Abstract
Uric acid is the metabolic end product of purine metabolism in humans. It has antioxidant properties that may be protective but can also be pro-oxidant, depending on its chemical microenvironment. Hyperuricemia predisposes to disease through the formation of urate crystals that cause gout, but hyperuricemia, independent of crystal formation, has also been linked with hypertension, atherosclerosis, insulin resistance, and diabetes. We discuss here the biology of urate metabolism and its role in disease. We also cover the genetics of urate transport, including URAT1, and recent studies identifying SLC2A9, which encodes the glucose transporter family isoform Glut9, as a major determinant of plasma uric acid levels and of gout development.
Introduction
Uric acid, a weak organic acid with a pKa of 5.75, is present principally as monosodium urate (MSU) at physiological pH values. Whereas in humans and the great apes, uric acid is the end product of purine degradation, in other mammals, it is further degraded into allantoin by uricase, an enzyme that is mostly found in the liver. The gene encoding uricase underwent mutational silencing during hominid evolution (1). The consequence of uricase inactivation is the appearance of urate levels that are much higher in humans (≈240–360 μM) in comparison to other mammals (≈30–50 μM in mice). It has been proposed that higher serum levels of urate may be of selective advantage in the evolution of hominids because of its antioxidant effects. On the other hand, hyperuricemia is associated with multiple diseases in humans and points to the deleterious effects of high concentrations of urate (Figure 1). Here, we review the information available on the role of uric acid as anti- or pro-oxidant, on the epidemiological link between hyperuricemia and disease, and on the molecular mechanisms of kidney urate transport.
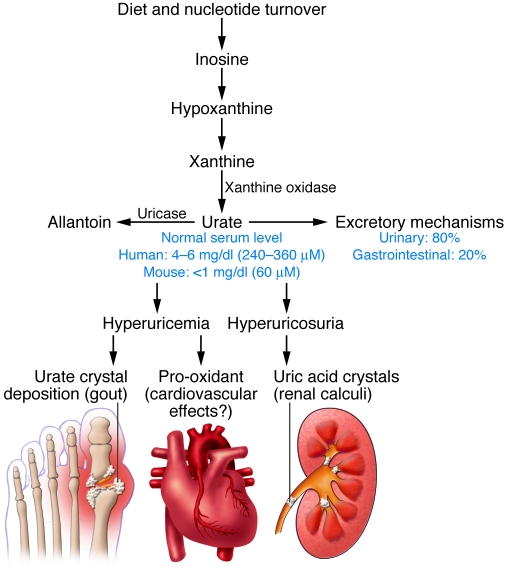
Figure 1. Pathways of urate homeostasis.
Summary scheme of the pathways to produce uric acid, to convert it into allantoin by the liver enzyme uricase, and to excrete it. The balance between these pathways regulates blood urate concentrations, which are higher in humans and apes due to inactivation of the uricase genes. Hyperuricemia can lead to gout and possibly to cardiovascular effects, whereas hyperuricosuria may leads to uric acid crystal–induced pathologies.
Clinical consequences of hyperuricemia
Epidemiology of gout and hyperuricemia
There is strong epidemiological evidence that the prevalence of gout and hyperuricemia is on the increase worldwide. Gout is a disease that affects mainly males, with a clear increase in prevalence in both sexes with aging, and is caused by the tissue deposition of urate crystals that elicit an intense self-limiting inflammatory reaction. The epidemiological link between hyperuricemia and gout was established more than 150 years ago (2, 3). Recent studies have shown an increasing prevalence of gout, especially in the older age group. In a U.S. study, the prevalence doubled in the population over 75 years of age between 1990 and 1999, from 21 per 1,000 to 41 per 1,000 (4). In a second study, the prevalence of gout in the U.K. adult population was estimated to be 1.4%, with a peak of more than 7% in men aged over 75 years old (5). This trend has also been observed in studies in developing countries in Asia (6, 7). Although the studies focused on gout, it is inferred that hyperuricemia incidence has also increased over the same period. Potential explanations for these findings include lifestyle and dietary changes brought about by increasing prosperity, increased life expectancy, the increased use of hypertensive agents, as well as aging of the overall population.
Hyperuricemia, insulin resistance, hypertension, and cardiovascular disease
Hyperuricemia is a common finding in patients with the metabolic syndrome (8), and an inverse correlation was noted between insulin resistance and decreased renal uric acid clearance, which is itself associated with elevated uricemia (9). Obesity, in particular visceral adiposity, is also positively associated with hyperuricemia, which can be reduced by body weight loss (10–12). Hyperuricemia is also frequently observed in patients with cardiovascular diseases. The question of whether hyperuricemia is an independent risk factor for cardiovascular disease was raised more than five decades ago (13). Recently, there has been renewed interest in hyperuricemia and its association with hypertension and cardiovascular mortality, and studies suggest that it indeed may have a direct vascular effect (reviewed in refs. 14 and 15). In hypertensive children with normal renal function, there is a strong correlation between hyperuricemia and blood pressure (16), and in a controlled trial, treatment of these subjects with the xanthine oxidase inhibitor allopurinol significantly lowered blood pressure in a short-term study (17). In the last 20 years, over 10 studies have reported that hyperuricemia is an independent risk factor for the development of hypertension (reviewed in ref. 14).
Epidemiological studies have also evaluated the association between hyperuricemia and cardiovascular disease. In the Framingham cohort, investigators concluded that hyperuricemia was a covariable of other known cardiovascular risk factors for cardiac deaths and coronary heart disease (18). More recently, data appear to be swinging the interpretation the other way, indicating that hyperuricemia predisposes to plaque formation and endothelial dysfunction, as assessed by ultrasonography (19–21). Hyperuricemia was also reported to be an independent risk factor for cardiovascular mortality (22, 23). In patients with established cardiovascular disease, elevated urate levels were an independent predictor of cardiovascular events (24); and finally, in a meta-analysis of the association between hyperuricemia and stroke, a small but increased risk was found even after adjustment for known cardiovascular risk factors (25). In most studies, the increased risk of hyperuricemia for cardiovascular morbidity (by odds ratio or hazard ratio) was around 1.2–1.4.
If hyperuricemia is an etiological factor in cardiovascular morbidity, what are the mechanisms, and will its modification affect outcome? Data from a rodent model suggested that uric acid–mediated vasoconstriction leads to endothelial dysfunction, activation of the renin-angiotensin system, and hypertension (26). Critics will, however, argue that it is impossible to disentangle hyperuricemia from hypertension and that hyperuricemia is a surrogate marker for early subclinical renal dysfunction, and the cardiovascular complications are secondary. In the absence of more compelling experimental data, this debate can only be resolved by large-scale intervention studies in a hyperuricemic population analyzing the effects on blood pressure and cardiovascular events over time.
Uric acid: antioxidant or pro-oxidant?
The relative hyperuricemia in humans has raised questions about its evolutionary advantages, and its association with diseases requires understanding how it can become deleterious at high concentrations. Initially, uric acid was considered an inert waste product that crystallizes at high concentrations to form renal stones and provoke gouty arthritis. Subsequently, uric acid was recognized to be a powerful antioxidant that scavenges singlet oxygen, oxygen radicals, and peroxynitrite and chelates transition metals, to reduce, for instance, iron ion–mediated ascorbic acid oxidation. Urate thus accounts for approximately half of the antioxidant capacity of human plasma, and its antioxidant properties are as powerful as those of ascorbic acid (27, 28). As illustrated in Figure 2A, uric acid can prevent peroxynitrite-induced protein nitrosation (29), lipid and protein peroxidation (30), and inactivation of tetrahydrobiopterin (31), a cofactor necessary for NOS. Uric acid also protects LDL from Cu2+-mediated oxidation (Figure 2B). Together, these antioxidant actions underlie the protective effects of uric acid action in cardiovascular diseases, aging, and cancer (27).
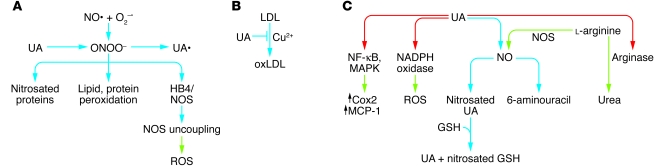
Figure 2. Antioxidant and pro-oxidant effect of uric acid.
Antioxidant activities. (A) Peroxynitrites (ONOO–) are produced from the reaction of nitric oxide (NO•) with superoxide (O2–•). Peroxynitrites can induce protein nitrosation and lipid and protein peroxidation and block tetrahydrobiopterin (HB4), a cofactor necessary for NOS activity. In the absence of HB4, NOS produces ROS. Uric acid (UA) can directly inactivate peroxynitrite by a reaction that generates uric acid radicals (UA•); these can be rapidly eliminated by plasma ascorbic acid. (B) Uric acid can also prevent Cu2+-induced oxidation of LDL, a reaction that may protect against atherosclerosis development. (C) By enhancing arginase activity, uric acid diverts l-arginine from NO production to urea production. Uric acid can also directly react with NO to generate nitrosated uric acid, and the nitroso group can then be transferred to glutathione (GSH) for transport to another recipient molecule. In the presence of oxygen, uric acid reacts with NO to produce the stable species 6-aminouracil. Uric acid uptake in adipocytes activates NADPH oxidase and increase production of ROS, which can initiate an inflammatory reaction. In vascular smooth muscle cells, uric acid can activate the NF-κB and MAPK pathway and increase cyclooxygenase and MCP-1 production. Blue arrows, chemical reactions; green arrows, products from enzymatic or signaling pathways; red arrows, activation of enzymatic activities.
In vitro and cellular studies have nevertheless demonstrated that depending on its chemical microenvironment, uric acid may also be pro-oxidant. For instance, although uric acid can protect native LDL particles against Cu2+-induced oxidation, it also increases the oxidation of already oxidized LDLs, which contain lipid peroxidation products (32, 33), and this dual role appears to depend on the presence of transition metals. As illustrated in Figure 2A, when uric acid is oxidized by peroxynitrites, urate radicals are produced that could propagate the pro-oxidant state (34), but in the plasma they are rapidly inactivated by reaction with ascorbic acid (31).
NO, described initially as an endothelial cell–derived relaxing factor, is an important regulatory molecule in the cardiovascular system, and reduced NO levels are associated with hypertension and insulin resistance (35–37). Urate can react directly with NO under aerobic conditions to generate an unstable nitrosated uric acid product that can transfer NO to other molecules such as glutathione (ref. 38 and Figure 2). Under anaerobic conditions, urate is converted in the presence of NO into stable 6-aminouracil (39). The possibility that increased urate plasma levels can reduce NO bioavailability has been tested in rats treated with the uricase inhibitor oxonic acid. The consequent increase in plasma uric acid was indeed associated with a decrease in plasma nitrites/nitrates (NOx). Similarly, direct exposure of endothelial cells to uric acid slightly reduces basal or VEGF-stimulated NO production (40). Thus, uric acid can dose-dependently reduce NO bioavailability. Although a direct chemical reaction of urate with NO could explain the decrease in plasma NOx, there is evidence that in vivo urate can decrease NO production by interfering with its biosynthesis. For instance, in pulmonary endothelial cells, uric acid reduces NO production by a mechanism that depends on uric acid increasing the activity of arginase, which diverts l-arginine to urea production instead of to NO production by eNOS (Figure 2C and ref. 41).
Another pro-oxidant action of urate has been described during adipogenic differentiation of 3T3-L1 cells (Figure 2). When these cells are induced to differentiate into adipocytes, addition of uric acid at physiological concentrations further increases ROS production by a mechanism that involves activation of NADPH oxidase (42). This effect in adipocytes may participate in the induction of inflammation and insulin resistance of adipose tissue observed in obesity (43). In vascular smooth muscle cells, uric acid has been reported to stimulate MCP-1 production following activation of NF-κB, MAPKs, and cyclooxygenase 2 (44).
Together, the available information indicates that uric acid has complex chemical and biological effects and that its pro-oxidant or NO-reducing properties may explain the association among hyperuricemia, hypertension, the metabolic syndrome, and cardiovascular disease (45). In addition, when hyperuricemia leads to the formation of microcrystals, it leads to joint and renal inflammation. Chronic inflammation (as in tophaceous gout) leads to bone and cartilage destruction, and chronic hyperuricemia and hyperuricosuria in gouty patients are also frequently associated with tubulointerstitial fibrosis and glomerulosclerosis, signs of local renal inflammation (46). Part of this is explained by the activation of the NALP3 inflammasome to process and secrete IL-1β (47), but other pathways of inflammation have also been demonstrated (47–49).
There is thus no simple explanation for the possible protective or pathogenic effect of hyperuricemia, and there is clearly a need for more animal models to study this link.
Urate transporting proteins and genetics of urate transporter pathologies
Urate homeostasis depends on the balance between production and complex processes of secretion and reabsorption in the kidney tubule and excretion in the intestine. It is estimated that approximately 30% of uric acid excretion is by the intestine by mechanisms that have so far not been investigated in detail. Renal mechanisms of urate excretion account for the other 70% and are key to the understanding of hyperuricemia. In patients presenting with gout and primary hyperuricemia, the majority underexcrete urate when the fractional clearance of urate is measured (50). Urate transport by the kidney has been investigated for many years, in part to search for uricosuric drugs to decrease plasma urate levels. So far, several classes of uricosuric drugs have been identified that decrease plasma urate levels, such as benzbromarone, probenecid, sulfinpyrazone, or losartan, whereas other pharmacological agents such as pyrazinoate, the active metabolite of pyrazinamide, nicotinate, and lactate, are antiuricosuric.
In the human kidney, urate handling involves urate glomerular filtration followed by a complex array of reabsorptive and secretory mechanisms taking place in the proximal tubule. In the mouse, both the proximal and distal convoluted tubule appear to be involved in urate reabsorption and secretion, as determined by the localization of the various urate carriers that are discussed below and depicted in Figure 3. It has to be noted that the relative importance of the reabsorption and secretion mechanisms differ among species. Humans, mice, and rats predominantly reabsorb uric acid, whereas pigs, rabbits, reptiles, and birds have more active secretory mechanisms.
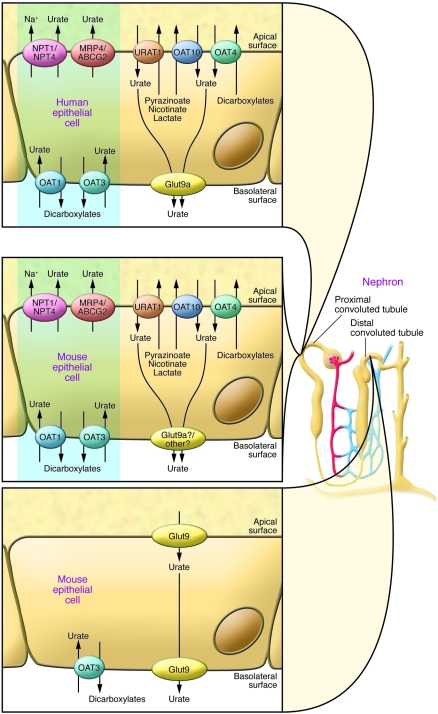
Figure 3. Urate transporters in kidney epithelial cells of humans and mice.
In humans (upper left panel), the urate reabsorption pathway involves the apical exchanger proteins URAT1, OAT4, and OAT10; intracellular urate is released through basolateral Glut9. Urate uptake by URAT1 and OAT10 is accelerated by intracellular monocarboxylates such as lactate, pyrazinoate, and nicotinate and by dicarboxylates for OAT4. Several apical monocarboxylate transporters are required to favor urate reabsorption, such as MCT9 and SMCT1 and -2 (see text). The excretion pathway (blue box) involves the basolateral urate/dicarboxylate exchangers OAT1 and OAT3 and the apical ATP-binding cassette proteins MRP4 and ABCG2, as well as the sodium/phosphate cotransporters NPT1 and NPT4. Functional organization of the apical transporters is regulated by interactions with PDZ domains present in URAT1, NPT1, OAT4, and the sodium/monocarboxylate cotransporter SMCT1 and with PDZK1 and NHERF1; and influenced by changes in actin polymerization regulated by the protein CARMIL, as determined by biochemical and genetic studies (see text). Urate transport in the mouse kidney involves both the proximal and distal convoluted tubules (middle and lower left panels). The same urate-transporting proteins present in humans are found in the mouse proximal tubules, except for Glut9, which is present at an extremely low levels. In mice, in contrast to humans, Glut9 is present at very high levels in both the apical and basolateral poles of distal convoluted tubule cells. However, it is not known which isoform of Glut9 is present in the apical and basolateral membranes.
The organic anion transporters (SLC22A family)
URAT1.
The urate/anion exchanger URAT1 (SLC22A12 gene) was first identified in a search for organic anion transporter–like (OAT-like) molecules in both gene databases and expression/functional studies in Xenopus oocytes (51). URAT1 is a 12-transmembrane domain–containing protein found in the apical membrane of proximal tubule epithelial cells and transports urate in exchange for Cl– or organic anions. The antiuricosuric agents lactate, pyrazinoate, and nicotinate can serve as substrate for the antiporter activity of URAT1 to increase urate reabsorption. On the other hand, URAT1 is inhibited by the classical uricosuric agents benzbromarone, probenecid, and losartan. Inactivating mutations in URAT1 have been found in Japanese patients with idiopathic renal hypouricemia (51, 52, 53). These patients have plasma uric acid levels lower than 60 μM (or <1 mg/dl) that are associated with urate fractional excretion rates of nearly 100%. These patients are mostly asymptomatic but may develop exercise-induced acute renal failure (54).
The mouse URAT1 is 74% identical to the human homolog; it shows similar transport properties, although its Km for urate is higher than that of human URAT1 (1200 μM vs. 370 μM); and it is also located in the apical membrane of proximal tubule epithelial cells (55). Knockout of the Urat1 gene in the mouse leads to increased urate excretion but no significant hypouricemia, indicating that in the mouse, this transporter plays a less important role than in humans for the control of uricemia (56).
OAT4 and OAT10.
OAT4 (encoded by the SLC22A11 gene) is a multispecific anion transporter present in the apical membrane of epithelial cells from the proximal tubule (57, 58). It is involved in luminal urate reabsorption by a mechanism that is transactivated by intracellular dicarboxylates but not by the antiuricosuric agents; it is also affected by the diuretic hydrochlorothiazide (59).
OAT10 (SLC22A13) is a urate and high-affinity nicotinate transporter expressed in brush border membrane vesicles from proximal tubules and, interestingly, also in cortical collecting ducts in rats (60).
OAT1 and OAT3.
The organic anion and urate transporters OAT1 (SLC22A6) and OAT3 (SLC22A8) can function as urate/dicarboxylate exchangers (61–64) and are found on the basolateral side of the same cells that express Oat4 (58). However, Oat3 is also found in all segments of the rat nephron from the proximal tubule to the collecting duct (65). Gene knockout studies in the mouse indicate that absence of OAT1 or OAT3 slightly decreases uricosuria, suggesting that their principal function is in urate excretion (56).
Multidrug resistance proteins
MRP4.
The multidrug resistance protein MRP4 (ATP-binding cassette family, ABCC4) is present in the apical membrane of proximal tubule epithelial cells. It appears to control ATP-dependent urate extrusion from the cells into the tubule lumen and thus contribute to urate excretion (66–68).
ABCG2.
Genome-wide association studies for hyperuricemia and gout identified the ABCG2 locus (69). Functional studies (70) demonstrated that ABCG2, which is expressed in the apical membrane of proximal collecting duct cells (71), functions as a urate efflux transporter. Furthermore, a common SNP that introduces a Q141K mutation was found to reduce the transport rate by half when tested in Xenopus oocytes. In a human cohort, the presence of this allele was associated with significantly increased plasma uric acid levels and the risk for gout. The data indicated that at least 10% of all gout cases in individuals of European descent are attributable to this causal variant (69).
Other genes identified by genetic association studies
In the studies mentioned above (69), the sodium/phosphate cotransporter NPT4 (SLC17A3), present in the apical membrane of proximal tubule epithelial cells (72) was also found associated with uric acid levels and gout. This multispecific organic anion transporter is the human homolog of the pig OTAv1 found to be involved in urate efflux (73). In a meta-analysis of over 28,000 individuals (74), several genetic loci were found to be associated with urate plasma levels. These include the SCL17A1 gene encoding NPT1 (a neighboring gene of SLC17A3), URAT1, OAT4, ABCG2, and SCL2A9 (Glut9, see below). In addition, the monocarboxylate transporter MCT9 (SLC16A9), PDZ domain–containing protein 1 (PDZK1), and the protein CARMIL (LRRC16A) were identified. The apical urate transporters URAT1, NPT1, and OAT4 are known to bind to PDZK1 through their C-terminal PDZ domain (75, 76). CARMIL is a protein highly expressed in the kidney and binds actin-capping proteins, thereby increasing actin filament polymerization (77).
The association of PDZK1, NHERF1 (another PDZ-containing protein), CARMIL, and the urate transporters has been suggested to form an apical transportasome complex implicated in the regulation of urate transport (78). This complex also includes the sodium monocarboxylate cotransporters (SMCT1, SLC5A8 and SMCT2, SLC2A12); SMCT1 can also bind PDZK1 (78). Coexpression studies indeed indicated that PDZK1 and NHERF1 overexpression in the presence of URAT1 increases its transport activity (75). These genetic and biochemical data thus indicate that very complex regulatory processes may control the magnitude and direction of urate fluxes across the proximal tubule epithelium. Much more work is necessary to understand them in detail and to determine whether they are regulated by various hormones or metabolic states.
The same meta-analysis (74) also identified the glucokinase regulatory protein (GCKR) locus with hyperuricemia. The role of GCKR in urate transport is, however, unclear. This protein is known for its role in the control of glucokinase activity and glucose utilization by liver, and other genetic association studies have found the GCKR locus to influence triglyceride levels (79, 80).
Glucose transporter family member SLC2A9
GLUT9 (SLC2A9) was initially identified by sequence similarity with members of the glucose transporter (Glut) family (81). GLUT9 has the structure of a type II Glut isoform, with 12 transmembrane domains, a large extracellular loop between the first and second transmembrane domains, and both amino- and carboxyterminal ends on the cytoplasmic side (82). In both humans and mice, GLUT9 exists as two alternatively spliced variants that encode different aminoterminal cytoplasmic tails (83, 84). Human GLUT9a has 540 amino acids and is encoded by 12 exons, whereas GLUT9b is 512 amino acids long and encoded by 13 exons, spread over an approximately 250-kb genomic region. In both humans and mice, GLUT9b expression is restricted to liver and kidney, whereas GLUT9a has a broad tissue distribution including liver, kidney, intestine, leukocytes, and chondrocytes (85), where its expression is upregulated by inflammatory cytokines (86) (Figure 4). In polarized epithelial cells, human GLUT9a is expressed in the basolateral membrane, whereas GLUT9b is targeted to the apical pole (84), and in human kidney, GLUT9 is present in the proximal tubule (84). In the mouse, Glut9 is present in the distal convoluted tubule (83), both in the basolateral and apical membranes (87) (Figure 3), but it is not yet known which isoform is present in each pole of the cells, in particular since both mouse isoforms have been reported to be targeted to the basolateral membrane of MDCK cells (83).
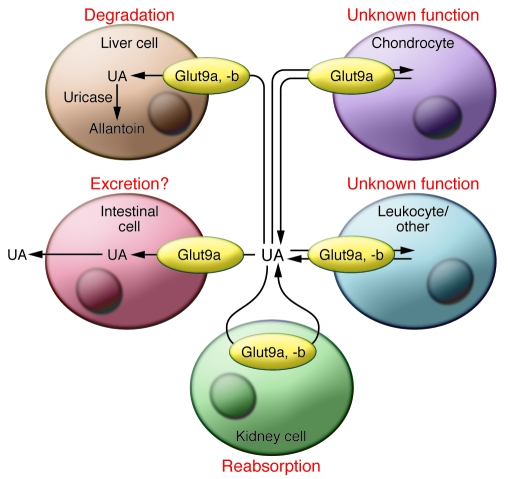
Figure 4. Summary of Glut9 sites of expression and function.
Glut9 plays an important role in the control of urate homeostasis by its role in several organs. In kidney, evidence strongly supports a major role of Glut9 in uric acid reabsorption; in intestine, Glut9 may participate in uric acid excretion, although there has been no direct testing of this hypothesis; in the liver of animals with active uricase, Glut9 is required for hepatic uric acid uptake and conversion to allantoin for excretion. Absence of uricase in humans raises the question of the role of hepatic Glut9 in humans. There is good evidence for Glut9 expression in chondrocytes and leukocytes, but so far there is no indication whether this transporter is required for uptake or secretion.
Functionally, Glut9 was initially reported to be a glucose (88) and/or fructose (89) transporter that, in contrast to other members of the Glut family, could not be inhibited by cytochalasin B. However, the glucose and fructose transport activity reported in these publications was very low and could not be observed in other studies (90, 91). Furthermore, genetic inactivation of the major liver glucose and fructose transporter (Glut2) completely suppressed glucose uptake by hepatocytes (92), even though Glut9 is still highly expressed on their cell surface, indicating that sugar transport is most probably not a physiologically relevant function of Glut9.
Remarkably, the function of GLUT9 was revealed by human genetic studies. Indeed, genome-wide association studies found that the major locus associated with uric acid plasma levels in human cohorts was the SLC2A9 gene, explaining up to 3.5% of serum uric acid level variations (93). This initial study was rapidly followed by several similar reports that replicated, in other cohorts, the observed association of SLC2A9 with uricemia and demonstrated that human GLUT9 is a urate transporter (69, 94–97). Detailed transport studies of the mouse Glut9a and Glut9b splice variants confirmed that the forms have indistinguishable kinetic properties, with a Km for urate of approximately 0.6 mM, and that transport cannot be competed by excess glucose or fructose. Furthermore, transport is electrogenic and independent of Na+ or Cl– concentrations but dependent on membrane potential (90). Importantly, urate transport can be inhibited by the uricosuric agents benzbromarone (90% inhibition of transport) and losartan (50% inhibition) but only marginally by pyrazinoate. The general glucose transporter inhibitor phloretin inhibited transport by approximately 50%, but cytochalasin B was inactive.
Glut9 in genome-wide association studies
In addition to the association of SLC2A9 with serum uric acid levels, a significant association with gout was reported (95). This study showed a greater impact of the minor allele on decreasing plasma uric acid with higher BMI (98). The SLC2A9 (and ABCG2) SNPs that were associated with gout were, however, not linked with coronary artery disease in the German MI Family Study (99), and no association of the SLC2A9 SNPs could be found with hypertension (96). All studies found a higher impact of the SLC2A9 SNPs in females than in males, and in one study (95) the level of expression of the mRNAs for both GLUT9 isoforms was evaluated in leukocytes, and a significant relationship was found between increased expression of the GLUT9b, but not GLUT9a, isoform and plasma uric acid levels.
Monogenic mutations in SLC2A9
Monogenic forms of hypouricemia have now been linked with mutations in the SLC2A9 gene. Anzai et al. found a P412R mutation in a hypouricemic patient (91); Dinour et al. reported an L75R mutation and a 36-kb deletion present in two different families of hypouricemic patients (100); and Matsuo et al. found two patients with GLUT9 mutations (R198W and R380C). In the latter study, these two patients were identified from a group of 70 hypouricemic patients, 47 of whom had mutations in URAT1 (101). When tested in Xenopus oocyte expression systems, the GLUT9 mutations severely impaired urate transport activity. The individuals with the L75R mutation had mean serum uric acid concentrations of 0.17 ± 0.2 mg/dl and a fractional excretion of uric acid (FE urate) of approximately 150%, a much more severe phenotype compared with individuals carrying a URAT1 mutation. Moreover, a FE urate greater than 100% is suggestive of active secretion of uric acid in the lumen, by an unclear mechanism. Three of these individuals with SLC2A9 mutations had nephrolithiasis, and three had a history of exercise-induced acute renal failure.
Dalmatian dogs excrete large quantities of urate and often develop renal uric acid crystals and nephropathy. They show a defect in uric acid conversion to allantoin, which is not due to a uricase mutation but rather associated with impaired liver urate uptake and renal reabsorption in the proximal tubule (102). Genetic studies have now identified a single mutation of a highly conserved cysteine in transmembrane 5 of Glut9, C188F, as the mutation causing both liver urate uptake and renal reabsorption defects (103). This phenotype is very similar to that observed in mice with genetic inactivation of the Glut9 gene.
Genetic inactivation of Glut9 in mice (104) induces moderate hyperuricemia and massive renal excretion of urate, with a fractional excretion of approximately 100% in males and approximately 150% in females. This is the result of a combined defect in urate conversion into allantoin in the liver and of renal reabsorption. Because Glut9 is also present in the mouse intestine, its genetic inactivation may also prevent intestinal excretion of urate, although this has not been formally tested. These mice display an early-onset nephropathy, characterized by obstructive lithiasis, tubulointerstitial inflammation, and progressive inflammatory fibrosis of the cortex. They also show a mild renal insufficiency, increased water intake, and approximately 5-fold-increased urine volume, with impaired urine concentration capacity.
Selective inactivation of Glut9 in the liver by tamoxifen injection of adult Alb-CreERT2;Glut9lox/lox mice (Lglut9–/– mice) induces severe hyperuricemia, reaching approximately 200 μM in males as compared with a control value of approximately 40 μM and a daily urate excretion rate that was similar to that of the systemic Glut9–/– mice with a fractional excretion of 25%–35%. This was also associated with a urine concentration defect but with only a small increase in urine volume. Together, these data indicate that Glut9 is required for urate access to hepatic uricase and conversion to allantoin and for urate reabsorption in the kidney.
It is also interesting to note that the Lglut9–/– mice showed no nephropathy or kidney morphological abnormalities, even though the daily urate excretion was the same as in the Glut9–/– mice. This suggests that hyperuricosuria-induced nephropathy requires specific conditions, as found in neonates, which have more acidic urine and which cannot compensate increased urine volume by increased fluid intake. Interestingly, the uricase-knockout mice also show hyperuricemia and massive uricosuria and development of nephropathy with accumulation of urate crystals in the kidney (105), confirming that hyperuricosuria present from the time of birth can induce nephropathy.
Impaired urate homeostasis in the Glut9–/– mice is much more severe than in Urat1–/–, Oat1–/–, or Oat3–/– mice (56), indicating that Glut9 is a major regulator of urate homeostasis. Also, it is important to note that in mice, Glut9 is mostly present in the distal convoluted tubule, whereas Urat1 and the other urate transporting proteins are present in the proximal tubule, as in humans (see Figure 3). This suggests that apical and basolateral expression of Glut9 in the same cells may be sufficient for transepithelial urate reabsorption. This also suggests that in the mouse, urate reabsorption takes place in the distal convoluted in addition to the proximal tubule. This has, however, never been tested directly in the mouse to our knowledge. If Glut9 is absent from the proximal tubule, where the other apical urate transport proteins are found, and since Glut9a is so far the only known basolateral urate protein involved in urate efflux, this suggests that another, as-yet-unidentified basolateral urate transporter may exist in the proximal tubule. Indeed, as noted above, Oat1 and Oat3 double-knockout mice have decreased uricosuria, indicating that these transporters are involved in urate secretion, and, on the other hand, Urat1–/– mice have increased uricosuria, indicating that this transporter, and the proximal tubule, must also be involved in urate reabsorption in the mouse. One cannot, however, exclude low levels of Glut9 expression in the proximal tubule that are not detected by immunofluorescence microscopy, because Glut9a mRNA is observed in this tubule segment (90).
Finally, it is not known whether expression of Glut9 in the distal tubule is unique to the mouse or whether it is also present in this nephron segment in other species. This observations nevertheless provides an indication that urate renal handling by Glut9 can proceed independent of the presence of the other urate-transporting proteins, in particular Urat1.
As discussed above, it is not yet established whether uric acid is causally linked with hypertension, atherosclerosis, or insulin resistance because there is a lack of animal models to study the role of hyperuricemia. The availability of mice with genetic induction of hyperuricemia in the adult, such as the Lglut9–/– mice, may be of great help in investigating these associations.
Future perspectives
The increasing understanding of urate transport mechanisms sheds light on the causes of hyperuricemia. On one hand, it provides a basis to dissect out the genetic influences on hyperuricemia and to start understanding the complex regulation of urate bidirectional fluxes in tubular cells. On the other hand, it will help researchers achieve a better understand the pharmacological basis for drug action, in relation to drugs that predispose to hyper- or hypouricemia. Indeed, development of uricosuric drugs that act on specific transporters has already started, and it is hoped that this will eventually lead to a wider selection of effective treatments for hyperuricemia. Finally, although efforts have concentrated on the renal transport mechanisms, it is intriguing that they may have a function in other organs or tissues. For example, what is the function of the Glut9 transporter in the leukocytes and chondrocytes? Are transporter mechanisms important in the response of endothelial cells to urate, and can they explain the vascular effects of urate? The availability of mice that are deficient for different transporters will certainly facilitate investigations in the future.
Acknowledgments
The authors thank Frédéric Preitner and Olivier Bonny for their critical review of the manuscript. The work was supported by grants from the Swiss National Science Foundation (31003A-113525), the European Union FP6 program on Hepatic and Adipose Tissue and Functions in the Metabolic Syndrome (EU-FP6 HEPADIP), and the FP7 program on European Drug Initiative on Channels and Transporters (EU-FP7 EDICT) to B. Thorens.
Footnotes
Conflict of interest: Alexander So has acted as a consultant and advisor for Novartis.
Citation for this article: J Clin Invest. 2010;120(6):1791–1799. doi:10.1172/JCI42344.
References
- 1.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 2.Campion EW, Glynn RJ, Delabry LO. Asymptomatic hyperuricemia: risks and consequences in the normative aging study. Am J Med. 1987;82(3):421–426. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 3.Garrod A. Observations on certain pathological conditions of the blood and urine, in gout, rheumatism and Bright’s disease. Med Chir Trans. 1848;31:83–97. doi: 10.1177/095952874803100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–1587. [PubMed] [Google Scholar]
- 5.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR, Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990-1999. Ann Rheum Dis. 2005;64(2):267–272. doi: 10.1136/ard.2004.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc. 2006;69(11):512–516. doi: 10.1016/S1726-4901(09)70320-X. [DOI] [PubMed] [Google Scholar]
- 7.Yoo TW, et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J. 2005;69(8):928–933. doi: 10.1253/circj.69.928. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 9.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011. doi: 10.1001/jama.266.21.3008. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism. 1997;46(10):1162–1165. doi: 10.1016/S0026-0495(97)90210-9. [DOI] [PubMed] [Google Scholar]
- 11.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998;8(4):250–261. doi: 10.1016/S1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 12.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–748. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 13.Gertler MM, Garn SM, Levine SA. Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann Intern Med. 1951;34(6):1421–1431. doi: 10.7326/0003-4819-34-6-1421. [DOI] [PubMed] [Google Scholar]
- 14.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaffo AL, Edwards NL, Saag KG. Gout. Hyperuricemia and cardiovascular disease: how strong is the evidence for a causal link? Arthritis Res Ther. 2009;11(4):240. doi: 10.1186/ar2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 19.Tavil Y, et al. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis. 2008;197(1):159–163. doi: 10.1016/j.atherosclerosis.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Pacifico L, et al. Serum uric acid and its association with metabolic syndrome and carotid atherosclerosis in obese children. Eur J Endocrinol. 2009;160(1):45–52. doi: 10.1530/EJE-08-0618. [DOI] [PubMed] [Google Scholar]
- 21.Neogi T, Ellison RC, Hunt S, Terkeltaub R, Felson DT, Zhang Y. Serum uric acid is associated with carotid plaques: the National Heart, Lung, and Blood Institute Family Heart Study. J Rheumatol. 2009;36(2):378–384. doi: 10.3899/jrheum.080646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH, Group MR. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168(10):1104–1110. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 23.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 24.Okura T, et al. Elevated serum uric acid is an independent predictor for cardiovascular events in patients with severe coronary artery stenosis: subanalysis of the Japanese Coronary Artery Disease (JCAD) Study. Circ J. 2009;73(5):885–891. doi: 10.1253/circj.CJ-08-0828. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RJ, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 27.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235(3):747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann N Y Acad Sci. 2002;962:242–259. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 30.Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93(6):284–289. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70(3):343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Bagnati M, Perugini C, Cau C, Bordone R, Albano E, Bellomo G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem J. 1999;340(pt 1):143–152. doi: 10.1042/0264-6021:3400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson RA, Horsley ET, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. J Lipid Res. 2003;44(3):512–521. doi: 10.1194/jlr.M200407-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372(2):285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 35.Cook S, et al. Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. Diabetes. 2004;53(8):2067–2072. doi: 10.2337/diabetes.53.8.2067. [DOI] [PubMed] [Google Scholar]
- 36.Cook S, et al. Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly. 2003;133(25–26):360–363. doi: 10.4414/smw.2003.10239. [DOI] [PubMed] [Google Scholar]
- 37.Duplain H, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104(3):342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T. Nitrosation of uric acid induced by nitric oxide under aerobic conditions. Nitric Oxide. 2007;16(2):266–273. doi: 10.1016/j.niox.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27(8):967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khosla UM, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 41.Zharikov S, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 44.Kanellis J, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 45.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25(1):39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Kang DH, Nakagawa T. Uric acid and chronic renal disease: possible implication of hyperuricemia on progression of renal disease. Semin Nephrol. 2005;25(1):43–49. doi: 10.1016/j.semnephrol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 48.So A. Developments in the scientific and clinical understanding of gout. Arthritis Res Ther. 2008;10(5):221. doi: 10.1186/ar2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19(6):615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Pascual E, Perdiguero M. Gout, diuretics and the kidney. Ann Rheum Dis. 2006;65(8):981–982. doi: 10.1136/ard.2005.049023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enomoto A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi T, et al. Recurrent URAT1 gene mutations and prevalence of renal hypouricemia in Japanese. Pediatr Nephrol. 2005;20(5):576–578. doi: 10.1007/s00467-005-1830-z. [DOI] [PubMed] [Google Scholar]
- 53.Ichida K, et al. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet. 2008;74(3):243–251. doi: 10.1111/j.1399-0004.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 54.Kaneko K, Taniguchi N, Tanabe Y, Nakano T, Hasui M, Nozu K. Oxidative imbalance in idiopathic renal hypouricemia. Pediatr Nephrol. 2009;24(4):869–871. doi: 10.1007/s00467-008-1032-6. [DOI] [PubMed] [Google Scholar]
- 55.Hosoyamada M, Ichida K, Enomoto A, Hosoya T, Endou H. Function and localization of urate transporter 1 in mouse kidney. J Am Soc Nephrol. 2004;15(2):261–268. doi: 10.1097/01.ASN.0000107560.80107.19. [DOI] [PubMed] [Google Scholar]
- 56.Eraly SA, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics. 2008;33(2):180–192. doi: 10.1152/physiolgenomics.00207.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cha SH, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275(6):4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 58.Ekaratanawong S, et al. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94(3):297–304. doi: 10.1254/jphs.94.297. [DOI] [PubMed] [Google Scholar]
- 59.Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol. 2007;18(2):430–439. doi: 10.1681/ASN.2006040415. [DOI] [PubMed] [Google Scholar]
- 60.Bahn A, et al. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem. 2008;283(24):16332–16341. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
- 61.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272(30):18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- 62.Bakhiya A, Bahn A, Burckhardt G, Wolff N. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem. 2003;13(5):249–256. doi: 10.1159/000074539. [DOI] [PubMed] [Google Scholar]
- 63.Kusuhara H, et al. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem. 1999;274(19):13675–13680. doi: 10.1074/jbc.274.19.13675. [DOI] [PubMed] [Google Scholar]
- 64.Cha SH, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59(5):1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 65.Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol. 2002;13(4):848–857. doi: 10.1681/ASN.V134848. [DOI] [PubMed] [Google Scholar]
- 66.El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. 2008;155(7):1066–1075. doi: 10.1038/bjp.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13(3):595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 68.Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288(2):F327–F333. doi: 10.1152/ajprenal.00133.2004. [DOI] [PubMed] [Google Scholar]
- 69.Dehghan A, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huls M, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73(2):220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 72.Ishibashi K, Matsuzaki T, Takata K, Imai M. Identification of a new member of type I Na/phosphate co-transporter in the rat kidney. Nephron Physiol. 2003;94(1):p10–p18. doi: 10.1159/000071070. [DOI] [PubMed] [Google Scholar]
- 73.Jutabha P, et al. Identification of a novel voltage-driven organic anion transporter present at apical membrane of renal proximal tubule. J Biol Chem. 2003;278(30):27930–27938. doi: 10.1074/jbc.M303210200. [DOI] [PubMed] [Google Scholar]
- 74.Kolz M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyazaki H, et al. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J Am Soc Nephrol. 2005;16(12):3498–3506. doi: 10.1681/ASN.2005030306. [DOI] [PubMed] [Google Scholar]
- 76.Anzai N, et al. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem. 2004;279(44):45942–45950. doi: 10.1074/jbc.M406724200. [DOI] [PubMed] [Google Scholar]
- 77.Yang C, et al. Mammalian CARMIL inhibits actin filament capping by capping protein. Dev Cell. 2005;9(2):209–221. doi: 10.1016/j.devcel.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19(2):151–157. doi: 10.1097/BOR.0b013e328032781a. [DOI] [PubMed] [Google Scholar]
- 79.Beer NL, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18(21):4081–4088. doi: 10.1093/hmg/ddp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orho-Melander M, et al. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57(11):3112–3121. doi: 10.2337/db08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phay JE, Hussain HB, Moley JF. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics. 2000;66(2):217–220. doi: 10.1006/geno.2000.6195. [DOI] [PubMed] [Google Scholar]
- 82.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, seuquence characteristics, and potential function of its novel members. Mol Membr Biol. 2001;18(4):247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 83.Keembiyehetty C, et al. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol. 2006;20(3):686–697. doi: 10.1210/me.2005-0010. [DOI] [PubMed] [Google Scholar]
- 84.Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279(16):16229–16236. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 85.Mobasheri A, et al. Expression of the GLUT1 and GLUT9 facilitative glucose transporters in embryonic chondroblasts and mature chondrocytes in ovine articular cartilage. Cell Biol Int. 2005;29(4):249–260. doi: 10.1016/j.cellbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 86.Shikhman AR, Brinson DC, Valbracht J, Lotz MK. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J Immunol. 2001;167(12):7001–7008. doi: 10.4049/jimmunol.167.12.7001. [DOI] [PubMed] [Google Scholar]
- 87.Preitner F, et al. GLUT9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106(36):15501–15506. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH. GLUT9 is differentially expressed and targeted in the preimplantation embryo. Endocrinology. 2004;145(3):1435–1443. doi: 10.1210/en.2003-1264. [DOI] [PubMed] [Google Scholar]
- 89.Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24(5–6):455–463. doi: 10.1080/09687680701298143. [DOI] [PubMed] [Google Scholar]
- 90.Bibert S, et al. Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Renal Physiol. 2009;297(3):F612–F619. doi: 10.1152/ajprenal.00139.2009. [DOI] [PubMed] [Google Scholar]
- 91.Anzai N, et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40):26834–26838. doi: 10.1074/jbc.C800156200. [DOI] [PubMed] [Google Scholar]
- 92.Guillam MT, Burcelin R, Thorens B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc Natl Acad Sci U S A. 1998;95(21):12317–12321. doi: 10.1073/pnas.95.21.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S, et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007;3(11):e194. doi: 10.1371/journal.pgen.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vitart V, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40(4):437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 95.Doring A, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40(4):430–436. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 96.Caulfield MJ, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5(10):e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallace C, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82(1):139–149. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brandstatter A, et al. Sex-specific association of the putative fructose transporter SLC2A9 variants with uric acid levels is modified by BMI. Diabetes Care. 2008;31(8):1662–1667. doi: 10.2337/dc08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stark K, et al. Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS ONE. 2009;4(11):e7729. doi: 10.1371/journal.pone.0007729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dinour D, et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010;21(1):64–72. doi: 10.1681/ASN.2009040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsuo H, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–751. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simkin PA. The Dalmatian defect: a hepatic endocrinopathy of urate transport. Arthritis Rheum. 2005;52(8):2257–2262. doi: 10.1002/art.21241. [DOI] [PubMed] [Google Scholar]
- 103.Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet. 2008;4(11):e1000246. doi: 10.1371/journal.pgen.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Preitner F, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106(36):15501–15506. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu X, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91(2):742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]