Abstract
Retinoic acid receptor alpha (RARα)-deficient mice are sterile, with abnormalities in the progression of spermatogenesis and spermiogenesis. In the present study, we investigated whether defective retinoid signaling involved at least in part, disrupted cell-cell interactions. Hypertonic fixation approaches revealed defects in the integrity of the Sertoli-cell barrier in the tubules of RARα-deficient testes. Dye transfer experiments further revealed that coupling between cells from the basal to adluminal compartments was aberrant. There were also differences in the expression of several known retinoic acid (RA)-responsive genes encoding structural components of tight junctions and gap junctions. Immunostaining demonstrated a delay in the incorporation of zonula occludens (ZO-1), a peripheral component protein of tight junctions, into the Sertoli cell tight junctions. Markedly reduced expression of connexin-40 in mutant pachytene spermatocytes and round spermatids was found by in situ hybridization. An ectopic distribution of vimentin and disrupted cyclic expression of vimentin, which is usually tightly regulated during spermiogenesis, was found in RARα-deficient testes at all ages examined. Thus, the specific defects in spermiogenesis in RARα-deficient testes may correlate with a disrupted cyclic expression of RA-responsive structural components, including vimentin, a down-regulation of connexin-40 in spermatogenic cells, and delayed assembly of ZO-1 into Sertoli cell tight junctions. Interestingly, bioinformatic analysis revealed that many genes that are components of tight junctions and gap junctions contained potential retinoic acid response element binding sites.
Keywords: cell-cell interactions, retinoid signaling, spermatogenesis
INTRODUCTION
Spermatogenesis is a highly regulated and complex process of differentiation that is controlled in part by Sertoli cells that physically interact with germ cells (Byers, et al., 1993; Cheng and Mruk, 2002). This intercellular communication between and among somatic cells and germ cells involves both gap junctions (Byers, et al., 1993; Cheng and Mruk, 2002) and tight junctions (Pelletier and Byers, 1992; Russell, et al., 1990). Tight junctions help form a micro-environment suitable for germ cell development by creating an immunological barrier for the germ cells in the adluminal compartment, allowing the diffusion of small ions but restricting the diffusion of large molecules such as immunoglobulins and lymphocytes. They also disassemble and reassemble to allow the passage of germ cells into the adluminal compartment for further differentiation into haploid spermatids. The inter-Sertoli cell tight junctions appear before the formation of the lumen of the tubule, which in the mouse, develops at 2 weeks postnatally (Flickinger, 1967). From 16 days to adult, tight junctions develop around the entire circumference of the tubules and the blood-testis barrier is established (Nagano and Suzuki, 1976; Vitale, et al., 1973).
It has been recognized for decades that signaling through vitamin A metabolites is indispensable for normal spermatogenesis (Howell, et al., 1963; Wolbach and Howe, 1925; Chung and Wolgemuth, 2004; de Rooij, et al., 1994; Eskild and Hansson, 1994; Griswold, et al., 1989). In particular, vitamin A deficiency (VAD) has been reported to cause spermatid degeneration, along with a disruption of Sertoli cell-spermatid association, a delay in spermiation, and a disruption of Sertoli cell tight junctions. The effects of all trans-retinoic acid (ATRA) are mediated through the retinoic acid receptors (RARs) and retinoid X receptors (RXRs), each with three subtypes, α, β, and γ (Chambon, 1996; Mangelsdorf and Evans, 1995; Piedrafita and Pfahl, 1999). These receptors function as ligand-dependent transcription factors that modulate expression of target genes (Chambon, 1996; Piedrafita and Pfahl, 1999). Targeted mutagenesis of several genes involved in retinoid synthesis, transport, nuclear and cytoplasmic binding, and degradation revealed only disruption of retinoic acid receptor alpha (RARα) function resulted in male sterility and aberrant spermatogenesis that resembled VAD (Lufkin, et al., 1993).
To understand the molecular and mechanistic basis for the aberrant spermatogenesis in RARα-deficient mice, a detailed analysis of the morphological properties and the time of onset of the abnormalities has been undertaken (Chung, et al., 2004; Chung, et al., 2005; Chung and Wolgemuth, 2004; Wolgemuth and Chung, 2007). These observations revealed prominent abnormalities in spermiogenesis (Chung, et al., 2004; Chung, et al., 2005), particularly in spermiation, that were similar to those in VAD testes. These studies further revealed the critical role of RARα in the establishment of normal progression of spermatogenesis and the subsequent formation of correct cellular associations. Using genetically manipulated animal models, restoring RARα expression in haploid spermatids of otherwise RARα-deficient mice rescued spermatogenic differentiation and fertility was partially restored (Chung, et al., 2009). This study provided new insight into the distinct requirement of RARα-mediated retinoid signaling specifically in germ cells.
The question of whether defective retinoid signaling could affect cell-cell interactions is of great interest. Leakage in the inter-Sertoli cell barrier has been observed by several investigators in VAD rat testes (Huang, et al., 1988; Ismail and Morales, 1992; Morales and Cavicchia, 2002). As the microenvironment in the adluminal compartment of the tubules is crucial for spermatogenic cell maturation, it would be of interest to examine whether tight junctions of RARα-deficient testes are affected. Similarly, gap junction permeability has been shown to be modulated by retinoid signaling in other cells (Ara, et al., 2002; Brummer, et al., 1991; Mehta, et al., 1986; Pitts, et al., 1986; Vine and Bertram, 2005; Vine, et al., 2005; Walder and Lutzelschwab, 1984; Weiler, et al., 1999), but the nature of the gap junctions in either VAD testis or in RARα-deficient testis remains unknown. As such, we have also attempted to ask whether the genes involved in the formation and function of tight junctions and gap junctions are direct targets of retiniod signaling via RAR’s (and RARα in particular) using bioinformatic analysis.
MATERIALS AND METHODS
Source of Animals and Tissues
All procedures were performed in accordance with guidelines of the Institutional Animal Care and Use Committee of the Columbia University Medical Center. Testes of RARα-deficient (Lufkin, et al., 1993) and control mice were dissected from animals that had been perfused with phosphate buffered saline (PBS) and then with 4 % paraformaldehyde (PFA) in PBS and fixed in 4% PFA overnight at 4° C. Testes were either embedded in paraffin or frozen in liquid nitrogen as described previously (Chung, et al., 2004). For frozen sections, testes were frozen in liquid nitrogen and sections were cut in a cryostat, mounted on coverslips and air-dried. They were post-fixed with cold acetone for 3 minutes at room temperature.
Hypertonic Fluid Test
The integrity of the Sertoli cell barrier of RARα-deficient testes was assessed by a modified hypertonic fluid test using hypertonic fixatives during perfusion-fixation procedures (Russell, et al., 1990; Weber, et al., 1988). In brief, the animals were perfused with 1x PBS and then with either 4% PFA or Bouin’s fixatives. The testicular morphology of adult testes from control mice treated with either 4% PFA or Bouin’s fixatives was examined and Bouin’s fixative, which gave better cellular morphology, was used in subsequent experiments. To titrate the hypertonic conditions in adult mice, 10, 15, or 20 % dextrose in 1x PBS was used and the duration for the hypertonic solution to permeate the tissue in order to give desired results in wild-type testes were assessed ranging from 20 minutes to 3 hours. The testes were then fixed overnight in Bouin’s fixative and dextrose in the corresponding concentrations. All subsequent procedures were identical to those described above.
In Situ Dye Coupling Assay of Testicular Tissues
The dye coupling assays were performed to visualize cell-cell coupling/communication in seminiferous tubules by the transfer of dye from one cell to another via gap junctions based on the cut end-loading method as described previously (Batias, et al., 2000; Decrouy, et al., 2004; el-Fouly, et al., 1987). In brief, freshly dissected, whole testes of 8 to 9-week-old control and RARα-deficient mice were cut in half transversely with a razor blade and placed in PBS solution containing 0.5% (w/v) Lucifer yellow (MW 475.2 kDa, Sigma, St. Louis, MO) and 0.5% (w/v) of Rhodamine-dextran (10,000 kDa, Sigma) at 32 °C for 10 minutes. Alternatively, whole testes were placed in dye solution and were poked with a 27 gauge 1/4″ needle (BD, Becton Dickinson, Franklin Lakes, NJ) for 2 minutes and then incubated with the dye at 32°C for an additional 10 minutes. After the incubation period, the tissue was rinsed five times in PBS and fixed in 4% PFA at 4°C. The fixed tissue was embedded in paraffin and the sections (5μm) were cut in a plane perpendicular to that of the razor blade cutting. After hydration of the slides, sections were incubated in 100mM DAPI for 10 minutes and were mounted with DAKO glycerol mounting media (DAKO®, Hamburg, Germany). They were viewed on a Nikon Eclipse 800 photomicroscope under fluorescent light to determine the cellular distribution of the fluorescent dyes and to identify dye-coupled cells. Photomicrographs were taken by using digital SPOT camera (Diagnostic Instruments Inc, Sterling Heights, MI). About 100 sections from five different testes were examined. Sections in which cells injured by cutting were loaded with both Lucifer yellow and dextran–rhodamine were selected. As for controls, about 100 seminiferous tubule sections in which at least one cell was labeled with RD and LY were examined.
Immunohistochemistry
Perfused, fixed tissues were embedded in paraffin, sectioned at 5 μm, mounted on Superfrost slides (Fisher) and immunostained using a Vectastain ABC kit (Vector Lacboratories, Burlingame, CA) as previously described (Chung, et al., 2004; Liu, et al., 1998). Mouse monoclonal IgM antibodies to vimentin (Sigma-Aldrich, Inc) were diluted 1:100 in 1x PBS. For controls, the slides were incubated with normal corresponding IgG or preimmune serum instead of primary antibody. For comparison of the relative levels of expression between samples, great caution was made to minimize the variation in fixation, thickness of sections, specific activity of the antibodies, etc. In addition, tissues from different experimental groups (for example, mutant versus normal; different ages, etc.) were sectioned onto the same slides to enable relative quantitative comparisons with greater confidence (Mutter, et al., 1988). The sections were viewed on a Nikon photomicroscope under bright-field optics and the cell types were identified according to Russell et al. (Russell, et al., 1990).
Immunofluorescent Detection
Slides were processed following the procedures described above and then incubated with primary antibody overnight at 4 °C in a humidified chamber using anti-ZO-1 (1:300) (Zymed; frozen sections gave better background for anti-ZO-1 antibody). After incubation, slides were washed for 30 minutes in PBST and were incubated with an anti-rabbit IgG-Alexa Fluor 594 secondary antibody (Molecular probe) for 30 minutes. Excess antiserum was removed; the slides were washed for 20 minutes in PBST, and then were counterstained with DAPI for 10 minutes and mounted using DAKO Glycergel mounting medium. They were viewed on a Nikon photomicroscope under fluorecent-field optics with excitation/emission at maximal wavelength of 590/617 nm, respectively.
Primer design for RT-PCR, real-time PCR and in situ hybridization analyses
Connexins belong to a family of gap junction proteins and contain areas of high homology. Therefore, protein sequences of the connexin genes were aligned and primers were chosen in non-homologous regions that were specific to the connexin gene of interest. The protein sequences were obtained from NCBI and the sequences were aligned using the sequence analysis program, DNAstar Megalign (DNA, Inc., Madison, WI). The alignment of the selected connexins was shown in supplementary figure S1. The specificity of the primers was confirmed by direct nucleotide sequencing of the PCR products. The primers were designed with Primer3 program (http://frodo.wi.mit.edu/cgibinprimer3/primer3_www.cgi) and synthesized by Sigma-Genosys (supplementary Table S1). The primers were designed to yield amplicons ranging from 300 to 600bp in length. Real time PCR requires amplicon sizes between 50–150bp for optimal assays, the antisense primers were redesigned for each gene to yield amplicons to be within the range of 50–150bp in length (supplementary Table S1).
RNA extraction, RT-PCR and real-time PCR analyses
RNA was extracted using TRIzol® Reagent (Invitrogen, Carlsbad, CA) from testes of 8 to 9-week-old Rara+/+ and Rara−/− mice. Reverse transcription (RT)-PCR was performed by using the ONE-Step RT-PCR kit from Invitrogen using total testicular RNA as described previously (Chung, et al., 1998a; Shang, et al., 2007). Real-time RT-PCR was performed as described previously on Smart Cycler II (Cepheid, Sunnyvale, CA) with OmniMix HS lyophilized PCR master mix kit and SYBR green following the manufacturer’s protocol (Shang, et al., 2007). The list of optimal annealing temperatures and reading temperatures for each connexin gene to avoid multiple bands was shown in Supplementary table S2. After the assay was conducted, the PCR products were loaded and run on a 1.2% agarose gel to confirm the presence of single, specific bands. The fold induction differences between the Rara+/+ and Rara−/− connexin expression levels were calculated using the equation: Fold induction = 2^-[ΔΔCt], as previously described (Shang, et al., 2007; Winer, et al., 1999).
Preparation and labeling of riboprobes for in situ hybridization
In situ hybridization studies of expression of the cDNA in the testes were performed using digoxigenin-labeled riboprobes essentially as described previously (Batourina, et al., 2001; Chung, et al., 1998b; Mendelsohn, et al., 1999; Packer, et al., 2000). In brief, a fragment of cDNA coding for connexin-40 was amplified from 8-week-old Rara+/+ mice testes using RT-PCR using specific primer pairs as shown in supplementary Table S1. cDNA with the expected size (supplementary Table S1) were then gel purified and subcloned and transformed into DH5α™ competent cells using TOPO TA Cloning® kit (Invitrogen, Carlsbad, CA). Authenticity and orientation of the PCR-synthesized cDNA were determined by direct nucleotide sequencing using either T7 or SP6 primer. For antisense probes, the cDNA in pCRII-TOPO vector was linearized with BamHI, and antisense transcripts were generated with T7 polymerase. For preparation of sense probes, the same cDNA was linearized with EcoRV, and sense transcripts were generated with SP6 polymerase. Samples were resolved in a 1.2% agarose gel and extracted using the QIAquick® (Qiagen, Valencia, CA) gel extraction kit. The probes were labeled with non-radioactive Diogoxygenin (DIG) (Roche, Indianapolis, IN). To make DIG labeled in situ probes, 2.5μg of digested DNA plasmid templates were mixed with 10μl of 5x transcription buffer, 10μl of 0.1M DTT, 0.5μl of Rnasin (20units/μl), 5μl of 10x DIG RNA labeling mix, 3μl of SP6 or T7 RNA polymerase and filled with DEPC H20 for a total reaction volume of 50μl. The reaction was incubated for 2 hours at 42°C. After 1 hour, 1μl of RNase-free DNase I (Roche, Indianapolis, IN) was added and incubated for 15 minutes at 37°C. 50μl of TE was added, and the labeled RNA probe was precipitated with 3M NaOAc and cold absolute ethanol at −80°C overnight. The pellet obtained was then washed with 70% ethanol, dried and resuspended in 50μl of TE. The concentration was tested by DIG quantification teststrips (Roche, Indianapolis, IN) and probe concentration of 100–500 ng/ml was used fro in situ hybridization.
In situ Hybridization
In situ hybridization studies of expression of the cDNA in the testes were performed using digoxigenin-labeled riboprobes essentially as described previously (Batourina, et al., 2001; Chung, et al., 1998b; Mendelsohn, et al., 1999; Packer, et al., 2000). In brief, 5μm sections of control and RARα-deficient mice testes were deparaffinized in histoclear and re-hydrated. The sections were then washed in 0.85% saline, and then equilibrated in 1x PBS for 5 minutes each. The sections were post-fixed in 4% PFA-PBS then washed twice in 1x PBS to remove the PFA. The sections were treated with 10μg/ml proteinase K (Roche, Indianapolis, IN) for 7 minutes. The sections were acetylated with 3.3ml of 0.1M triethanolamine and 625μl of acetic anhydride in 250μl of DEPC-treated water for 10 minutes. The sections were washed in 0.85% saline, 1xPBS for 5 minutes and dipped in DEPC-treated water, and overlaid with hybridization buffer (50% formamide, 4x SSC, 5x Denhardt solution, 500μg/ml of denatured salmon sperm DNA, and 250 μg/ml of yeast RNA) for 2 hours in 55°C. The sections were then incubated overnight in 55°C with the digoxigenin-labeled riboprobe diluted in hybridization buffer. After hybridization, the sections were washed in 50% formamide in 1x SSC for 1 hour in 50°C and 0.5x SSC for 30 minutes in 37°C and then treated with 10mg/ml of RNase (Roche, Indianapolis, IN) in 3.5x SSC for 30 minutes at room temperature. Then the sections were washed twice in 3.5x SSC at room temperature and washed in 0.1x SSC for 1 hour at 65°C. After the washes, the sections were incubated with the blocking solution consisting of bovine serum and maleic acid buffer (DIG Wash and Block Buffer Set, Roche, Indianapolis, IN) for 1 hour at room temperature. Then the sections were overlaid with the Anti-diogoxigenin-AP antibody (Roche, Indianapolis, IN) diluted 1:300 in blocking solution overnight in 4°C. After the incubation period, the sections were washed in washing buffer (DIG Wash and Block Buffer Set, Roche, Indianapolis, IN). After the washes, the sections were covered in detection buffer (DIG Wash and Block Buffer Set, Roche, Indianapolis, IN) for at least 3 minutes. The signal was visualized using a substrate solution (10% Polyvinyl alcohol in detection buffer with nitroblue tetrazolium [NBT] chloride and 5-bromo-4-chloro-3-indolyl-phosphate toluidine [BCIP]) (Roche, Indianapolis, IN) in 4°C for 1–3 days in the dark. The slides were counterstained with 1% neutral red dye and dehydrated and mounted in DAKO Glycergel mounting media.
RARE Transcription Factor Binding Site Analysis
3,000 bp of the 5′ upstream sequences and all 5′UTR sequences, including intronic sequences within the 5′UTR region, of those connexin genes found to be expressed in testes and genes that are components of tight junctions were analyzed to see if they contained RARE sites. Since transcription factor binding sites can generally be found upstream of the promoter sites, we chose to analyze up to 3,000 bp of the 5′ upstream region and all 5′ UTR sequences upstream of the coding start site. The sequences of these genes were found using NCBI (www.ncbi.nlm.nih.gov/entrez/) and ensembl! mouse (www.ensembl.org). Ensembl!mouse provided fully annotated gene sequences including coding and non-coding regions. The non-coding upstream sequences provided by Ensembl! mouse were used to identify RARE sites. Some genes had multiple transcripts because they had variations in their annotation. NCBI provided mRNA sequences of the genes and were used as a reference.
The upstream non-coding sequences were scanned for RARE transcription factor binding sites using the program Genomatix MatInspector. MatInspector identifies RARE sites by filtering through sequences to locate binding sites for RAR and RXR heterodimers. MatInspector is a highly useful and powerful tool for preliminary studies to identify binding sites (Quandt et al., 1995; Cartharius et al. 2005). This program utilizes a library of nucleotide distribution matrices of consensus patterns for known and published transcription factor binding sites. In nucleotide distribution matrices, each nucleotide position is weighed and a core sequence is defined. Therefore, MatInspector utilizes the information of core sequences and the overall consensus sequence to scan sequences of unlimited length. MatInspector is highly effective due to its criteria of optimized matrix thresholds and grouping families of transcription factors into single matrices. Optimized matrix thresholds decrease the possibility of false negatives and false positives because MatInspector only allows three matches within 10,000 bp that contain an overall high core sequence and consensus sequence similarity to be considered as potential binding sites. Also, the use of family matrices decreases the redundancy of matches due to similar transcription factors containing similar binding sites.
RESULTS
Disruption of the Sertoli cell barrier detected by treatment with hypertonic fixatives in Rara−/− mutant testes
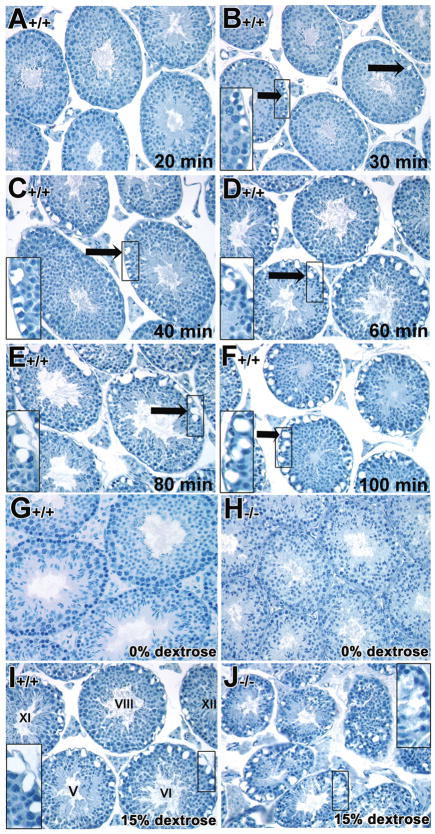
The integrity of the Sertoli cell barrier of mutant testes was assessed by a hypertonic fluid test using modified hypertonic fixatives during perfusion-fixation procedures (Russell, et al., 1990; Weber, et al., 1988). As noted in the Materials and Methods, 15% dextrose, 3 hour prior to fixation was selected for the experiments comparing control and mutant testes based on preliminary titration experiments on adult testes (data not shown). Since vacuolar-like spaces developed with age in the tubules of mutant mice (Chung, et al., 2004), we used younger mice (6–7 weeks of age) of both control and mutant testes in all subsequent studies. Wild type testes treated with 15% dextrose before fixation resulted in the predicted shrinkage of Sertoli cells and germ cells within the basal but not the adluminal compartment, with resulting exaggerated extracellular spaces, and concomitantly, there were no enlarged extracellular spaces surrounding germ cells in the adluminal compartment (arrows in Figs. 1B–F). The extracellular spaces in the basal compartment developed gradually as length of treatment with hypertonic solution increased from 30 minutes to 100 minutes (Fig. 1A–F), while the extracellular spaces surrounding germ cells in the adluminal compartment remained unchanged (Figs. 1B to F).
Fig. 1. Time frame of treatment with hypertonic fixatives in control testes and disruption of the Sertoli cell barrier detected by treatment with hypertonic fixatives in Rara−/− mutant testes.
A–F: Hypertonic fixatives were employed to test the integrity of the Sertoli cell barrier in 7 week-old control testes. 15% dextrose was used to treat the testes at different time point ranged from 20, 30, 40, 60, 80 and 100 minutes before fixation in Bouin’s. The extracellular spaces (arrows in B–F) in the basal compartment developed gradually as length of treatment with hypertonic solution increased. Inserts showed high magnification of the base of the tubules (B–F). G–J: Representative histological sections of control (G, I) and mutant (H, J) testes treated with isotonic fixative (G, H, respectively) and with 15% dextrose hypertonic buffer and fixative (I and J, respectively), each for 60 minutes. A–J, x40. Roman numerals indicate the stage of the tubules (Russell, et al., 1990). These findings are representative data from 3 independent experiments.
The 60-minute time point was selected as the reference time point for subsequent studies comparing control and mutant tubules. Testes of both control and mutant mice perfused with isotonic buffer and fixative exhibited intact cellular architecture within the tubules and intercellular spaces were not exaggerated (Fig. 1G and H, respectively). With hypertonic buffer and fixatives, testes from both control and mutant mice exhibited extensive exaggerated intercellular spaces within the basal compartment of all seminiferous tubules at all stages of the spermatogenic cycle (Fig. 1I and J, respectively). However, the shrinkage of adluminal germ cells concomitant with the appearance of exaggerated extracellular spaces in the adluminal compartment of the tubules was observed only in the mutant testes (Fig. 1J; 90% of 100 tubules, n=3) and not in the adluminal compartment of control testes (Fig. 1I; n=3). This observation was similar to a previously study using cytochalasin D treatment (Russell, et al., 1990; Weber, et al., 1988).
Coupling between cells from the basal to adluminal compartment is disrupted in Rara−/− mutant testicular tubules
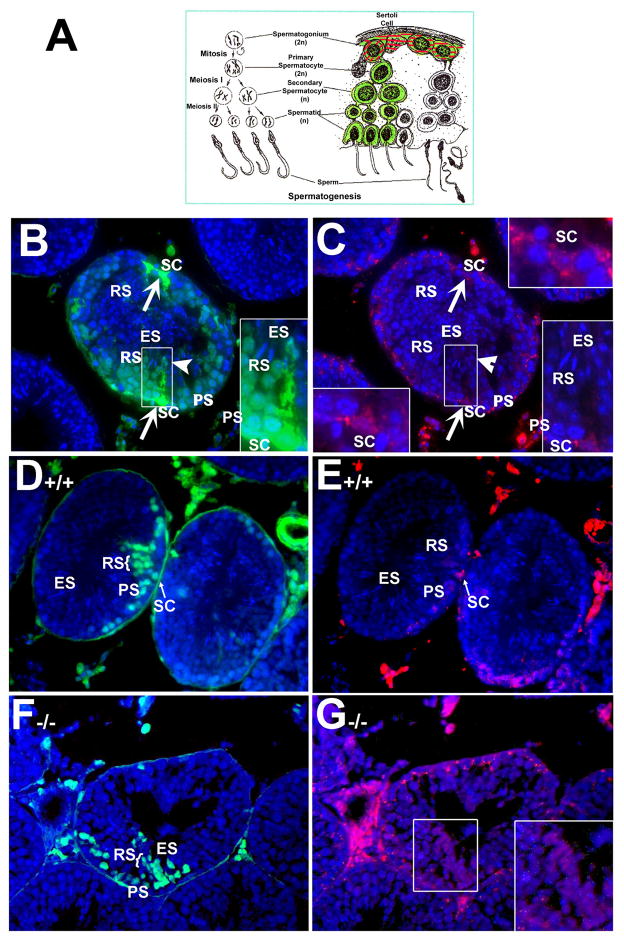
To better understand the function of gap junctions in the seminiferous tubules, we used a previously described dye-coupling assay together with morphological analyses (Batias, et al., 2000; Decrouy, et al., 2004; el-Fouly, et al., 1987). A schematic representation of this assay of gap junction intercellular communication in the seminiferous epithelium is shown in Fig. 2A. Gap junction-mediated coupling occurs when Lucifer yellow (LY, green fluorescence) diffuses into the cut surfaces of cells in the basal compartment and then is transferred between cells inward towards the spermatocytes and the round spermatids located in the adluminal compartment. Due to its low molecular weight, LY is transferable between cells since gap junctions allow the diffusion of molecules less than 1,000 Daltons. Rhodamine-dextran (RD), due to its high molecular weight, is used as a negative control because this dye molecule is too large to pass through gap junctions.
Fig. 2. Fluorescent micrographs of Lucifer yellow dye coupling in normal mouse testicular tubules and coupling between cells from the basal to adluminal compartment was disrupted in Rara−/− mutant testicular tubules.
A: Schematic representation of gap junction intercellular communication in the seminiferous epithelium. Rhodamine-dextran (RD, red) can diffuse into the cut surface of cells but is too large to be transmitted across cells or pass through gap junctions. Lucifer yellow (LY, green), however, can diffuse between Sertoli cells and spermatogonia, spermatocytes, round spermatids and elongated spermatids via cell coupling. B: Cellular-coupling via gap junctions. Gap junction coupling between Sertoli cells, identified by diffusion of LY, was observed in the 4-month-old wild-type seminiferous epithelium. LY diffused btween Sertoli cells (arrows pointed to the cells in the basal lamina) and spermatogonia at the basal lamina, spermatocytes, round spermatids and elongated spermatids (arrowhead). This is the merged photograph with the nuclear staining (DAPI, blue) showing clearly the cell types and position within the tubule. C: RD can only be found in Sertoli cells in the basal lamina but is too large to be transmitted across cells. Inserts in C (upper right and bottom left corners) showed the clearly triangular-shaped nucleus (DAPI, blue) of Sertoli cells situated at the basal compartment. Inserts with high magnification in B and C (bottom right corners) illustrated the coupling between the different cell types identified by diffusion of LY (B) but not by RD (C). PS, pachytene spermatocytes; RS, round spermatids; ES, elongated spermatids; SC, Sertoli cells. B–C, x40. D: LY coupling in control testes occurs from the basal to the adluminal compartment in an inward direction. E: RD is only found in the basal compartment and cannot be transferred through the gap junctions due to its high molecular weight. F: LY is found among adluminal germ cells (RS, ES, PS) but not in Sertoli cells in the mutant testes. G: RD is found in both the adluminal and basal compartments in the mutant testes. B, D, F: DAPI merged with LY; C, E, G: DAPI merged with RD; A–D x40. RS, round spermatid; PS, pachytene spermatocyte; ES, elongated spermatid; SC, Sertoli cell.
Sertoli cells loaded with both LY (green fluorescence) and RD (red fluorescence) were easily observed in the seminiferous tubules (with arrows pointed, Fig. 2A, B, respectively). Gap junction coupling between Sertoli cells, identified by diffusion of LY (green) between cells, was observed in the 4-month-old wild-type seminiferous epithelium (Fig. 2B). LY was diffused between Sertoli cells (arrows pointed to the cells in the basal lamina) and spermatogonia at the basal lamina, spermatocytes, round spermatids and elongated spermatids (arrowhead) via cell coupling. Although gap junctions have been found to be localized on germ cells in both the basal and adluminal compartments in the seminiferous tubules by freeze-fracture (Lee, et al., 2007; McGinley, et al., 1977; McGinley, et al., 1979) and Pelletier (Pelletier, 1988) in 1988 reported the presence of gap junctions between mink Sertoli cells and spermatogonia, spermatocytes and round spermatids, elongated spermatid-Sertoli gap junctions were rarely observed as spermatids begin the elongation phase of development (McGinley, et al., 1979). While whether our observed coupling to elongated spermatids is due to gap junction or intercellular bridges remains unknown; however, we clearly observed the LY in elongated spermatids (Fig. 2B), in parallel with the picture of the head of spermatids stained with DAPI (Fig. 2C, bottom right corners). LY showed similar signal intensity in the adjacent connected cells, indicating that dye coupling may occur via the intercellular bridges between cells since intercellular bridges are present in all different types of spermatogenic cells (Ren and Russell, 1991; Weber and Russell, 1987). In contrast, RD (red) can only be found in Sertoli cells in the basal lamina but is too large to be transmitted across cells (Fig. 2C). Counter-staining with DAPI clearly showed the nuclei of the cells and their position within the tubule (Fig. 2B, C) and facilitated their identification according the Russell et al (Russell, et al., 1990).
The dye-coupling assay was then performed in testes of young control and mutant mice at 8.5 weeks of age. In the control testes. LY was observed in Sertoli cells (SC, arrows pointed, Fig. 2D) and in more adluminal cells, including spermatocytes (PS in Fig. 2D) and round spermatids (RS in Fig. 2D). Coupling is also observed laterally via intercellular bridges between adjacent pachytene spermatocytes (PS in Fig. 2D). In contrast, RD (red) can diffuse into the cut surface of cells in the basal compartment but is too large to be transmitted across cells or pass through gap junctions (Fig. 2E).
In the mutant testes, there appeared to be LY fluorescence in pachytene spermatocytes into the adluminal compartment, suggesting possible inward coupling (Fig. 2F). However, there was no indication of true coupling occurring between cells in the basal and adluminal compartment. RD was not restricted to the cut borders of the basal compartment, but was also in the germ cells located in the adluminal compartment (insert with higher magnification in Fig. 2G). The presence of RD in both compartments may indicate improper function of the tight junctions formed by Sertoli cells in the basal compartment, essentially permitting RD to leak into the adluminal compartment via the disrupted tight junction barrier. Further, comparatively few germ cells may be present within the mutant tubules which allow the illustration of the Sertoli cell cytoplasm filling with RD (Chung, et al., 2004; Chung, et al., 2009). This would also apply to the entrance of LY into the adluminal compartment, and further suggests that true coupling was disrupted.
Delay in the assembly of the peripheral component protein, ZO-1, into the Sertoli cell barrier of the Rara−/− mutant mice
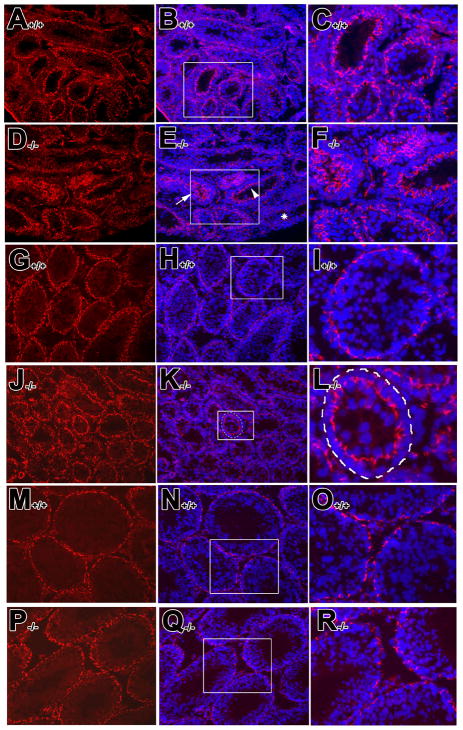
The expression of ZO-1, a tight junction peripheral adaptor protein, has been suggested as a biochemical marker to monitor the establishment of inter-Sertoli cell tight junctions both in vitro (Chung, et al., 1999; Li, et al., 2009; Wong, et al., 2000) and in vivo (Wong, et al., 2004). Of particular note in the present studies, Z0-1 has been reported to be an RA-responsive gene (Kubota, et al., 2001). In 2 week-old control testis, ZO-1 was found in the apical region of Sertoli cells (98.5±1.03%, n=3) (Fig. 3A–C), consistent with previous observation (Byers, et al., 1991; Mruk and Cheng, 2004; Moroi, et al., 1998). In the mutant testis (Fig. 3D–F), some tubules looked much like controls (45.67±1.15%, n=3) (arrowhead in Fig. 3E and insert in 3F), some exhibited a barely detectable signal (17.0±1.0%, n=3) (* in Fig. 3E), while in others, ZO-1 remained in the apical compartment and was not yet localized to the basal region of borders between adjacent Sertoli cells (37.33±2.08% (arrow in Fig. 3E and insert in 3F). In the 4 week-old control testis, ZO-1 was localized to the basal region of borders between adjacent Sertoli cells (Fig. 3G–I), while in the mutant testis, ZO-1 remained localized in the apical region of Sertoli cells (Fig. 3J–L, insert in Fig. 3K and L). At postnatal 8 weeks, ZO-1 was localized to the basal region of Sertoli cells in both control (Fig. 3M–O, see also Moroi, et al., 1998) and mutant testes (Fig. 3P–R). These results suggest that there is a delay in the appearance and localization of components of Sertoli cell tight junctions in the mutant testes.
Fig. 3. Delay in the assembly of the peripheral component protein, ZO-1, into the Sertoli cell barrier of the Rara−/− mutant mice.
Immununofluorescent staining of ZO-1 was detected in the 2, 4, 8 week-old testis from control (A–C, G–I, and M–O) and mutant mice (D–F, J–L and P–R), respectively. The expression of ZO-1 is shown in left panels while the corresponding nuclear staining are shown in middle panels as merged figure with ZO-1 distribution, respectively, using DAPI counterstaining. Right panels show the enlarged figures of the inserts in middle panels illustrating the localization of ZO-1 distribution and the corresponding nuclear staining. A–R, x40.
Reduced expression of connexin-40 in Rara−/− mutant testes as detected by in situ hybridization
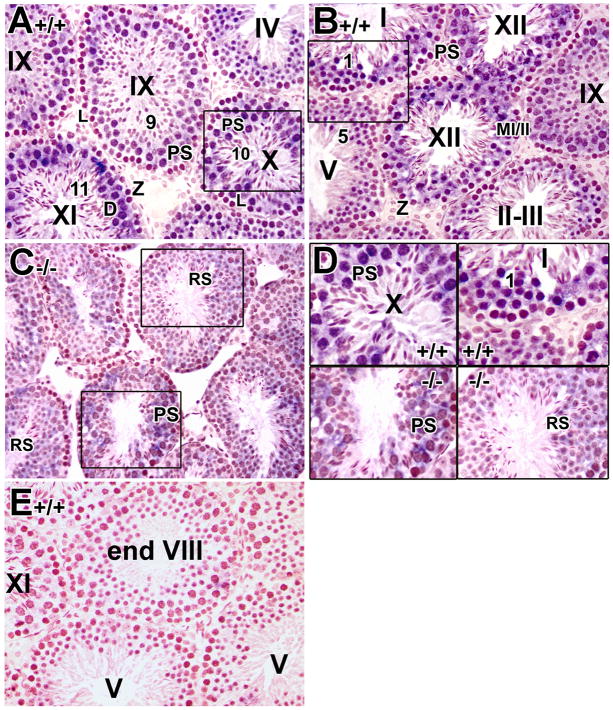
Several gap junction component genes have been shown to be RA-regulated, including connexin 43 and 26 (Ara, et al., 2002; Vine and Bertram, 2005; Vine, et al., 2005). In preliminary RT-PCR and real-time RT-PCR analysis of total testicular RNA from control and mutant testes for all the connexin genes expressed in the testis, connexin 40 appeared in both assays to exhibit a trend of reduced expression in the mutant animals (supplementary Figs. S1–2 and Tables S1–2). Interestingly, connexin-40 also has more than one RARE binding site in its promoter region according to the Genomatix MatInspector results (supplementary Table S3). In lieu of the availability of antibodies suitable for immunohistochemical detection in our hands, we performed in situ hybridization to examine more precisely the expression pattern of connexin-40 in the mutant testes. In control testes, the expression of connexin-40 was first observed in pachytene spermatocytes at stage IV and onward (Fig. 4A, B). Although colorimetric in situ hybridization detection methods are not quantitative, the signal clearly became more obvious in late pachytene spermatocytes at stage X (Fig. 4A and 4D, upper left panel). Connexin-40 expression appeared strongest in diplotene spermatocytes at stage XI (Fig. 4A), MI/II spermatocytes at stage XII (Fig. 4B) and step 1 spermatids at stage I (Fig. 4B and 4D, upper right panel). The expression then dropped drastically from step 2 to step 9 spermatids (Fig. 4A, B) and no detectable connexin-40 expression was found in step 10 spermatids and onwards in all tubules examined (Fig. 4A), consistent with the observation of connexin-40 in different cell types by RT-PCR (Risley, 2000). No detectable signal was observed in testis using the control sense probe (Fig. 4E). Interestingly, in the mutant testes, the expression of connexin-40 appeared to be significantly reduced in the late pachytene spermatocytes (lower middle tubule in Fig. 4C and 4D, bottom left panel) and slightly reduced in round spermatids (Fig. 4C and 4D, bottom right panel), suggesting the possible down-regulation of connexin-40. Although this technique is not particularly quantitative, the mutant and control testis sections were processed side by side on the same slide, so relative intensity differences are likely to be meaningful.
Fig. 4. Reduced expression of connexin-40 in Rara−/− mutant testes as detected by in situ hybridization.
Representative histological sections of 8-week-old control (A, B, E) and mutant testes (C) were subjected to in situ hybridization with a DIG-labeled probe for connexin-40. The expression of connexin-40 was detected as a blue color, while the nucleus was counterstained with neutral red. Upper panel D represents a higher magnification of the insert in panels A and B while the bottom panel D shows higher magnification of the inserts in panel C. No detectable signal observed in testis using sense probe (Fig. 4E) indicating the specificity of the probe. L, leptotene spermatocytes; Z, zygotene spermatocytes; PS, pachytene spermatocytes; D, diplotene spermatocytes; MI/II, meiosis I/II; RS, round spermatids. Roman numerals indicate the stage of the seminiferous tubules and arabic numerals indicate the step of spermatids (Russell, et al., 1990). A–E, x40
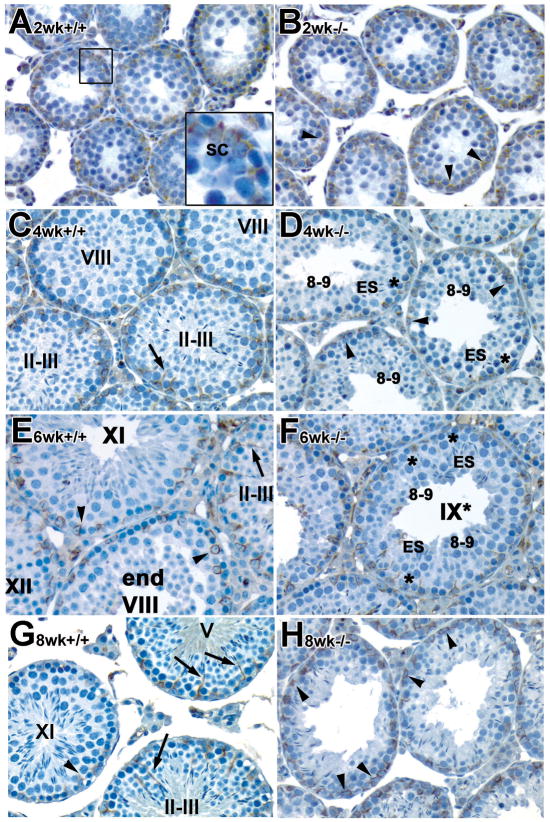
Ectopic distribution of vimentin in the developing and young adult Rara−/− mutant testes
Vimentin is an intermediate filament protein that is tightly associated with the localization of elongated spermatid heads, through a transient association with elongated spermatids. It is also known to be a RA-responsive intermediate filament (Benazzouz and Duprey, 1999). Since both failure of spermatid orientation and alignment to the tubular lumen were found in mutant testes (Chung, et al., 2004; Chung, et al., 2005), the distribution of vimentin was then examined. Distinct cyclic changes in the expression of vimentin in the cytoplasm of rat Sertoli cells that tightly associated with elongated spermatid heads during the seminiferous epithelium cycle have been reported (Amlani and Vogl, 1988; Vogl, 1989; Vogl, et al., 2000; Vogl, et al., 1991; Zhu, et al., 1997). In control testes, vimentin was mainly located at the basal region surrounding Sertoli cell nuclei at postnatal 2 weeks (insert in Fig. 5A), consistent with previous observations in rat testis (Amlani and Vogl, 1988; Vogl, 1989; Vogl, et al., 2000; Vogl, et al., 1991; Zhu, et al., 1997). Supranuclear or apical regions were stained at 4 weeks of age and staining intensity gradually increased during development, with highest expression at stage II–III (arrows in Fig. 5C, E and G). In the young adult, bundles of vimentin were present not only in the perinuclear region, but also ran between adjacent spermatocytes and round spermatids and in the main stalk apical to the Sertoli cell nuclei, extending to the elongated spermatids in tubules at stage I–VI (arrows in Fig. 5E and G). These apical extensions became shorter and were found only in the perinuclear region of Sertoli cells in tubules at stage VII to IX, just before and after spermiation (arrowheads in Fig. 5C and E) (Amlani and Vogl, 1988; Vogl, 1989; Vogl, et al., 2000; Vogl, et al., 1991; Zhu, et al., 1997). Short apical extensions reappeared at stage XI (arrowheads in Fig. 5E and G).
Fig. 5. Ectopic distribution of vimentin (intermediate filaments) in the developing and young adult Rara−/− mutant testes.
Histological sections of paraformaldehyde-fixed paraffin-embedded testes from control (A, C, E, G) and mutant males (B, D, F, H) were immunostained with anti-vimentin antibody. A–H, x40. Asterisk: although the asynchronous cell associations complicate staging, an attempt was made to stage the mutant tubules using the acrosomal system (Russell et al., 1990), and we refer to these approximately staged tubules with a roman numeral followed by an asterisk. ES, elongated spermatids; SC, Sertoli cells. Roman numerals indicate the stage of the seminiferous tubules and arabic numerals indicate the step of spermatids (Russell, et al., 1990).
Similar to controls, vimentin was detectable at the perinuclear region of Sertoli cells in 2 and 4 week old mutant testes (arrowheads in Fig. 5B, D, respectively). At 4 weeks, most of the cells were arrested at step 8–9 spermatids in mutant testes, although there were some elongated spermatids embedded among the Sertoli cells. However, vimentin was not tightly associated with the heads of these elongated spermatids (asterisks in Fig. 5D). In 6-week-old mutant testes, at stage IX*, after spermiation should have occurred, some elongated spermatids were retained (Fig. 5F). Interestingly, the bundles of vimentin were not tightly associated with the heads of these elongated spermatids at stage IX* (asterisks in Fig. 5F). At 8 weeks of age, sloughing of germ cell layers was observed and vimentin was found in the perinuclear region of Sertoli cells and was not closely associated with the heads of the remaining elongated spermatids (arrowheads in Fig. 5H). Thus, the stage-specific localization of intermediate filaments was completely disrupted in the mutant testis (Fig. 5D, F, H).
Tight and gap junction component genes potentially contain RARE sites
Several studies have suggested the involvement of retinoid signaling in the establishment of intercellular communication via both tight and gap junctions (reviewed in Chung and Wolgemuth, 2004). To begin to ask whether the genes involved in the formation and function of tight and gap junctions are direct targets of retinoid signaling via RARs (and RARα in particular), we initiated a database search for a prediction of putative RAREs in genes that are involved in junctional formation and function, using the Genomatix Software (MatInspector) as described in Quandt et al. (Quandt, et al., 1995) and Cartharius et al. (Cartharius, et al., 2005). The initial phase of this search, which focused on components of tight and gap junctions, is summarized in the data presented in supplementary Table S3. The program was directed to utilize the RXR matrix family which allows the location of heterodimers of RXR with several other nuclear receptors including RAR and RXR heterodimer binding sites, initially in selected connexin genes. That is, all the connexin genes that are known to be expressed in the testes were scanned, which included connexin-26, -31, -31.1, -32, -33, -36, -37, -40, -43, -45, -46, -50, and -57 (Risley, 2000); reviewed in (Byers, et al., 1993; Cheng and Mruk, 2002; Pointis, et al., 2005) for RARE sites. Interestingly, all these connexins except for connexin-36 were shown to contain potential RARE sites (supplementary Table S3). The analysis was then extended to tight junction genes, which were also shown to contain potential RARE sites, including the integral membrane proteins occludin, claudin-1, -4, -5, -7, -8, -11, as well as the associated proteins ZO-1, -3, symplekin, and Src (supplementary Table S3). Unexpectedly, vimentin contains no RARE binding site (supplementary Table S3).
DISCUSSION
In the present study, hypertonic fixation procedures and a dye-coupling assay revealed that the Sertoli barrier was disrupted and coupling between cells from the basal to adluminal compartments was aberrant in RARα-deficient testes, respectively. In mutant testes, “coupling” as indicated by the presence of LY was only evident either between spermatogonia in the basal compartment or germ cells in the adluminal compartment, but not between cells in the two compartments. In the adluminal compartment, only directed transfer from pachytene spermatocytes and onward into round spermatids was observed, along with coupling between pachytene spermatocytes, suggesting that the dyes entered cells in the adluminal compartment through defects in the Sertoli barrier. This was supported by the observation of the presence of RD in the adluminal compartment. RD has a relative molecular weight that is too high for it to pass through gap junctions let alone tight junctions. Therefore the apparent “coupling” between the pachytene spermatocytes observed in mutant testes may have been the result of random diffusion rather than true coupling.
Intercellular communication between the somatic cells and germ cells and between cells in the same lineage via gap junctions is critical for normal progression of spermatogenesis. Disruption of this communication could impair differentiation, including the abnormal cellular associations observed in the seminiferous epithelium of mutant testes. We were therefore interested to further examine whether the connexin genes known to be expressed in the testis and to be involved in the formation and function of gap junctions (reviewed in Byers, et al., 1993; Cheng and Mruk, 2002; Pointis, et al., 2005) were targets of retiniod signaling via RARs (and RARα in particular).
By in situ hybridization analysis, the expression of connexin-40 was high in control late pachytene spermatocytes but appeared to be reduced in the late pachytene spermatocytes of mutant testes, suggesting the connexin-40 mRNA levels may be regulated via an RARα-mediated signaling pathway. This regulation could be at the level of transcription as has been documented for many genes as either direct targets of RARα complexes or as indirect, downstream targets. Alternatively, RARα could regulate connexin-40 post-transcriptionally, similar to connexin-43, whose expression was regulated by increasing the stability of connexin-43 mRNA (Clairmont and Sies, 1997). In addition, it was unanticipated to not detect expression of connexin-40 in Sertoli cells in the control testes, as its expression had been reported in isolated Sertoli cells by RT-PCR (Risley, 2000). Since these isolated Sertoli cells from 20-day-old rat testes involved manual separation using enzyme digestion and gradient cell separation, the broken cellular junctions may have induced the expression of genes in cells that typically have very low or undetectable levels of expression. Further, the expression patterns for Sertoli cells and peritubular cells in premature rats in the previous study could differ from our current one since we used adult, mature mice. Interestingly, reduced expression of connexin-43 was reported in RXRβ-deficient mouse testis by in situ hybridization; however, there was no assessment of junctional integrity in these studies (Batias, et al., 2000).
With the exception of connexin-43, deletion of single connexin genes showed no alteration of spermatogenesis while targeted double deletions displayed impaired spermatogenesis, with defects similar to those seen in RARα-deficient testes (Brehm, et al., 2007; Pointis, et al., 2005; Sridharan, et al., 2007). Although there was no detailed examination of the seminiferous tubules of connexin-40 knockout mice, they were reported to be fertile (Kirchhoff, et al., 1998). However, targeted double connexin mutants defective in Cx37 and Cx40 displayed impaired testicular function with vacuolar degeneration in spermatogonia and necrosis of the testicular tissue (Simon and McWhorter, 2002). Furthermore, when connexin-40 was knocked into the connexin-43 locus, the mice were infertile, exhibiting a Sertoli-cell-only phenotype, with seminiferous tubules totally depleted of germ cells (Plum, et al., 2000). Our findings of altered Sertoli cell barriers and aberrant cellular coupling from the basal to the adluminal compartment and along with lowered expression of connexin-40 in pachytene spermatocytes and round spermatids in mutant mice, support the idea that connexin-40 may be an additional connexin that plays a critical role in intercellular communication in the testes.
We have previously reported that elongated spermatids cannot properly align at the surface of tubular lumen for spermiation in RARα-deficient testes (Chung, et al., 2004). Vimentin, a major intermediate filament protein in fibroblasts and endothelial cells, has been shown to play important roles in deformability, migration, and contractility (Wang and Stamenovic, 2000). Intermediate filaments of the vimentin type are abundant in the cytoplasm of Sertoli cells (Vogl, et al., 1993), and may be associated with actin filaments in the elongated spermatid (Amlani and Vogl, 1988). Ultrastructural studies demonstrated that intermediate filaments appeared to pass through holes in the cistern of the endoplasmic reticulum, penetrate into the ectoplasmic specialization, a testis-specific adherens junction, and associate with the microfilament bundles (Zhu, et al., 1997). The distinct cyclic expression of vimentin and its close association with elongated spermatid heads have suggested the possible role of intermediate filaments not only in anchoring the crypts and elongated spermatids, but also in controlling their migration during spermiogenesis and spermiation (Franke, et al., 1979; Zhu, et al., 1997).
The action of follicle-stimulating hormone (FSH) has been implicated in altering the distribution of intermediate filaments and induced changes from a flat to a stellate Sertoli cell morphology in culture, possibly via phosphorylation of vimentin (Spruill, et al., 1983). It is possible that a similar retinoid-mediated mechanism acts on the elaborate cytoskeletal network in Sertoli cells in vivo. In the present study, the disrupted cyclic expression of vimentin was observed in testis lacking RARα. Interestingly, it has been shown that FSH inhibited ATRA-induced RARα nuclear localization and transcriptional activation in mouse Sertoli cell lines (Braun, et al., 2000). A 1710-bp vimentin promoter was shown to be activated in F9 embryonal carcinoma cells that were induced to differentiate by ATRA and cAMP (Benazzouz and Duprey, 1999), suggesting that the vimentin gene is RA-responsive. However, since no RARE binding sites were found in the vimentin gene by Genomatix analysis, it could be that a putative action of FSH on vimentin expression is acting through a downstream target of RARα.
Several studies have suggested the involvement of retinoid receptor-mediated signaling for induction of both tight junction-associated molecules and barrier function. Stra6, an RA-inducible gene identified in P19 cells (Bouillet, et al., 1995; Bouillet, et al., 1997), appears to encode a novel integral membrane protein whose expression localized to blood-organ barriers in several tissues (Bouillet, et al., 1997). In the testis, Stra6 was exclusively expressed in the basal lamina membranes of Sertoli cells in a stage-dependent manner, particularly in stage VI–VII tubules. In RARα-deficient testes, Stra6 is expressed in almost all the tubules, suggesting that RARα is required for the selective expression of Stra6 at stage VI–VII (Bouillet, et al., 1997). Stra6 was recently found to be a specific membrane receptor of retinol binding protein, representing a major physiological mediator of cellular vitamin A uptake (Kawaguchi, et al., 2007). Further, mutations in the human STRA6 gene are associated with Matthew-Wood syndrome, manifested by pleiotropic, multisystem developmental malformations (Chassaing, et al., 2009; Pasutto, et al., 2007).
It has been reported that ATRA induced tight junction formation and expression of several tight junction-associated molecules, such as ZO-1, occludin, claudin-6, and claudin-7, as well as a barrier function in F9 cells mediated by specific RXR-RAR pairs (Kubota, et al., 2001). Another aspect of the disruption of correct cell-cell interactions due to loss of RARα activity or VAD may also be the induction of a premature detachment from the seminiferous tubules of pachytene spermatocytes and round spermatids. Retionid signaling, via RA and the RARs in particular, has been implicated in the maintenance of cell-cell adhesion and the deposition of extracellular proteins (Marinos, et al., 1995; Ricci, et al., 1999; Smith, et al., 1995; Vasios, et al., 1989; Wang, et al., 1992b). Our findings of abnormalities in the expression in RARα-deficient testes of several structural components, vimentin, connexin-40 and ZO-1, strongly support the involvement of RARα in mediating cell adhesion events. In addition, a delay in the assembly of the peripheral component protein, ZO-1, into the Sertoli cell barrier was observed in the mutant testes. This was concomitant with an immature appearance of some of the mutant testes at 4 weeks of age, with the most advanced spermatogenic cells only at mid- to late-pachytene spermatocytes (more similar to a 2-week-old normal testis).
Sertoli cell tight junctions are the major component of blood-testis barrier, which provides for the stable microenvironment required for normal spermatogenesis (Pelletier and Byers, 1992; Russell, et al., 1990). In VAD testis, leakage in the Sertoli cell tight junctions was also reported (Huang, et al., 1988). Disturbance in the Sertoli cell barrier may allow serum factors which are usually excluded by these junctions to enter the lumen of seminiferous tubules, resulting in an abnormal microenvironment (Huang, et al., 1988). Under these circumstances, spermatogenic cells cannot develop normally, thereby resulting in the loss of differentiating cells during the chronic stages of VAD or in the absence of RARα. Electron microscopic examination of the Sertoli cell barrier in both models will confirm these observations and allow the examination of this apparent breakdown in the Sertoli cell barrier.
In conclusion, we suggest that sterility in the RARα-deficient males is due at least in part to specific defects in spermiogenesis which may in turn correlate with disrupted cellular junctions. We observed alterations in the cyclic expression of vimentin, a down-regulation of connexin-40 in spermatogenic cells, and the delayed assembly of ZO-1 into Sertoli cell tight junctions. Interestingly, bioinformatic analysis revealed that many genes that are components of tight and gap junctions contained potential RARE binding sites. These genes are thus prime candidates for examining changes in their gene expression levels, by in situ hybridization, real-time PCR and immunohistochemical analysis.
Supplementary Material
Supplementary Fig. S1. Alignment of protein sequences of connexin genes of interest. Amino acid sequences of selected connexins were aligned to design primers to target specific connexin genes. Primers were selected in non-homologous areas which are indicated by the red circles.
Supplementary Fig. S2. Variation in connexin expression in Rara−/− mutant testes as detected by RT-PCR and real time PCR. (A) Differences in connexin expression levels between Rara+/+ and Rara−/− testes by RT-PCR. RT-PCR assays were conducted as a preliminary assay to detect the differences in connexin expression levels in Rara+/+ and Rara−/− testes. (B) Connexin 40 expression is downregulated in Rara−/− testes. Real-time reverse transcription (RT)-PCR was used to quantify changes in the levels of expression of selected connexin genes from control and Rara−/− testes. The expression levels are presented as relative levels of the wild-type expression and the wild-type expression levels are set as one. The results, corrected with β-actin expression, are means ± s.e.m. of at least three experiments in three pairs of animals. Connexin-26 was up-regulated about 0.4 fold and connexin-40 was down regulated about 0.4 fold as well.
Supplementary Table S1. The primers used for RT-PCR, real-time RT-PCR and in situ hybridization for connexin genes were listed.
Supplementary Table S2. List of optimal annealing temperatures and reading temperatures for each connexin gene to avoid multiple bands.
Supplementary Table S3. Junctional component genes containing putative RARE sites.
Acknowledgments
This work was supported in part by NIH grant, U01-HD060479 to D.J.W and S.S.W.C. and the CONRAD Foundation, CIG-05-105 and CIG-05-107 to D.J.W.
Footnotes
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/XXX/suppmat.
References
- Amlani S, Vogl AW. Changes in the distribution of microtubules and intermediate filaments in mammalian Sertoli cells during spermatogenesis. Anat Rec. 1988;220(2):143–60. doi: 10.1002/ar.1092200206. [DOI] [PubMed] [Google Scholar]
- Ara C, Massimi M, Devirgiliis Conti L. Retinoic acid modulates gap junctional intercellular communication in hepatocytes and hepatoma cells. Cell Mol Life Sci. 2002;59(10):1758–65. doi: 10.1007/PL00012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batias C, Siffroi JP, Fenichel P, Pointis G, Segretain D. Connexin43 gene expression and regulation in the rodent seminiferous epithelium. J Histochem Cytochem. 2000;48(6):793–805. doi: 10.1177/002215540004800608. [DOI] [PubMed] [Google Scholar]
- Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet. 2001;27(1):74–8. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Duprey P. The vimentin promoter as a tool to analyze the early events of retinoic acid-induced differentiation of cultured embryonal carcinoma cells. Differentiation. 1999;65(3):171–80. doi: 10.1046/j.1432-0436.1999.6530171.x. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier JM, Schuhbaur B, Dolle P, Chambon P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2) Dev Biol. 1995;170(2):420–33. doi: 10.1006/dbio.1995.1226. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63(2):173–86. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Braun KW, Tribley WA, Griswold MD, Kim KH. Follicle-stimulating hormone inhibits all-trans-retinoic acid-induced retinoic acid receptor alpha nuclear localization and transcriptional activation in mouse Sertoli cell lines. J Biol Chem. 2000;275(6):4145–51. doi: 10.1074/jbc.275.6.4145. [DOI] [PubMed] [Google Scholar]
- Brehm R, Zeiler M, Ruttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lecureuil C, Steger K, Konrad L, Biermann K, Failing K, Bergmann M. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171(1):19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer F, Zempel G, Buhle P, Stein JC, Hulser DF. Retinoic acid modulates gap junctional permeability: a comparative study of dye spreading and ionic coupling in cultured cells. Exp Cell Res. 1991;196(2):158–63. doi: 10.1016/0014-4827(91)90245-p. [DOI] [PubMed] [Google Scholar]
- Byers S, Graham R, Dai HN, Hoxter B. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am J Anat. 1991;191(1):35–47. doi: 10.1002/aja.1001910104. [DOI] [PubMed] [Google Scholar]
- Byers S, Pelletier RM, Suarez-Quian C. Sertoli-Sertoli cell junctions and the seminiferous epithelium barrier. In: Griswold MD, Russell LD, editors. The Sertoli Cell. Clearwater. FL: Cache River Press; 1993. pp. 431–446. [Google Scholar]
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–42. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10(9):940–54. [PubMed] [Google Scholar]
- Chassaing N, Golzio C, Odent S, Lequeux L, Vigouroux A, Martinovic-Bouriel J, Tiziano FD, Masini L, Piro F, Maragliano G, Delezoide AL, Attie-Bitach T, Manouvrier-Hanu S, Etchevers HC, Calvas P. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum Mutat. 2009;30(5):E673–81. doi: 10.1002/humu.21023. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82(4):825–74. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Chung SS, Lee WM, Cheng CY. Study on the formation of specialized inter-Sertoli cell junctions in vitro. J Cell Physiol. 1999;181(2):258–72. doi: 10.1002/(SICI)1097-4652(199911)181:2<258::AID-JCP8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chung SS, Mo MY, Silvestrini B, Lee WM, Cheng CY. Rat testicular N-cadherin: its complementary deoxyribonucleic acid cloning and regulation. Endocrinology. 1998a;139(4):1853–62. doi: 10.1210/endo.139.4.5958. [DOI] [PubMed] [Google Scholar]
- Chung SS, Sung W, Wang X, Wolgemuth DJ. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn. 2004;230(4):754–66. doi: 10.1002/dvdy.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Wang X, Wolgemuth DJ. Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalities in spermiogenesis. Differentiation. 2005;73(4):188–98. doi: 10.1111/j.1432-0436.2005.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Wang X, Wolgemuth DJ. Expression of retinoic acid receptor alpha in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development. 2009;136(12):2091–100. doi: 10.1242/dev.020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res. 2004;105(2–4):189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Zhu LJ, Mo MY, Silvestrini B, Lee WM, Cheng CY. Evidence for cross-talk between Sertoli and germ cells using selected cathepsins as markers. J Androl. 1998b;19(6):686–703. [PubMed] [Google Scholar]
- Clairmont A, Sies H. Evidence for a posttranscriptional effect of retinoic acid on connexin43 gene expression via the 3′-untranslated region. FEBS Lett. 1997;419(2–3):268–70. doi: 10.1016/s0014-5793(97)01468-3. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, van Pelt AMM, Van de Kant HJG, van der Saag PT, Peters AHFM, Heyting C, de Boer P. Role of retinoids in spermatogonial proliferation and differentiation and the meiotic prophase. In: Bartke A, editor. Function of somatic cells in the testis. Berlin Heidelberg New York: Springer; 1994. p. 345. [Google Scholar]
- Decrouy X, Gasc JM, Pointis G, Segretain D. Functional characterization of Cx43 based gap junctions during spermatogenesis. J Cell Physiol. 2004;200(1):146–54. doi: 10.1002/jcp.10473. [DOI] [PubMed] [Google Scholar]
- el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168(2):422–30. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Eskild W, Hansson V. Vitamin A functions in the reproductive organs. In: Blomhoff R, editor. Vitamin A in health and disease. New York: Dekker; 1994. pp. 531–559. [Google Scholar]
- Flickinger CJ. The postnatal development of the Sertoli cells of the mouse. Z Zellforsch Mikrosk Anat. 1967;78(1):92–113. doi: 10.1007/BF00344405. [DOI] [PubMed] [Google Scholar]
- Franke WW, Grund C, Schmid E. Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol. 1979;19(3):269–75. [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–72. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- Howell JM, Thompson JN, Pitt GAJ. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid, I. The male rat. J Reprod Fertil. 1963;5:159–67. doi: 10.1530/jrf.0.0050159. [DOI] [PubMed] [Google Scholar]
- Huang HF, Yang CS, Meyenhofer M, Gould S, Boccabella AV. Disruption of sustentacular (Sertoli) cell tight junctions and regression of spermatogenesis in vitamin-A-deficient rats. Acta Anat (Basel) 1988;133(1):10–15. doi: 10.1159/000146606. [DOI] [PubMed] [Google Scholar]
- Ismail N, Morales CR. Effects of vitamin A deficiency on the inter-Sertoli cell tight junctions and on the germ cell population. Microsc Res Tech. 1992;20(1):43–9. doi: 10.1002/jemt.1070200106. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820–5. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Nelles E, Hagendorff A, Kruger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol. 1998;8(5):299–302. doi: 10.1016/s0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- Kubota H, Chiba H, Takakuwa Y, Osanai M, Tobioka H, Kohama G, Mori M, Sawada N. Retinoid X receptor alpha and retinoic acid receptor gamma mediate expression of genes encoding tight-junction proteins and barrier function in F9 cells during visceral endodermal differentiation. Exp Cell Res. 2001;263(1):163–72. doi: 10.1006/excr.2000.5113. [DOI] [PubMed] [Google Scholar]
- Lee NP, Yeung WS, Luk JM. Junction interaction in the seminiferous epithelium: regulatory roles of connexin-based gap junction. Front Biosci. 2007;12:1552–62. doi: 10.2741/2168. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009;106(25):10213–8. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nature Genetics. 1998;20(4):377–80. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993;90(15):7225–9. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Marinos E, Kulukussa M, Zotos A, Kittas C. Retinoic acid affects basement membrane formation of the seminiferous cords in 14-day male rat gonads in vitro. Differentiation. 1995;59(2):87–94. doi: 10.1046/j.1432-0436.1995.5920087.x. [DOI] [PubMed] [Google Scholar]
- McGinley D, Posalaky Z, Provaznik M. Intercellular junctional complexes of the rat seminiferous tubules: a freeze-fracture study. Anat Rec. 1977;189(2):211–31. doi: 10.1002/ar.1091890208. [DOI] [PubMed] [Google Scholar]
- McGinley DM, Posalaky Z, Porvaznik M, Russell L. Gap junctions between Sertoli and germ cells of rat seminiferous tubules. Tissue Cell. 1979;11(4):741–54. doi: 10.1016/0040-8166(79)90028-4. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Bertram JS, Loewenstein WR. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986;44(1):187–96. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126(6):1139–48. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- Morales A, Cavicchia JC. Spermatogenesis and blood-testis barrier in rats after long-term Vitamin A deprivation. Tissue Cell. 2002;34(5):349–55. doi: 10.1016/s0040816602000356. [DOI] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, Tsukita S. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am J Physiol. 1998;274(6 Pt 1):C1708–17. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25(5):747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Grills GS, Wolgemuth DJ. Evidence for the involvement of the proto-oncogene c-mos in mammalian meiotic maturation and possibly very early embryogenesis. Embo J. 1988;7(3):683–9. doi: 10.1002/j.1460-2075.1988.tb02863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Suzuki F. The postnatal development of the junctional complexes of the mouse Sertoli cells as revealed by freeze-fracture. Anat Rec. 1976;185(4):403–17. doi: 10.1002/ar.1091850403. [DOI] [PubMed] [Google Scholar]
- Packer AI, Jane-Wit D, McLean L, Panteleyev AA, Christiano AM, Wolgemuth DJ. Hoxa4 expression in developing mouse hair follicles and skin. Mech Dev. 2000;99(1–2):153–7. doi: 10.1016/s0925-4773(00)00471-8. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80(3):550–60. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier RM. Cyclic modulation of Sertoli cell junctional complexes in a seasonal breeder: the mink (Mustela vison) Am J Anat. 1988;183(1):68–102. doi: 10.1002/aja.1001830105. [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20(1):3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- Piedrafita FJ, Pfahl M. Nuclear retinoid receptors and mechanisms of action. In: Nau H, Blaner WS, editors. Retinoids: The Biochemical and Molecular Basis of Vitamin A and Retinoid Action. 1999. Berlin Heidelberg New York: Springer-Verlag; 1999. pp. 153–184. [Google Scholar]
- Pitts JD, Hamilton AE, Kam E, Burk RR, Murphy JP. Retinoic acid inhibits junctional communication between animal cells. Carcinogenesis. 1986;7(6):1003–10. doi: 10.1093/carcin/7.6.1003. [DOI] [PubMed] [Google Scholar]
- Plum A, Hallas G, Magin T, Dombrowski F, Hagendorff A, Schumacher B, Wolpert C, Kim J, Lamers WH, Evert M, Meda P, Traub O, Willecke K. Unique and shared functions of different connexins in mice. Curr Biol. 2000;10(18):1083–91. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochim Biophys Acta. 2005;1719(1–2):102–16. doi: 10.1016/j.bbamem.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23(23):4878–84. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren HP, Russell LD. Clonal development of interconnected germ cells in the rat and its relationship to the segmental and subsegmental organization of spermatogenesis. Am J Anat. 1991;192(2):121–8. doi: 10.1002/aja.1001920203. [DOI] [PubMed] [Google Scholar]
- Ricci G, Catizone A, Scarcella MF, Galdieri M. Vitamin A modulation of basement membrane production by purified testicular myoid cells. Exp Cell Res. 1999;249(1):102–8. doi: 10.1006/excr.1999.4444. [DOI] [PubMed] [Google Scholar]
- Risley MS. Connexin gene expression in seminiferous tubules of the Sprague-Dawley rat. Biol Reprod. 2000;62(3):748–54. doi: 10.1095/biolreprod62.3.748. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, SinhaHikim AP, Clegg ED. In: Histological and histopathological evaluation of the testis. Russell LD, Ettlin RA, SinhaHikim AP, Clegg ED, editors. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development. 2007;134(19):3507–15. doi: 10.1242/dev.004481. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251(2):206–20. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- Smith SM, Kirstein IJ, Wang ZS, Fallon JF, Kelley J, Bradshaw-Rouse J. Differential expression of retinoic acid receptor-beta isoforms during chick limb ontogeny. Dev Dyn. 1995;202(1):54–66. doi: 10.1002/aja.1002020106. [DOI] [PubMed] [Google Scholar]
- Spruill WA, Steiner AL, Tres LL, Kierszenbaum AL. Follicle-stimulating hormone-dependent phosphorylation of vimentin in cultures of rat Sertoli cells. Proc Natl Acad Sci U S A. 1983;80(4):993–7. doi: 10.1073/pnas.80.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod. 2007;76(5):804–12. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- Vasios GW, Gold JD, Petkovich M, Chambon P, Gudas LJ. A retinoic acid-responsive element is present in the 5′ flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989;86(23):9099–103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine AL, Bertram JS. Upregulation of connexin 43 by retinoids but not by non-provitamin A carotenoids requires RARs. Nutr Cancer. 2005;52(1):105–13. doi: 10.1207/s15327914nc5201_13. [DOI] [PubMed] [Google Scholar]
- Vine AL, Leung YM, Bertram JS. Transcriptional regulation of connexin 43 expression by retinoids and carotenoids: similarities and differences. Mol Carcinog. 2005;43(2):75–85. doi: 10.1002/mc.20080. [DOI] [PubMed] [Google Scholar]
- Vitale R, Fawcett DW, Dym M. The normal development of the blood-testis barrier and the effects of clomiphene and estrogen treatment. Anat Rec. 1973;176(3):331–44. doi: 10.1002/ar.1091760309. [DOI] [PubMed] [Google Scholar]
- Vogl AW. Distribution and function of organized concentrations of actin filaments in mammalian spermatogenic cells and Sertoli cells. Int Rev Cytol. 1989;119:1–56. doi: 10.1016/s0074-7696(08)60648-8. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63(1):1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Redenbach DM. Ectoplasmic (“junctional”) specializations in mammalian Sertoli cells: influence on spermatogenic cells. Ann N Y Acad Sci. 1991;637:175–202. doi: 10.1111/j.1749-6632.1991.tb27310.x. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Redenbach DM, Grove BD. Sertoli cell cytoskeleton. In: Russell LD, Griswold MD, editors. The Sertoli cell. Clearwater, FL: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- Walder L, Lutzelschwab R. Effects of 12-O-tetradecanoylphorbol-13-acetate (TPA), retinoic acid and diazepam on intercellular communication in a monolayer of rat liver epithelial cells. Exp Cell Res. 1984;152(1):66–76. doi: 10.1016/0014-4827(84)90230-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cao Y, D’Urso CM, Ferrone S. Differential susceptibility of cultured human melanoma cell lines to enhancement by retinoic acid of intercellular adhesion molecule 1 expression. Cancer Res. 1992b;52(17):4766–72. [PubMed] [Google Scholar]
- Weber JE, Russell LD. A study of intercellular bridges during spermatogenesis in the rat. Am J Anat. 1987;180(1):1–24. doi: 10.1002/aja.1001800102. [DOI] [PubMed] [Google Scholar]
- Weber JE, Turner TT, Tung KS, Russell LD. Effects of cytochalasin D on the integrity of the Sertoli cell (blood-testis) barrier. Am J Anat. 1988;182(2):130–47. doi: 10.1002/aja.1001820204. [DOI] [PubMed] [Google Scholar]
- Weiler R, He S, Vaney DI. Retinoic acid modulates gap junctional permeability between horizontal cells of the mammalian retina. Eur J Neurosci. 1999;11(9):3346–50. doi: 10.1046/j.1460-9568.1999.00799.x. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270(1):41–9. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–77. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth DJ, Chung SS. Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor alpha. Soc Reprod Fertil Suppl. 2007;63:11–23. [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Chung SS, Grima J, Zhu LJ, Mruk D, Lee WM, Cheng CY. Changes in the expression of junctional and nonjunctional complex component genes when inter-sertoli tight junctions are formed in vitro. J Androl. 2000;21(2):227–37. [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117(Pt 5):783–98. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Zong SD, Phillips DM, Moo-Young AJ, Bardin CW. Changes in the distribution of intermediate filaments in rat Sertoli cells during the seminiferous epithelium cycle and postnatal development. Anat Rec. 1997;248(3):391–405. doi: 10.1002/(SICI)1097-0185(199707)248:3<391::AID-AR12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Alignment of protein sequences of connexin genes of interest. Amino acid sequences of selected connexins were aligned to design primers to target specific connexin genes. Primers were selected in non-homologous areas which are indicated by the red circles.
Supplementary Fig. S2. Variation in connexin expression in Rara−/− mutant testes as detected by RT-PCR and real time PCR. (A) Differences in connexin expression levels between Rara+/+ and Rara−/− testes by RT-PCR. RT-PCR assays were conducted as a preliminary assay to detect the differences in connexin expression levels in Rara+/+ and Rara−/− testes. (B) Connexin 40 expression is downregulated in Rara−/− testes. Real-time reverse transcription (RT)-PCR was used to quantify changes in the levels of expression of selected connexin genes from control and Rara−/− testes. The expression levels are presented as relative levels of the wild-type expression and the wild-type expression levels are set as one. The results, corrected with β-actin expression, are means ± s.e.m. of at least three experiments in three pairs of animals. Connexin-26 was up-regulated about 0.4 fold and connexin-40 was down regulated about 0.4 fold as well.
Supplementary Table S1. The primers used for RT-PCR, real-time RT-PCR and in situ hybridization for connexin genes were listed.
Supplementary Table S2. List of optimal annealing temperatures and reading temperatures for each connexin gene to avoid multiple bands.
Supplementary Table S3. Junctional component genes containing putative RARE sites.