Abstract
Sulfated progesterone metabolite (P4-S) levels are raised in normal pregnancy and elevated further in intrahepatic cholestasis of pregnancy (ICP), a bile acid-liver disorder of pregnancy. ICP can be complicated by preterm labor and intrauterine death. The impact of P4-S on bile acid uptake was studied using two experimental models of hepatic uptake of bile acids, namely cultured primary human hepatocytes (PHH) and Na+-taurocholate co-transporting polypeptide (NTCP)-expressing Xenopus laevis oocytes. Two P4-S compounds, allopregnanolone-sulfate (PM4-S) and epiallopregnanolone-sulfate (PM5-S), reduced [3H]taurocholate (TC) uptake in a dose-dependent manner in PHH, with both Na+-dependent and -independent bile acid uptake systems significantly inhibited. PM5-S-mediated inhibition of TC uptake could be reversed by increasing the TC concentration against a fixed PM5-S dose indicating competitive inhibition. Experiments using NTCP-expressing Xenopus oocytes confirmed that PM4-S/PM5-S are capable of competitively inhibiting NTCP-mediated uptake of [3H]TC. Total serum PM4-S + PM5-S levels were measured in non-pregnant and third trimester pregnant women using liquid chromatography-electrospray tandem mass spectrometry and were increased in pregnant women, at levels capable of inhibiting TC uptake. In conclusion, pregnancy levels of P4-S can inhibit Na+-dependent and -independent influx of taurocholate in PHH and cause competitive inhibition of NTCP-mediated uptake of taurocholate in Xenopus oocytes.
Keywords: Lipid/Bile Acid, Reproduction, Tissue/Organ Systems/Hepatocyte, Tissue/Organ Systems/Liver, Transport, Hepatocyte, Steroid Hormone, Cholestasis, Hypercholanemia, Progesterone
Introduction
Normal pregnancy is characterized by altered bile acid homeostasis, and two recent studies have reported asymptomatic hypercholanemia of pregnancy in 10–40% of pregnant women (1, 2). Approximately 25% of women with asymptomatic hypercholanemia of pregnancy subsequently develop intrahepatic cholestasis of pregnancy (ICP),4 a liver disorder that presents with pruritus, raised serum bile acids, and liver transaminases (3). In pregnancies complicated by maternal serum bile acid levels of >40 μm, there are increased rates of spontaneous preterm labor, fetal asphyxial events, and meconium-stained amniotic fluid (3). ICP may also be complicated by third trimester intrauterine death (3, 4).
ICP has a complex etiology with genetic and endocrine components. Parous sisters have a 12-fold increased risk (5), and in ∼10% of ICP cases, mutations have been identified in genes that encode biliary transporters (6–9) and the primary bile acid sensor farnesoid X receptor (NR1H4) (10). Furthermore, the V444A polymorphism in the ABCB11 gene is a population risk factor for ICP (8), and ICP patients homozygous for the polymorphism have been shown to have increased bile acid levels when compared with controls (11).
Cholestatic metabolites of estrogen and progesterone influence bile acid metabolism and transport. A subgroup of women with a history of ICP who take the oral contraceptive pill or exogenous estrogens develop pruritus and hepatic impairment (4). In addition, administration of oral micronized natural progesterone for prophylaxis of preterm labor causes increased rates of ICP (12). Moreover, there is experimental evidence demonstrating that cholestatic reproductive hormone metabolites can influence the major and minor bile acid uptake transporters, the sodium-dependent Na+-taurocholate co-transporting polypeptide (NTCP), and the Na+-independent organic anion transporting proteins (13, 14), respectively, as well as the major canalicular bile salt export pump (BSEP) (14–16). Hepatocytes extracted from pregnant rats have been shown to have reduced Na+-dependent uptake of bile acids, indicating that pregnancy-specific factors are able to modulate bile acid transporter levels/activity (17, 18). Ethinylestradiol and the cholestatic estrogen metabolite estradiol-17β-d-glucuronide cause impaired bile flow (14, 15), reduced mRNA, and protein expression of biliary transporters in rodents (13, 14). These changes were abrogated in mice deficient of the estrogen receptor α (14). Estradiol-17β-d-glucuronide has been shown to induce submembrane internalization of the canalicular bile salt export pump in rats (15) and to trans-inhibit BSEP-mediated bile acid efflux from Xenopus laevis oocytes (16).
Levels of serum progesterone and its metabolites increase significantly throughout the course of pregnancy (19), and in particular, sulfated progesterone metabolites (P4-S) are raised (20). In ICP cases, urinary and serum P4-S profiles are markedly higher than in normal pregnancy, whereas unconjugated progesterone and glucuronidated progesterone metabolites remain unchanged (21, 22). Moreover, increased P4-S levels precede pruritus and abnormal liver function tests in ICP (21) indicating that these metabolites play a role in the etiology of ICP.
Allopregnanolone-sulfate, a P4-S, reduces bile output in perfused whole rat livers and trans-inhibits BSEP-mediated bile acid efflux in X. laevis oocytes, whereas the nonsulfated form did not have any significant impact (16). Based on patient and experimental data, P4-S levels may be involved in the pathogenesis of hypercholanemia in ICP. In this study, we characterized normal bile acid uptake kinetics in a primary human hepatocyte (PHH) experimental model and the impact P4-S levels have on bile acid uptake. We further investigated the mechanisms by which P4-S levels impair influx of taurocholate using NTCP-transfected Xenopus oocytes. We also measured total serum levels of a specific P4-S in non-pregnant and third trimester pregnant women.
EXPERIMENTAL PROCEDURES
Reagents
Progesterone, allopregnanolone (PM4), epiallopregnanolone (PM5), allopregnanolone-sulfate (PM4-S), epiallopregnanolone-sulfate (PM5-S), and sodium taurocholate (TC) were purchased from Steraloids; [3H]taurocholic acid ([3H]TC; specific activity 5 Ci/mmol) and UltimaGold scintillation mixture were purchased from PerkinElmer Life Sciences. All chemicals were purchased from Sigma, unless otherwise stated.
Human Serum Samples
This study conformed to the guidelines outlined by the 1975 Declaration of Helsinki, and permission was obtained from the Ethics Committee of the Hammersmith Hospitals NHS Trust, London, UK (REC 97/5197). Fasting serum samples were collected from 10 non-pregnant (not using hormone-based contraception) and 8 pregnant women (between 30 and 38 weeks with no history of cholestasis or liver disease).
Cell Culture
Human liver tissue was taken (with informed consent and local research ethics approval (RFH38–2000)) at tumor-free resection margins following surgical intervention for secondary liver tumors. PHH were isolated by collagenase perfusion (23). Isolated hepatocytes were plated into 24-well type I collagen-coated plates (BD Biosciences) using hepatocyte basal media with supplements (Lonza).
RNA Isolation and Quantitative PCR
Total RNA was isolated from cultured PHH 24 h after seeding and from pulverized snap-frozen human liver using the Qiagen RNeasy kit. 0.5 μg of RNA was reverse-transcribed using the Advantage RT-for-PCR kit (Takara Biosciences). Expression levels of L19, NTCP (SLC10A1), OATP1B1 (SLC01B1), and OATP1B3 (SLC01B3) were assayed in snap-frozen human liver and PHH by quantitative PCR (see supplemental tables for sequences) using SYBR Green Jumpstart Readymix (Sigma) and calculated using the ΔΔCt method.
PHH [3H]TC Uptake Assay
PHH seeded into 24-well plates were incubated at 4/37 °C with 250 μl of influx media consisting of 0.2 μm [3H]TC and 0–99.8 μm TC in Dulbecco's phosphate-buffered saline (Invitrogen) for 5–60 min with 0–50 μm PM4-S/PM5-S. Influx activity was stopped by washing with 500 μl of ice-cold 1 mm TC, and cells were lysed with 250 μl of RIPA buffer (Sigma), 175 μl of which was mixed with 2 ml of scintillation mixture for radioactivity counting, and the remainder was used to assay protein quantity (Pierce BCA) for normalization. Where Na+-dependent and Na+-independent influxes were measured, a sodium chloride or choline chloride buffer was used instead of Dulbecco's phosphate-buffered saline (24).
GC-MS Analysis of PHH PM5-S Intracellular Quantity
PHH seeded into 24-well plates were incubated at 4/37 °C with 250 μl of 0–50 μm PM5-S for 10 min, washed three times with ice-cold Dulbecco's phosphate-buffered saline, and scraped in 250 μl of ice-cold methanol. Lysates were sonicated in a Bioruptor-200 for 30 s. Samples dissolved in methanol underwent solvolysis with tetrahydrofurane and trifluoroacetic acid and were analyzed by GC-MS (25).
Animals
Mature female frog oocytes (X. laevis; Regine Olig, Hamburg, Germany) were used according to protocols approved by the Ethical Committee for Laboratory Animals, University of Salamanca, Spain.
Uptake Studies in X. laevis Oocytes
Harvesting and preparation of oocytes were carried out as described previously (26). Human NTCP cRNA synthesis was performed using the T7 mMessage mMachine ultra kit (Ambion) and the appropriate plasmid. Oocytes were microinjected with TE buffer (1 mm EDTA, 10 mm Tris, pH 8.0) alone (control) or containing 9 ng of NTCP cRNA. Uptake studies were performed as described (26) 2 days post-RNA injection using 10 oocytes/data point. Experiments were repeated 3–4 times using oocytes obtained from 3 to 4 frogs. After incubation with [3H]TC, PM4-S/PM5-S at 25 °C for the desired time, uptake was stopped by washing with 4 ml of ice-cold uptake medium, and oocytes were digested with 200 μl of 10% SDS and placed individually in vials to measure [3H]TC by liquid scintillation.
HPLC-MS/MS Analysis of PM4-S/PM5-S and Serum Bile Acid Concentrations in Human Serum and Oocyte Cavity
PM4-S/PM5-S and serum bile acids were analyzed by liquid chromatography-electrospray tandem mass spectrometry using a modified and extended methodology initially described for bile acid analysis in human serum by Tagliacozzi et al. (27). For the analysis of oocytes, these were incubated as above and lysed with 90% methanol containing 0.5 μm taurosulfolithocholic acid (internal standard) to determine PM4-S and PM5-S by HPLC-MS/MS. This was first used in MS2Scan mode to select the precursor ions (supplemental Fig. 1, A and B), which was 397.1 m/z for both epimers PM4-S and PM5-S (chemical structure in supplemental Fig. 1C). Negative ionization (electrospray ionization) was performed with the following conditions: gas temperature 350 °C, gas flow 10 liters/min, nebulizer 20 p.s.i., capillary voltage 2500 V. Precursor ions were filtered, and abundance was determined in multiple reaction monitoring mode (supplemental Fig. 1, A and B). To determine taurosulfolithocholic acid, the specific m/z transition 280.7 to 464 was followed. HPLC was performed using 60:40 methanol/water, containing 5 mm ammonium acetate, 0.01% formic acid, pH 4.6. The flow rate was 0.3 ml/min at 35 °C using a Zorbax C18 column (30 mm × 2.1 mm, 3.5 μm). Recovery from the lysis/extraction procedure, as calculated from taurosulfolithocholic acid measurements, was 88 ± 7%.
Graphing and Statistics Composition
Specific PHH uptake was calculated by subtracting 4 °C uptake values from 37 °C values. OriginPro 8 software (OriginLab Corp.) was used to generate graphical representations and to calculate transport kinetics (Hill fit; a routinely used method for the calculation of transporter stoichiometry but is limited to producing only a measure of the degree of cooperativity in the binding process), IC50 values (dose-response fit), and statistical significance using a one-tailed Mann-Whitney statistical analysis (untreated test sample values were consistently greater than treated samples values) for the PHH studies. Specific oocyte NTCP-mediated uptake was calculated by subtracting the amount of nonspecific wild-type oocyte uptake from total uptake measured in NTCP-transfected oocytes. Statistical significance of the differences between groups was determined by Student's or paired t test. Minimal squares method was used to calculate regression lines. Results are expressed as mean ± S.E., unless otherwise stated.
RESULTS
Functional Characterization of Bile Acid Uptake in Cultured PHH
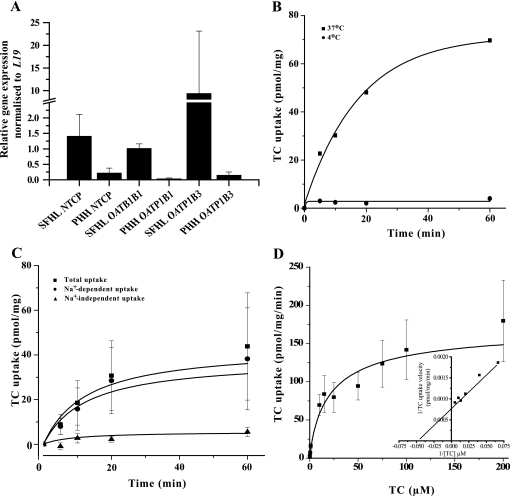
Gene expression levels of the bile acid influx transporters NTCP, OATP1B1, and OATP1B3 were ascertained in cultured PHH 24 h after seeding (time point when the transport studies are performed) and compared with snap-frozen human liver. Overall, levels of the uptake transporters in the different cultures are relatively consistent but lower than snap-frozen human liver samples (Fig. 1A); there were several snap-frozen human liver samples that possessed NTCP and OATP1B3 expression levels that were approximately the same or lower than PHH (supplemental Table 2). PHH were characterized for their ability to take up [3H]TC at different time points at 4/37 °C. PHH incubated with 0.2 μm [3H]TC uptake media at 4 °C showed no significant uptake over time compared with PHH incubated at 37 °C (Fig. 1B).
FIGURE 1.
Characterization of cultured PHH bile acid transporters. A, relative gene expression levels (PHH relative to snap-frozen human liver (SFHL)) of hepatic bile acid uptake transporters NTCP, OATP1B1, and OATP1B3 normalized to L19 in PHH cultured for 24 h and in snap-frozen human liver. Values represent mean ± S.D. of n ≥3. B, representation of typical [3H]TC (0.2 μm) uptake time course. C, time course of PHH temperature-sensitive Na+-dependent and Na+-independent [3H]TC uptake (0.2 μm) in PHH. Na+-dependent uptake was calculated as the difference between total uptake and Na+-independent uptake. D, TC dose-response curve. PHH were incubated with fixed 0.2 μm [3H]TC and increasing concentrations of unlabeled TC. Inset graph is a representation of typical double-reciprocal plot of TC uptake velocity versus TC concentration focusing on the 15–200 μm TC concentration range. Values represent mean ± S.E. of n ≥3.
To establish the relative contribution of Na+-dependent versus Na+-independent uptake in cultured PHH, cultures were incubated with a sodium chloride or choline chloride-based uptake medium that contained 0.2 μm [3H]TC at different time points. Na+-dependent and Na+-independent uptakes were time-dependent and began to lose linearity after 10 and 5 min, respectively. The 5-min incubation time point was subsequently used, as it represented the earliest time point with acceptable signal-to-noise ratio and approximate match of the requirement for kinetic studies of initial velocity conditions. Na+-dependent uptake accounted for an average of 89% of total uptake at all the time points studied (Fig. 1C). PHH incubated with 0.2 μm [3H]TC with varying unlabeled TC concentrations showed that total uptake was a saturable process that could be described using the Michaelis-Menten affinity constant (Km). The apparent Km value was 14.6 μm, whereas the maximal velocity (Vmax) was 190 pmol/mg/min (calculated from Fig. 1D, inset, double reciprocal plot). 1 μm TC was used as the incubation concentration in subsequent studies, as uptake is linear at this concentration (Fig. 1D).
P4-S Inhibits Bile Acid Uptake in PHH
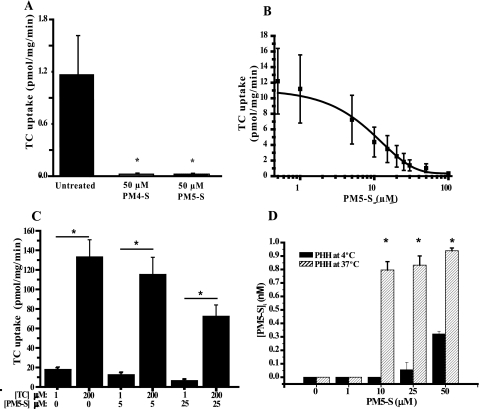
50 μm PM4-S and PM5-S significantly inhibited bile acid uptake in PHH by 98 and 97.5%, respectively (p < 0.05) (Fig. 2A). Because both compounds acted equally, PM5-S was used in subsequent PHH studies. To investigate the effects of PM5-S on temperature-sensitive uptake of bile acids, PHH were co-incubated with 1 μm TC and a range of PM5-S concentrations. This resulted in a dose-dependent reduction of TC uptake (Fig. 2B) and a calculated PM5-S IC50 of 9.3 μm. When uptake values from PM5-S-treated cells are expressed as a percentage of untreated controls, PM5-S mediated reduction of TC uptake reaches statistical significance at ≥5 μm (p < 0.05) (supplemental Fig. 2). The 5–10 μm PM5-S dose increase resulted in the greatest reduction of TC uptake (39%). To study the impact of PM5-S on Na+-dependent and -independent TC uptake, PHH were co-incubated in TC uptake media containing PM5-S with sodium or choline chloride. PM5-S significantly inhibited (p < 0.05) both Na+-dependent and -independent bile acid influx components at 20 min (Table 1) by 98 and 92%, respectively.
FIGURE 2.
Impact of P4-S on PHH bile acid uptake. A, PHH incubated with 0.2 μm [3H]TC and 50 μm PM4-S/PM5-S for 5 min. *, p < 0.05 for total uptake with PM4-S/PM5-S versus total uptake. B, PM5-S dose-response curve. PHH incubated with 1 μm TC (0.2 μm [3H]TC + 0.8 μm TC) and variable PM5-S concentrations for 5 min. C, mode of inhibition of TC uptake mediated by PM5-S. PHH were incubated with 0.2 μm [3H]TC and unlabeled TC to make a final concentration of 1 or 200 μm, with/without 5 or 25 μm PM5-S for 5 min. *, p < 0.05 for PHH incubated with 200 μm TC versus 1 μm TC at all PM5-S concentrations. D, intracellular concentrations of PM5-S in PHH after incubation with PM5-S. PHH were incubated with 0–50 μm PM5-S for 10 min. GC-MS was used to quantify intracellular PM5-S levels. *, p < 0.05 for PM5-S-treated groups versus untreated control. Values represent mean ± S.E. of n = 3.
TABLE 1.
Impact of PM5-S on Na+-dependent and Na+-independent TC uptake in PHH
PHH were incubated with 1 μm TC (0.2 mm [3H]TC + 0.8 μm TC) for 20 min with/without 50 μm PM5-S in sodium/choline chloride-based uptake media. Values represent mean ± S.E. of n = 3.
| Bile acid uptake component and treatment | Uptake (±S.E.) |
|---|---|
| pmol/mg | |
| Total untreated | 67.3 ± 21.5 |
| Total + 50 mm PM5-S | 1.3 ± 0.7a |
| Na+-dependent untreated | 64.2 ± 21.8 |
| Na+-dependent + 50 mm PM5-S | 1.0 ± 0.7a |
| Na+-independent untreated | 3.1 ± 0.8 |
| Na+-independent + 50 mm PM5-S | 0.3 ± 0.2a |
a p < 0.05 for PM5-S-treated versus control untreated.
If PM5-S is a noncompetitive inhibitor, it should maintain a relatively constant inhibition of uptake regardless of TC concentration applied. Conversely, if inhibition was competitive, an increase in TC concentration versus a fixed PM5-S concentration should result in an increase in TC uptake. By keeping the PM5-S concentration constant at 5 or 25 μm, but increasing the TC concentration from 1 to 200 μm, there was a significant increase (p < 0.05) in TC uptake of ≥90% in all comparisons (Fig. 2C).
There have been no published studies showing the temperature-sensitive uptake of P4-S into the human hepatocyte, which is an important determinant in the mode of bile acid uptake inhibition. To address this, we incubated primary human hepatocytes for 10 min with 0, 1, 10, 25, and 50 μm PM5-S at 4/37 °C to capture the relative contribution of temperature-sensitive uptake. Using GC-MS to measure intracellular levels of PM5-S levels, PHH incubated with 10, 25, and 50 μm PM5-S resulted in 0.8, 0.78, and 0.62 nmol of temperature-sensitive uptake of intracellular PM5-S, respectively (Fig. 2D). These levels represent 32, 12, and 5% of the PM5-S taken up from the 10, 25, and 50 μm PM5-S media over the 10-min incubation period, respectively. Incubation with 1 μm PM5-S did not result in detectable intracellular PM5-S levels.
P4-S-mediated TC Uptake Inhibition in NTCP-expressing X. laevis Oocytes
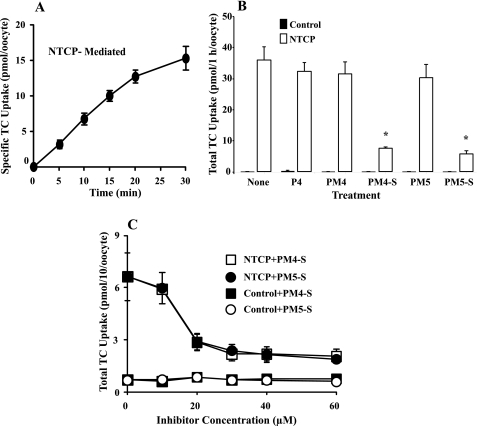
Xenopus oocytes transfected with NTCP were used to assess the ability of PM4-S and PM5-S to inhibit NTCP-mediated TC transport. NTCP-transfected oocytes were incubated with 10 μm TC to study uptake over time. Uptake was linear for more than 10 min, and hence this time was selected for future studies (Fig. 3A).
FIGURE 3.
Impact of P4-S on NTCP-transfected oocyte bile acid uptake. A, time course of specific NTCP-mediated uptake of [3H]TC in NTCP-transfected Xenopus oocytes incubated with 10 μm TC at 25 °C. B, total uptake of TC by wild-type and NTCP-expressing oocytes that were incubated with 10 μm TC in the absence or presence of 30 μm progesterone (P4)/metabolite at 25 °C for 1 h. C, PM4-S/PM5-S dose response; Xenopus oocytes were incubated with 10 μm TC and variable PM4-S/PM5-S concentrations. Values are mean ± S.D. of n = 3, 10 oocytes/experiment (from three frogs). *, p < 0.05 for oocytes incubated with inhibitor versus those without as determined by the Bonferroni method for multiple range testing.
The impact of 30 μm PM4-S and PM5-S as well as the nonsulfated forms (PM4 and PM5) and progesterone on TC uptake was ascertained by incubating the oocytes for 1 h in the presence of the compounds. None of the compounds tested significantly impacted nonspecific TC uptake in wild-type cells (control). In NTCP-expressing oocytes, progesterone and nonsulfated PM4 and PM5 were unable to inhibit TC uptake. In contrast, PM4-S and PM5-S markedly reduced TC uptake by 80 and 85%, respectively (Fig. 3B). Oocytes were incubated with 0–60 μm PM4-S/PM5-S and 10 μm TC. This resulted in a dose-dependent decrease in NTCP-mediated TC uptake demonstrating a similar magnitude of TC uptake inhibition by both compounds. Control cell nonspecific TC uptake was unaffected by PM4-S/PM5-S (Fig. 3C). PM4-S/PM5-S-mediated inhibition of TC uptake reaches saturation at 30 μm, and therefore this concentration was used in subsequent kinetic studies.
P4-S-mediated Inhibition Is Competitive
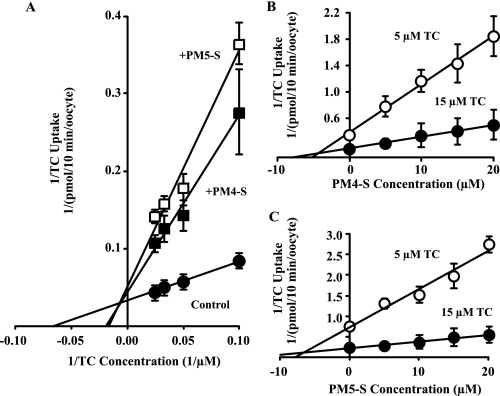
Using 30 μm PM4-S/PM5-S with varying TC concentrations, NTCP-mediated TC uptake was measured to determine the mode of inhibition. A double-reciprocal plot of TC concentration versus TC uptake (Fig. 4A) indicates that inhibition induced by both PM4-S and PM5-S is mainly competitive, as the inhibitors have a mild effect on the Vmax of transport (Vmax, reciprocal of y axis intercept), but markedly increases the apparent Km value (Km, absolute value of the reciprocal of x axis intercept) (Fig. 4A). This resulted in a marked decrease in the efficiency of transport defined as Vmax/Km (Table 2).
FIGURE 4.
Mode of PM4-S/PM5-S-mediated bile acid uptake inhibition in NTCP-transfected oocytes. A, representative double-reciprocal plot of specific NTCP-mediated uptake of [3H]TC by NTCP-expressing oocytes. Oocytes were incubated with varying concentrations of TC without inhibitor or with PM4-S/PM5-S (B and C). Dixon's plots of specific NTCP-mediated uptake of [3H]TC by NTCP-expressing oocytes with varying concentrations of PM4-S (B), PM5-S (C) and 5 or 15 μm TC at 25 °C for 10 min. Values are mean ± S.D., 10 oocytes/data point. All regression lines were p < 0.001.
TABLE 2.
Effect of P4-S on kinetic parameters of NTCP-mediated TC uptake in X. laevis oocytes
Values are calculated from double-reciprocal (Vmax and Km values and efficiency of transport) and Dixon's plots. Specific uptake was calculated as the difference of [3H]TC acid uptake between oocytes expressing NTCP and wild-type oocytes incubated with the same substrate and inhibitor concentrations.
| Inhibitor | Vmax (pmol/10 min/oocyte) | % | Km | % | Efficiency of transport (Vmax/Km) | % | Ki |
|---|---|---|---|---|---|---|---|
| μm | μm | ||||||
| None | 13.2 ± 7.5 | 100 | 10.1 ± 2.1 | 100 | 1.11 ± 0.45 | 100 | |
| PM4-S | 10.7 ± 5.4 | 82 | 38.6 ± 8.9a | 383 | 0.25 ± 0.09a | 23 | 7.7 ± 1.1 |
| PM5-S | 10.9 ± 4.4 | 82 | 40.1 ± 5.4a | 397 | 0.25 ± 0.08a | 23 | 6.4 ± 1.6 |
a p < 0.05, paired t test of efficiency of transport with inhibitor versus efficiency of transport with no inhibitor is shown. Values represent mean ± S.D. of three independent experiments, 10 oocytes/data point/experiment.
The apparent inhibitor constant (Ki) of PM4-S/PM5-S was ascertained by measuring uptake (v) in NTCP-transfected oocytes incubated with 5 and 15 μm TC and varying concentrations of PM4-S/PM5-S. From this, it was possible to plot 1/v versus inhibitor concentration (Fig. 4, B and C). The results of both Dixon's plots were consistent with a strong competitive component for PM4-S/PM5-S-induced inhibition of TC uptake. The calculated apparent Ki values of both compounds were similar and in the same order of the Km value calculated for TC in the absence of inhibitor (Table 2).
NTCP Is Able to Mediate Transport of P4-S into the Intracellular Environment
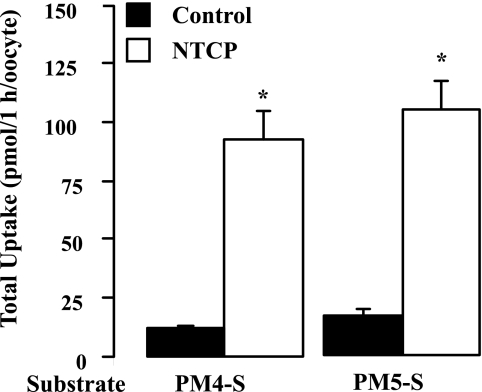
The GC-MS analysis of PHH incubated with PM5-S indicated that uptake transporters were able to influx PM5-S in a temperature-sensitive manner (Fig. 2C). To further dissect this result, NTCP-transfected or wild-type oocytes were incubated for 1 h with 30 μm PM4-S/PM5-S, and using HPLC-MS/MS, the intracellular PM4-S/PM5-S content was measured. The expression of NTCP significantly increased the ability of the oocytes to take up PM4-S and PM5-S relative to the wild-type oocytes by 8- and 6.4-fold, respectively (Fig. 5).
FIGURE 5.
NTCP-expressing oocytes are able to uptake PM4-S and PM5-S. Total uptakes of PM4-S/PM5-S by wild-type and NTCP-expressing Xenopus oocytes were incubated with 30 μm of each P4-S at 25 °C for 1 h. Measurements were carried out by HPLC-MS/MS in 15 individual oocytes/data point (from three different frogs). Determinations of PM4-S and PM5-S content were corrected by the recovery of internal standard (taurosulfolithocholic acid) added to the lysis/extraction solution. Values are mean ± S.D. *, p < 0.05 for NTCP-expressing oocytes versus control oocytes as determined by the Student's t test.
Third Trimester Serum PM4-S/PM5-S Serum Concentrations Reach Levels Capable of Inhibiting PHH TC Uptake
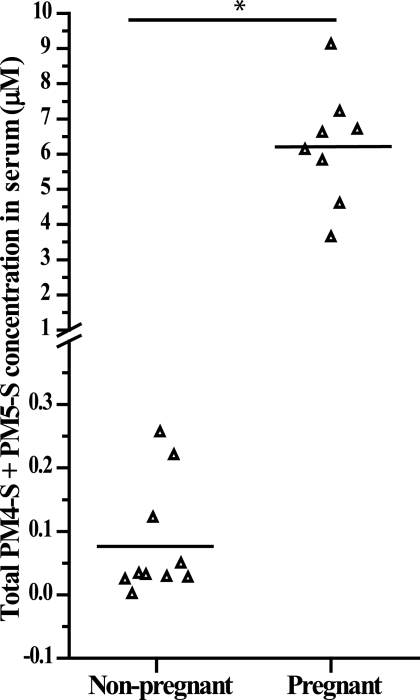
Liquid chromatography-electrospray tandem mass spectrometry was used to assay total PM4-S + PM5-S serum concentrations in fasted non-pregnant and pregnant women (30–38 weeks). Serum from non-pregnant and pregnant women had mean PM4-S + PM5-S serum concentrations of 0.08 and 6.2 μm, respectively (Fig. 6). This equates to an 80-fold increase in total PM4-S + PM5-S levels during pregnancy. There was no difference in total fasting bile acid concentration in the pregnant group (mean ± S.D., 2.7 ± 1.48 μm) relative to the non-pregnant group (2 ± 1.34 μm).
FIGURE 6.
Serum concentrations of total PM4-S + PM5-S in fasted non-pregnant and 30–38-week pregnant women. Black line represents mean serum concentrations of total PM4-S + PM5-S. Measurements were carried out by liquid chromatography-electrospray tandem mass spectrometry on a minimum of n = 8 samples. *, p < 0.05 for non-pregnant versus pregnant serum samples as determined by the Student's t test.
DISCUSSION
We provide the first demonstration that P4-S, at serum concentrations found in normal pregnancy, are able to inhibit Na+-dependent bile acid uptake, the predominant uptake mechanism for bile acids in PHH. Using an NTCP-expressing oocyte model, the inhibition of uptake was shown to be mediated specifically by the sulfated forms of the progesterone metabolites, indicating substrate specificity by NTCP. Furthermore, the mechanism of P4-S-mediated inhibition of bile acid uptake appeared to be mainly competitive in nature in PHH, and this was confirmed in the NTCP-expressing oocytes. These findings are of likely physiological relevance as P4-S rises in normal pregnancy (19), and our data are consistent with these metabolites contributing to the asymptomatic hypercholanemia that is observed in some pregnant women with otherwise normal biochemical liver tests (1, 2). Serum levels of P4-S rise further in pregnancies complicated by ICP (21, 22) and therefore they may play a role in the hypercholanemia that is reported in this condition. Furthermore, total monosulfated P4-S levels in ICP occur at concentrations consistent with the concentrations used in this study (30–50 μm) (21). Raised P4-S levels are unlikely to explain the full phenotype of ICP as genetic studies have implicated the bile acid transporters ABCB11 (8, 9), ABCB4 (6, 7, 11, 28), and ATP8B1 (29) as well as the primary bile acid sensor farnesoid X receptor (10) in the etiology of the disease. However, raised serum P4-S levels occur at concentrations where it is likely that they contribute to the hypercholanemia of ICP. This may be another “hit” that exacerbates the phenotype of ICP in susceptible women.
It is not known why P4-S levels are elevated in ICP patients. It has been postulated that levels of P4-S in ICP are increased due to either a reduction of hepatobiliary excretion or alternatively due to an increase in overall steroid sulfation or a decrease in steroid sulfatase activity. However, it has yet to be established whether elevated P4-S are a cause or consequence of ICP. It is noteworthy that increased P4-S levels were shown to precede pruritus and abnormal liver function tests in two ICP patients (21). The fetal complications of ICP occur more commonly in cases with serum bile acid levels >40 μm (3). Thus, raised levels of P4-S in ICP cases may enhance the fetal risk due to an exacerbation of hypercholanemia. Ursodeoxycholic acid has been shown to ameliorate cholestasis and in parallel decrease P4-S levels in urine and serum (30).
In agreement with a previous report (31), the expression level of the main hepatic bile acid importer NTCP is variable in snap-frozen human liver across different patient samples. This was also the case for the organic anion importer OATP1B3. NTCP and OATP1B3 expression levels ranged from several orders of magnitude higher to levels lower than PHH. Although the PHH bile acid transporter Ct values indicate lower expression levels than the majority of snap-frozen human liver samples studied, the Ct values for the PHH samples indicate relatively high expression levels (taking into account L19 housekeeping control Ct values; supplemental Table 2). It is possible that the higher levels of NTCP in snap-frozen human liver compared with PHH may result in higher Km and Vmax values, thereby reducing the competitive nature of P4-S in vivo. All three bile acid importers appear to be expressed consistently across the different PHH cultures. This indicates that extra-hepatic factors may contribute to the heterogeneic gene expression profiles observed in snap-frozen human liver (Fig. 1A). PHH transporter kinetic studies indicate that the uptake of TC is a time- and temperature-dependent process, which can be characterized by a Km value of 14.6 μm and Vmax of 190 pmol/mg/min (Fig. 1D). The observed Km value in PHH is similar to that generated from the NTCP-transfected oocyte model (Km 10.1 μm) used in this study (Fig. 4A and Table 2) and to other previously reported values (32, 33). A screen of PM4-S and PM5-S, in a PHH bile acid uptake assay, revealed that both compounds are able to significantly inhibit TC uptake (Fig. 2A), and this was confirmed in NTCP-transfected oocytes (Fig. 3B). Interestingly, progesterone and nonsulfated PM4 and PM5 did not significantly reduce TC uptake in NTCP-transfected oocytes indicating NTCP substrate specificity for PM4-S/PM5-S at the concentrations tested in this study (Fig. 3B). Progesterone has previously been reported to inhibit NTCP uptake of TC (31, 34). A possible explanation for the difference in results is the concentration ratio of TC and progesterone used in other studies, which were considerably greater than the 10 μm TC versus 50 μm progesterone used in this oocyte study (i.e. compared with 1 μm TC to 200 μm progesterone (34) and 5 μm TC to 100 μm progesterone (31)). When PHH and NTCP-transfected oocytes were treated with PM5-S at varying concentrations, TC uptake was inhibited in a dose-dependent manner (Fig. 2B and Fig. 3C). PM5-S had an IC50 of 9.2 μm in PHH (Fig. 2B). PM4-S and PM5-S had Ki values 7.7 and 6.4 μm, respectively, in NTCP-transfected oocytes (Fig. 4, A–C, Table 2). The IC50 and Ki values for the P4-S compounds studied are similar to the total levels of PM4-S + PM5-S observed in third trimester pregnant serum (6.2 μm; Fig. 6) and within the documented total P4-S levels in the serum of ICP patients (21), typifying the physiological importance of pregnancy levels of P4-S in women with raised serum bile acid levels.
In agreement with previous reports (35), our study has revealed that Na+-dependent uptake is responsible for the majority of temperature-sensitive bile acid influx into the hepatocyte that is mediated by the NTCP transporter (Fig. 1C). We showed that PM4-S and PM5-S significantly inhibit Na+-dependent bile acid uptake (Table 1 and Fig. 3, B and C). Our data are consistent with elevated P4-S levels reducing NTCP-mediated bile acid uptake capacity and increased competition between bile acids for uptake (Fig. 2C, Fig. 3C, and Fig. 4). The Na+-independent uptake component of influx is also significantly inhibited by PM5-S (Table 1). It is reasonable to infer that Na+-independent influxers are also involved in the uptake of P4-S but to a smaller degree than Na+-dependent transporters. We showed that PM5-S is efficiently taken up into the intracellular environment of PHH (Fig. 2D), with uptake efficiencies reaching 32% for PHH incubated for 10 min in 10 μm PM5-S. This supports the hypothesis that P4-S can compete with bile acids for uptake into the human hepatocyte.
It is interesting to note that we show that non-pregnant serum possesses 80-fold less total PM4-S + PM5-S (Fig. 6) than in third-trimester pregnant serum. This may explain why pregnant women with hypercholanemia appear to have normal bile acid levels outside of pregnancy. Serum bile acid levels were not significantly different between the two groups. This may be due to the fact that the study participants were fasted prior to venipuncture.
P4-S have been shown to reduce bile flow and output from whole rat perfused livers, and this was attributed to a trans-inhibitory effect on BSEP based on a Xenopus oocyte model transfected with rat BSEP (16). We have shown that PM5-S inhibits bile acid uptake in primary human hepatocytes, in a competitive manner, indicating that P4-S can impact the major routes of uptake and efflux of bile acids in the liver.
It has been shown that there is a reduction in Na+-dependent bile acid uptake in hepatocytes taken from pregnant rats (17), and in another study, the pregnancy environment was shown to transcriptionally down-regulate rat Ntcp (18). However, the majority of research investigating the endocrine component of ICP has focused on the role of estrogen and its metabolites (14, 15).
Dexamethasone has been shown to up-regulate human NTCP (36). Mechanistically, this may increase the “uptake capacity” of the liver, thus reducing the competition between P4-S and bile acids. Some studies have reported that ICP cases respond to dexamethasone treatment (37) but other studies have not (38, 39). Screening compound libraries for potential molecules that are able to up-regulate NTCP levels/activity would be of benefit to not only ICP patients but to those with other forms of cholestasis.
In summary, our results show that treating PHH and NTCP-transfected oocytes with pregnancy levels of P4-S can markedly inhibit, in a competitive manner, bile acid uptake by the major pathway accounting for this process, i.e. NTCP, although a minor contribution by Na+-independent mechanisms may be also affected. Taken together, these results provide further insights into the mechanism by which raised sulfated progesterone metabolite levels may contribute to the pathogenesis of hypercholanemia in normal pregnancy and in cholestasis in pregnancy.
Supplementary Material
Acknowledgments
We thank Dr. Peter J. Meier, Dr. Bruno Stieger, and Dr. Bruno Hagenbuch (Zurich University Hospital, Switzerland) for NTCP expression plasmids.
This work was supported in part by Action Medical Research Grant SP4005, Women for Women Charity, Biomedical Research Centre at Imperial College Hospitals NHS Trust, Junta de Castilla y Leon Grants GR75/2008, SA033A08, SA03508, SA03608, and SAN 673/SA07/08 (Spain), Ministerio de Ciencia y Tecnologia, Plan Nacional de Investigacion Cientifica, and Desarrollo e Innovacion Tecnologica Grants BFU2006-12577 and BFU2007-30688-E/BFI (Spain).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2 and Tables 1 and 2.
- ICP
- intrahepatic cholestasis of pregnancy
- P4-S
- sulfated progesterone metabolite
- PHH
- primary human hepatocyte
- TC
- taurocholate
- PM4-S
- allopregnanolone-sulfate
- PM5-S
- epiallopregnanolone-sulfate
- NTCP
- Na+-taurocholate co-transporting polypeptide
- GC-MS
- gas chromatography-mass spectrometry
- HPLC
- high pressure liquid chromatography
- MS/MS
- tandem mass spectrometry
- BSEP
- bile salt export pump.
REFERENCES
- 1.Pascual M. J., Serrano M. A., El-Mir M. Y., Macias R. I., Jiménez F., Marin J. J. (2002) Clin. Sci. 102, 587–593 [PubMed] [Google Scholar]
- 2.Castaño G., Lucangioli S., Sookoian S., Mesquida M., Lemberg A., Di Scala M., Franchi P., Carducci C., Tripodi V. (2006) Clin. Sci. 110, 459–465 [DOI] [PubMed] [Google Scholar]
- 3.Glantz A., Marschall H. U., Mattsson L. A. (2004) Hepatology 40, 467–474 [DOI] [PubMed] [Google Scholar]
- 4.Williamson C., Hems L. M., Goulis D. G., Walker I., Chambers J., Donaldson O., Swiet M., Johnston D. G. (2004) BJOG 111, 676–681 [DOI] [PubMed] [Google Scholar]
- 5.Dixon P. H., Williamson C. (2008) Obs. Med. 1, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon P. H., Weerasekera N., Linton K. J., Donaldson O., Chambers J., Egginton E., Weaver J., Nelson-Piercy C., de, Swiet M., Warnes G., Elias E., Higgins C. F., Johnston D. G., McCarthy M. I., Williamson C. (2000) Hum. Mol. Genet. 9, 1209–1217 [DOI] [PubMed] [Google Scholar]
- 7.Müllenbach R., Linton K. J., Wiltshire S., Weerasekera N., Chambers J., Elias E., Higgins C. F., Johnston D. G., McCarthy M. I., Williamson C. (2003) J. Med. Genet. 40, e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon P. H., van Mil S. W., Chambers J., Strautnieks S., Thompson R. J., Lammert F., Kubitz R., Keitel V., Glantz A., Mattsson L. A., Marschall H. U., Molokhia M., Moore G. E., Linton K. J., Williamson C. (2009) Gut 58, 537–544 [DOI] [PubMed] [Google Scholar]
- 9.Pauli-Magnus C., Lang T., Meier Y., Zodan-Marin T., Jung D., Breymann C., Zimmermann R., Kenngott S., Beuers U., Reichel C., Kerb R., Penger A., Meier P. J., Kullak-Ublick G. A. (2004) Pharmacogenetics 14, 91–102 [DOI] [PubMed] [Google Scholar]
- 10.Van Mil S. W., Milona A., Dixon P. H., Mullenbach R., Geenes V. L., Chambers J., Shevchuk V., Moore G. E., Lammert F., Glantz A. G., Mattsson L. A., Whittaker J., Parker M. G., White R., Williamson C. (2007) Gastroenterology 133, 507–516 [DOI] [PubMed] [Google Scholar]
- 11.Meier Y., Zodan T., Lang C., Zimmermann R., Kullak-Ublick G. A., Meier P. J., Stieger B., Pauli-Magnus C. (2008) World J. Gastroenterol. 14, 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacq Y., Sapey T., Bréchot M. C., Pierre F., Fignon A., Dubois F. (1997) Hepatology 26, 358–364 [DOI] [PubMed] [Google Scholar]
- 13.Geier A., Dietrich C. G., Gerloff T., Haendly J., Kullak-Ublick G. A., Stieger B., Meier P. J., Matern S., Gartung C. (2003) Biochim. Biophys. Acta 1609, 87–94 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y., Moore R., Hess H. A., Guo G. L., Gonzalez F. J., Korach K. S., Maronpot R. R., Negishi M. (2006) J. Biol. Chem. 281, 16625–16631 [DOI] [PubMed] [Google Scholar]
- 15.Crocenzi F. A., Mottino A. D., Cao J., Veggi L. M., Pozzi E. J., Vore M., Coleman R., Roma M. G. (2003) Am. J. Physiol. Gastrointest. Liver Physiol. 285, G449–G459 [DOI] [PubMed] [Google Scholar]
- 16.Vallejo M., Briz O., Serrano M. A., Monte M. J., Marin J. J. (2006) J. Hepatol. 44, 1150–1157 [DOI] [PubMed] [Google Scholar]
- 17.Ganguly T., Hyde J. F., Vore M. (1993) J. Pharmacol. Exp. Ther. 267, 82–87 [PubMed] [Google Scholar]
- 18.Arrese M., Trauner M., Ananthanarayanan M., Pizarro M., Solís N., Accatino L., Soroka C., Boyer J. L., Karpen S. J., Miquel J. F., Suchy F. J. (2003) J. Hepatol. 38, 148–155 [DOI] [PubMed] [Google Scholar]
- 19.Kancheva R., Hill M., Cibula D., Vceláková H., Kancheva L., Vrbíková J., Fait T., Parízek A., Stárka L. (2007) J. Endocrinol. 195, 67–78 [DOI] [PubMed] [Google Scholar]
- 20.Sjövall K. (1970) Ann. Clin. Res. 2, 393–408 [PubMed] [Google Scholar]
- 21.Sjövall J., Sjövall K. (1970) Ann. Clin. Res. 2, 321–337 [PubMed] [Google Scholar]
- 22.Meng L. J., Reyes H., Palma J., Hernandez I., Ribalta J., Sjövall J. (1997) J. Hepatol. 27, 346–357 [DOI] [PubMed] [Google Scholar]
- 23.Selden C., Mellor N., Rees M., Laurson J., Kirwan M., Escors D., Collins M., Hodgson H. (2007) J. Gene Med. 9, 67–76 [DOI] [PubMed] [Google Scholar]
- 24.Vicens M., Medarde M., Macias R. I., Larena M. G., Villafaina A., Serrano M. A., Marin J. J. (2007) Bioorg. Med. Chem. 15, 2359–2367 [DOI] [PubMed] [Google Scholar]
- 25.Marschall H. U., Broomé U., Einarsson C., Alvelius G., Thomas H. G., Matern S. (2001) J. Lipid Res. 42, 735–742 [PubMed] [Google Scholar]
- 26.Briz O., Serrano M. A., Rebollo N., Hagenbuch B., Meier P. J., Koepsell H., Marin J. J. (2002) Mol. Pharmacol. 61, 853–860 [DOI] [PubMed] [Google Scholar]
- 27.Tagliacozzi D., Mozzi A. F., Casetta B., Bertucci P., Bernardini S., Di Ilio C., Urbani A., Federici G. (2003) Clin. Chem. Lab. Med. 41, 1633–1641 [DOI] [PubMed] [Google Scholar]
- 28.Wasmuth H. E., Glantz A., Keppeler H., Simon E., Bartz C., Rath W., Mattsson L. A., Marschall H. U., Lammert F. (2007) Gut 56, 265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müllenbach R., Bennett A., Tetlow N., Patel N., Hamilton G., Cheng F., Chambers J., Howard R., Taylor-Robinson S. D., Williamson C. (2005) Gut 54, 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glantz A., Reilly S. J., Benthin L., Lammert F., Mattsson L. A., Marschall H. U. (2008) Hepatology 47, 544–551 [DOI] [PubMed] [Google Scholar]
- 31.Kim R. B., Leake B., Cvetkovic M., Roden M. M., Nadeau J., Walubo A., Wilkinson G. R. (1999) J. Pharmacol. Exp. Ther. 291, 1204–1209 [PubMed] [Google Scholar]
- 32.Ho R. H., Leake B. F., Roberts R. L., Lee W., Kim R. B. (2004) J. Biol. Chem. 279, 7213–7222 [DOI] [PubMed] [Google Scholar]
- 33.Kullak-Ublick G. A., Ismair M. G., Kubitz R., Schmitt M., Häussinger D., Stieger B., Hagenbuch B., Meier P. J., Beuers U., Paumgartner G. (2000) Cytotechnology 34, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerli B., Valantinas J., Meier P. J. (1989) J. Pharmacol. Exp. Ther. 250, 301–308 [PubMed] [Google Scholar]
- 35.Sandker G. W., Weert B., Olinga P., Wolters H., Slooff M. J., Meijer D. K., Groothuis G. M. (1994) Biochem. Pharmacol. 47, 2193–2200 [DOI] [PubMed] [Google Scholar]
- 36.Eloranta J. J., Jung D., Kullak-Ublick G. A. (2006) Mol. Endocrinol. 20, 65–79 [DOI] [PubMed] [Google Scholar]
- 37.Hirvioja M. L., Tuimala R., Vuori J. (1992) Br. J. Obstet. Gynaecol. 99, 109–111 [DOI] [PubMed] [Google Scholar]
- 38.Glantz A., Marschall H. U., Lammert F., Mattsson L. A. (2005) Hepatology 42, 1399–1405 [DOI] [PubMed] [Google Scholar]
- 39.Diac M., Kenyon A., Nelson-Piercy C., Girling J., Cheng F., Tribe R. M., Goodman J., Shennan A., Williamson C. (2006) J. Obstet. Gynaecol. 26, 110–114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.