switching and activation in smooth-muscle cells of occlusive vascular diseases. Biochem Soc Trans. 2007;35:890–894. doi: 10.1042/BST0350890. [DOI] [PubMed] [Google Scholar]
- 22.Tagawa N, Tamanaka J, Fujinami A, Kobayashi Y, Takano T, Fukata S, Kuma K, Tada H, Amino N. Serum dehydroepiandrosterone, dehydroepiandrosterone sulfate, and pregnenolone sulfate concentrations in patients with hyperthyroidism and hypothyroidism. Clin Chem. 2000;46:523–528. [PubMed] [Google Scholar]
- 23.Suzuki E, Handa K, Toledo MS, Hakomori S. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci U S A. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur S, Constable PD, Eppley RM, Tumbleson ME, Smith GW, Tranquilli WJ, Morin DE, Haschek WM. Fumonisin B(1) increases serum sphinganine concentration but does not alter serum sphingosine concentration or induce cardiovascular changes in milk-fed calves. Toxicol Sci. 2001;60:379–384. doi: 10.1093/toxsci/60.2.379. [DOI] [PubMed] [Google Scholar]
- 25.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 27.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 28.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 30.Radaelli A, Loardi C, Cazzaniga M, Balestri G, DeCarlini C, Cerrito MG, Cusa EN, Guerra L, Garducci S, Santo D, Menicanti L, Paolini G, Azzellino A, Lavitrano ML, Mancia G, Ferrari AU. Inflammatory activation during coronary artery surgery and its dose-dependent modulation by statin/ACE-inhibitor combination. Arterioscler Thromb Vasc Biol. 2007;27:2750–2755. doi: 10.1161/ATVBAHA.107.149039. [DOI] [PubMed] [Google Scholar]
- 31.Mayr M, Zampetaki A, Sidibe A, Mayr U, Yin X, De Souza AI, Chung YL, Madhu B, Quax PH, Hu Y, Griffiths JR, Xu Q. Proteomic and metabolomic analysis of smooth muscle cells derived from the arterial media and adventitial progenitors of apolipoprotein E-deficient mice. Circ Res. 2008;102:1046–1056. doi: 10.1161/CIRCRESAHA.108.174623. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β/NFAT axis. Circ. 2009;120:1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 33.Ii M, Hoshiga M, Negoro N, Fukui R, Nakakoji T, Kohbayashi E, Shibata N, Furutama D, Ishihara T, Hanafusa T, Losordo DW, Ohsawa N. Adrenal androgen dehydroepiandrosterone sulfate inhibits vascular remodeling following arterial injury. Atherosclerosis. 2009;206:77–85. doi: 10.1016/j.atherosclerosis.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.