Abstract
Because issues of cost and bioavailability have hampered the development of gene-encoded antimicrobial peptides to combat infectious diseases, short linear peptides with high microbial cell selectivity have been recently considered as antibiotic substitutes. A new type of short antimicrobial peptide, designated temporin-SHf, was isolated and cloned from the skin of the frog Pelophylax saharica. Temporin-SHf has a highly hydrophobic sequence (FFFLSRIFa) and possesses the highest percentage of Phe residues of any known peptide or protein. Moreover, it is the smallest natural linear antimicrobial peptide found to date, with only eight residues. Despite its small size and hydrophobicity, temporin-SHf has broad-spectrum microbicidal activity against Gram-positive and Gram-negative bacteria and yeasts, with no hemolytic activity. CD and NMR spectroscopy combined with restrained molecular dynamics calculations showed that the peptide adopts a well defined non-amphipathic α-helical structure from residue 3 to 8, when bound to zwitterionic dodecyl phosphocholine or anionic SDS micelles. Relaxation enhancement caused by paramagnetic probes showed that the peptide adopts nearly parallel orientations to the micelle surface and that the helical structure is stabilized by a compact hydrophobic core on one face that penetrates into the micelle interior. Differential scanning calorimetry on multilamellar vesicles combined with membrane permeabilization assays on bacterial cells indicated that temporin-SHf disrupts the acyl chain packing of anionic lipid bilayers, thereby triggering local cracks and microbial membrane disintegration through a detergent-like effect probably via the carpet mechanism. The short length, compositional simplicity, and broad-spectrum activity of temporin-SHf make it an attractive candidate to develop new antibiotic agents.
Keywords: Antimicrobial Peptides, Cell Permeabilization, Evolution, Peptide Biosynthesis, Peptide Conformation, Calorimetry, Circular Dichroism, Phylogeny
Introduction
Cationic amphipathic antimicrobial peptides (AMPs)2 are central effector molecules of innate immunity that are conserved in all forms of life (1–3). AMPs rapidly kill a broad range of bacteria, yeasts, fungi, and protozoa by permeating and destroying their plasma membrane and/or inactivating intracellular targets (4). Because these peptides are less susceptible to the development of bacterial resistance, they have attracted considerable interest as a possible new generation of antibiotics, especially for antibiotic-resistant pathogens. One main drawback that hinders the therapeutic development of AMPs is their large size, comprising typically 12–100 amino acids. Short peptides that can be efficiently produced by chemical synthesis have emerged as prospective candidates in the clinical use of AMPs to produce large quantities of peptide at competitive costs, to enhance bioavailability and stability, and to reduce immunogenicity.
In vitro and in vivo studies have demonstrated that antimicrobial activities of AMPs are mediated by a complex and sensitive balance between various interrelated peptide parameters (including length, net charge, hydrophobicity, amphipathicity, degree of structure formation, and flexibility) and that the role of each parameter changes from one peptide to another (5–12). Thus, engineering peptides with low toxicity and high antimicrobial activity is actually restricted to a case-by-case study. Moreover, several studies have shown that some membrane activities of AMPs, such as permeabilization, are not dependent on specific amino acid sequences or three-dimensional structures, but to the interfacial activity of the peptide, i.e. its ability to partition into the membrane-water interface and to alter the packing and organization of lipids (13). This led to the hypothesis that a structure-based rational design of potent AMPs will not be efficient and that peptides might be more effectively selected from libraries with varying sequence composition rather than libraries designed with a particular structure in mind (13).
Another current and foreseeable strategies for discovering potent and short AMPs is to exploit the amazing properties of the skin peptidome of hylid and ranid frogs to produce gene-based combinatorial libraries of AMPs that are grouped into a superfamily termed dermaseptins (14–17). Peptides of the dermaseptin superfamily are genetically related, with a remarkable identity in signal sequences and intervening sequences of their prepro forms but have markedly diverged to yield several families of AMPs with different lengths, sequences, net charges, hydrophobicities, amphipathicities, and antimicrobial spectra. The impressive divergence between and within frog species is such that generally no single AMP from one species has been found with an identical amino acid sequence in another species; orthologous peptides, as well as paralogous peptides, have different amino acid sequences in different species, and often display differential biological activity.
Herein, we have used the conservation of the preproregion sequences of the preprodermaseptin transcripts to identify eight new members of the dermaseptin superfamily in the North African ranid frog Pelophylax saharica (family: Ranidae). One of these peptides, temporin-SHf, with sequence FFFLSRIFa, kills Gram-positive and Gram-negative bacteria and yeasts by permeating/disrupting the microbial membrane but does not resemble any antimicrobial peptide identified to date: (i) temporin-SHf is the smallest (8-mer) among the linear AMPs hitherto found in nature, (ii) it possesses the highest percentage of Phe residues (50%) of any known peptide or protein, and (iii) once bound parallel to the microbial membrane surface, it folds into a non-amphipathic hydrophobic α-helix. Owing to its short length, simple composition, and broad spectrum of antimicrobial activity, temporin-SHf is a promising candidate for the development of a new class of antimicrobial drugs.
EXPERIMENTAL PROCEDURES
Molecular Cloning of cDNA Precursors
One adult specimen of P. saharica frog (Béja, Tunisia) was anesthetized on ice and killed by decapitation according to the Association for Research in Vision and Ophtalmology resolution of the use of animals in research and the guidelines of the INSERM ethical committee on animal research. The skin was immediately removed, and small pieces of skin (130 mg) were pulverized in liquid nitrogen using a mortar and pestle. Poly(A)+ RNA was isolated from the frozen powder (Micro-FastTrack mRNA isolation kit, Invitrogen) and transcribed into cDNAs with an oligo(dT) primer (Advantage RT-for-PCR kit, Clontech) according to the protocol of the manufacturer. Different sets of PCR-specific primers were used: (a) 5′-TTCCTTGGGACCATCAACTTATCTC-3′/5′-GACATCTGTTGAGCATTTAGCCAAA-3′, matching the conserved signal peptide-coding region and the 3′-UTR of temporin precursors (18), (b) 5′-ATACACATTTGTGGTCCCAGTGAAA-3′/5′-GCATTTAGCTAAATGATATTCCACATCA-3′ (5′-UTR of brevinin precursors and conserved 3′-UTR of preprodermaseptins) (19), and (c) 5′-GTCTACATTCTCAGCACCAACTGAA-3′/5′-GCATTTAGCTAAATGATATTCCACATCA-3′ (conserved 5′- and 3′-UTR of preprodermaseptins). PCR was performed using the following cycling procedure: initial denaturation at 94 °C for 2 min; 30 cycles of 94 °C for 45 s; primer annealing for 1 min (56 °C for a and b, and 54 °C for c), extension at 72 °C for 3 min; final extension at 72 °C for 10 min. PCR products were gel-purified (NucleoSpin Extract, Macherey-Nagel Eurl, Hordt, France), cloned into the pGEM-T easy vector system (Promega, Madison, WI), and sequenced on both strands (Genome Express, Meylan, France).
Molecular Phylogeny
The predicted amino acid sequences of 51 known antimicrobial peptide precursors from the following ranid frogs were obtained from GenBankTM: Odorrana versabilis, Odorrana livida, Pelophylax plancyi, Pelophylax lessonae/ridibunda, Rana temporaria, Rana ornativentris, Rana sakuraii, Rana tagoi, Rana amurensis, Glandirana rugosa, Lithobates pipiens, Lithobates palustris, Lithobates catesbeianus, and P. saharica. For P. saharica, we used the predicted amino acid sequences of preprotemporin-SHa, -SHb, and -SHc that we have previously identified (18) and those of preprotemporin-SHd, -SHe, and -SHf obtained from this study. We aligned the predicted amino acid sequences of the different domains of the precursor polypeptides separately with ClustalW (20). Then the different aligned regions were joined manually, and final adjustments were made by eye. Molecular phylograms were constructed using maximum likelihood with PHYML (21). Levels of support for branches were estimated with bootstrapping methods.
Purification and Identification of Temporin-SHf
Peptides were extracted from the skin of P. saharica frog at 4 °C by homogenization in 10% acetic acid using a Polytron homogenizer. The homogenate was clarified from insoluble material by performing two centrifugations (10,000 × g, 20 min, 4 °C). The supernatant was lyophilized, dissolved in 0.1% trifluoroacetic acid/H2O, and centrifuged for 10 min at 13,000 × g, at 4 °C. The supernatant was then filtered (0.45 μm) and subjected to a rapid separation on a Sep-Pak C-18 cartridge eluted with 60% acetonitrile (ACN) in 0.1% trifluoroacetic acid/H2O. The prepurified extract (20 mg) was lyophilized, reconstituted in 0.1% trifluoroacetic acid/H2O, and fractionated by reversed phase-HPLC on a semi-preparative Nucleosil C-18 column (5 μm, 250 × 10 mm, Interchim SA, Montluçon, France) eluted at 4 ml/min with a 0–60% linear gradient of acetonitrile in 0.1% trifluoroacetic acid/water (1% ACN/min). Fractions corresponding to 4 ml were collected, lyophilized, and reconstituted in 500 μl of H2O. The prepurified skin extract was also analyzed by analytical HPLC on a Lichrospher C-18 column (modulo-cart QS, 5 μm, ODS2, 250 × 4.6 mm, Interchim SA) using the same conditions with a flow rate of 0.75 ml/min. Absorbance was monitored at 220 nm. Temporin-SHf was identified in semi-preparative HPLC fractions by MALDI-TOF mass spectrometry (Voyager DE-PRO Applied Biosystems, Courtaboeuf, France) using the matrix α-cyano-4-hydroxycinnamic acid. The mass spectrometry-positive ion spectra were carried out in the reflector mode with external calibration using the 4700 Standard kit. The primary structure of temporin-SHf was confirmed by MALDI-TOF-TOF (4700 Proteomic Analyzer, Applied Biosystems). The protonated molecule of the precursor was selected in the window (−5u,+5u) and fragmented using N2 as the collision gas (2 × 10−7 Torr, collision energy of 1 keV).
Solid-phase Peptide Synthesis of Temporin-SHf
Carboxamidated temporin-SHf was synthesized using a solid-phase FastMoc chemistry procedure on an Applied Biosystems 433A automated peptide synthesizer as described (22). The peptide was purified by reversed phase-HPLC on a Waters RCM compact preparative cartridge module (300 Å, 25 × 100 mm) eluted at a flow rate of 8 ml/min by a 20–70% linear gradient of ACN (0.07% trifluoroacetic acid) in 0.1% trifluoroacetic acid/water (1% ACN/min). The homogeneity and identity of the synthetic peptide were assessed by MALDI-TOF mass spectrometry (Voyager DE-PRO Applied Biosystems) and analytical reversed phase-HPLC on a Lichrospher 5-μm C-18 column using the above conditions with a flow rate of 0.75 ml/min.
Antimicrobial and Hemolytic Activities of Temporin-SHf
Gram-positive bacteria (Bacillus megaterium, Staphylococcus aureus, and Enterococcus faecalis), Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), yeasts (Candida albicans, C. parapsilosis, and Sacharomyces cerevisiae), and the fungus Aspergillus flavus were cultured as described previously (18). Logarithmic phase cultures of bacteria and yeasts were diluted in LB to an A630 of 0.01 (106 cfu/ml). For the fungal strain (A. flavus), spores were obtained after maceration and filtration of the filament, counted, and diluted to 106 cfu/ml in YPD (1% yeast extract, 2% peptone, 2% glucose). The minimal inhibitory concentrations (MICs) of peptides were determined by measuring the absorbance at 630 nm in 96-well microtitration plates by growing 50 μl of the microorganism suspension (106 cfu/ml) with 50 μl of 2-fold serial dilutions of synthetic temporin-SHf (200–1 μm) overnight at 37 °C (30 °C for the fungal strain) (18). Positive (0.7% formaldehyde) and negative (H2O) inhibition controls were also determined. MIC well contents (S. aureus and B. megaterium) were spread on agar plates and incubated overnight at 37 °C to distinguish between bacteriostatic and bactericidal effects. The hemolytic activity on human erythrocytes was also determined, as detailed previously (18). Results were expressed as the average value from three independent experiments performed in triplicate.
Temporin-SHf-induced Permeabilization of the Cytoplasmic Membrane of E. coli
The E. coli strain ML-35p was used to evaluate the ability of temporin-SHf to induce permeabilization of the cytoplasmic membrane of Gram-negative bacteria. E. coli ML-35p constitutively expresses cytoplasmic β-galactosidase and is lactose permease-deficient, which thus prevents the uptake of o-nitrophenyl-β-d-galactopyranoside (ONPG). Peptide-induced permeabilization of the cytoplasmic plasma membrane was measured by the rate of production of o-nitrophenol (ONP) at 405 nm, following hydrolysis of ONPG by β-galactosidase (23). Bacteria were grown to stationary phase at 37 °C in LB medium, centrifuged (1000 × g, 10 min, 4 °C), resuspended in phosphate-buffered saline buffer (10 mm phosphate, 100 mm NaCl, pH 7.3), washed twice, and diluted to an A630 of 0.35 in phosphate-buffered saline. 15 μl of the washed bacterial suspension was incubated with 135 μl of phosphate-buffered saline buffer containing 2.5 mm ONPG and different concentrations of peptide in 96-well microtitration plates. The hydrolysis of ONPG was followed by measuring the absorbance at 405 nm at 5-min intervals for 120 min at 37 °C using a Fluostar Galaxy plate reader (BMG Labtech, Champigny-sur-Marne, France). Complete permeabilization was induced in the presence of melittin (25 μm) or dermaseptin B2 (50 μm) as positive controls, and wells lacking peptides served as negative control. Results were expressed as the mean ± S.E. and were representative of three experiments carried out in triplicates.
CD Spectroscopy
CD spectra were recorded at 20 °C with a Jobin Yvon CD6 spectropolarimeter using a quartz cell of 1-mm path length. Spectra were acquired between 185 and 260 nm with a spectral bandwith of 2 nm and 0.5 nm steps. The instrument outputs were calibrated with d(+)-10-camphorsulfonic acid. Peptide was solubilized in H2O milliQ (concentration range from 10 to 100 μm), or in 80 mm SDS, or in 120 mm DPC micelles. Each spectrum was the average of four scans, and solvent spectra (H2O, 80 mm SDS, or 120 mm DPC) were subtracted, followed by baseline correction and smoothing. CD measurements are reported as Δϵ/n, where Δϵ is the dichroic increment and n is the number of residues in the peptide.
NMR Spectroscopy
All NMR samples were prepared in 550 μl of H2O/D2O (90:10, v/v) or D2O. Detergents were added at a concentration of 80 mm for SDS-d25 or 120 mm for DPC-d38 (Eurisotop, Saint-Aubin, France). The peptide concentration was 1.4 mm in water and micellar SDS and 2 mm in micellar DPC. Assuming an average number of 60 detergent molecules per micelle, this corresponds to a peptide:micelle ratio of around 1:1. Sodium 2,2-dimethyl-2-silapentane-5-sulfonate (Isotec, Sigma-Aldrich) was used as an internal reference for chemical shift calibration. The pH was set to 5.4 using microliter amounts of HCl 0.1 m. The NMR experiments were recorded on a Bruker Avance III spectrometer equipped with a 1H/13C/15N/2H Z-gradient TCI cryoprobe and operated at a 1H frequency of 500 MHz. Spectra were recorded at temperatures between 16 and 37 °C. NMR experiments were recorded and processed with Bruker TOPSPIN 2.0 program as described (24).3JHN-Hα coupling constants could not be measured in detergent solutions because of the large linewidths of amide protons. 3JHα-Hβ coupling constants were measured on F2 rows of two-dimensional total correlation spectroscopy experiments recorded in D2O with a long acquisition time. A zero-quantum filter was employed to provide essentially pure absorption lineshapes of total correlation spectroscopy cross-peaks (25). The 1H and 13C chemical shifts of temporin-SHf have been deposited in the BioMagResBank (www.brmb.wisc.edu) under accession number 20113. The chemical shift deviations (CSDs) of 1H and 13C resonances were calculated using a set of random coil values reported in water (26). The NMR samples used to monitor paramagnetic attenuations contained 1.1 mm temporin-SHf in the presence of 66 mm DPC-d38 in D2O. No significant chemical shift differences were observed with the previous samples used for the structure determination, showing the absence of concentration effect on peptide conformation and interaction with micelle. Titrations with Mn2+ ion were performed by stepwise additions of microliter amounts of a 50 mm solution in D2O, to a final concentration of 0.7 mm. Titrations with 16-doxylstearic acid (Sigma-Aldrich) were performed by stepwise additions of microliter amounts of a 650 mm stock solution in CD3OD, to a final concentration of 3.2 mm. Two-dimensional 1H-13C heteronuclear single quantum coherence spectra were recorded in the absence and in the presence of paramagnetic agents. A longer relaxation delay was used (3 s instead of 1 s in other two-dimensional experiments) to minimize the contribution of longitudinal relaxation enhancement that may cause an increase in cross-peak volumes for the first additions of paramagnetic reagent. Cross-peak volumes were integrated using the TOPSPIN program.
Molecular Mechanics Calculations
Interproton distance restraints were estimated from NOE cross-peak volumes on nuclear Overhauser effect spectroscopy spectra recorded with a short mixing time (50 ms) to limit spin diffusion. Five upper limit distance classes of 2.8, 3.3, 3.8, 4.3, and 5 Å were defined following cross-peak integration in XEASY and distance calibration within DYANA. χ1 torsion angles restraints were derived for a few residues from the analysis of intraresidual and sequential NOEs together with 3JHα-Hβ coupling constants. For each peptide, a set of 50 structures was calculated by torsion angle dynamics in DYANA using standard parameters (27). The best 25 structures having the lowest target function were then minimized using the XPLOR-NIH program (28) and CHARMM22 forcefield. Non-bond terms consisted of a Lennard-Jones potential and an electrostatic potential with a distance-dependent dielectric of ϵ = 4r. Structures were analyzed using InsightII (Accelrys, San Diego, CA) and PROCHECK-NMR programs (29). The distance restraints and NMR structures of temporin-SHf have been deposited within the BioMagResBank under accession number 20113.
DSC
DMPC and DMPG were purchased from Avanti Polar Lipids (Alabaster, AL). Differential scanning calorimetry (DSC) experiments were performed according to the procedure previously described (24). Briefly, 1 mg of DMPC or DMPG was dissolved in chloroform and chloroform/methanol (1:1), respectively. Lipid films were obtained by removing the solvent with a stream of nitrogen and drying the samples in vacuo for 3 h at 45 °C. MLVs (1 mg/ml) were formed by hydrating the dry lipid films with phosphate-buffered saline buffer (10 mm phosphate, 100 mm NaCl, pH 7.3) at 35 °C and vortexing until a homogenous suspension was formed. The multilamellar vesicles were frozen in liquid nitrogen and thawed in a water bath at ∼10 °C above Tm, and this process was repeated seven times. Different peptide/lipid molar ratios (1:100, 1:50, and 1:20) were tested. The DSC thermograms were recorded on a Nano III calorimeter (Calorimetry Sciences Corp., Lindon, UT) using a temperature range of 0–35 °C and heating and cooling rates of 0.5 °C/min and 1.5 °C/min, respectively. Ten scans were run for each sample, with a 10-min equilibration time between each scan. The raw data corresponding to the heating scans were converted to molar heat capacity using the corresponding average lipid molecular weight and a partial specific volume of 0.73 ml/g. Thermodynamic values (Tm and ΔH) were estimated with the software CpCalc.
RESULTS
Molecular Cloning and Identification of Temporin-SHf from the Skin Secretion of P. saharica
Reverse transcription-PCR analysis of skin mRNA from P. saharica using primers based on the conserved coding region or 5′-UTR, and 3′-UTR of known preprodermaseptins revealed eight cDNAs encoding 50- to 64-amino acid residue sequences ending with a stop codon. Five of these cDNA sequences (GenBankTM accession numbers FN557009, FN557010, FN557011, FN557012, and FN557013) encoded novel brevinins-1 whose primary structures were unknown (Fig. 1A). These were designated brevinin-1SHa, -1SHb, -1SHc, and -1SHd, according to the recommended nomenclature (30). A search in the Antimicrobial Peptide Data base, APD (aps.unmc.edu/AP/main.php) revealed that brevinin-1SHa and -1SHb share only 50% similarity with other members of the brevinin-1 family. In contrast, brevinin-1SHc and -1SHd display high similarities (87 and 96%, respectively) with brevinin-1E isolated from Pelophylax lessonae/ridibundus (previously, Rana esculenta) (31) and also found in P. saharica (32). Two of the other cDNAs encoded new putative antimicrobial peptides that are structurally related to the temporin family. Because we have previously isolated temporin-SHa, -SHb, and -SHc from P. saharica (18), the novel temporins were designated temporin-SHd (AM903075) and temporin-SHe (FN557008), respectively.
FIGURE 1.
A, predicted amino acid sequence (single-letter code) of precursors of temporin-SHd to -SHf from the skin of P. saharica. The open reading frame contains a part of the signal peptide (gray) followed by the acidic propiece, and the mature peptide (underlined). The G residue (italics) at the C-terminal of the mature peptide sequence serves as an amide donor after removal of the Lys residue (italics) with a carboxypeptidase. Deduced amino acid sequence of brevinin-1 precursor cDNAs from P. saharica are also indicated with the predicted mature brevinins-1(underlined). B, multiple sequence alignment of the amino acid sequences of polypeptide precursors representative of the esculentin-1 (E-1), esculentin-2 (E-2), brevinin-2 (B-2), ranatuerin-2 (Rt-2), palustrin-1 (P-1), brevinin-1 (B-1), nigrocin-2 (N-2), ranacyclin (Rc), and temporin (T) families of antimicrobial peptides from ranid frogs. Gaps (−) have been introduced to maximize sequence similarities. Identical (grey background) amino acid residues are highlighted. Cysteine residues that form disulfide bonds are indicated (black background). Residues G or GK (italics) at the end of the mature peptide sequence serve for C-terminal amidation.
The remaining cDNA sequence (AM903076) encoded a short, Phe-rich peptide that does not resemble any of the antimicrobial peptides identified to date (Fig. 1, A and B). The corresponding prepropeptide has the characteristic signature of the preproforms of the dermaseptin superfamily, including a signal peptide ending with a Cys residue, followed by a 25-residue acidic propiece that ends in a typical prohormone processing signal Lys-Arg that precedes the single downstream copy of the mature peptide progenitor sequence, FFFLSRIFGK. The progenitor sequence was terminated by a Gly-Lys dipeptide, suggesting that C-terminal amidation may be involved in production of the final peptide. Extracts from the skin of P. saharica were analyzed by reversed phase-HPLC to assess the presence of the predicted Phe-rich peptide and the status of its C terminus in vivo (Fig. 2A). MALDI-TOF analysis of semi-preparative HPLC fractions revealed the presence of anion product ([M+H]+ = 1075.6) eluting at ∼49% acetonitrile (fraction at 52 min) (Fig. 2B and Table 1). The primary structure of the corresponding peptide, FFFLSRIFamide, was established by MALDI-TOF-TOF from collision-induced dissociation of the parent ion ([M+H]+, 1075.6 m/z) and confirmed by the presence of the two-fragmentation series b and y (data not shown). The nature of Leu and Ile residues was determined by the presence of w ions (m/z 575 and 233).
FIGURE 2.
Identification of temporin-SHf in the skin of P. saharica frog. A, fractionation of an acidic extract of P. saharica skin by reversed-phase HPLC on an analytical Lichrospher 5-μm C-18 column. The solvent system used was composed of H2O-0.1% trifluoroacetic acid (solvent A) and ACN containing 0.07% trifluoroacetic acid (solvent B). After 3-min equilibration with solvent A, separation was performed with a 0–60% linear gradient of solvent B (1%/min) at a flow rate of 0.75 ml/min. Absorbance was monitored at 220 nm. B, MALDI-TOF mass spectrometry analysis of semi-preparative HPLC fractions. HPLC fractions (4 ml) were obtained after separation of the acidic extract of P. saharica skin on a reversed-phase Nucleosil 5-μm C-18 column eluted with a 0–60% linear gradient of solvent B (1%/min) at a flow rate of 4 ml/min. The mass spectrometry spectrum of fraction at 52 min (∼49% ACN) is shown and reveals a mass product ([M+H]+, 1075.6 m/z) corresponding to temporin-SHf indicated by a circle.
TABLE 1.
Amino acid sequence and physico-chemical properties of temporin-SHf
a Deduced from the cDNA precursor and confirmed by MALDI-TOF-TOF. Hydrophobic residues are colored in gray. a: amide.
b Calculated and
c observed monoisotopic masses. Mobs value is deduced from the protonated molecule ([M+H]+).
d Mean relative hydrophobic moment and
e mean hydrophobicity were calculated using the HydroMCalc software by Alex Tossi and Luca Sandri (www.bbcm.univ.trieste.it/∼tossi/HydroCalc/HydroMCalc.html) with the CSS scale.
f Temporin-SHf forms an α-helix in a membrane environment (see “Results”), but polar and apolar residues are not segregated on opposing surfaces along the long axis of the helix. Hydrophobic residues are colored in gray.
We examined the relationships between this new Phe-rich AMP and AMPs of ranid frogs by constructing phylogenetic trees with the maximum likelihood method from alignments of antimicrobial peptide precursors from ranids of the genera Odorrana (O. versabilis and O. livida), Pelophylax (P. plancyi, P. lessonae/ridibunda, and P. saharica), Rana (R. temporaria, R. ornativentris, R. sakuraii, R. tagoi, R. amurensis, and R. chensinensis), Glandirana (G. rugosa), and Lithobates (L. pipiens, L. palustris, and L. catesbeiana). As shown in Fig. 1B, all these antimicrobial peptide precursors belonging to the dermaseptin superfamily (14, 19) have a strongly conserved preproregion, but the sequences of the mature antimicrobial peptides are highly variable both between and within species. The phylogenic reconstruction shown in Fig. 3 indicates that the 50 precursor sequences fall into 9 clades corresponding to known antimicrobial peptide families (30), i.e. nigrocin-2, brevinin-1, temporin, brevinin-2, ranatuerin-2, ranacyclin, esculentin-1, palustrin-1, and esculentin-2. The Phe-rich peptide and all temporins from P. saharica cluster together monophyletically in a single group (bootstrap value of 85) within the temporin clade, suggesting that they arose from multiple gene duplication within this lineage (16). Despite the atypical amino acid sequence of the Phe-rich peptide, the phylogenetic reconstruction is consistent with the proposal that this peptide should be regarded as a new member of the temporin family of antimicrobial peptides (33) and therefore designated temporin-SHf.
FIGURE 3.
Molecular phylogram of antimicrobial peptide precursors from ranid frogs constructed with the maximum likelihood method. Maximum parsimony and LogDet analyses yielded the same ordinal phylogeny. Numbers at nodes are bootstrap values based on 100 replicates. Preprodermaseptin B2 from Phyllomedusa bicolor was assigned as the out-group (16). Frogs belonging to the Ranidae family are described by the species name set out in research.amnh.org/herpetology/amphibia/index.php (D. R. Frost, 2007, Amphibian Species of the World: an Online Reference. Version 5.1, American Museum of Natural History, New York). Nomenclature adopted for antimicrobial peptides follows recent guidelines (36). Note that the names lividin-1, chensirin-2, ranalexin, and gaegurin-5 are synonymous with brevinin-1. AMA polypeptide-1, -2, and -3, amurin-2, chensirin-1, and pelophylaxin-4 are synonymous with temporin. The name peptide LR is synonymous with ranacyclin.
Sequence Analysis of Temporin-SHf
Although genetically related to other members of the temporin family and to other family members of the dermaseptin superfamily, temporin-SHf exhibits unique structural characteristics (Table 1). A comparison of the sequence of temporin-SHf with those of temporins from other ranid frogs revealed no more than 38% identity. This cationic peptide (net charge of +2) is the smallest (8-mer) of the naturally occurring linear AMPs. It is unusually rich in Phe (50%) and has a highly hydrophobic amino acid sequence with 6 out of 8 bulky aliphatic and aromatic residues. Furthermore, the amino acid sequence of temporin-SHf exhibits neither primary amphipathicity nor secondary amphipathicity (5). When plotted on a Schiffer-Edmunson α-helical wheel, this peptide had a non-amphipathic helical structure with polar and apolar residues that do not segregate on opposite faces of the helical cylinder.
Antimicrobial and Hemolytic Activities of Temporin-SHf
The antimicrobial activity of temporin-SHf was assayed against Gram-negative and Gram-positive bacteria, yeasts, fungi, and human protozoa. As shown in Table 2, temporin-SHf displays a broad-spectrum activity against Gram-positive and Gram-negative bacteria and yeasts. The peptide was highly active against B. megaterium, S. aureus, E. coli (ATCC25922 and ML-35p), and S. cerevisiae with MIC in the range 3–30 μm, and also, but to a lesser extent, against E. faecalis, C. albicans, and C. parapsilosis (MIC = 50 μm). No visible colony was observed when MIC well contents (S. aureus and E. coli ATCC 25922) were spread on agar plates (37 °C, overnight), indicating that the peptide was bactericidal. However, the peptide was only weakly active, or devoid of activity against the Gram-negative bacteria E. coli D21, E. coli ATCC 35218, and P. aeruginosa and toward the filamentous fungus A. flavus. Temporin-SHf had no activity against the promastigote (insect stage) and axenic amastigote (mammalian stage) forms of the human protozoan parasite Leishmania infantum, in the peptide concentration range tested (0.94–60 μm) (Table 2). Interestingly, temporin-SHf is not cytotoxic against human erythrocytes (LC50 = 200 μm), with a hemolytic activity detected only at peptide concentration far above the MIC values determined for the sensitive strains (3–50 μm) (Table 2).
TABLE 2.
Antimicrobial and hemolytic activities of temporin-SHf
| MICa | |
|---|---|
| μm | |
| Gram-negative bacteria | |
| E. coli ATCC 25922 | 25 |
| E. coli ATCC 35218 | >200 |
| E. coli D21 | 100 |
| E. coli ML-35p | 30 |
| P. aeruginosa ATCC 27853 | >200 |
| Gram-positive bacteria | |
| S. aureus ATCC 25923 | 12.5 |
| E. faecalis ATCC 29212 | 50 |
| B. megaterium | 3 |
| Yeasts | |
| C. albicans ATCC 90028 | 50 |
| C. parapsilosis ATCC 22019 | 50 |
| S. cerevisiae | 12.5 |
| Fungus | |
| A. flavus | >200 |
| Leishmania protozoan | |
| L. infantumpromastigotes | NAb |
| L. infantumamastigotes | NA |
| Human erythrocytes | LC50a (μm) 200 |
a MICs and LC50 (concentration of peptide producing 50% hemolysis) are expressed as average values from three independent experiments performed in triplicate.
b NA, not active in the range of 0.94–60 μm.
Permeabilization/Disruption of the Bacterial Cytoplasmic Membrane
Temporin-SHf-induced permeabilization of the bacterial cytoplasmic membrane was revealed by incubating E. coli ML-35p with the peptide (2–100 μm) and by measuring the time-dependent hydrolysis of the small chromogenic substrate ONPG into ONP by cytoplasmic β-galactosidase (Fig. 4). Dermaseptin B2 (50 μm) and melittin (25 μm) used as positive controls induced rapid and similar kinetics of ONP production. At peptide concentrations equal to, or above the MIC, temporin-SHf permeabilized the cytoplasmic membrane of E. coli ML-35p, as shown by the increasing hydrolysis of ONPG with time. At the MIC (30 μm), ∼85% of hydrolysis was observed after 120 min incubation compared to the positive controls. To test whether the bacterial cytoplasmic membrane was disrupted by temporin-SHf and became leaky to large cytoplasmic components such as β-galactosidase, E. coli ML-35p cells were incubated with the peptide (30 and 100 μm) for 60 min, and the β-galactosidase activity was measured after removing bacteria by centrifugation and adding ONPG to the supernatant. As above, ONP production was observed at peptide concentrations equal to, or above the MIC (data not shown), suggesting that membrane permeabilization/disruption of bacteria by temporin-SHf induces the leakage of the intracellular content and is concomitant with cell death. A similar behavior was observed for dermaseptin B2 (10 μm) but not for melittin (10 μm), two antimicrobial peptides known to act via distinct mechanisms (detergent effect and pore formation, respectively).
FIGURE 4.
Kinetics of the cytoplasmic membrane leakage of E. coli ML-35p after treatment with increasing concentrations (2, 4, 10, 15, 30, and 100 μm) of temporin-SHf (top) and with 25 μm melittin or 50 μm dermaseptin B2 as positive controls (bottom). The negative control (without peptide) is also shown. The membrane leakage was followed by measuring the hydrolysis of ONPG at 405 nm by the cytoplasmic bacterial β-galactosidase after incubation with the peptides (see “Experimental Procedures”). Data are expressed as the mean ± S.E.
Comparing the Interaction of Temporin-SHf with Zwitterionic and Anionic Model Membranes by DSC
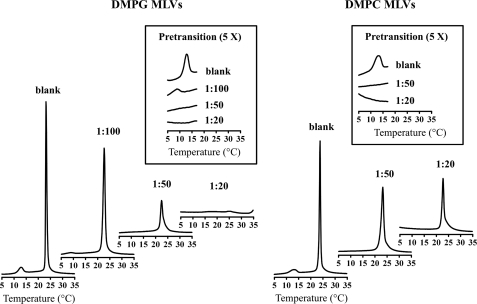
DSC was used to study the thermotropic behavior of multilamellar vesicles upon addition of temporin-SHf. DMPC, a zwitterionic phospholipid, was used as a model for mammalian cell membranes. DMPG was chosen as a model for negatively charged bacterial membranes. In the absence of peptide, DMPC and DMPG MLVs exhibit two endothermic events on heating, i.e. a weakly energetic pretransition near 13 °C (conversion of the ordered lamellar gel phase Lβ′, with tilted hydrocarbon chains, to the ordered rippled gel phase Pβ′) and a strongly energetic and highly cooperative main transition near 23 °C (DMPG) or 24 °C (DMPC) (conversion of the rippled gel phase to the fluid lamellar liquid-crystalline phase Lα) (35, 36) (Fig. 5). The pretransition is due to interactions between the headgroups of the phospholipids, and increasing the distance between the headgroups eliminates these interactions and causes the pretransition peak to disappear. The main phase transition (chain melting) is mainly due to trans-gauche isomerization of the acyl chains, which decreases the acyl chain packing of the lipid molecules and increases fluidity of the membrane. Therefore, the effect of added peptide on the temperature (Tm), enthalpy (ΔH), and cooperativity(ΔT½) of the pretransition, and the main transition was monitored as an indicator of the ability of the peptide to interact with lipid headgroups and to perturb the packing of the lipid acyl chains, respectively (37–39). The addition of increasing quantities of peptide onto the DMPC and DMPG bilayers lowers the temperature, the enthalpy, and the cooperativity of the pretransition, abolishing it entirely at peptide/lipid ratios starting from 1:50 (Fig. 5 and Table 3). This suggests that the interaction of the peptides with the lipid headgroups abolishes hydrocarbon chain tilt in the gel phase bilayer, causing the replacement of the Lβ′ and Pβ′ phases with a disordered Lβ-like phase.
FIGURE 5.
High sensitivity DSC heating scans illustrating the effect of temporin-SHf on anionic DMPG (left panel) and zwitterionic DMPC (right panel) MLVs. The corresponding pretransition regions are shown on a larger scale (insets). Scans were acquired without peptide (blank) and at different peptide/lipid molar ratios (1:100, 1:50, and 1:20).
TABLE 3.
Thermotropic behavior of anionic DMPG and zwitterionic DMPC multilamellar vesicles upon addition of temporin-SHf
Phase transition temperature (Tm) and total enthalpy for gel-to liquid crystalline transition of MLVs with and without (blank) temporin-SHf are indicated. Phase transition temperature and total enthalpy were estimated by a peak-fitting procedure using CPCalc software. Values were obtained from six scans and represent the mean ± S.E.
| Pretransition |
Main transition |
|||
|---|---|---|---|---|
| Tm | ΔH | Tm | ΔH | |
| °C | kcal/mol | °C | kcal/mol | |
| DMPG blank | 12.8 ± 0.02 | 0.9 ± 0.02 | 23.2 ± 0.02 | 7.6 ± 0.02 |
| Peptide/DMPG, 1:100 | 8.8 ± 0.02 | 0.2 ± 0.00 | 22.6 ± 0.00 | 7.9 ± 0.27 |
| Peptide/DMPG, 1:50 | 22.4 ± 0.00 | 3.3 ± 0.02 | ||
| Peptide/DMPG, 1:20 | ||||
| DMPC blank | 13.0 ± 0.02 | 0.9 ± 0.05 | 23.9 ± 0.00 | 9.2 ± 0.36 |
| Peptide/DMPC, 1:50 | 23.2 ± 0.00 | 10.5 ± 0.18 | ||
| Peptide/DMPC, 1:20 | 22.8 ± 0.02 | 6.7 ± 0.07 | ||
Temporin-SHf has very different perturbing effects on the main lipid-phase transition of zwitterionic and negatively charged MLVs. As shown in Fig. 5 and Table 3, the peptide affects only slightly the main transition of DMPC vesicles, with a small decrease (27%) in the enthalpy of the main peak with a peptide/lipid molar ratio up to 1:20. Conversely, at the same peptide/lipid ratio, the main lipid-phase transition was completely abolished for DMPG MLVs, and a pronounced decrease (57%) of the enthalpy was already observed at a peptide:lipid molar ratio of 1:50, compared with DMPC. These results indicate that temporin-SHf strongly and selectively perturbs anionic bilayers membranes by interacting with the polar headgroups and the glycerol backbone region of the phospholipids, hence disrupting the acyl chain packing of the bilayer.
Secondary Structure of Membrane-bound Temporin-SHf Determined by CD Spectroscopy
To investigate the secondary structure of temporin-SHf, we first performed a CD study of the peptide in water, micellar SDS, and micellar DPC solutions (Fig. 6). The CD spectrum of temporin-SHf in aqueous solution shows a strong negative band around 191 nm that is characteristic of unordered peptides. A quite intense positive band is also observed around 219 nm, which might suggest a significant population of the polyproline II helix conformation (40). However, the dichroic signal in the region around 220 nm is likely to be dominated by a positive contribution of Phe side-chain chromophores (41), due to the large number of these aromatic residues in the sequence (4 Phe out of 8 residues).
FIGURE 6.
CD spectra of temporin-SHf (100 μm) in H2O (thin solid line), 80 mm SDS solution (dotted line), and 120 mm DPC solution (thick solid line) at 20 °C.
The CD spectra in membrane-mimetic environments (SDS or DPC solutions) exhibit very distinct shapes from that observed in aqueous solution, with a strong positive band around 195 nm and a strong negative band near 206 nm, that are indicative of the presence of ordered conformations. Such signatures are reminiscent of the spectra of helical peptides, but the expected second minimum around 222 nm is not clearly visible. As aforementioned, this atypical signature might arise from a strong positive contribution of aromatic Phe side chain in the 220 nm region that partially cancels the negative contribution of the peptidic chromophore in a helical environment.
Three-dimensional Structure of Membrane-bound Temporin-SHf by NMR Spectroscopy
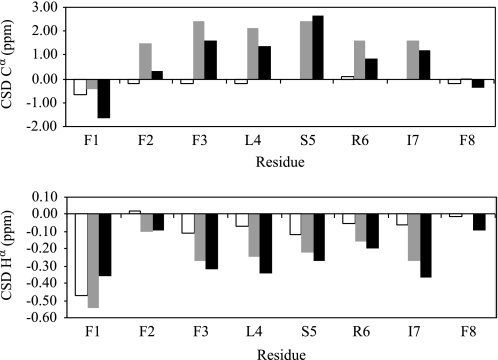
Because the CD spectra exhibited rather atypical signatures, the conformation of temporin-SHf in water and in micellar environments was further investigated by NMR spectroscopy. 1H and 13C sequence-specific resonance assignments were obtained from the analysis of two-dimensional total correlation spectroscopy and nuclear Overhauser effect spectroscopy homonuclear experiments, and two-dimensional heteronuclear1H-13C heteronuclear single quantum coherence spectra. The CSDs of 1Hα and 13Cα resonances, corresponding to the differences between observed chemical shifts and values observed for the same residues in random coil state, were used as indicators of local conformational preferences of each residue (Fig. 7). Most residues of temporin-SHf show near zero CSD values in water, indicating that the peptide does not adopt preferential conformations. In contrast, most residues exhibit in micellar environments an upfield shift of their 1Hα resonance (negative CSDs below −0.1 ppm) and a downfield shift of their 13Cα resonance (positive CSDs above 1 ppm), indicative of turn or helical conformations.
FIGURE 7.
1Hα and 13Cα CSDs of temporin-SHf in water (white bars), in 80 mm SDS (gray bars), and in 120 mm DPC (black bars).
The structure of temporin-SHf in micellar environments was further investigated by analysis of intraresidual, sequential, and medium-range NOEs in DPC (Fig. 8). N-terminal residues Phe1 and Phe2 mainly adopt extended conformations as shown by strong sequential Hαi-HNi+1 NOE correlations. Residues in segment 3–8 are characterized by weaker sequential Hαi-HNi+1 NOE correlations together, with stronger intraresidual Hαi-HNi NOEs and stronger sequential HNi-HNi+1 NOEs, indicating that they preferentially explore the α-helical region of the Ramachandran diagram. Furthermore several medium-range NOEs are observed throughout the 3–8 segment, in particular HNi-HNi+2, Hαi-HNi+3, and Hαi-Hβi+3 correlations. These NOEs are typical of a helical conformation encompassing the 3–8 segment. The monitoring of proton exchange in D2O revealed that the amide proton of Ile7 has slow exchange kinetics in comparison with other amide protons, because it is the only proton still observed after several hours in D2O (2- to 8-h interval at 16.5 °C). This suggests that the amide proton of Ile7 is engaged in a more stable hydrogen bond than other amide protons. Temporin-SHf exhibited very similar NOEs in SDS micelles (not shown). However, the NMR spectra were of lower quality owing to signal broadening.
FIGURE 8.
NOE connectivities of temporin-SHf (2 mm) in 120 mm DPC. The relative intensity of NOEs is indicated by horizontal bars of varying thickness.
The NMR structures of temporin-SHf in DPC (Fig. 9) were calculated by restrained molecular dynamics in DYANA using a set of 85 NOEs (22 intraresidual, 34 sequential, and 29 medium-range) and subsequently minimized within XPLOR-NIH with the CHARMM22 forcefield. The root mean square deviation of all backbone atoms is 0.85 Å, and the structures do not display distance violations greater than 0.1 Å. Segment 3–8 forms a well defined helix, whereas the N-terminal Phe1 and Phe2 residues have disordered conformations. In the calculated structures, the amide protons of Ile7 and Phe8 are engaged in hydrogen bonds with the carbonyl groups of Phe3 and Leu4, respectively. The helical turn is stabilized by numerous van der Waals interactions between Phe3, Leu4, Ile7, and Phe8 side chains, as shown by 18 NOEs involving their side-chain protons. The analysis of 3JHα-Hβ coupling constants of residues Leu4, Ile7, and Phe8 showed that these residues adopt a major rotamer around the χ1 angle, corresponding to a gauche− conformation (χ1 ∼ −60°).
FIGURE 9.
NMR structure family of temporin-SHf in micellar DPC. A, all backbone atoms; B, all backbone atoms and heavy atoms of residues 3–8. Structures were superimposed by best fitting of N, Cα, and C′ atoms of residues 3–8. Atoms are shown with different colors (amide hydrogen in white, carbon in green, nitrogen in blue, and oxygen in red). N and C, N and C termini.
The position of temporin-SHf relative to the DPC micelle surface was investigated using two paramagnetic probes, Mn2+, which is located in the aqueous phase, and 16-doxylstearic acid, which is embedded in the micelle interior. The spatial proximity of nuclear spins with the paramagnetic agent leads to resonance broadening due to strong enhancements of the transversal relaxation. These paramagnetic enhancements were monitored by measuring the residual volumes of 1H-13C cross-peaks on two-dimensional heteronuclear single quantum coherence spectra. Under our experimental conditions, the Mn2+ ion selectively affected the resonances of DPC molecule in the polar headgroup and the first methylene groups of the alkyl chain (residual volumes smaller than 40%, data not shown). In contrast 16-doxylstearic acid affected mainly the alkyl chain of DPC with marginal effect on the polar headgroup (residual volumes larger than 60%). The 16-doxylstearic acid probe causes broadening of temporin-SHf resonances indicating that the peptide strongly interacts with DPC micelle. The residues of temporin-SHf show different sensitivities to the paramagnetic probes, depending on their sequence position (Fig. 10). Residues Phe3, Leu4, and Phe8 are strongly affected by the 16-doxylstearic acid probe, with residual volumes smaller than 40%, indicating that these residues are embedded within the DPC micelle. The largest relaxation effect observed on residue Phe3 shows that this residue is able to deeply penetrate in the micelle interior. The resonances of temporin-SHf are also sensitive to the water-soluble Mn2+ probe (Fig. 10), indicating that the peptide remains close to the surface. The largest attenuation is observed for the C-terminal Phe8 residue, other residues being less affected. Nevertheless small periodic variations are observed in segment 1–7, the residual volume profile showing slightly increased sensitivity on positions 2 and 5.
FIGURE 10.
Attenuations of temporin-SHf resonances induced by paramagnetic probes. The relaxation enhancements induced by Mn2+ (0.7 mm) or 16-doxylstearic acid (3.2 mm) are shown in blue and red, respectively. The volumes of CHα and CHβ cross-peaks on two-dimensional 1H-13C heteronuclear single quantum coherence spectra were compared with corresponding correlations on a reference spectrum in the absence of paramagnetic agent. For diastereotopic methylene groups, the average of both peaks is given. Missing attenuations are due to peak overlap.
DISCUSSION
Aromatic residues appear to play special roles in protein-membrane interactions because of their interface-seeking properties. Clusters of basic and aromatic residues in general are found in a wide range of membrane-associated proteins involved in signal transduction, and Phe residues are often associated with basic residues in peripheral membrane proteins (42). Compared with Leu, Phe has ∼12% larger hydrophobic surface and, as opposed to Trp and Tyr, has no preference for the interface over the core of the membrane. For instance, Phe has much higher occurrence than Trp and Tyr in the transmembrane segment of membrane proteins (42–44). In addition, the aromatic ring of Phe can participate in cation-π interactions.
Statistical analysis of AMPs has shown that they exhibit varying sequence length (12–100 amino acids) and contain, on the average, close to 50% polar/charged and 50% apolar residues (5). Leu, Ala, Gly, Cys, and Lys are particularly well represented in AMPs. Some AMPs or AMPs families distinguish themselves from other AMPs, because they are rich in one amino acid, i.e. His, Gly, Trp, Pro, Cys, and Glu (The Antimicrobial Peptide Data base, aps.unmc.edu/AP/main.php). The high aromatic content in these AMPs is essentially due to the Trp residue, such as indolicidin, a Trp-rich antimicrobial tridecapeptide containing 5 Trp residues (45). In contrast, the relative frequency of Phe is low, showing no predisposition of this residue for AMPs. For instance, clavanin, which is considered as a Phe-rich peptide, contains only 5 Phe out of 23 residues, and the role this amino acid plays in the membrane-binding and antimicrobial properties of this peptide is still unclear (46). Prophenin, a 79-residue AMP originally isolated from porcine leukocytes, has a particularly high content of Pro (42 residues) and is also considered as Phe-rich with only 15 Phe (47). Temporin-SHf (8-mer) is the smallest linear AMP isolated from a natural source, presents a highly hydrophobic sequence (75% apolar residues) with a prominent apolar patch in its N-terminal part, and possesses the highest percentage of Phe residues (50%) of any known peptide or protein. As a first family member of Phe-rich AMPs, temporin-SHf is an unexpected addition to the structurally diverse peptides encoded by genes belonging to the dermaseptin superfamily.
The NMR data clearly indicate that, despite its small size, temporin-SHf adopts a well defined helix encompassing residues 3–8 when bound to DPC micelles. The structure is stabilized by a compact hydrophobic core on one face that penetrates in the micelle interior, as shown by NMR studies with paramagnetic agents. The comparison of CD spectra and NMR parameters, including CSDs of Hα and Cα resonances and characteristic NOEs, shows that temporin-SHf adopts similar structures in micellar SDS and DPC. A minor difference is observed for the Cα CSD of Phe2 that may indicate that this residue explores less extended conformations in SDS. The observation of similar structures in both types of micelles indicates that the secondary structure stabilization is mainly driven by interactions with polar headgroups and hydrophobic interactions with the alkyl chains and that electrostatic interactions with anionic headgroups are not a prerequisite for structure stabilization. The relaxation enhancements induced by the water-soluble and the lipophilic paramagnetic probes give information on the depth of immersion of temporin-SHf residues within the micelle. Residues Phe3, Leu4, and Phe8 are able to deeply penetrate into the micelle interior, as shown by their high sensitivity to the lipophilic paramagnetic probe. In contrast, the sensitivity of the C-terminal Phe8 residue to Mn2+ probe shows that this residue lies closest to the surface. Information on the peptide orientation with respect to the micelle surface can be obtained from the paramagnetic relaxation enhancements. However, these effects must be interpreted cautiously, because the time averaging makes them sensitive to the peptide conformational flexibility and to dynamic equilibria affecting the peptide orientation and depth of immersion within the micelle. Thus, the sensitivity of Phe8 to both probes can be interpreted on the basis of a dynamical equilibrium modifying its position at the water-micelle interface. The paramagnetic enhancements could therefore be explained by a parallel orientation of temporin-SHf with respect to the surface, with translational motions outward causing the C terminus to lie closer to the surface. Alternatively, the peptide may adopt more tilted orientations that would position the Phe3 and Leu4 residues deeply inside the micelle.
Despite the fact that the peptide chain of temporin-SHf is very short, its mode of antimicrobial action involves binding and penetration into the bilayer, causing disruption of acyl chain packing, efflux of large molecules and microbial cell death, similar to that of many long AMPs (23, 48, 49). In terms of a mechanistic model, temporin-SHf is not long enough to form toroidal pores through the target membrane. Instead, results from DSC and NMR spectroscopy studies suggest that the peptide destabilizes anionic lipids through a detergent-like effect, probably via the carpet mechanism (50, 51), i.e. the peptide accumulates on the surface of the membrane oriented parallel to the plane of the membrane until a threshold concentration has been reached, followed by membrane permeation/disintegration. Penetration into the bilayer is necessary but not sufficient to cause membrane disruption. Small highly hydrophobic peptides that partition deeply into bilayers have been shown not to cause membrane leakage even at high peptide concentrations, because they were not able to disrupt lipid packing (34). Placing the amino acid sequence of temporin-SHf on a Schiffer-Edmunson wheel projection reveals the peptide helix to be very poorly amphipathic, with the two polar Ser5 and Arg6 residues not gathered in the same part of the hydrophobic surface of the helical cylinder (Table 1). Introduction of two polar residues within a highly hydrophobic peptide helix that can otherwise insert deep into the acyl core region of the membrane allows modification of size and shape of the helix apolar patch. This would presumably reinforce the perturbing efficacy of temporin-SHf, hence its membrane-damaging activity (42).
Temporin-SHf has low micromolar broad-spectrum, sterilizing antimicrobial activity with no hemolytic activity. Its small size and compositional simplicity make it a promising model compound for the rational development of new antibiotics.
Acknowledgments
We thank Dr. G. Bolbach and G. Clodic (Plate-forme de Spectrométrie de Masse et Protéomique, IFR83, Université Pierre et Marie Curie, France) for tandem mass spectrometry analysis and Drs. B. Oury and D. Sereno (UR16, Institut de Recherche pour le développement Montpellier, France) for leishmanicidal tests. The E. coli strain ML-35p was kindly provided by Prof. S. Rebuffat (FRE3206 CNRS/Muséum National d'Histoire Naturelle, Paris, France).
- AMP
- antimicrobial peptide
- ACN
- acetonitrile
- CSD
- chemical shift deviation
- DSC
- differential scaning calorimetry
- DMPG
- dimyristoyl phosphatidylglycerol
- DMPC
- dimyristoyl phosphatidylcholine
- DPC
- dodecyl phosphocholine
- MALDI-TOF
- matrix-assisted laser desorption-time of flight
- MIC
- minimal inhibitory concentration
- MLV
- multilamellar lipid vesicle
- HPLC
- high performance liquid chromatography
- ONPG
- o-nitrophenyl-β-d-galactopyranoside
- ONP
- o-nitrophenol
- NOE
- nuclear Overhauser effect.
REFERENCES
- 1.Radek K., Gallo R. (2007) Semin. Immunopathol. 29, 27–43 [DOI] [PubMed] [Google Scholar]
- 2.Jenssen H., Hamill P., Hancock R. E. (2006) Clin. Microbiol. Rev. 19, 491–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zasloff M. (2002) Nature 415, 389–395 [DOI] [PubMed] [Google Scholar]
- 4.Nicolas P. (2009) FEBS J. 276, 6483–6496 [DOI] [PubMed] [Google Scholar]
- 5.Dennison S. R., Wallace J., Harris F., Phoenix D. A. (2005) Protein Pept. Lett. 12, 31–39 [DOI] [PubMed] [Google Scholar]
- 6.Papo N., Shai Y. (2003) Peptides 24, 1693–1703 [DOI] [PubMed] [Google Scholar]
- 7.Powers J. P., Hancock R. E. (2003) Peptides 24, 1681–1691 [DOI] [PubMed] [Google Scholar]
- 8.Dathe M., Meyer J., Beyermann M., Maul B., Hoischen C., Bienert M. (2002) Biochim. Biophys. Acta 1558, 171–186 [DOI] [PubMed] [Google Scholar]
- 9.Tachi T., Epand R. F., Epand R. M., Matsuzaki K. (2002) Biochemistry 41, 10723–10731 [DOI] [PubMed] [Google Scholar]
- 10.Giangaspero A., Sandri L., Tossi A. (2001) Eur. J. Biochem. 268, 5589–5600 [DOI] [PubMed] [Google Scholar]
- 11.Bechinger B. (1999) Biochim. Biophys. Acta 1462, 157–183 [DOI] [PubMed] [Google Scholar]
- 12.Blondelle S. E., Lohner K., Aguilar M. (1999) Biochim. Biophys. Acta 1462, 89–108 [DOI] [PubMed] [Google Scholar]
- 13.Rathinakumar R., Wimley W. C. (2008) J. Am. Chem. Soc. 130, 9849–9858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolas P., El Amri C. (2009) Biochim. Biophys. Acta 1788, 1537–1550 [DOI] [PubMed] [Google Scholar]
- 15.Amiche M., Ladram A., Nicolas P. (2008) Peptides 29, 2074–2082 [DOI] [PubMed] [Google Scholar]
- 16.Nicolas P., Vanhoye D., Amiche M. (2003) Peptides 24, 1669–1680 [DOI] [PubMed] [Google Scholar]
- 17.Vanhoye D., Bruston F., Nicolas P., Amiche M. (2003) Eur. J. Biochem. 270, 2068–2081 [DOI] [PubMed] [Google Scholar]
- 18.Abbassi F., Oury B., Blasco T., Sereno D., Bolbach G., Nicolas P., Hani K., Amiche M., Ladram A. (2008) Peptides 29, 1526–1533 [DOI] [PubMed] [Google Scholar]
- 19.Amiche M., Seon A. A., Pierre T. N., Nicolas P. (1999) FEBS Lett. 456, 352–356 [DOI] [PubMed] [Google Scholar]
- 20.Labarga A., Valentin F., Anderson M., Lopez R. (2007) Nucleic Acids Res. 35, W6–W11; web server issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guindon S., Lethiec F., Duroux P., Gascuel O. (2005) Nucleic Acids Res. 33, W557–W559; web server issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanth C., Abbassi F., Lequin O., Ayala-Sanmartin J., Ladram A., Nicolas P., Amiche M. (2009) Biochemistry 48, 313–327 [DOI] [PubMed] [Google Scholar]
- 23.Lequin O., Ladram A., Chabbert L., Bruston F., Convert O., Vanhoye D., Chassaing G., Nicolas P., Amiche M. (2006) Biochemistry 45, 468–480 [DOI] [PubMed] [Google Scholar]
- 24.Abbassi F., Galanth C., Amiche M., Saito K., Piesse C., Zargarian L., Hani K., Nicolas P., Lequin O., Ladram A. (2008) Biochemistry 47, 10513–10525 [DOI] [PubMed] [Google Scholar]
- 25.Thrippleton M. J., Keeler J. (2003) Angew Chem. Int. Ed. Eng. 42, 3938–3941 [DOI] [PubMed] [Google Scholar]
- 26.Wishart D. S., Bigam C. G., Holm A., Hodges R. S., Sykes B. D. (1995) J. Biomol. NMR 5, 67–81 [DOI] [PubMed] [Google Scholar]
- 27.Güntert P., Mumenthaler C., Wüthrich K. (1997) J. Mol. Biol. 273, 283–298 [DOI] [PubMed] [Google Scholar]
- 28.Schwieters C. D., Kuszewski J. J., Tjandra N., Clore G. M. (2003) J. Magn Reson. 160, 65–73 [DOI] [PubMed] [Google Scholar]
- 29.Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 30.Conlon J. M. (2008) Peptides 29, 1815–1819 [DOI] [PubMed] [Google Scholar]
- 31.Simmaco M., Mignogna G., Barra D., Bossa F. (1994) J. Biol. Chem. 269, 11956–11961 [PubMed] [Google Scholar]
- 32.Marenah L., Flatt P. R., Orr D. F., Shaw C., Abdel-Wahab Y. H. (2006) J. Endocrinol. 188, 1–9 [DOI] [PubMed] [Google Scholar]
- 33.Mangoni M. L. (2006) Cell Mol. Life Sci. 63, 1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wimley W. C., Hristova K., Ladokhin A. S., Silvestro L., Axelsen P. H., White S. H. (1998) J. Mol. Biol. 277, 1091–1110 [DOI] [PubMed] [Google Scholar]
- 35.Lewis R. N., Mak N., McElhaney R. N. (1987) Biochemistry 26, 6118–6126 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y. P., Lewis R. N., McElhaney R. N. (1997) Biophys. J. 72, 779–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McElhaney R. N. (1986) Biochim. Biophys. Acta 864, 361–421 [DOI] [PubMed] [Google Scholar]
- 38.Lohner K., Prenner E. J. (1999) Biochim. Biophys. Acta 1462, 141–156 [DOI] [PubMed] [Google Scholar]
- 39.Henzler-Wildman K. A., Martinez G. V., Brown M. F., Ramamoorthy A. (2004) Biochemistry 43, 8459–8469 [DOI] [PubMed] [Google Scholar]
- 40.Woody R. W. (1992) Adv. Biophys. Chem. 2, 37–79 [Google Scholar]
- 41.Banerjee R., Basu G. (2002) FEBS Lett. 523, 152–156 [DOI] [PubMed] [Google Scholar]
- 42.Zhang W., Crocker E., McLaughlin S., Smith S. O. (2003) J. Biol. Chem. 278, 21459–21466 [DOI] [PubMed] [Google Scholar]
- 43.Eilers M., Patel A. B., Liu W., Smith S. O. (2002) Biophys. J. 82, 2720–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun P., von Heijne G. (1999) Biochemistry 38, 9778–9782 [DOI] [PubMed] [Google Scholar]
- 45.Selsted M. E., Novotny M. J., Morris W. L., Tang Y. Q., Smith W., Cullor J. S. (1992) J. Biol. Chem. 267, 4292–4295 [PubMed] [Google Scholar]
- 46.van Kan E. J., Demel R. A., van der Bent A., de Kruijff B. (2003) Biochim. Biophys. Acta 1615, 84–92 [DOI] [PubMed] [Google Scholar]
- 47.Harwig S. S., Kokryakov V. N., Swiderek K. M., Aleshina G. M., Zhao C., Lehrer R. I. (1995) FEBS Lett. 362, 65–69 [DOI] [PubMed] [Google Scholar]
- 48.Lequin O., Bruston F., Convert O., Chassaing G., Nicolas P. (2003) Biochemistry 42, 10311–10323 [DOI] [PubMed] [Google Scholar]
- 49.Mor A., Nicolas P. (1994) J. Biol. Chem. 269, 1934–1939 [PubMed] [Google Scholar]
- 50.Shai Y. (2002) Biopolymers 66, 236–248 [DOI] [PubMed] [Google Scholar]
- 51.Strahilevitz J., Mor A., Nicolas P., Shai Y. (1994) Biochemistry 33, 10951–10960 [DOI] [PubMed] [Google Scholar]