Abstract
Intercellular channels formed by connexin proteins play a pivotal role in the direct movement of ions and larger cytoplasmic solutes between vascular endothelial cells, between vascular smooth muscle cells, and between endothelial and smooth muscle cells. Multiple genetic and epigenetic factors modulate connexin expression levels and/or channel function, including cell type-independent and cell type-specific transcription factors, posttranslational modification and localized membrane targeting. Additionally, differences in protein-protein interactions, including those between connexins, significantly contribute to both vascular homeostasis and disease progression. The biophysical properties of the connexin channels identified in the vasculature, those formed by Cx37, Cx40, Cx43 and/or Cx45 proteins, are discussed in this review in the physiological and pathophysiological context of vessel function.
Keywords: Connexin, Endothelial Cells, Hemichannel, Heterocellular Communication, Myoendothelial Junction, Smooth Muscle Cells
1 INTRODUCTION
The coordination of vascular responses is essential for normal vessel function. Long distance communication networks in the vessel wall achieve the dynamic modulation of vascular resistance and blood flow to match different tissue oxygen requirements. Morphologically distinct gap junctions are a vital component in this process.
While ionic coupling by gap junctions enables changes in membrane potential to be propagated electrotonically between adjacent cells, they uniquely allow the cellular exchange of metabolites, ions and other small molecules, such as second messengers, which play important roles in transverse and longitudinal signaling in the vascular bed, and processes including angiogenesis, vascular cell growth, cell differentiation and development (Chadjichristos et al., 2008; Coutinho et al., 2003; Liao et al., 2007).
The expression of different gap junction proteins is dynamically regulated throughout different types of vascular bed in healthy vessels and in vascular disease states such as atherosclerosis and hypertension (Brisset et al., 2009; Kwak, 2002).
2 THE VASCULATURE
Direct electrical (ionic) coupling between cells has been observed for over half a century (Holland and Dunn, 1954), a process requiring intimate focal contact structures bridging inter-membrane ‘gaps’ (Dewey and Barr, 1964). The nature of these interactions was resolved by thin section and freeze fracture electron microscopy of heart and nonexcitable liver tissue. En face views of the focal plaque-like regions showed there to be densely packed hexagonal intramembrane particles in the membranes of both apposing cells where two cells remained separated by a uniform gap of ~2nm. These particles had diameters of ~7nm and an electron opaque region of ~1nm at their centre (Revel and Karnovsky, 1967). Such observations, having subsequently been structurally refined by biochemical, biophysical and crystallographic study, support the working/current model of ‘gap junction’ structure as an array of aqueous intercellular channels directly connecting the cytoplasms of adjacent cells. .
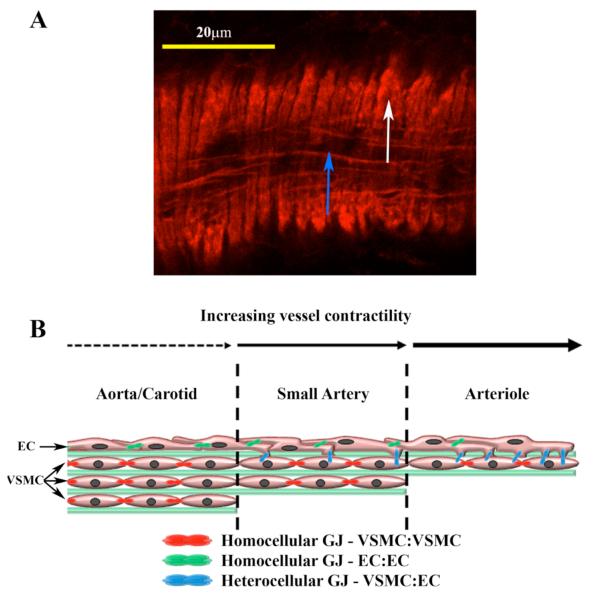
Gap junctions can be morphologically identified within the vasculature at sites of endothelial cell (EC), vascular smooth muscle cell (VSMC), and EC and VSMC contact, the latter a specialized region referred to as the ‘myoendothelial junction’ (MEJ) (de Wit et al., 2006a; de Wit et al., 2006b; Isakson and Duling, 2005; Rhodin, 1967; Simionescu et al., 1976) (Figure 1).
FIGURE 1. VESSEL COMPOSITION AND FUNCTIONAL INTERACTIONS.
A mouse cremasteric arteriole is stained with phalloidin (A). Endothelial cells align in the direction of flow (blue arrow) and vascular smooth muscle cells align perpendicular to the endothelial cells (white arrow). Gap junctions form homocellular (green and red symbols) and heterocellular (blue) contact points between VSMC and EC in the vasculature depending on the vessel bed studied (B).
The involvement of gap junctions, and their constituent connexin proteins (abbreviated Cx), is vital to the coordination of cellular activities within the wall of the vasculature (Figueroa and Duling, 2008; Segal and Jacobs, 2001), and most other tissues (Harris and Locke, 2009).
A total of 21 human connexin genes and 20 mouse connexin genes have been identified, with each cell generally containing multiple connexin isoforms. Each isoform contributes a particular set of functional properties to a gap junction channel that is essential, either intact or as modified by interaction with other connexins, for normal cellular development and function (Harris and Locke, 2009).
In most tissues, including the vasculature, functional deletion of a connexin isoform produces distinct and severe pathophysiology, and genetic knock-in replacement of one connexin by another isoform fails to fully compensate for the loss (Winterhager et al., 2007; Zheng-Fischhofer et al., 2006); specifically, this reflects the need for different intercellular signaling pathways mediated by different connexin channels.
The vasculature can be divided into large conduit vessels (macrovessels; e.g., veins, aorta or carotid arteries) and smaller highly contractile vessels (microvessels; e.g., venules, mesenteric arteries and cremasteric arterioles). Anatomically, EC line the lumen of all vessels and align with the direction of blood flow (Brisset et al., 2009). EC are separated by extracellular matrix (ECM), the internal elastic lamina (IEL), from layers of VSMC patterned perpendicularly and arranged helically to the EC on the outside of the vessel (Cornhill et al., 1980; Hirano et al., 2007; Ohtani et al., 1983; Wolinsky and Glagov, 1967). This assembly permits efficient flow within the vessel across the EC, and control of vessel tone by VSMC.
In the larger vessels, ECM separates VSMC layers into ‘lamellar units’ that vary in number, proportionate to vessel size and flow rate, e.g., eight in the ascending and four in the descending aorta (Hirano et al., 2007). Here, the VSMC are important in maintenance of tone, whereas in the smaller vessels, such as the arterioles, single layers of EC and VSMC are more responsive to contractile stimuli, and so play an integral role in the maintenance of blood pressure.
2.1 HOMOCELLULAR GAP JUNCTIONS
Gap junctions facilitate the movement of small molecules and ions at homocellular contact points, i.e., EC:EC or VSMC:VSMC (Beny and Gribi, 1989; Ebong et al., 2006; Figueroa et al., 2003; Hoffmann et al., 2003; Isakson et al., 2006a; Ko et al., 1999; Little et al., 1995a; Rummery and Hill, 2004; Sandow et al., 2003; Segal and Beny, 1992).
Macrovessel EC are extensively coupled by these intercellular connections, as shown by laying out the vessel en face (an exposed luminal face) and microinjecting a single EC with a gap junction-permeable fluorescent dye that quickly diffuses to surrounding cells (Ebong et al., 2006; Segal and Duling, 1986). Homocellular dye transfer between VSMC in vivo has been demonstrated (Little et al., 1995a; Straub et al., 2009), though not to the same extent as between EC, due in large part to the orientation of the VSMC making these experiments difficult.
2.2 HETEROCELLULAR GAP JUNCTIONS
At perforations of the IEL in microvessels, EC and VSMC make heterocellular contact at MEJ (Rhodin, 1967). These unique structures are widely distributed throughout the vasculature, though are less abundant in the larger arteries and veins than in the arterioles and venules, where they are numerous (Heberlein et al., 2009). It is estimated that one MEJ is found every ~5μm along the length of first-order arterioles, which represents six MEJ for every EC or three MEJ for every VSMC (Heberlein et al., 2009; Sokoya et al., 2007). The MEJ are small, ~0.5μm wide by ~0.5μm in length, making their detection by light microscopy extremely difficult.
The complexity of the MEJ environment has also made difficult and limiting any standard in vivo techniques, and as such has required innovative new approaches, which have only recently begun to show some interesting results. Evolving experimental evidence shows gap junctions at the MEJ play a pivotal role in coordinating microvessel function (Duling et al., 2003; Haddock et al., 2006; Heberlein et al., 2009; Isakson, 2008; Isakson et al., 2008).
3 CONNEXIN CHANNELS
Only four connexin proteins, Cx37, Cx40, Cx43 and Cx45, have been robustly documented within the different layers of the vasculature (Brisset et al., 2009; Hill et al., 2001; Isakson et al., 2008; Isakson et al., 2006a) (Table 1). Prior to the realization that there was more than one connexin isoform, morphological studies had shown that the size and abundance of gap junctions varied in different regions of the vascular tree and changed during disease. The distribution of connexins is currently known to vary between individual vascular beds of one species, between the same vascular bed of different species as well as during embryological development and progression of disease (Figueroa et al., 2004; Severs, 2001).
TABLE 1. PHYSICAL PROPERTIES OF VASCULAR GAP JUNCTIONS.
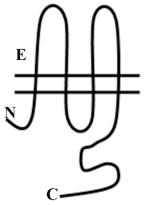
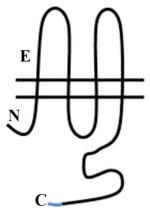
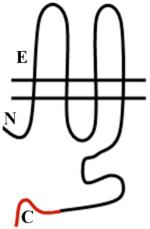
Vascular Cx37, Cx40, C43 and Cx45 show significant sequence homology. Major sequence divergence is observed primarily in the cytoplasmic loop (CL) and tail (CT) domains. Images are characterizations of membrane topology with colors highlighting the regions at which there are significant differences in amino acid sequence or length. The amino terminus in most α subgroup connexins is ~23aa, but is shorter in Cx45 at ~18aa. The CL of Cx45 is elongated compared to Cx37, Cx40 or Cx43. The lengths of the CT domains are based on hydropathy plots.
| Cx37 | Cx40 | Cx43 | Cx45 | |
|---|---|---|---|---|
 |
 |
 |
 |
|
| chromosome | 1p35.1 | 1q21.1 | 6q22-q22.3 | 17q21.31 |
| gene | GJA4 | GJA5 | GJA1 | GJC1 |
| subgroup | α | α | α | γ |
| protein (kDa) | 37 | 40 | 43 | 45 |
| protein (aa) | 332 | 357 | 382 | 396 |
| NT domain (aa)* | 19 | 18 | 12 | 22 |
| CL (aa)* | 49 | 67 | 55 | 80 |
| CT domain (aa)* | 103 | 132 | 159 | 148 |
| pore diameter (Å) | 10-14Å (Veenstra et al., 1994b) | 6.6Å (Beblo and Veenstra, 1997) | 10Å** (Heyman and Burt, 2008) | 10Å (Veenstra et al., 1994a) |
| pore length (Å) | nd | nd | 160Å (Heyman and Burt, 2008) | nd |
| protein expression | EC/VSMC (Krattinger et al., 2007; Yeh et al., 2000b) | EC/VSMC | VSMC/EC (Krattinger et al., 2007; Yeh et al., 2000b) | VSMC/EC |
Abbreviations: sizes and approximate regions were determined from data available in http://www.uniprot.org/;
radius ~6Å has been determined, but is inconsistent with dye transfer so is thus inferred to be ~10Å (Heyman, and Burt, 2008); nd, not demonstrated.
The primary role for connexin proteins is the formation of intercellular gap junctions, which are unique in the type of intercellular signaling they mediate. However, connexins may also be involved in a regulated nonjunctional permeability pathway, one allowing ions and some (larger) molecules to passively cross the plasma membrane (Spray et al., 2006). Additionally, gap junctions are now realized to be focal sites for interaction with accessory proteins, these often linked to the cytoskeleton (Giepmans, 2004); such nonchannel behavior is a common feature of other membrane channels and transporters and provides mechanisms for both ‘outside-in’ and ‘inside-out’ signaling.
3.1 CONNEXINS
In the absence of a clear understanding of the functional differences between different connexins, a logical nomenclature for distinguishing the various members of the gene family was to add the molecular weight of each protein variant as a suffix to the descriptor ‘Cx’. Hence, a protein composing gap junctions in heart that runs at ~43kDa by reducing SDS-PAGE is known as Cx43.
Connexins may be further subclassified based on their phylogenetic origins (Eastman et al., 2006). Of the four vascular connexins, three belong to the alpha (α) subgroup (Cx37, Cx40 and Cx43) while Cx45 is a member of the gamma (γ) subgroup (Saez et al., 2003). Subgroup designation plays a major role in determining the ability of these proteins to interact with one another (Beyer et al., 2000; Gemel et al., 2004) (see below) (Table 2).
TABLE 2. VASCULAR GAP JUNCTION CHANNEL ASSEMBLY.
Table summarizes studies of the (homotypic and) heterotypic compatibility between connexins expressed in vascular tissue.
| Cx37 | Cx40 | Cx43 | Cx45 | |
|---|---|---|---|---|
| Cx37 | Y | Y (Rackauskas et al., 2007) | Y (Brink et al., 1997) | Y (Elfgang et al., 1995) |
| Cx40 | - | Y | Y/N* (Cottrell et al., 2002; Rackauskas et al., 2007; Valiunas et al., 2000) |
Y (Martinez et al., 2002) |
| Cx43 | - | - | Y | Y (Bukauskas et al., 2002) |
| Cx45 | - | - | - | Y |
Abbreviations: Y, channels formed; N, channels not formed;
conflicting literature.
Irrespective of such differences, all connexins share the same overall protein topology. There are four transmembrane domains (M1-4), two extracellular loops (E1, E2), cytoplasmic amino-terminal (NT) and carboxyl-terminal (CT) domains, and a cytoplasmic loop (CL) between M2 and M3 (Evans and Martin, 2002; Kovacs et al., 2007).
The NT is relatively conserved, though its exact length has not been conclusively demonstrated [the NT domain of most α subgroup connexins is ~23aa (Kyle et al., 2008), but is shorter in Cx45 at ~18aa (Essner et al., 1996)]; it is predicted to lie within the channel pore (Lin et al., 2006; Purnick et al., 2000) and is an important region involved in channel gating in response to changes in membrane voltage (Kyle et al., 2008).
Disulfide bridge interactions between spatially conserved cysteine residues in the extracellular loops, in E1 that connects M1-M2 and in E2 that connects M3-M4 (Dahl et al., 1992; Yilla et al., 1992), are necessary for the docking process by which oligomerized connexin proteins in each apposing cell form gap junctions.
The extracellular docking forms a tight seal to prevent the loss of cytoplasmic ions and molecules to the extracellular space. The pore itself is formed by one or two of the four hydrophobic transmembrane domains (Maeda et al., 2009), can accommodate molecules of up to ~2kDa (subjective), while nonuniform pore diameters ranging from ~14Å to ~40Å have been described (Beblo and Veenstra, 1997; Fleishman et al., 2004; Heyman and Burt, 2008; Maeda et al., 2009; Oshima et al., 2007; Unger et al., 1999; Veenstra et al., 1995).
The CL and CT domains, which constitute the majority of the cytoplasmic surface are more divergent in sequence homology [the CL of Cx37 and Cx40 varies in length by 20aa (Duffy et al., 2002; Seki et al., 2004); there is an unusually elongated CL in Cx45 as compared to the other three vascular connexin isoforms (Kumai et al., 2000; Manthey et al., 1999); the CT domains range in length from 103aa in Cx37 to 159aa in Cx43 (Goodenough et al., 1996; Kyle et al., 2008; Stergiopoulos et al., 1999)], are less organized in tertiary structure and, in general, contain sites for intramolecular and intermolecular protein-protein binding and for posttranslational modifications, each of which allow for different regulatory mechanisms for fine tuning the permeation pathway.
For example, phosphorylation dynamically modulates Cx43 trafficking to, and internalization from, junctional plaques, the channel open probability and its ion conductance (Solan and Lampe, 2007). Intermolecular interactions between Cx43 and tight junction proteins are implicated in determining the size of the junctional plaque (Hunter et al., 2005). Additionally, intramolecular interactions between CL and CT of Cx43 are responsible for pH dependent channel gating (Duffy et al., 2002; Sosinsky and Nicholson, 2005). Related intermolecular interactions occur between Cx40 and Cx43 in hemi gap junction channels; the CT of Cx40 can regulate a truncated Cx43 channel, and the CT of Cx43 can rescue the pH sensitivity of a truncated Cx40 (Stergiopoulos et al., 1999).
3.2 HEMICHANNELS
Connexins oligomerize in the ER/Golgi, or at a later stage in the trans-Golgi network (TGN) (Koval, 2006), to form hexameric ‘hemichannels’ (also known as ‘connexons’). While most connexins, including Cx37, Cx40 and Cx45, oligomerize in the ER, Cx43 is not assembled into oligomers until the TGN (Maza et al., 2005). The chaperone Erp29 has been implicated in delayed Cx43 oligomerization (Das et al., 2009).
Oligomerization is thought to spontaneously occur through non-covalent interaction (Ahmad and Evans, 2002). The hemichanel structure may be further stabilized through shared sites between neighboring connexins within the same hexamer (Maeda et al., 2009). The NT and M3 regions are implicated in the oligomerization process, with residues within the NT thought to play a prominent role in dictating interaction between members of different phylogenetic subgroups, i.e., α:α and β:β interactions are allowed, but α:β interactions are generally not (Beyer et al., 2000; Gemel et al., 2004); of note, Cx45 (γ) is promiscuous.
As such, interactions between connexins in hemichannels are of two described types: homomeric hemichannels are composed of six monomers of a single connexin isoform; heteromeric hemichannels are composed by at least two different isoforms.
It is becoming apparent that isoform mixing within hemichannels may be a means for fine tuning the permeability character of gap junction channels (Ayad et al., 2006; Bevans and Harris, 1999a; Bevans and Harris, 1999b; Bevans et al., 1998; Brink et al., 1997; He et al., 1999; Locke et al., 2007; Locke et al., 2004; Weber et al., 2004).
3.2.1 HEMICHANNEL TRAFFICKING TO PLASMA MEMBRANE
At the light microscope level and by biochemical study, there is some evidence for small domains of aggregated nonjunctional hemichannels in plasma membrane. Hemichannels are considered to remain closed until docked with an appositional hemichannel (Evans et al., 2006; Spray et al., 2006), although regulated openings have been suggested (Saez et al., 2005); however, the involvement of connexins as the composition of such functional plasma membrane pores has been brought into question (see below).
Transport of hemichannels to the plasma membrane is largely dependent on an intact microtubule and Golgi network (Falk et al., 1997; Falk and Gilula, 1998; George et al., 1999; Martin et al., 2001; Thomas et al., 2005). The functional significance of interactions between connexins and the actin cytoskeleton are much less clear. There are reports that Cx43 can bypass the Golgi if cells are treated with brefeldin A and cotreated with cyclic AMP or low density lipoprotein (LDL) (Paulson et al., 2000). In part, this suggests that alternative plasma membrane trafficking pathways for hemichannels may exist under certain (patho)physiological conditions; for example, Cx26 may bypass the Golgi en route to the plasma membrane in hepatocytes (Ahmad and Evans, 2002; George et al., 1999; Martin et al., 2001).
Less is known about the trafficking of Cx37, Cx40 and Cx45 hemichannels specifically, though posttranslational modifications, in particular the phosphorylation of the CT domain, may be implicated (Solan and Lampe, 2005; Solan and Lampe, 2007). Many connexins, including Cx37, Cx40 and Cx45, are phosphoproteins as shown by either a phosphatase-sensitive shift in their electrophoretic mobility, direct incorporation of [32P]-phosphate or by mass spectrometry. As analyzed by SDS-PAGE, Cx43 shows multiple electrophoretic isoforms including a faster migrating form that is nonphosphorylated (termed ‘P0’ or ‘NP’), and at least two phosphorylated, and therefore slower migrating, forms termed ‘P1’ and ‘P2’. The Triton X100 detergent solubility of P0 and P1 and insolubility of P2 forms (Musil and Goodenough, 1991) have been used extensively to study differences between nonjunctional and junctional pools of Cx43, respectively.
Protein-protein interactions have also been implicated in the trafficking process and the targeting and removal of hemichannels and gap junction channels in the plasma membrane (Giepmans, 2004; Laird, 2006). Most in-depth studies on the binding of connexins to other cell proteins, and the functional consequences of these interactions, have largely focused on Cx43.
In the plasma membrane, gap junction plaques grow, at least in part, by the lateral movement of unpaired hemichannels to the periphery of a gap junction plaque (Gaietta et al., 2002). Interactions between Cx43 and ZO-1, a tight junction protein, may control the rate of (hemi)channel accretion at the periphery of the plaque and limit the occupied surface area (Hunter et al., 2005).
One emerging mode for plasma membrane localization, and possible regulation of channel activity, exists in the form of lipid raft movement and segregation of hemichannels. Some connexin isoforms are differentially localized to detergent soluble and insoluble lipid rafts, and show movement into and out of caveolin-1 based rafts (Langlois et al., 2008; Locke et al., 2005; Simek et al., 2009). There are well-documented lipid rafts unique to EC and VSMC [e.g., extensive caveolin-1 present in EC, and absent in VSMC (Callera et al., 2007; Raghu et al., 2007)], so it is conceivable that connexin localization or redistribution into different types of lipid raft might alter membrane localization of hemichannels and influence hemichannel/gap junction function in a cell-type specific manner.
3.2.2 CONNEXIN HEMICHANNELS AND PANNEXIN CHANNELS
As a large membrane pore, even brief opening of unapposed/nonjunctional hemichannels in plasma membranes must be tightly regulated to maintain cellular integrity (Contreras et al., 2003; Contreras et al., 2004). Connexin hemichannels (‘connexons’) have been implicated in a regulated plasma membrane pathway allowing the uptake and release of large biological and nonbiological molecules. However, the protein composition of this pathway has been of dispute.
The most prominent non-connexin pathway is that formed by pannexin channels (‘pannexons’). Pannexins (Panx), closely related to innexins, which form gap junctions in invertebrates, share the same transmembrane topology as connexins, but no primary sequence homology (Baranova et al., 2004; Yen and Saier, 2007). Pannexins do not form intercellular channels because one of their extracellular loops is glycosylated, this modification adding substantial bulk to the extracellular-facing aspect of the protein, preventing the formation of a patent cell-cell channel [insertion of glycosylation sites into the extracellular loop domains of connexins also blocks formation of junctional channels when these sites are glycosylated (Boassa et al., 2007)].
Only three pannexin isoforms have been described; Panx1 (47.6kDa), Panx2 (69.5kDa) and Panx3 (44.7kDa). The cellular and tissue expression of pannexins, notably Panx1, overlaps with that of most all connexin isoforms, so it is now believed that many of the phenomena that have been attributed to unpaired plasma membrane connexons may in fact be mediated by pannexons. Pannexin channels are permeable to everything that a connexin hemichannel should be (generally) permeable to, but, in fact, may be wider than the widest connexons (Bao et al., 2007; Locovei et al., 2007). Moreover, pannexin channels are blocked by several of the pharmacological agents commonly used to inhibit connexin hemichannels.
For these reasons, identifying which protein/‘hemi’/‘channel’ is involved in a particular cellular physiology requires careful evaluation of the specific experimental conditions and biological requirements in each case (Parthasarathi et al., 2006; Scemes, 2008; Scemes et al., 2007; Spray et al., 2006).
3.3 THE GAP JUNCTION CHANNEL
At the plasma membrane, two hexameric hemichannels align across the extracellular space to form a dodecameric intercellular channel. The gap junction plaque is a focal area of membrane containing accreted connexin channels. The composition of the plaque is unique; a requirement for other proteins has not been demonstrated and the exogenous expression of connexins in a wide variety of model systems is sufficient to result in the formation of membrane structures that are functionally and morphologically indistinguishable from gap junction plaques observed in native tissues.
Interactions between hemichannels when forming junctional channels are of two described types: homotypic junctional channels are formed by twelve identical connexin subunits; heterotypic channels are formed by two hemichannels that are each homomeric for different isoforms.
As with the oligomerization of connexins into hemichannels, members of the α and β subgroups, e.g., Cx43 and Cx26, do not readily form heterotypic channels, despite their extracellular domains being conserved in structure (Oshima et al., 2007). This incompatibility is suspected to result from different hydrogen bonding signatures in E2 (Yeager and Harris, 2007). Again, Cx45 (γ) is rather immoral and can form heterotypic channels with members from α and β subfamilies.
Other connexin domains are also involved in regulating heterotypic compatibility (Maza et al., 2005). In studies of heterotypic Cx37-Cx43 junctions, substitutions of ~12aa in the NT domain prevented channel formation (Lagree et al., 2003), yet smaller substitutions of between 2-8aa in the same region were sufficient to permit formation of heterotypic gap junctions (Kyle et al., 2008). The intact CL domain is also important; studies looking at heterotypic pairing of hemichannels formed by CL deletion mutants of Cx43 with Cx37, Cx40 and Cx45 showed there was reduced heterotypic gap junction formation with Cx37 and Cx45, and heterotypic Cx40-Cx43 junctions did not form at all (Wang et al., 2005).
4 GAP JUNCTIONAL COMMUNICATION PATHWAYS IN THE VASCULATURE
Homocellular and heterocellular contact is commonplace; EC and VSMC connect in all combinations and morphologically distinct gap junctions are observed between both cells in vivo. In mouse aortas and arterioles, Cx37, Cx40 and Cx43 are typically expressed in EC whereas Cx43, and possibly Cx37, are expressed in VSMC (Haefliger et al., 2004; Isakson et al., 2006a; Johnstone et al., 2009; Li and Simard, 2002; Saitongdee et al., 2004). However there are conflicting data on the gap junctional connexin content in the MEJ in rat, with claims that both Cx37 and Cx40 are expressed or that only Cx40 is present (Haddock et al., 2006). In mouse, Cx40 and Cx43 are present in the apposed membranes between EC and renin-secreting cells as well as in the mouse cremasteric arterioles (Isakson et al., 2008; Straub et al., 2009). However, as noted above, connexin expression in EC, VSMC or at the MEJ between EC and VSMC is both vascular bed and species specific (Isakson et al., 2008; van Kempen and Jongsma, 1999).
4.1 BETWEEN ENDOTHELIAL CELLS IN LARGE VESSELS
Immunohistochemistry, transmission and scanning electron microscopy demonstrate that EC of the large vessels are particularly well coupled by gap junctions, with all four vascular connexins expressed to various degrees (Isakson et al., 2006a; Kwak, 2002; Yeh et al., 2000a; Yeh et al., 2000b; Yeh et al., 2001).
In general, Cx40 and Cx37 are abundantly expressed in elastic (aorta) and muscular (coronary) arteries of various species, while the expression of Cx43 is typically restricted to EC in regions of turbulent flow such as vessel branch regions, particularly in the rat aortic and carotid arteries (Dai et al., 2004; Gabriels and Paul, 1998; Hill et al., 2001; Saitongdee et al., 2004). In other tissues, Cx43 is integral to cell proliferation (Chadjichristos et al., 2006b; Johnstone et al., 2009; Kwak et al., 2003; Liao et al., 2007) and apoptotic control (Kalvelyte et al., 2003), so Cx43 may act to protect EC from improper proliferation or cell death due to elevated sheer stress at these regions (Feaver et al., 2008; Johnson and Nerem, 2007).
Currently, there is no evidence for heterocellular contact between VSMC and EC in large vessels; thus, gap junctional communication in large vessels is likely to be entirely homocellular. However, while immunocytochemistry demonstrates junctional colocalization of Cx37, Cx40 and Cx43 in the EC of mouse aortas, this does not prove that heteromeric or heterotypic channels are present in vivo (Yeh et al., 2000a; Yeh et al., 2000b); though, functional heterotypic gap junction channels are observed in vitro (Isakson and Duling, 2005).
Several studies demonstrate that intracellular signaling/conducted responses within aortic EC occur bidirectionally through gap junctions (Simon and McWhorter, 2003; Wolfle et al., 2007). For example, ablation of Cx40 (Cx40−/−) from the mouse genome results in reduced acetylcholine induced vasodilation (Figueroa and Duling, 2008) and dye transfer between EC (Simon and McWhorter, 2003). That any dye coupling is observed between the EC of Cx40−/− mice shows that some junctional communication remains; upregulation of Cx37 has been demonstrated in this instance (Kruger et al., 2002; Simon and McWhorter, 2003), however this is disputed. Interestingly, neither Cx37 or Cx45 appear to play significant roles in the acetylcholine induced conductive responses in EC, suggesting that they may have other, as yet unestablished, functions (Figueroa and Duling, 2008; Hou et al., 2008; Kumai et al., 2000; Wolfle et al., 2007).
Nonbiological tracer dyes indicate the presence of extensive intercellular communication compartments, and several studies have detected changes in dye transfer pathways in response to different arterial flow patterns across the EC (Ebong et al., 2006), the biological significance of which is not yet known.
Enhanced gap junctional communication between EC is typically observed as vessel flow rate increases. Using EC in culture, Cx37 and Cx40, but not Cx43, were upregulated in response to elevated flow rates (Ebong et al., 2006). In atheroprone sheer stress models, both Cx37 and Cx43 gap junctions are redistributed to intracellular regions of cultured EC; there is increased plasma membrane organization of gap junctions under atheroprotective flow rates (Dai et al., 2004). As Cx43 is upregulated at sites of turbulent flow in vivo, with no alterations to Cx37 or Cx40 expression (Gabriels and Paul, 1998), these studies demonstrate the need for careful consideration of the model system under investigation.
4.2 BETWEEN VASCULAR SMOOTH MUSCLE CELLS
Gap junctions between VSMC are usually between small sections of plasma membrane and do not resemble those between EC, i.e., these being large gap junction plaques between tightly sealed opposing plasma membranes. As with the EC layers of the large vessels, it is generally accepted that VSMC communication is homocellular, and bidirectional conducted responses by gap junctions may be involved in acetylcholine induced conducted vasoconstriction (Figueroa and Duling, 2009), or maintaining cell quiescence (Zhang et al., 2003). Following vascular damage through insertion of indwelling catheters in mice, sites distal to the injury in the direction opposite to blood flow showed enhanced cell proliferation (Reidy, 1990). Numerous studies have shown that alterations in VSMC connexin expression are associated with cell proliferation (Brisset et al., 2009).
Only Cx43 and Cx45 are identifiable in the VSMC of healthy aortic and carotid tissues (Haefliger et al., 2004; Isakson et al., 2006a; Li and Simard, 1999; Saitongdee et al., 2004). Heteromeric and heterotypic interactions between Cx43 and Cx45 have been demonstrated in vitro (Rackauskas et al., 2007), despite their belonging to different phylogenetic/compatibilty subgroups (α and γ respectively). This association is mediated, in part, by the binding of Cx43 and Cx45 to the PDZ domains of ZO-1 (Kausalya et al., 2001; Laing et al., 2001). Homotypic Cx43 and Cx45 versus heterotypic association of Cx43 and Cx45 hemichannels may be a means by which the permeability characteristics of the VSMC gap junction pathway can be modulated in vivo, as suggested by in vitro study (Desplantez et al., 2004; Elenes et al., 2001).
Several studies have also identified expression of Cx37 in VSMC (Kwak, 2002; Simon and McWhorter, 2002; Simon and McWhorter, 2003), although it is not normally expressed in healthy adult aortas or carotids (Cai et al., 2004; Saitongdee et al., 2004); this more likely reflects species differences (van Kempen and Jongsma, 1999) or changes occurring in response to the progression of vascular disease (Cai et al., 2004; Kwak, 2002).
4.3 IN RESISTANCE VESSELS
As mentioned, connexin expression in resistance vessels is dependant on the vascular bed and species studied. Typically, Cx40 is found in EC, with Cx37, Cx43 and Cx45 being subject to more variable expression both within EC and VSMC (Bruzzone et al., 1993; Cai et al., 2004; Cai et al., 2001; Hakim et al., 2008; Hwan Seul and Beyer, 2000; Isakson, 2008; Isakson et al., 2008; Isakson et al., 2006a; Isakson and Duling, 2005; Little et al., 1995a; van Kempen and Jongsma, 1999).
Initial work to address the presence of gap junctions in resistance vessels demonstrated that tracer dyes rapidly moved between EC in hamster cheek pouch arterioles (Segal and Beny, 1992). Anatomical evidence and immunohistochemistry further shows that EC of the resistance vessels are highly coupled, similar to those between EC of large arteries (Bruzzone et al., 1993; Hakim et al., 2008; Isakson et al., 2006a; van Kempen and Jongsma, 1999).
Although in vivo morphological verification of gap junction plaques in VSMC is difficult, arteriolar VSMC are electrically coupled (Beny and Connat, 1992). However, experiments to show dye coupling between VSMC within resistance vessels have given mixed results (Beny and Connat, 1992; Little et al., 1995b; Segal and Beny, 1992; Straub et al., 2009).
The unique aspect of resistance vessel physiology is the degree to which EC and VSMC are integrated. While such integration may occur via paracrine mechanisms (see later), gap junctions play important roles at the MEJ in small vessels.
Extensive ultrastructural evidence for gap junctions has been demonstrated by transmission electron microscopy. Connexin expression is dependant on vessel location and species (Heberlein et al., 2009); recent data have found Cx37 and Cx40 in rat brain arterioles, Cx40 and Cx37 separately or in combination in rat mesentery, Cx40 and Cx43 in mice arterioles and Cx43 in human subcutaneous resistance arteries (Haddock et al., 2006; Isakson et al., 2006a; Isakson and Duling, 2005; Sandow and Garland, 2006; Sandow et al., 2003). Further, Cx37, Cx40 and Cx43 but not Cx45 have been localized at the MEJ sites in vitro (Isakson and Duling, 2005) and in vivo (Haddock et al., 2006; Isakson et al., 2008; Sandow and Garland, 2006). However, data from rat studies suggests Cx37 expression at these sites to be more variable than that of Cx40 and Cx43, or absent altogether (Isakson et al., 2008; Isakson and Duling, 2005).
Studies of the ‘endothelium derived hyperpolarizing factor’ (EDHF) have provided the vast majority of the evidence for functional gap junction signaling from EC to VSMC at the MEJ (de Wit et al., 2006a; Sandow et al., 2009). The EDHF phenomenon is based on observations that stimulation of EC with acetylcholine produces a vascular dilatory ‘factor’ (i.e., EDHF) that moves from EC to VSMC through gap junctions (presumably), even when prostaglandin and nitric oxide (NO) synthase inhibitors are present. This dilatory process is characterized by increases in intracellular Ca2+ levels ([Ca2+]i) in EC. As might be expected for a pathway involving gap junctions, VSMC would display a corresponding increase in [Ca2+]i and respond by constricting. However, the converse has been repeatedly demonstrated; VSMC display a corresponding decrease in [Ca2+]i and the vessel responds by dilating (Sandow et al., 2009).
Signaling from VSMC to EC through gap junctions at the MEJ has not been studied to the same extent as EDHF. Stimulation of VSMC with phenylephrine produces an increase in [Ca2+]i via IP3, which, in isolated vessels and in vitro, moves to EC through gap junctions present at the MEJ to produce an increase in EC [Ca2+]i and subsequent release of NO (Dora et al., 1997; Isakson, 2008; Isakson et al., 2007; Lamboley et al., 2005). This signaling process is highly dependant on the location of the receptor for IP3 (IP3-R) at the MEJ; it has been suggested that localization of IP3-R on the EC side of the MEJ, rather than the VSMC side, is the reason that the Ca2+ signal is not observed moving from EC to VSMC (Isakson, 2008).
Evidence has also indicated that gap junctions at the MEJ may not always be functional/open during acetylcholine induced conduction (Budel et al., 2003). Recent work has suggested that closure may result from posttranslational modification of the CT domain of Cx43 (Isakson et al., 2008). Phosphorylation of serine 368 (Ser368) in the CT domain of Cx43 has been observed in mouse cremaster MEJ, but not rat mesenteric MEJ, correlating with an absence of carboxyfluoresceine dye transfer in cremasteric vessels but its abundant transfer in the mesenteric vessels (Isakson et al., 2008). In fact, if the cAMP analog, pCPT, was applied to mouse cremaster, Ser368 phosphorylation was no longer detected and dye readily transfered from VSMC to EC (Straub et al., 2009).
4.4 IN CAPILLARIES AND VEINS
Strikingly, little is known about connexin expression in capillaries and veins, and thus the functional roles remain to be elucidated.
Capillary EC are ionically/electrically coupled (Beach et al., 1998) despite connexins not being readily identified (Looft-Wilson et al., 2004). This may be a consequence of the relative sensitivity of immunofluorescent and electrophysiological techniques used (Looft-Wilson et al., 2004) or explained by differences in expression levels between vessels beds as determined by signaling requirements of the tissue.
Cx40 and Cx43 have been identified in the EC and VSMC of saphenous veins, with especially high amounts of Cx43 within the VSMC (Deglise et al., 2005). It is thought this may regulate VSMC proliferation, as previously described for VSMC in larger vessels, above.
5 PERMEABILITY OF VASCULAR CONNEXIN CHANNELS
Demonstrations of junctional coupling have been established by electrophysiological measurements and observations of the intercellular transfer of small membrane-impermeable fluorescent dyes or cellular metabolites (Table 3, Table 4). Typically, gap junctions were the only identifiable intimate contact between adjacent cells. Particularly, the observation of intercellular metabolite permeability sparked broad interest in gap junction channels as mediators of intercellular molecular signaling and as key players in the processes of development, physiology and disease (Harris and Locke, 2009).
TABLE 3. NONBIOLOGICAL MOLECULAR PERMEANTS.
Typical fluorescent probes used to assess the function of gap junctions.
| size | charge | ||||
|---|---|---|---|---|---|
| mass (Da) | X (Å) | Y (Å) | Z (Å) | ||
| DAPI (Heyman and Burt, 2008) | 279 | 15.4 | 6.0 | - | +2 |
| PI (Contreras et al., 2003; Retamal et al., 2006; Valiunas, 2002) |
661 | 12.0 | 9.3 | 4.5 | +2 |
| EB (Contreras et al., 2003; Retamal et al., 2006; Valiunas, 2002) |
394 | 11.6 | 9.3 | 4.3 | +1 |
| NBD-TMA (Ek-Vitorin et al., 2006; Heyman and Burt, 2008; Simon and McWhorter, 2003) |
280 | 11.7 | 6.0 | 3.85 | +1 |
| Biocytin (Simon and McWhorter, 2003) | 372 | - | - | - | 0 |
| A350 (Weber et al., 2004) | 326 | 13.0 | 5.2 | 3.2 | −1 |
| CF | 376 | 12.6 | 12.7 | 8.5 | −2 |
| LY (Heyman and Burt, 2008) | 457 | 12.6 | 14.0 | 5.5 | −2 |
| A488 (Heyman and Burt, 2008; Weber et al., 2004) |
546 | 11.3 | 10.5 | 9.0 | −2 |
| A594 (Heyman and Burt, 2008; Weber et al., 2004) |
734 | 16.6 | 13.8 | 9.3 | −2 |
| IP3* (Ayad et al., 2006) | 180-492 | 5.5-10.6 | 5.4-9.9 | 3.1-7.9 | −6 |
Abbreviations: -, not determined;
IP3 has several structural conformers, so the approx. largest and smallest dimensions are reported (Ayad et al., 2006).
TABLE 4. RELATIVE CONDUCTANCE OF VASCULAR GAP JUNCTIONS AND HEMICHANNELS.
| Cx37 | Cx40 | Cx43 | Cx45 | |
|---|---|---|---|---|
| unitary conductance (pS) | ||||
| junction (Vj) | 300, 347 (Reed et al., 1993; Veenstra et al., 1994b; Veenstra et al., 1995) | 142-198 (Beblo and Veenstra, 1997; Veenstra et al., 1995) | 60-120 (Veenstra et al., 1995; Wang and Veenstra, 1997) | 26-37 (Valiunas, 2002; Veenstra et al., 1994a; Veenstra et al., 1995) |
| hemichannel (Vj) | 600* (Banach et al., 2000; Hu et al., 2006) |
300 (Rackauskas et al., 2007) | 220 (Contreras et al., 2003) | 57 (Valiunas, 2002) |
| substate | 63 (Veenstra et al., 1994b) | 30-36 | 30-60 | 23 |
| relative tracer permeability | ||||
| EB or PI | Cx37 = Cx40 | |||
| LY | Cx43 >> Cx40 > Cx45 = Cx40/Cx45 = Cx43/45** > Cx37 (Heyman and Burt, 2008; Rackauskas et al., 2007) | |||
| A350 | Cx45 > Cx40 > Cx43 > Cx37 = Cx37/Cx43 (Weber et al., 2004) | |||
| Cx43 >> Cx40 >> Cx40/45 = Cx43/Cx45 > Cx37 (Heyman and Burt, 2008; Rackauskas et al., 2007) | ||||
| A488 | Cx43 > Cx40 > Cx45 >> Cx37 = Cx37/Cx43 (Weber et al., 2004) | |||
| A594 | Cx43 > Cx45 > Cx40 >> Cx37/Cx43 > Cx37(nd) (Weber et al., 2004) | |||
Abbreviations: predicted hemichannel conductance of Cx37, which has not been demonstrated due to a lack of voltage dependent saturation (Banach et al., 2000; Veenstra et al., 1994b); nd, not demonstrated.
The most important point to make at the onset is of the large diversity in the permeability and modulatory properties of connexin channels. Homomeric/homotypic and heteromeric/heterotypic channels have been described, and each is of unique character; when two connexins are expressed in the same cell, several hemichannel stoichiometries and/or radial connexin arrangements around the central lumen are made possible. Thus, the relevant permeabilities of connexin channels can extend from charge selectivity among atomic ions through the size and charge selectivity among nonbiological tracer molecules (i.e., dyes) to highly specific selectivity amongst cytoplasmic molecules (i.e., second messengers) (Harris and Locke, 2009).
The properties of junctional channels have so far been reasonably well predicted from those of their component hemichannels. The intercellular channels, behave, for the most part, as if each hemichannel is a functionally independent unit, acting in series with the other docked hemichannel. This does, occasionally, give rise to unexpected permeability character and/or gating behavior (Bukauskas et al., 1995; Oh et al., 1999; Suchyna et al., 1999), not so much reflecting allosteric changes occurring to the pore itself upon hemichannel docking so much as the functional superimposition of hemichannels with different biophysical, regulatory and/or pharmacological properties (Harris, 2001).
Individual studies bearing directly on the permeability of Cx37, Cx40, Cx43 and Cx45 channels are too numerous to be discussed individually. However, a sampling of the relevant data are integrated here, in particular when there is information on the relative permeability to different ions and molecules through each connexin isoform, and when the permeability to a single ion or molecule through the different vascular connexin channels are known, where it is possible to rigorously allow such comparisons to be made. For the latter, differences in the number of functional junctional channels may have introduced error [connexin expression can vary greatly between species, tissue studied (i.e., different vascular beds) or cell lines, be affected by posttranslational modifications, as discussed below], as would intracellular distribution, cytoplasmic/nuclear binding, cell volume and concentration of tracer studied (i.e., assay procedure).
5.1 ION PERMEABILITY
Channel unitary conductance, in picoSiemens (pS), being the ease or difficulty with which ions pass through single channels when a voltage is imposed across the channel, are remarkably different for different connexin isoforms (Beblo and Veenstra, 1997; Hu et al., 2006; Moreno et al., 1994a; Moreno et al., 1994b; Puljung et al., 2004; Reed et al., 1993; Valiunas, 2002; Veenstra et al., 1994a; Veenstra et al., 1994b); itself this means the internal pore topography of gap junction channels formed by different connexin isoforms are different. The relative unitary conductance of homotypic channels is in the order; Cx37 (300ps) > Cx40 (140pS) > Cx43 (100pS) > Cx45 (30pS).
Speaking generally, greater ion selectivity is not observed in channels with smaller conductance, as would be expected for a narrower pore. In fact, connexin channels have a larger pore than the classical ion selective channels, with more than one ion able to enter the channel at the same time. In most cases, the permeability to current carrying atomic ions is high and the ionic selectivity of connexin channels are unremarkable. However, for Cx43 and Cx40 the ionic selectivity is influenced by interactions between anions and cations within the pore itself (Cottrell and Burt, 2001; Weber et al., 2004); in particular, the electrical conductance of the Cx40 pore is specifically reduced by anions affecting cation permeation.
For Cx37, significant alterations in unitary conductance are also attributed to NT domain truncations (Francis et al., 1999; Kumari et al., 2000). Polymorphic variability in this region increases the susceptibility to altherosclerotic plaque formation, and has been attributed in part to greater nonjunctional hemichannel permeability (Boerma et al., 1999; Derouette et al., 2009).
In the vasculature, gap junctions link EC:EC, VSMC:VSMC and EC:VSMC compartments, and, as there is cell-type specific expression of Cx37, Cx40, Cx43 and Cx45, there is great potential for the formation of heterotypic junctions (Burt et al., 2001; Cottrell and Burt, 2001; Desplantez et al., 2004). The realities are that the electrophysiological properties of heteromeric/heterotypic channels cannot often reliably be predicted from homomeric/homotypic data (see below) (Elfgang et al., 1995).
In vitro systems demonstrate heterotypic Cx37-Cx40 (Rackauskas et al., 2007), Cx37-Cx43 (Brink et al., 1997), Cx43-Cx45 (Elenes et al., 2001; Martinez et al., 2002; Rackauskas et al., 2007) and Cx40-Cx45 junctions (Bukauskas et al., 2006). In vivo studies have suggested formation of Cx40-Cx43 heterotypic channels (Cottrell et al., 2002; Valiunas et al., 2000).
The most compelling demonstration for heteromeric channels by electrophysiological means was obtained for Cx37 and Cx43 in transfected cells (Brink et al., 1997), as it was possible to directly compare homotypic and heterotypic single channel properties. The measured unitary conductances could not be explained solely by the formation of heterotypic or homotypic channels. Similar studies using Cx40 in addition to Cx37 and Cx43, in the same transfected cells, confirmed similar behavior for heteromeric Cx37/Cx43 and Cx40/Cx43 channels (Beyer et al., 2000). The unitary conductances of heterotypic channels, where calculated from conductances of homomeric hemichannels (the conductivity of a homomeric hemichannel is approx. twice that of the homomeric junctional channel) are (shown here as predicted/actual): for Cx40-Cx43, 127/100-150pS; for Cx43-Cx46, 75/120pS; for Cx43-Cx45, 51/52pS.
5.1.1 ELECTRICAL COUPLING
Voltage is generated by the flow of ionic current between two adjacent cells via gap junctions. These electrical connections are typically bidirectional, but may not be symmetrical in their flow. Ionic currents resulting from interconnecting cells with different potentials are clearly seen in the heart and nervous system, but are now evident in the ear, lens and in developing embryos.
Conducted responses along vessels require changes of the membrane potential; depolarization results in local and conducted vasoconstriction and hyperpolarization the dilatory conducted response. Although these electrical signals are opposite in their polarity, they both make use of gap junctions, the low resistance electrical conduction pathway in the vascular wall. Heterotypic junctions formed by Cx37 and Cx40 are rectifying, showing a macroscopic asymmetric voltage-conductance relationship, meaning that junctional currents elicited by hyperpolarization or depolarization of either coupled cell are not identical; ionic flow may thus be unidirectional.
5.1.2 VOLTAGE AND CHEMICAL GATING
All intercellular channels are sensitive to ‘transmembrane’ (Vi-o or Vm) and ‘transjunctional’ voltage (Vj) differences. These types of voltage gating are intrinsic to hemichannels, so in an intercellular channel each apposed hemichannel contains separate gating structures arranged in series. Evidence for this was shown with Cx37 and/or Cx40 channels (as mentioned above); when heterotypic channels were formed from homomeric hemichannels, one polarity of junctional voltage induces a response like that of Cx40 and the other like that of Cx37 (Bruzzone et al., 1993; Hennemann et al., 1992).
The most well described case of Vm gating is for Cx45, for which there may be species differences; human Cx45 is more sensitive to membrane potential differences than mouse Cx45. Typically, Cx45 channels open upon depolarization (Barrio et al., 1997), whereas Cx43 channels close (White et al., 1994) and Cx40 channels are unresponsive (White et al., 1994). Vm and Vj sensitivity processes are different, as was shown for Cx43 channels (Revilla et al., 2000), suggesting independent sensors and gating mechanisms.
The molecular determinants and the mechanism of polarity determination of ‘Vj’ or ‘fast’ gating (<1msec) have been studied extensively for non-vascular connexins, but differences in gating polarity different channels result from differences in the charged residues of the NT, which form the entry of the channel pore (Oh et al., 2004). Polymorphic variability in the NT region of Cx37 has been implicated in increased susceptibility to altherosclerotic plaque formation (Boerma et al., 1999; Derouette et al., 2009). Data also support involvement of the CT domain in the Vj gating mechanism of Cx43 (Bukauskas et al., 2001).
Some channels also have bipolar Vj/fast gating mechanisms, meaning that the channel may close via intermediate substates (Banach et al., 2000; Oh et al., 2004). This mechanism can involve a proline kink in TM2, an interaction between the CL and CT, posttranslational modification, especially phosphorylation (Cx43 in particular), or results from the movement of a cytoplasmic domain as a gating particle.
Less is known about the molecular determinants and mechanisms of ‘loop’ or ‘slow’ gating (>5msec), which is also sensitive to Vj, Vi-o/Vm. The most defined mechanism for of loop/slow gating is the closure of gap junction channels in response to acidification of the intracellular space, i.e., ‘pH gating’ or ‘chemical gating’.
Chemical gating of Cx43 involves pH-dependent intramolecular interactions between specific segments of Cx43 CT and CL, a mechanism that presents itself as a target for chemical or genetic manipulation (see below) (Delmar et al., 2004). This mechanism is present and absent in other connexin channels. For example, a functional interaction between Cx40 and Cx43 that modulates the pH sensitivity of junctional channels is almost certainly explained by their presence in the same hemichannels (Gu et al., 2000) - in fact, the Cx43 CT can induce pH sensitivity in CT truncated (i.e., pH insensitive) Cx40, and vice versa (Stergiopoulos et al., 1999); however, truncation of the CT of Cx37 or Cx45 has little effect on their pH sensitivity (Stergiopoulos et al., 1999).
In a rare example of a ‘dominant positive’ effect, ‘heterotypic’ docking of Cx45 with a CT truncation mutant of Cx45 that does not form homotypic junctional channels enables to the truncated Cx45 hemichannel to open (Hulser et al., 2001).
5.2 MOLECULAR PERMEABILITY
Uncharged and relatively chemically unreactive tracers have been used sparingly to infer the diameter of connexin pore lumens at their narrowest point (the ‘size-selectivity filter’) (Harris, 2001). In only one study, using sugars as tracers, mannitol (a linear monosaccharide alcohol) and stachyose (a branched tetrasaccharide) were found to be impermeable through Cx40 channels, but mannitol and not raffinose (a branched trisaccharide) or stachyose were permeable through Cx43 channels (Wang and Veenstra, 1997), suggesting that Cx40 channels have a narrower limiting diameter than Cx43 channels.
Connexin channel molecular permeability and the physical size constraints of the pore have been more widely assessed using several types of nonbiological molecules, usually fluorescent dyes of different sizes, charge and chemistry. Dye molecules report the existence and extent of junctional communication pathways, while, at their most basic level, providing some information about the relative abilities of diverse, large permeants to pass through pores formed by different connexins. This literature is vast and, typically, unitary conductance and channel permeability to these larger molecules do not correlate well with each other, meaning the former do not allow easy inferences or extrapolations about the latter (Veenstra et al., 1995).
Dyes are less informative as tools with which to investigate the biological nature of the permeability pathway itself. Only a few studies have revealed differences in permeation by different biological molecules through a particular type of connexin channel, or differences in permeation by a particular biological molecule through different types of connexin channels. In large part, the perceived absence of useful data is because these experiments are technically challenging; the pore entrance and exit are cytoplasmic, so (applied, biological) ‘permeant’ species cannot typically be altered with impunity.
Introduction of most biologically active compounds into cells can have direct or indirect downstream effects on gap junction channels. Further, biological compounds can be degraded, a factor that does not typically affect assessment of permeability using dyes, and whose rate of degradation can be dependent upon metabolic state and cell type. Critically, the ability to quantitatively detect intercellular transfer is often constrained by available detection methods, such as by chemical modification of the molecules (i.e., adding fluorescent tags) that could affect their permeability. Further, posttranslational modifications of connexin may affect biological molecule permeability, either by direct modification of the permeation pathway or by altered voltage gating leading to occupation of conductance substates [this has been demonstrated for Cx43 (Qu and Dahl, 2002)].
It is easy to believe that the permeation through connexin channels, in part, would depend on the limiting pore width of the channel lumen, i.e., its diameter at its narrowest point; historically, a molecular weight limit of ~2kDa and ~10Å had been in place, but permeant charge and chemistry are now known to play important roles, particularly as the channel lumen is not featureless - instead size, shape and chemical interactions between permeant and pore walls influence molecular permeation.
The few data available do make clear that different connexin channels can have highly distinct and differential permeabilities among cytoplasmic molecules, and that these bear little discernible relation to the channels’ ionic charge selectivities, to the permeabilities of nonbiological tracer/dye molecules or, in part, to permeants’ size.
Where possible, available studies with charged and uncharged nonbiological tracers rank the limiting pore diameter of vascular connexins, widest to narrowest (unitary conductance, in picoSiemens (pS), is in parentheses): Cx43 (90pS) >> Cx40 (180pS) ≈ Cx45 (30pS) > Cx37 (300pS). Of note, Cx37 channels are the narrowest pore in spite of having the largest unitary conductance. In fact, for these four connexins, there is almost an inverse correlation in the magnitudes of these channels’ conductances and the sizes of the permeants; larger nonbiological tracer molecules permeate channels with low conductances (e.g., Cx45), but are unable to permeate channels with much higher conductances (e.g., Cx37, Cx40).
To the extent that the limited data also allow comparison between pore width and ionic charge selectivity, the data suggests that the narrower channels (i.e., Cx37, Cx45 and Cx40) are also significantly more charge selective (than Cx43, the widest). Their permeability to large cationic tracers is Cx45 (30pS) and Cx43 (90pS) > Cx37 (300pS), and their permeability to large anionic tracers is Cx43 (90pS) > Cx40 (180pS) > Cx45 (30pS) >> Cx37 (300pS) (Bedner et al., 2006; Cottrell and Burt, 2001). One piece of data conflicts with this, that is, the movement of Alexa350 dye (A350), a small anionic dye, through Cx43 channels is specifically reduced by a unique interaction between A350 and the Cx43 pore that does not occur for other connexin channels (Weber et al., 2004).
In fact, while Cx43 channels are equally permeable to large anionic and cationic dyes, Cx43 displays a remarkable and unpredictable divergence with regard to permeation by large, anionic biological molecules, thus favoring ADP/ATP over AMP and adenosine (Goldberg et al., 2002). It is difficult to account for this selectivity without invoking some kind of specific affinity between the channel and these permeants.
Remarkable and unexpected findings also involve Cx43 channel permeation by RNA and peptides. Single-stranded interfering RNAs, which one would expect to be physically too large to permeate junctional channels, can do so. This property is connexin specific, with permeability through homotypic Cx43 channels documented (Valiunas et al., 2005). Linear/unstructured peptides of length up to ~10aa can also permeate homotypic Cx43 junctional channels, which means gap junctions potentially play important roles in cell-cell transfer of peptides in antigen presentation and cross-presentation in the immune system (Neijssen et al., 2005)
5.2.1 COMMUNICATION COMPARTMENTS
Dye transfer studies establish the presence or absence of gap junctions. The least quantitative studies are the most common; these assess the number of cells to which dye spreads from a ‘donor’ cell loaded with tracer. More qualitative clues about relative tracer permeabilities can be inferred if spread is normalized to the number of channels (i.e., ‘junctional conductance’ divided by ‘unitary conductance’), factors such as ‘frictional force’ or various incarnations of ‘affinity’ are taken into account, or tracer permeability is normalized to the permeability of a second tracer applied at the same time.
Lucifer yellow (LY, charge −2) is the most commonly used gap junction permeable dye. Early studies demonstrated EC:EC transfer of LY, and the absence of EC:VSMC or VSMC:VSMC transfer in cheek pouch arterioles (Segal and Beny, 1992; Welsh and Segal, 1998).
In large vessels, biocytin (charge neutral) and NBD-TMA (charge +1) transfer between EC in aortas. Ex vivo, dye injections of mouse aortic endothelium from Cx40−/− and Cx37−/− mice demonstrated significant reductions in dye transfer compared to wildtype (Simon and McWhorter, 2003; Simon et al., 2004). These studies, wildtype aortic EC were more permeable to biocytin than NBD-TMA, and knockout of Cx40 but not Cx37 in these layers significantly reduced transfer of both biocytin and NBD-TMA compared to control mice (Simon and McWhorter, 2003). Later, it was found that treatment of aortas from Cx37 knockout animals with lipopolysaccharide reduced expression of Cx40 resulting in a decrease in dye transfer (Simon et al., 2004).
5.2.2 SIGNALING KINETICS
The relative junctional permeabilities of different biological molecules are key factors in any coordinated signaling or regulatory system that has a kinetic component, particularly where the rate of change or ‘oscillation’ in the levels of a compound are important. Oscillatory changes in signaling molecules (e.g., Ca2+, cAMP, ATP) occur across systems of cells coupled by gap junctions convey information distinct from changes in steady-state levels. Even modest differences or changes in selectivity at cellular junctions have major impact on the strength, character and location of the intercellular signaling, under both steady state and kinetic conditions.
Essentially, for two molecules having different junctional permeabilities, it appears as though cells are better coupled with regard to one compound. Whenever there is a difference or a change in synthesis or degradation (e.g., in response to hormone receptor activation or difference/change in connexin channel composition), the profile of concentration of a compound will change with kinetics that also includes their rates of junctional flux. The consequence of having an intercellular pathway is that not only can the steady state levels of the compounds be different, but the kinetics of change in those levels are different for each compound in not one, but a population of cells.
5.2.3 SELECTIVE PERMEABILITY
The naive view is that gap junction channels, as large membrane pores, have little selective molecular permeability, so that any permeant biological molecules readily equilibrate within a population of coupled cells. Instead, relative and absolute differences in junctional permeability of different biological molecules is important, and produce profound differences in tissue response, for example, to periodic release of second messengers.
A variety of experimental approaches have been used, some involving direct measurements of molecular flux through unambiguously identified connexin channels and others relying on indirect methods to infer the identity of the transferred compounds or that the transfer is through connexin channels.
The results make clear that different connexin channels can have highly distinct and differential permeabilities among dye molecules, and that these bear little discernible relation to the atomic ion charge selectivities or to the permeabilities to biological fluorescent tracers (c.f., Cx43 permeability of Alexa dyes versus adenosine compounds).
The vast majority of work documents all-or-none permeability. With only a few exceptions, commonly studied second messengers, i.e., ATP, IP3/Ca2+, cAMP and cGMP, are permeable to some degree through each type of connexin channel studied, and all are important in modulating vessel function (Faigle et al., 2008; Kanaporis et al., 2008). Selectivity has been shown only for cAMP which diffuses more readily through Cx43 than Cx45 (Bedner et al., 2006) or Cx40 junctions (Kanaporis et al., 2008).
In the vasculature, it has been demonstrated that the gap junctions facilitate the movement of IP3 between EC (Boitano et al., 1992) and heterocellular movement of IP3 through gap junctions at the MEJ (Isakson et al., 2007), the latter independent of connexin composition (Isakson, 2008). In VSMC, gap junctions facilitate the propagation of oscillatory [Ca2+]i waves (Christ et al., 1992), and heterocellular movement of Ca2+ across the MEJ was demonstrated bidirectionally using in vitro vascular cell co-culture for EC and VSMC (Isakson et al., 2007). These responses were independent of IP3 levels, directly indicating Ca2+ movement through gap junctions at the MEJ.
Where established to be junctional, as opposed to paracellular, Ca2+ signaling is usually considered as evidence that IP3 permeates junctional channels. However, junctional Ca2+ flux may also be involved. In some cases, it is clear that regenerative Ca2+ signaling is mediated by IP3 flux and that the contribution of junctional Ca2+ flux is minor (Leybaert et al., 1998). In certain cases it appears that junctional Ca2+ flux is a major factor and in others that both are involved (Faigle et al., 2008; Isakson, 2001; Isakson et al., 2007; Kanaporis et al., 2008; Saez et al., 1989). Computational modeling has supported each scenario (Iacobas et al., 2006).
5.2.4 HEMICHANNEL-MEDIATED PARACRINE SIGNALING
Typically, Ca2+ wave propagation involves two pathways: direct intercellular flux of IP3 through gap junction channels and an extracellular pathway involving ATP release and purinergic receptors. In EC, release of ATP through vascular ‘hemichannels’ is thought to propagate oscillatory Ca2+ wave conduction in arterioles and capillaries (Bennett et al., 2003; de Wit et al., 2006a; de Wit et al., 2006b; Parthasarathi et al., 2006). This promotes vessel contraction and additional Ca2+ release from intracellular stores, which regenerates the signal, along the vessel length (Haddock et al., 2006). The paracine release of ATP may be a compensatory mechanism for reduction in gap junctional permeability of ATP (De Vuyst et al., 2006). However, in vascular disease, the release of ATP is chemoprotective, preventing leukocyte adhesion and accumulation at atherosclerotic regions (Wong et al., 2006a).
A genetic polymorphism in GJA4, the human gene encoding Cx37, is a potential prognostic marker for atherosclerosis. Cx37 expression is altered in mouse and human atherosclerotic lesions; it is absent from the endothelium of advanced plaques but is present in macrophages recruited to the lesions. It is suggested Cx37 hemichannel activity (i.e., release of ATP into the extracellular space) in macrophages inhibits leukocyte adhesion and initiation of the development of atherosclerotic plaques. Macrophages expressing either of the two Cx37 proteins encoded by a polymorphism in the GJA4 gene (Cx37-Ser319, Cx37-Pro319) show differential ATP dependent adhesion (Wong et al., 2006a).
However, when considering of the involvement of ‘hemichannels’ in normal vessel cell physiology and disease, one must be careful; the paracrine/extracellular release of ATP or Ca2+ described above might occur via any of several connexin and nonconnexin (e.g., pannexin) candidate mechanisms. In many studies, correlation of extracellular release of biological molecules with connexin expression is taken to indicate that the release is through connexin hemichannels.
A large literature documents the multifaceted biological effects of connexin expression. Altered connexin expression has been shown to have dramatic effects on the expression of hundreds of other gene products, which may have downstream effects on non-connexin plasma membrane proteins and other mechanisms of cell release of signaling molecules. Such changes could alter expression or regulation of the other candidate (ATP or Ca2+) release channels.
Panx1 has been identified in vascular smooth muscle cells of rat cerebral arteries but not in the EC (Chen et al., 2008). Panx2 is expressed in both endothelial and smooth muscle cells layers, interestingly localizing at regions of gap junctional plaques (Chen et al., 2008).
Pannexin channels can exist alone in membranes, or be activated by purinergic P2X7 receptors. Panx1 channels are closed at negative potentials, open at positive potentials and inactivate over time. They are activated at normal resting membrane potentials by mechanical stress or by increases of cytoplasmic Ca2+. Panx1 channels are very permeable to ATP, so much so that current carried through the channel by ATP itself can be recorded. It is therefore extremely likely that pannexons are the prime candidates for the ATP release channel and/or source of paracrine Ca2+ wave propagation in the vasculature.
5.3 POSTTRANSLATIONAL MODIFICATIONS
Either by direct modification of the permeation pathway, by altered gating leading to occupation of conductance substate(s) or by changing the ability of the connexin to interact with other proteins, posttranslational modifications play an important role in control of gap junction permeability. This has been explicitly demonstrated for Cx43 and, to date, most studies, irrespective of connexin isoform involved, focus on the role of phosphorylation; modification of the CT domain may regulate unapposed hemichannel function (Lampe and Lau, 2004; Zeilinger et al., 2005), and there is differential Cx43 phosphorylation in both healthy and diseased vessels (Isakson et al., 2008; Isakson et al., 2006a; Johnstone et al., 2009).
The role of other posttranslational modifications affecting the channel permeability (and of phosphorylation of other connexins) is relatively under explored. For example, NO donors cause nitrosylation of Cx43 and enhance the probability of opening hemichannels [in astrocytes (Retamal et al., 2006)], while they lead to reduction in Cx37 junctional coupling [in HUVECs (Kameritsch et al., 2005)]. In both examples, NO donors were administered at supraphysiological concentrations.
Phosphorylation affects connexin trafficking and the gating properties of the channels in the plasma membrane (Ek-Vitorin et al., 2006; Solan and Lampe, 2008). Phosphorylation in the CT of Ser, Thr and Tyr residues of Cx43 has been described, both predicted and actual, involving, e.g., Src, MAPK, PKA and PKC, p34cdc and casein kinase 1 (CK1). Phosphorylation by PKA and CK1 enhance gap junctional communication by Cx43, whereas PKC, Src and MAPK reduce channel function (Pahujaa et al., 2007; Solan and Lampe, 2005; Solan and Lampe, 2008). Of interest, phosphorylation of Ser368 of Cx43, which is reported to reduce Cx43 involved communication pathways (Solan et al., 2007), has recently been identified in skeletal muscle beds, but not in mesenteric vessels (Isakson et al., 2008; Straub et al., 2009). Additionally, phosphorylation at one site (for example Ser365, which enhances communication) inhibits the phosphorylation of others (for example, of Ser368, which inhibits communication) (Solan et al., 2007). This suggests that site-specific connexin phosphorylation may be a means to influence both homocellular and heterocellular communication in the vasculature, although this requires further investigation.
There are also species specific differences in phosphorylation patterns; one example, rat Cx43 is regulated by PKG, which shifts the channel toward lower conductance states, but human Cx43 is not, as the PKG consensus phosphorylation site (Ser257) is absent from the CT domain (Kwak et al., 1995; Moreno et al., 1994b).
Effects of phosphorylation of other connexins are less well characterized. Phosphorylation of rat Cx45 by PKC has been reported to cause the appearance of a new conductance state (Kwak et al., 1995), but PKC-mediated phosphorylation of mouse Cx45 increases the occupancy time of existing states (van Veen et al., 2000). While Cx37 phosphorylation has not been demonstrated, sequence homology of the Cx43 and Cx37 CT domains suggest the following equivalent binding sites; for MAPK, Cx43-Ser282 and Cx37-Ser275; for PKC, Cx43-Ser368 and Cx37-Ser282 (Burt et al., 2008). The expression of Cx40 is also reportedly altered through signaling pathways involving PKA, implicating a change in the phosphorylation state and site of action (Hoffmann et al., 2003). Phosphorylation by PKA seems to favor the higher conductance states of Cx40 (van Rijen et al., 2000).
5.4 INTERACTION WITH OTHER PROTEINS
While the intercellular communication pathways formed are well defined, to some extent, the nonchannel functions of different connexin isoforms are less so. Gap junctions, as part of a larger organization once referred to as the ‘nexus’, serve as a network hub for intercellular and intracellular communication; connexins provide sites for attachment of cytoskeletal proteins, for components involved in multiple intracellular signaling pathways, or other cell junction complexes and membrane channels (Chanson et al., 2007; Giepmans, 2004).
Most studies on the direct binding of connexins to other proteins have focused on Cx43; it interacts with kinases such as Src, protein kinases A, C and G, MAPK, cyclin-dependent kinase cdc2 and phosphatases, such as receptor protein tyrosine phosphatase μ, as well as structural proteins such as zonula occludens-1 (ZO-1), catenins and cadherins, caveolin, tubulin and drebrin. For example, trafficking of Cx43 (also Cx45) is dependent on CT domain interactions with ZO-1, a tight junction protein (Laing et al., 2001). ZO-1 contains three PDZ domains, the second of which binds to the Cx43 CT domain; most proteins with PDZ domains are membrane-associated and are found at specialized membrane domains such as synapses, junctions and apical-basolateral interface regions.
While physiological and developmental defects have typically been attributed to defects in the permeation pathway itself, as for most membrane proteins, connexin mutations are more likely to result in trafficking, folding or assembly, and regulatory defects to produce a functional knockout. Additionally, ‘dominant-negative’ effects are sometimes observed where the expression of one mutant connexin inhibits the functional expression of others, largely via their heteromultimerization with wildtype connexins. In each case, the absence or reduced function of a large multiprotein signaling complex situated at the cell surface can be of just as much importance as the channel activity in influencing cell development and differentiation.
Of further interest, ZO-1 directly regulates gating of Cx43 channels, serving as a scaffold that connects a phospholipase directly to these gap junctions, thereby controlling local phosphatidylinositol levels (van Zeijl et al., 2007). Oxidized phospholipids have also been shown to directly alter Cx43 expression and phosphorylation (Isakson et al., 2006b).
5.5 PHARMACOLOGICAL BLOCK
A major problem in the gap junction field is the absence of specific pore blocking reagents for connexin channels, which are necessary for investigating the physiological roles of connexin channels and for structure-function studies of the permeation pathway; a predominance of the known modulators of gap junction communication are lipophilic and/or show some specificity with regard to membrane lipids. Further, the potency and specificity of these reagents are not optimal for long-term studies.
Halothane, an inhalation anesthetic, is widely used to rapidly and reversibly inhibit junctional communication (Spray and Burt, 1990). Halothane is not specific for connexin channels, and appears to be effective on all connexins, but Cx40 and Cx43 have slightly different sensitivities to halothane (He and Burt, 2000); heteromeric channels are significantly less sensitive than the corresponding homomeric channels. Halothane can bind with high affinity to appropriately sized hydrophobic cavities in proteins to stabilize helix-helix configurations, so its action on Cx40 and/or Cx43 channels could indicate structural differences for each connexin in a homomeric and heteromeric configuration.
In addition, peptides targeting the extracellular loop domains have been shown to effectively reduce the formation of gap junction channels (Evans and Leybaert, 2007). The potential utility of ‘mimetic’ peptides are highlighted by the ability to inhibit specific connexin subtypes simply by designing peptides unique to individual connexin isoforms, though there are several drawbacks; firstly, inhibition of junctional communication typically requires incubation of peptide with cells for several hours and, secondly, reasonably high concentrations are needed, though, thirdly, junctional coupling is not reduced completely (Dahl, 2007). In addition to inhibiting gap junctions, mimetic peptides also reduce cellular dye uptake, presumably mediated by connexons - though one cannot rule out their effect on pannexons. For example, in rat mesenteric arteries, connexin mimetic peptides caused a reduction in electrotonic coupling without nonjunctional effects (Matchkov et al., 2006), though a recent report indicates that connexin mimemtic peptides strongly reduce membrane currents in Xenopus oocytes expressing Panx1 at concentrations similar to those that inhibit connexin channels (Wang et al., 2007).
Peptides targeting the cytoplasmic domains have also emerged as an interesting approach for development of a gap junctional pharmacology; modification of both the chemical and voltage gating behavior of Cx43 can be achieved by a 34aa peptide (Shibayama et al., 2006) that interferes in the pH-dependent intramolecular interaction between CT and CL. Its application may be useful for maintaining gap junctional communication during acidification of the intracellular space, i.e., cardiac arrhythmia.
Thus, specific inhibitors of connexin channels may have therapeutic utility, and connexin channels are new and promising pharmacological targets for the treatment of many diseases and dysfunctional states, including wound healing, hypertension, and atherosclerosis (Herve and Dhein, 2006; Herve and Sarrouilhe, 2005).
6 INVOLEMENT OF CONNEXIN IN VASCULAR DISEASE
6.1 HYPERTENSION
Hypertension, the elevation of blood pressure, is one of the most important risk factors for coronary heart disease and a leading cause of chronic renal failure. Primary (essential) hypertension accounts for nearly all cases worldwide and results from the interaction of genetic and environmental factors that affect cardiac output, peripheral resistance, or both. However, in ~10% of cases (secondary), hypertension is the result of renal disease or atherosclerotic narrowing of the renal artery, both of which affect the renin-angiotensin system and/or Na+ homeostasis, itself central to blood pressure regulation. It is also clear that unregulated secretion of renin from kidneys is also an important part of the disease process for primary hypertension.
In the kidneys, renin producing cells are coupled to each other and EC by Cx40, while EC are themselves coupled to each other by Cx43 (Krattinger et al., 2007). Genetic replacement of Cx43 by Cx32 (knock-in, KI) protects Cx43KI32 mice from experimentall induced hypertension as there is decreased renin production (Haefliger et al., 2001; Haefliger et al., 2006), whereas knockout of Cx40 makes the animals hypertensive, due in part to excessive stimulation of renin biosynthesis and release, through a defect of conduction along EC leading to increased peripheral resistance cannot be ruled out (Kurtz et al., 2007; Wagner et al., 2007). Thus, renin secretion is likely controlled both by Cx40 signaling in the kidney cells that produce the hormone, and by Cx43 dependent signaling that originates in the vascular EC.
Conversely, hypotension has been observed by endothelial-specific knockout of Cx43 using an endothelial TIE-2 promotor; these mice demonstrated a significant decrease in plasma angiotensin II levels (Liao, 2001).
Neither Cx37 or Cx45 have been implicated in the development of hypertension (Just et al., 2009; Kurtz et al., 2009; Schweda et al., 2009), though genetic polymorphism of Cx37 (C1019T) has been implicated in vessel occlusion and narrowing (‘atherosclerosis’) and carotid artery intimal thickening (Collings et al., 2007; Lanfear et al., 2007), all of which may contribute to the progression of the disease.
6.2 ATHEROSCLEROSIS
Atherosclerosis, a progressive disease of the large arteries, typically leads to myocardial infarction and stroke. Knowledge of the mechanisms underlying atherosclerosis are significant, though many questions about the initiating factors of atherogenesis remain unanswered. Even so, atherogenesis appears to have a robust, connexin associated pathology as evidenced by studies on human blood vessels and mouse models; in particular, Cx37 has been identified as a major risk factor for development of atherosclerosis, for there are remarkable changes in its expression and function in the endothelium and smooth muscle of the larger arteries (e.g., the abdominal aorta, the coronary arteries, and the carotid arteries), the primary sites of atherogenesis (Derouette et al., 2009; Wong et al., 2006a).
These arteries have two main layers; the ‘tunica intima’ lining the lumen of the vessel comprising a single EC layer that is separated from VSMC in the ‘tunica media’ by the ECM rich IEL. In the earliest stages of atherogenesis, EC become more permeable to inflammatory cells, while quiescent VSMC become highly motile and lose their contractile ability. These VSMC accumulate in the tunica intima to form a ‘fatty streak’ that eventually forms a ‘fibrous cap’, the mature atherosclerotic plaque. In mouse and human aortas and carotids, Cx37, Cx40 and Cx43 are expressed in EC, whereas Cx43 is mainly expressed in VSMC. Expression of Cx37, Cx40 and Cx43 are differentially modified during atherosclerotic plaque development. Mouse models have shown that Cx43 has an atherogenic effect, whereas Cx37 and Cx40 seem to be atheroprotective (Chadjichristos et al., 2006a; Chadjichristos et al., 2006b; Chanson and Kwak, 2007; Johnstone et al., 2009; Kwak et al., 2003). Counteracting mechanisms for Cx37 and Cx43 have been shown in the vasculature in aging mice.
The first experimental support for the role of connexins in atherogenesis was obtained by study of atherosclerotic plaques in human and rabbit carotid arteries, with the observations that Cx43 is downregulated in VSMC underneath the plaque (Kwak, 2002) and that macrophage foam cells in the plaques expressed significant amounts of Cx43 mRNA, even though monocytes and macrophages, from which foam cells arise, did not. It was later found that Cx43 was first upregulated, and then downregulated in the VSMC of more advanced lesions (Brisset et al., 2009; Chadjichristos et al., 2008; Kwak, 2002). The exact mechanism by which this occurs is unknown, though many studies elucidate a role for Cx43 and its posttranslational phosphorylation in cell proliferation and migration (Sanchez-Alvarez et al., 2006; Tabernero et al., 2006; Xie et al., 1997), key stages in atherogenic development.
While these studies established that connexin expression might be modified during the progression of the disease, they did not establish causality between connexins and atherosclerosis. It was the finding that a single base pair change in the CX37 gene, C1019T resulting in the Cx37-Ser319 or Cx37-Pro319 polymorphism, either was a risk factor for atherosclerosis development or limited the extent of atherosclerosis, respectively, which greatly stimulated research into the association (Chanson and Kwak, 2007).
More extensive research has been conducted using mouse models created through interbreeding of specific connexin knockout mice and atherosclerosis-prone LDL receptor null (LDLR−/−) or apolipoprotein E null (ApoE−/−) mice. In mice with both homozygous knockout of LDLR and heterozygous knockout of Cx43, atherosclerotic plaque development was significantly increased (Kwak et al., 2003). Additionally, double knockout of Cx37 and ApoE lead to increased macrophage recruitment and plaque size (Wong et al., 2006b).
Atherosclerosis in coronary arteries results in ischemic heart disease. A common and effective mechanical treatment for coronary atherosclerosis is angioplasty, the disruption of a developing atherosclerotic plaque by balloon-catheter distension. However, renarrowing of the vessels (‘restenosis’) typically occurs at the site of intervention in over half of the patients after angioplasty, and up to ~30% of patients after subsequent implantation of a stent, which itself provides mechanical support to the vessel and extends its internal diameter to avoid reocclusion.
Restenosis mechanisms are not fully understood, but parietal trauma induced by the balloon-catheter distension typically triggers platelet aggregation and thrombus formation. In addition, the artery becomes stretched, exaggerating and accelerating the recruitment and infiltration of inflammatory cells. Re-endothelialization is necessary to repair the lining of the damaged vasculature and is critical to prevent thrombosis and restenosis.
Both gap junctional and purinergic signaling contribute to re-endothelialization of human EC by their effects on both intracellular and extracellular Ca2+ stores. The release of growth factors and proinflammatory cytokines by platelets, inflammatory cells and VSMC, in addition to the loss of endothelial anti-proliferating agents, such as NO, modulate the phenotype of VSMC, shifting them from a quiescent and ‘contractile’ phenotype to one that is ‘synthetic’, i.e., more proliferating and less differentiated (Bundy et al., 2000). VSMC proliferate shortly after angioplasty, and migrate towards the intima. VSCM synthesize ECM and further contribute to intimal hyperplasia. Intimal thickening is greatest after several months, after which ‘synthetic’ VSMC dedifferentiate, the wall is remodeled and intimal thickening stops (Brisset et al., 2009).
In the rat carotid artery after balloon-catheter insertion, Cx43 has been shown to be upregulated in intimal and medial VSMC concomitantly to their activation and phenotypic modulation (Chadjichristos et al., 2008). Enhanced Cx43 expression in macrophages of restenotic lesions has also been confirmed in different species (Chadjichristos et al., 2006b) Low density lipoprotein receptor-null mice crossbread to a heterozygous background of Cx43 (Cx43+/−LDLR−/−, thus expressing reduced levels of Cx43) display restricted intimal thickening after balloon catheter distension of the carotid artery, despite marked endothelial denudation and VSMC activation, as compared to control Cx43+/+LDLR−/− littermates (Chadjichristos et al., 2006b). Reduced expression of Cx43 results in decreased macrophage infiltration, VSMC migration and proliferation, and thus accelerated re-endothelialization, suggesting that modulation of Cx43 levels could be a novel therapeutic strategy to prevent restenosis after coronary angioplasty and stent implantation.
7 CONCLUDING REMARKS
The highly coordinated behavior of EC in the vascular bed is achieved by gap junctions formed by different connexin isoforms. In addition, gap junctions also couple VSMC, and interconnect EC and VSMC at the MEJ, a short distance transverse pathway allowing direct and reciprocal communication between the two anatomically distinct vascular cell types.
Significant advances have been made in the understanding of the biophysics of different connexin channels, in particular those found to play prominent roles in the vasculature. Each connexin isoform contributes a particular set of functional or regulatory properties to gap junction channels that appear essential, either intact or as modified by interaction with other connexins and nonconnexin proteins. Approaches using mice with knockout or cell-specific gene disruption for different connexins confirm that Cx37, Cx40, Cx43 and Cx43 serve specific biological functions in the microcirculation.
The function(s) and role(s) of connexins for both maintenance of vascular health and progression of vascular disease states in humans are areas of active and fruitful investigation.
ACKNOWLEDGMENTS
The Authors thank Walter Duran, Andrew L. Harris, Brian Duling, Adrian A. Anderson and Michael J. Rizzo for comments. This work is supported by NIH GM36044 and GM61406 (DL), an American Heart Association Scientist Development Grant, and by NIH HL088554 (both to BEI).
Abbreviations
- A350
Alexa350 dye
- A488
Alexa488 dye
- A594
Alexa594 dye
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- BFA
brefeldin A
- Ca2+/[Ca2+]i
calcium ion /intracellular concentration of
- CA
calcein
- cAMP
adenosine 3′, 5′-cyclic phosphate
- CF
6-carboxyflourescein
- CL
cytoplasmic loop domain
- cGMP
guanosine 3′, 5′-cyclic phosphate
- CT
carboxyl-terminal domain
- Cx
connexin
- Cx37
connexin37
- Cx40
connexin40
- Cx43
connexin43
- Cx45
connexin45
- DAPI
4, 6-diamidino-2-phenyl-indole dihydrochloride
- E1/E2
first/second extracellular loop domains
- EB
ethidium bromide
- EC
endothelial cell
- ECM
extracellular matrix
- IEL
internal elastic lamina
- IP3
inositol triphosphate
- IP3-R
receptor for I P3
- LY
Lucifer yellow
- M1/M2/M3/M4
connexin transmembrane domains
- MEJ
myoendothelial junction
- NB
neurobiotin
- NBD-TMA
2-(4-nitro-2,1,3-benzoxadiazol-7-yl)-aminoethyl trimethyalammonium
- NO
nitric oxide
- NT
amino-terminal domain
- P0 or NP
nonphosphorylated Cx43
- P1 and P2
phosphorylated Cx43
- Panx
pannexin
- Panx1
pannexin1
- Panx2
pannexin2
- Panx3
pannexin3
- PI
propidium iodide
- pS
picoSiemens
- siRNA
silencing/antisense RNA
- t1/2
half life
- TGN
trans-Golgi network
- Vi-o or Vm
transmembrane voltage
- Vj
transjunctional voltage
- VSMC
vascular smooth muscle cell
- ZO-1
zonula occludens-1
REFERENCES
- Ahmad S, Evans WH. Post-translational integration and oligomerization of connexin 26 in plasma membranes and evidence of formation of membrane pores: implications for the assembly of gap junctions. Biochem. J. 2002;365:693–9. doi: 10.1042/BJ20011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad WA, Locke D, Koreen IV, Harris AL. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J. Biol. Chem. 2006;281:16727–39. doi: 10.1074/jbc.M600136200. [DOI] [PubMed] [Google Scholar]
- Banach K, Ramanan SV, Brink PR. The influence of surface charges on the conductance of the human connexin37 gap junction channel. Biophys. J. 2000;78:752–760. doi: 10.1016/S0006-3495(00)76633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Samuels S, Locovei S, Macagno ER, Muller KJ, Dahl G. Innexins form two types of channels. FEBS Lett. 2007;581:5703–8. doi: 10.1016/j.febslet.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–16. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Barrio LC, Capel J, Jarillo JA, Castro C, Revilla A. Species-specific voltage-gating properties of connexin45 junctions expressed in Xenopus oocytes. Biophy. J. 1997;73:757–69. doi: 10.1016/S0006-3495(97)78108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach JM, McGahren ED, Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am. J. Physiol. 1998;275:H1489–96. doi: 10.1152/ajpheart.1998.275.4.H1489. [DOI] [PubMed] [Google Scholar]
- Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J. Gen. Physiol. 1997;109:509–522. doi: 10.1085/jgp.109.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedner P, Niessen H, Odermatt B, Kretz M, Willecke K, Harz H. Selective permeability of different connexin channels to the second messenger cyclic AMP. J. Biol. Chem. 2006;281:6673–81. doi: 10.1074/jbc.M511235200. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trend Neurosci. 2003;26:610–7. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny JL, Connat JL. An electron-microscopic study of smooth muscle cell dye coupling in the pig coronary arteries. Role of gap junctions. Circ. Res. 1992;70:49–55. doi: 10.1161/01.res.70.1.49. [DOI] [PubMed] [Google Scholar]
- Beny JL, Gribi F. Dye and electrical coupling of endothelial cells in situ. Tissue Cell. 1989;21:797–802. doi: 10.1016/0040-8166(89)90030-x. [DOI] [PubMed] [Google Scholar]
- Bevans CG, Harris AL. Direct high affinity modulation of connexin channel activity by cyclic nucleotides. J. Biol. Chem. 1999a;274:3720–5. doi: 10.1074/jbc.274.6.3720. [DOI] [PubMed] [Google Scholar]
- Bevans CG, Harris AL. Regulation of connexin channels by pH. Direct action of the protonated form of taurine and other aminosulfonates. J. Biol. Chem. 1999b;274:3711–9. doi: 10.1074/jbc.274.6.3711. [DOI] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J. Biol. Chem. 1998;273:2808–16. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Gemel J, Seul KH, Larson DM, Banach K, Brink PR. Modulation of intercellular communication by differential regulation and heteromeric mixing of co-expressed connexins. Braz. J. Med. Biol. Res. 2000;33:391–7. doi: 10.1590/s0100-879x2000000400004. [DOI] [PubMed] [Google Scholar]
- Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 2007;282:31733–43. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- Boerma M, Forsberg L, Van Zeijl L, Morgenstern R, De Faire U, Lemne C, Erlinge D, Thulin T, Hong Y, Cotgreave IA. A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J. Int. Med. 1999;246:211–8. doi: 10.1046/j.1365-2796.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–5. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Brink PR, Cronin K, Banach K, Peterson E, Westphale EM, Seul KH, Ramanan SV, Beyer EC. Evidence for heteromeric gap junction channels formed from rat connexin43 and human connexin37. Am. J. Physiol. 1997;273:C1386–96. doi: 10.1152/ajpcell.1997.273.4.C1386. [DOI] [PubMed] [Google Scholar]
- Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 2009;11:267–82. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol. Biol. Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ. Res. 2003;93:61–8. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys. J. 2001;81:137–52. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Elfgang C, Willecke K, Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pflugers Arch. 1995;429:870–2. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MV, Verselis VK, Willecke K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: implications for atrioventricular conduction in the heart. Proc. Natl. Acd. Sci. USA. 2006;103:9726–31. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy RE, Marczin N, Birks EF, Chester AH, Yacoub MH. Transplant atherosclerosis: role of phenotypic modulation of vascular smooth muscle by nitric oxide. Gen. Pharmacol. 2000;34:73–84. doi: 10.1016/s0306-3623(00)00047-1. [DOI] [PubMed] [Google Scholar]
- Burt JM, Fletcher AM, Steele TD, Wu Y, Cottrell GT, Kurjiaka DT. Alteration of Cx43:Cx40 expression ratio in A7r5 cells. Am. J. Physiol. Cell Physiol. 2001;280:C500–8. doi: 10.1152/ajpcell.2001.280.3.C500. [DOI] [PubMed] [Google Scholar]
- Burt JM, Nelson TK, Simon AM, Fang JS. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am. J. Physiol. Cell Physiol. 2008;295:C1103–12. doi: 10.1152/ajpcell.299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WJ, Kocsis E, Scholz D, Luo X, Schaper W, Schaper J. Presence of Cx37 and lack of desmin in smooth muscle cells are early markers for arteriogenesis. Mol. Cell. Biochem. 2004;262:17–23. doi: 10.1023/b:mcbi.0000038201.43148.20. [DOI] [PubMed] [Google Scholar]
- Cai WJ, Koltai S, Kocsis E, Scholz D, Schaper W, Schaper J. Connexin37, not Cx40 and Cx43, is induced in vascular smooth muscle cells during coronary arteriogenesis. J. Mol. Cell. Cardiol. 2001;33:957–67. doi: 10.1006/jmcc.2001.1360. [DOI] [PubMed] [Google Scholar]
- Callera GE, Montezano AC, Yogi A, Tostes RC, Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr. Opin. Nephrol. Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Derouette JP, Kwak BR. Connexins in atherosclerosis. Adv. Cardiol. 2006a;42:255–67. doi: 10.1159/000092574. [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Luscher TF, Chanson M, Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006b;113:2835–43. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- Chadjichristos CE, Morel S, Derouette JP, Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML, Kwak BR. Targeting connexin 43 prevents platelet-derived growth factor-BB-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ. Res. 2008;102:653–60. doi: 10.1161/CIRCRESAHA.107.170472. [DOI] [PubMed] [Google Scholar]
- Chanson M, Kotsias BA, Peracchia C, O’Grady SM. Interactions of connexins with other membrane channels and transporters. Prog. Biophys. Mol. Biol. 2007;94:233–44. doi: 10.1016/j.pbiomolbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M, Kwak BR. Connexin37: a potential modifier gene of inflammatory disease. J. Mol. Med. 2007;85:787–95. doi: 10.1007/s00109-007-0169-2. [DOI] [PubMed] [Google Scholar]
- Chen J, Burns AR, Phillips SC, Sokoya EM. Pannexin protein expression in the rat middle cerebral artery. FASEB J. 2008;22:1144. [Google Scholar]
- Christ GJ, Moreno AP, Melman A, Spray DC. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am. J. Physiol. 1992;263:C373–C383. doi: 10.1152/ajpcell.1992.263.2.C373. [DOI] [PubMed] [Google Scholar]
- Collings A, Islam MS, Juonala M, Rontu R, Kahonen M, Hutri-Kahonen N, Laitinen T, Marniemi J, Viikari JS, Raitakari OT, Lehtimaki TJ. Associations between connexin37 gene polymorphism and markers of subclinical atherosclerosis: the Cardiovascular Risk in Young Finns study. Atherosclerosis. 2007;195:379–84. doi: 10.1016/j.atherosclerosis.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acd. Sci. USA. 2003;100:11388–93. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res. Brain Res. Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornhill JF, Levesque MJ, Herderick EE, Nerem RM, Kilman JW, Vasko JS. Quantitative study of the rabbit aortic endothelium using vascular casts. Atherosclerosis. 1980;35:321–37. doi: 10.1016/0021-9150(80)90130-6. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am. J. Physiol. Cell Physiol. 2001;281:C1559–67. doi: 10.1152/ajpcell.2001.281.5.C1559. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/ heterotypic gap junction channel properties. Am. J. Physiol. Cell Physiol. 2002;282:C1469–82. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- Coutinho P, Qiu C, Frank S, Tamber K, Becker D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 2003;27:525–41. doi: 10.1016/s1065-6995(03)00077-5. [DOI] [PubMed] [Google Scholar]
- Dahl G. Gap junction-mimetic peptides do work, but in unexpected ways. Cell Commun. Adhes. 2007;14:259–64. doi: 10.1080/15419060801891018. [DOI] [PubMed] [Google Scholar]
- Dahl G, Werner R, Levine E, Rabadan-Diehl C. Mutational analysis of gap junction formation. Biophys. J. 1992;62:172–80. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acd. Sci. USA. 2004;101:14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Smith TD, Sarma JD, Ritzenthaler JD, Maza J, Kaplan BE, Cunningham LA, Suaud L, Hubbard MJ, Rubenstein RC, Koval M. ERp29 restricts Connexin43 oligomerization in the endoplasmic reticulum. Mol. Biol. Cell. 2009;20:2593–604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, Hoepfl B, Wolfle SE. Endothelial mediators and communication through vascular gap junctions. Biol. Chem. 2006a;387:3–9. doi: 10.1515/BC.2006.002. [DOI] [PubMed] [Google Scholar]
- de Wit C, Wolfle SE, Hopfl B. Connexin-dependent communication within the vascular wall: contribution to the control of arteriolar diameter. Adv. Cardiol. 2006b;42:268–83. doi: 10.1159/000092575. [DOI] [PubMed] [Google Scholar]
- Deglise S, Martin D, Probst H, Saucy F, Hayoz D, Waeber G, Nicod P, Ris HB, Corpataux JM, Haefliger JA. Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. J. Vasc. Surg. 2005;41:1043–52. doi: 10.1016/j.jvs.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of Connexin43 channels. Cardiovasc. Res. 2004;62:268–75. doi: 10.1016/j.cardiores.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Derouette JP, Desplantez T, Wong CW, Roth I, Kwak BR, Weingart R. Functional differences between human Cx37 polymorphic hemichannels. J. Mol. Cell. Cardiol. 2009;46:499–507. doi: 10.1016/j.yjmcc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Desplantez T, Halliday D, Dupont E, Weingart R. Cardiac connexins Cx43 and Cx45: formation of diverse gap junction channels with diverse electrical properties. Pflugers Arch. 2004;448:363–75. doi: 10.1007/s00424-004-1250-0. [DOI] [PubMed] [Google Scholar]
- Dewey MM, Barr L. A Study of the Structure and Distribution of the Nexus. J. Cell Biol. 1964;23:553–85. doi: 10.1083/jcb.23.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc. Natl. Acd. Sci. USA. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy HS, Sorgen PL, Girvin ME, O’Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J. Biol. Chem. 2002;277:36706–14. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- Duling BR, Day KH, Damon DN, Paul DL, Simon A, Liao YB. Connexins 43 and 40 mediate smooth muscle-endothelial cell intereactions. FASEB J. 2003;17:A65. [Google Scholar]
- Eastman SD, Chen TH, Falk MM, Mendelson TC, Iovine MK. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics. 2006;87:265–74. doi: 10.1016/j.ygeno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Ebong EE, Kim S, DePaola N. Flow regulates intercellular communication in HAEC by assembling functional Cx40 and Cx37 gap junctional channels. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2015–23. doi: 10.1152/ajpheart.00204.2005. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ. Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenes S, Martinez AD, Delmar M, Beyer EC, Moreno AP. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys. J. 2001;81:1406–18. doi: 10.1016/S0006-3495(01)75796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner JJ, Laing JG, Beyer EC, Johnson RG, Hackett PB., Jr. Expression of zebrafish connexin43.4 in the notochord and tail bud of wild-type and mutant no tail embryos. Dev. Biol. 1996;177:449–62. doi: 10.1006/dbio.1996.0177. [DOI] [PubMed] [Google Scholar]
- Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun. Adhes. 2007;14:265–73. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function. Mol. Membr. Biol. 2002;19:121–36. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM, Buehler LK, Kumar NM, Gilula NB. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J. 1997;16:2703–16. doi: 10.1093/emboj/16.10.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM, Gilula NB. Connexin membrane protein biosynthesis is influenced by polypeptide positioning within the translocon and signal peptidase access. J. Biol. Chem. 1998;273:7856–64. doi: 10.1074/jbc.273.14.7856. [DOI] [PubMed] [Google Scholar]
- Feaver RE, Hastings NE, Pryor A, Blackman BR. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1534–1541. doi: 10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2001–7. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009;11:251–66. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology. 2004;19:277–84. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ. Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- Fleishman SJ, Unger VM, Yeager M, Ben-Tal N. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol. Cell. 2004;15:879–88. doi: 10.1016/j.molcel.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Francis D, Stergiopoulos K, Ek-Vitorin JF, Cao FL, Taffet SM, Delmar M. Connexin diversity and gap junction regulation by pHi. Dev. Genet. 1999;24:123–36. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<123::AID-DVG12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ. Res. 1998;83:636–43. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Gemel J, Valiunas V, Brink P, Beyer E. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J. Cell Sci. 2004;117:2469–2490. doi: 10.1242/jcs.01084. [DOI] [PubMed] [Google Scholar]
- George CH, Kendall JM, Evans WH. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J. Biol. Chem. 1999;274:8678–85. doi: 10.1074/jbc.274.13.8678. [DOI] [PubMed] [Google Scholar]
- Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 2004;62:233–45. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J. Biol. Chem. 2002;277:36725–30. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Gu H, Ek-Vitorin JF, Taffet SM, Delmar M. Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions: a model for synergistic interactions among connexins. Circ. Res. 2000;86:E98–E103. [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int. 2001;60:190–201. doi: 10.1046/j.1523-1755.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, Plum A, Charollais A, Willecke K, Meda P. Connexin43-dependent mechanism modulates renin secretion and hypertension. J. Clin. Invest. 2006;116:405–13. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc. Res. 2004;62:345–56. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–14. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Qtly. Rev. Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Harris AL, Locke D. Connexins: A Guide. Springer; New York, NY: 2009. [Google Scholar]
- He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ. Res. 2000;86:E104–9. doi: 10.1161/01.res.86.11.e104. [DOI] [PubMed] [Google Scholar]
- He DS, Jiang JX, Taffet SM, Burt JM. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc. Natl. Acd. Sci. USA. 1999;96:6495–500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix. Microcirculation. 2009;16:307–22. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann H, Suchyna T, Lichtenberg-Frate H, Jungbluth S, Dahl E, Schwarz J, Nicholson BJ, Willecke K. Molecular cloning and functional expression of mouse connexin40, a second gap junction gene preferentially expressed in lung. J. Cell Biol. 1992;117:1299–310. doi: 10.1083/jcb.117.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve JC, Dhein S. Pharmacology of cardiovascular gap junctions. Adv. Cardiol. 2006;42:107–31. doi: 10.1159/000092565. [DOI] [PubMed] [Google Scholar]
- Herve JC, Sarrouilhe D. Connexin-made channels as pharmacological targets. Curr Pharm. Des. 2005;11:1941–58. doi: 10.2174/1381612054021060. [DOI] [PubMed] [Google Scholar]
- Heyman NS, Burt JM. Hindered diffusion through an aqueous pore describes invariant dye selectivity of Cx43 junctions. Biophys. J. 2008;94:840–854. doi: 10.1529/biophysj.107.115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med. Res. Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ. Res. 2007;101:523–531. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Gloe T, Pohl U, Zahler S. Nitric oxide enhances de novo formation of endothelial gap junctions. Cardiovasc. Res. 2003;60:421–30. doi: 10.1016/j.cardiores.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Holland WC, Dunn CE. Role of the cell membrane and mitochondria in the phenomenon of ion transport in cardiac muscle. Am. J. Physiol. 1954;179:486–90. doi: 10.1152/ajplegacy.1954.179.3.486. [DOI] [PubMed] [Google Scholar]
- Hou CJ, Tsai CH, Su CH, Wu YJ, Chen SJ, Chiu JJ, Shiao MS, Yeh HI. Diabetes Reduces Aortic Endothelial Gap Junctions in ApoE-deficient Mice: Simvastatin Exacerbates the Reduction. J. Histochem. Cytochem. 2008;56:745–752. doi: 10.1369/jhc.2008.950816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Ma M, Dahl G. Conductance of connexin hemichannels segregates with the first transmembrane segment. Biophys. J. 2006;90:140–150. doi: 10.1529/biophysj.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulser DF, Rutz ML, Eckert R, Traub O. Functional rescue of defective mutant connexons by pairing with wild-type connexons. Pflugers Archiv. Eur. J. Physiol. 2001;441:521–8. doi: 10.1007/s004240000460. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell. 2005;16:5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seul K. Hwan, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc. Res. 2000;59:140–8. doi: 10.1006/mvre.1999.2216. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Suadicani SO, Spray DC, Scemes E. A stochastic two-dimensional model of intercellular Ca2+ wave spread in glia. Biophys J. 2006;90:24–41. doi: 10.1529/biophysj.105.064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L221–8. doi: 10.1152/ajplung.2001.280.2.L221. [DOI] [PubMed] [Google Scholar]
- Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J. Cell Sci. 2008;121:3664–73. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2898–904. doi: 10.1152/ajpheart.91488.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am. J. Physiol. Heart Circ. Physiol. 2006a;290:H1199–205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ. Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Kronke G, Kadl A, Leitinger N, Duling BR. Oxidized phospholipids alter vascular connexin expression, phosphorylation, and heterocellular communication. Arterioscl. Thromb. Vasc. Biol. 2006b;26:2216–21. doi: 10.1161/01.ATV.0000237608.19055.53. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ. Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- Johnson TL, Nerem RM. Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium. 2007;14:215–26. doi: 10.1080/10623320701617233. [DOI] [PubMed] [Google Scholar]
- Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe P, Leitinger N, Isakson BE. Differential Cx43 phosphorylation and smooth muscle cell proliferation, in vivo. Am. J. Path. 2009 doi: 10.2353/ajpath.2009.090160. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just A, Kurtz L, de Wit C, Wagner C, Kurtz A, Arendshorst WJ. Connexin 40 Mediates the Tubuloglomerular Feedback Contribution to Renal Blood Flow Autoregulation. J. Am. Soc. Nephrol. 2009 doi: 10.1681/ASN.2008090943. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvelyte A, Imbrasaite A, Bukauskiene A, Verselis VK, Bukauskas FF. Connexins and apoptotic transformation. Biochem. Pharmacol. 2003;66:1661–72. doi: 10.1016/s0006-2952(03)00540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameritsch P, Khandoga N, Nagel W, Hundhausen C, Lidington D, Pohl U. Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J. Cell. Physiol. 2005;203:233–42. doi: 10.1002/jcp.20218. [DOI] [PubMed] [Google Scholar]
- Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J. Gen. Physiol. 2008;131:293–305. doi: 10.1085/jgp.200709934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Reichert M, Hunziker W. Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett. 2001;505:92–6. doi: 10.1016/s0014-5793(01)02786-7. [DOI] [PubMed] [Google Scholar]
- Ko YS, Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ. Connexin make-up of endothelial gap junctions in the rat pulmonary artery as revealed by immunoconfocal microscopy and triple-label immunogold electron microscopy. J. Histochem. Cytochem. 1999;47:683–92. doi: 10.1177/002215549904700510. [DOI] [PubMed] [Google Scholar]
- Kovacs JA, Baker KA, Altenberg GA, Abagyan R, Yeager M. Molecular modeling and mutagenesis of gap junction channels. Prog. Biophys. Mol. Biol. 2007;94:15–28. doi: 10.1016/j.pbiomolbio.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval M. Pathways and control of connexin oligomerization. Trend Cell Biol. 2006;16:159–66. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- Kruger O, Beny JL, Chabaud F, Traub O, Theis M, Brix K, Kirchhoff S, Willecke K. Altered dye diffusion and upregulation of connexin37 in mouse aortic endothelium deficient in connexin40. J. Vasc. Res. 2002;39:160–72. doi: 10.1159/000057764. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–12. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, Valiunas V, Ramanan SV, Christensen EA, Beyer EC, Brink PR. Functional expression and biophysical properties of polymorphic variants of the human gap junction protein connexin37. Biochem. Biophys. Res. Comm. 2000;274:216–24. doi: 10.1006/bbrc.2000.3054. [DOI] [PubMed] [Google Scholar]
- Kurtz L, Gerl M, Kriz W, Wagner C, Kurtz A. Replacement of Cx40 by Cx 45 Causes Ectopic Localization of Renin-Producing Cells in the Kidney but Maintains the in Vivo Control of Renin Gene Expression. Am. J. Physiol. Renal Physiol. 2009 doi: 10.1152/ajprenal.00176.2009. in press. [DOI] [PubMed] [Google Scholar]
- Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J. Am. Soc. Nephrol. 2007;18:1103–11. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- Kwak BR. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterio. Thromb. Vasc. Biol. 2002;22:225–30. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Saez JC, Wilders R, Chanson M, Fishman GI, Hertzberg EL, Spray DC, Jongsma HJ. Effects of cGMP-dependent phosphorylation on rat and human connexin43 gap junction channels. Pflugers Archiv. Eur. J. Physiol. 1995;430:770–8. doi: 10.1007/BF00386175. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Veillard N, Pelli G, Mulhaupt F, James RW, Chanson M, Mach F. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation. 2003;107:1033–9. doi: 10.1161/01.cir.0000051364.70064.d1. [DOI] [PubMed] [Google Scholar]
- Kyle JW, Minogue PJ, Thomas BC, Domowicz DA, Berthoud VM, Hanck DA, Beyer EC. An intact connexin N-terminus is required for function but not gap junction formation. J. Cell Sci. 2008;121:2744–50. doi: 10.1242/jcs.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM. Specific amino-acid residues in the N-terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J. Cell Sci. 2003;116:3189–201. doi: 10.1242/jcs.00604. [DOI] [PubMed] [Google Scholar]
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH. Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J. Biol. Chemistry. 2001;276:23051–5. doi: 10.1074/jbc.M100303200. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium. 2005;37:311–320. doi: 10.1016/j.ceca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–86. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear DE, Jones PG, Marsh S, Cresci S, Spertus JA, McLeod HL. Connexin37 (GJA4) genotype predicts survival after an acute coronary syndrome. Am. Heart J. 2007;154:561–6. doi: 10.1016/j.ahj.2007.04.059. [DOI] [PubMed] [Google Scholar]
- Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol. Biol. Cell. 2008;19:912–28. doi: 10.1091/mbc.E07-06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Paemeleire K, Strahonja A, Sanderson MJ. Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia. 1998;24:398–407. [PubMed] [Google Scholar]
- Li X, Simard JM. Multiple connexins form gap junction channels in rat basilar artery smooth muscle cells. Circ. Res. 1999;84:1277–84. doi: 10.1161/01.res.84.11.1277. [DOI] [PubMed] [Google Scholar]
- Li X, Simard JM. Increase in Cx45 gap junction channels in cerebral smooth muscle cells from SHR. Hypertension. 2002;40:940–6. doi: 10.1161/01.hyp.0000041882.39865.a8. [DOI] [PubMed] [Google Scholar]
- Liao Y. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc. Natl. Acd. Sci. USA. 2001;98:9989–94. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler. Thromb. Vasc. Biol. 2007;27:1037–42. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- Lin X, Fenn E, Veenstra RD. An amino-terminal lysine residue of rat connexin40 that is required for spermine block. J. Physiol. 2006;570:251–69. doi: 10.1113/jphysiol.2005.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am. J. Physiol. 1995a;268:H729–39. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ. Res. 1995b;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Locke D, Jamieson S, Stein T, Liu J, Hodgins MB, Harris AL, Gusterson B. Nature of Cx30-containing channels in the adult mouse mammary gland. Cell Tissue Res. 2007;328:97–107. doi: 10.1007/s00441-006-0301-6. [DOI] [PubMed] [Google Scholar]
- Locke D, Liu J, Harris AL. Lipid rafts prepared by different methods contain different connexin channels, but gap junctions are not lipid rafts. Biochemistry. 2005;44:13027–42. doi: 10.1021/bi050495a. [DOI] [PubMed] [Google Scholar]
- Locke D, Stein T, Davies C, Morris J, Harris AL, Evans WH, Monaghan P, Gusterson B. Altered permeability and modulatory character of connexin channels during mammary gland development. Exp. Cell Res. 2004;298:643–60. doi: 10.1016/j.yexcr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–8. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J. Appl. Physiol. 2004;97:1152–8. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- Manthey D, Bukauskas F, Lee CG, Kozak CA, Willecke K. Molecular cloning and functional expression of the mouse gap junction gene connexin-57 in human HeLa cells. J. Biol. Chem. 1999;274:14716–23. doi: 10.1074/jbc.274.21.14716. [DOI] [PubMed] [Google Scholar]
- Martin PE, Blundell G, Ahmad S, Errington RJ, Evans WH. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci. 2001;114:3845–55. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ. Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- Matchkov VV, Rahman A, Bakker LM, Griffith TM, Nilsson H, Aalkjaer C. Analysis of effects of connexin-mimetic peptides in rat mesenteric small arteries. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H357–67. doi: 10.1152/ajpheart.00681.2005. [DOI] [PubMed] [Google Scholar]
- Maza J, Das Sarma J, Koval M. Defining a minimal motif required to prevent connexin oligomerization in the endoplasmic reticulum. J. Biol. Chem. 2005;280:21115–21. doi: 10.1074/jbc.M412612200. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Rook MB, Fishman GI, Spray DC. Gap junction channels: distinct voltage-sensitive and -insensitive conductance states. Biophys. J. 1994a;67:113–9. doi: 10.1016/S0006-3495(94)80460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ. Res. 1994b;74:1050–7. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 1991;115:1357–74. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–8. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys. J. 2004;87:912–28. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J. Gen. Physiol. 1999;114:339–64. doi: 10.1085/jgp.114.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani O, Kikuta A, Ohtsuka A, Taguchi T, Murakami T. Microvasculature as studied by the microvascular corrosion casting/scanning electron microscope method. I. Endocrine and digestive system. Arch. Histol. Jpn. 1983;46:1–42. doi: 10.1679/aohc.46.1. [DOI] [PubMed] [Google Scholar]
- Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc. Natl. Acd. Sci. USA. 2007;104:10034–9. doi: 10.1073/pnas.0703704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp. Cell Res. 2007;313:4083–90. doi: 10.1016/j.yexcr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J. Clin. Invest. 2006;116:2193–200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson AF, Lampe PD, Meyer RA, TenBroek E, Atkinson MM, Walseth TF, Johnson RG. Cyclic AMP and LDL trigger a rapid enhancement in gap junction assembly through a stimulation of connexin trafficking. J. Cell Sci. 2000;113:3037–49. doi: 10.1242/jcs.113.17.3037. [DOI] [PubMed] [Google Scholar]
- Puljung MC, Berthoud VM, Beyer EC, Hanck DA. Polyvalent cations constitute the voltage gating particle in human connexin37 hemichannels. J. Gen. Physiol. 2004;124:587–603. doi: 10.1085/jgp.200409023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch. Biochem. Biophys. 2000;381:181–90. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- Qu Y, Dahl G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc. Natl. Acd. Sci. USA. 2002;99:697–702. doi: 10.1073/pnas.022324499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys. J. 2007;92:1952–65. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Varga L, Bottero V, Chandran B. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J. Virol. 2007;81:7941–59. doi: 10.1128/JVI.02848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KE, Westphale EM, Larson DM, Wang HZ, Veenstra RD, Beyer EC. Molecular cloning and functional expression of human connexin37, an endothelial cell gap junction protein. J. Clin. Invest. 1993;91:997–1004. doi: 10.1172/JCI116321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy MA. Proliferation of smooth muscle cells at sites distant from vascular injury. Arteriosclerosis. 1990;10:298–305. doi: 10.1161/01.atv.10.2.298. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acd. Sci. USA. 2006;103:4475–80. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla A, Bennett MV, Barrio LC. Molecular determinants of membrane potential dependence in vertebrate gap junction channels. Proc. Natl. Acd. Sci. USA. 2000;97:14760–5. doi: 10.1073/pnas.97.26.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J. Ultrastruct. Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Rummery NM, Hill CE. Vascular gap junctions and implications for hypertension. Clin. Exp. Pharmacol. Physiol. 2004;31:659–67. doi: 10.1111/j.1440-1681.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Saez JC, Gregory WA, Watanabe T, Dermietzel R, Hertzberg EL, Reid L, Bennett MV, Spray DC. cAMP delays disappearance of gap junctions between pairs of rat hepatocytes in primary culture. Am. J. Physiol. 1989;257:C1–11. doi: 10.1152/ajpcell.1989.257.1.1-a. [DOI] [PubMed] [Google Scholar]
- Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim. Biophys. Acta. 2005;1711:215–24. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitongdee P, Becker DL, Milner P, Knight GE, Burnstock G. Levels of gap junction proteins in coronary arterioles and aorta of hamsters exposed to the cold and during hibernation and arousal. J. Histochem. Cytochem. 2004;52:603–15. doi: 10.1177/002215540405200505. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alvarez R, Paino T, Herrero-Gonzalez S, Medina JM, Tabernero A. Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia. 2006;54:125–34. doi: 10.1002/glia.20363. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Garland CJ. Spatial association of K-Ca and gap junction connexins in rat mesenteric artery. FASEB J. 2006;20:A275. [Google Scholar]
- Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clin. Exp. Pharmacol. Physiol. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc. Res. 2003;60:643–53. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Scemes E. Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43. Glia. 2008;56:145–53. doi: 10.1002/glia.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweda F, Kurtz L, de Wit C, Janssen-Bienhold U, Kurtz A, Wagner C. Substitution of connexin40 with connexin45 prevents hyperreninemia and attenuates hypertension. Kidney Int. 2009;75:482–9. doi: 10.1038/ki.2008.637. [DOI] [PubMed] [Google Scholar]
- Segal SS, Beny JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am. J. Physiol. 1992;263:H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–70. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J. Physiol. 2001;536:937–46. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ. Res. 2004;95:e22–8. doi: 10.1161/01.RES.0000140737.62245.c5. [DOI] [PubMed] [Google Scholar]
- Severs NJ. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc. Res. Tech. 2001;52:301–22. doi: 10.1002/1097-0029(20010201)52:3<301::AID-JEMT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Shibayama J, Lewandowski R, Kieken F, Coombs W, Shah S, Sorgen PL, Taffet SM, Delmar M. Identification of a novel peptide that interferes with the chemical regulation of connexin43. Circ. Res. 2006;98:1365–72. doi: 10.1161/01.RES.0000225911.24228.9c. [DOI] [PubMed] [Google Scholar]
- Simek J, Churko J, Shao Q, Laird DW. Cx43 has distinct mobility within plasma-membrane domains, indicative of progressive formation of gap-junction plaques. J. Cell Sci. 2009;122:554–62. doi: 10.1242/jcs.036970. [DOI] [PubMed] [Google Scholar]
- Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J. Cell Biol. 1976;68:705–23. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 2002;251:206–20. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J. Cell Sci. 2003;116:2223–36. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR, Chen H, Jackson CL, Ouellette Y. Decreased intercellular communication and connexin expression in mouse aortic endothelium during lipopolysaccharide-induced inflammation. J. Vasc. Res. 2004;41:323–33. doi: 10.1159/000079614. [DOI] [PubMed] [Google Scholar]
- Sokoya EM, Burns AR, Marrelli SP, Chen J. Myoendothelial gap junction frequency does not account for sex differences in EDHF responses in rat MCA. Microvasc. Res. 2007;74:39–44. doi: 10.1016/j.mvr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta. 2005;1711:154–63. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun. Adhes. 2008;15:75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J. Cell Biol. 2007;179:1301–9. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Nicholson BJ. Structural organization of gap junction channels. Biochim. Biophys. Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Spray DC, Burt JM. Structure-activity relations of the cardiac gap junction channel. Am. J. Physiol. 1990;258:C195–205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–73. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos K, Alvarado JL, Mastroianni M, Ek-Vitorin JF, Taffet SM, Delmar M. Hetero-domain interactions as a mechanism for the regulation of connexin channels. Circ. Res. 1999;84:1144–55. doi: 10.1161/01.res.84.10.1144. [DOI] [PubMed] [Google Scholar]
- Straub AC, Johnstone SR, Rizzo MJ, Heberlein KR, Best AK, Boitano S, Isakson BE. Site specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J. Vasc. Res. 2009 doi: 10.1159/000265562. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Nitsche JM, Chilton M, Harris AL, Veenstra RD, Nicholson BJ. Different ionic selectivities for connexins 26 and 32 produce rectifying gap junction channels. Biophys. J. 1999;77:2968–87. doi: 10.1016/S0006-3495(99)77129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero A, Sanchez-Alvarez R, Medina JM. Increased levels of cyclins D1 and D3 after inhibition of gap junctional communication in astrocytes. J. Neurochem. 2006;96:973–82. doi: 10.1111/j.1471-4159.2005.03623.x. [DOI] [PubMed] [Google Scholar]
- Thomas T, Jordan K, Simek J, Shao Q, Jedeszko C, Walton P, Laird DW. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J. Cell Sci. 2005;118:4451–4462. doi: 10.1242/jcs.02569. [DOI] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-Specific Cell to Cell Transfer of Short Interfering RNA by Gap Junctions. J. Physiol. 2005;568:459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Weingart R, Brink PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ. Res. 2000;86:E42–9. doi: 10.1161/01.res.86.2.e42. [DOI] [PubMed] [Google Scholar]
- van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem. Cell Biol. 1999;112:479–86. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, van Veen TA, Hermans MM, Jongsma HJ. Human connexin40 gap junction channels are modulated by cAMP. Cardiovasc. Res. 2000;45:941–51. doi: 10.1016/s0008-6363(99)00373-9. [DOI] [PubMed] [Google Scholar]
- van Veen TA, van Rijen HV, Jongsma HJ. Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc. Res. 2000;46:496–510. doi: 10.1016/s0008-6363(00)00047-x. [DOI] [PubMed] [Google Scholar]
- van Zeijl L, Ponsioen B, Giepmans BN, Ariaens A, Postma FR, Varnai P, Balla T, Divecha N, Jalink K, Moolenaar WH. Regulation of connexin43 gap junctional communication by phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 2007;177:881–91. doi: 10.1083/jcb.200610144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink PR. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ. Res. 1995;77:1156–1165. doi: 10.1161/01.res.77.6.1156. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ. Res. 1994a;75:483–90. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Ramanan SV, Brink PR. Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys. J. 1994b;66:1915–28. doi: 10.1016/S0006-3495(94)80985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ. Res. 2007;100:556–63. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Veenstra RD. Monovalent ion selectivity sequences of the rat connexin43 gap junction channel. J. Gen. Physiol. 1997;109:491–507. doi: 10.1085/jgp.109.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am. J. Physiol. Cell Physiol. 2007;293:C1112–9. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- Wang M, Martinez AD, Berthoud VM, Seul KH, Gemel J, Valiunas V, Kumari S, Brink PR, Beyer EC. Connexin43 with a cytoplasmic loop deletion inhibits the function of several connexins. Biochem. Biophys. Res. Commun. 2005;333:1185–93. doi: 10.1016/j.bbrc.2005.05.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004;87:958–73. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am. J. Physiol. 1998;274:H178–86. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Goodenough DA, Paul DL. Voltage gating of connexins. Nature. 1994;371:208–9. doi: 10.1038/371208a0. [DOI] [PubMed] [Google Scholar]
- Winterhager E, Pielensticker N, Freyer J, Ghanem A, Schrickel JW, Kim JS, Behr R, Grummer R, Maass K, Urschel S, Lewalter T, Tiemann K, Simoni M, Willecke K. Replacement of connexin43 by connexin26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev. Biol. 2007;7:26. doi: 10.1186/1471-213X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ. Res. 2007;101:1292–9. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nature Med. 2006a;12:950–4. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- Wong RC, Dottori M, Koh KL, Nguyen LT, Pera MF, Pebay A. Gap junctions modulate apoptosis and colony growth of human embryonic stem cells maintained in a serum-free system. Biochem. Biophys. Res. Comm. 2006b;344:181–8. doi: 10.1016/j.bbrc.2006.03.127. [DOI] [PubMed] [Google Scholar]
- Xie H, Laird DW, Chang TH, Hu VW. A mitosis-specific phosphorylation of the gap junction protein connexin43 in human vascular cells: biochemical characterization and localization. J. Cell Biol. 1997;137:203–10. doi: 10.1083/jcb.137.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M, Harris AL. Gap junction channel structure in the early 21st century: facts and fantasies. Curr. Opin. Cell Biol. 2007;19:521–8. doi: 10.1016/j.ceb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HI, Chang HM, Lu WW, Lee YN, Ko YS, Severs NJ, Tsai CH. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J. Histochem. Cytochem. 2000a;48:1377–89. doi: 10.1177/002215540004801008. [DOI] [PubMed] [Google Scholar]
- Yeh HI, Lai YJ, Chang HM, Ko YS, Severs NJ, Tsai CH. Multiple connexin expression in regenerating arterial endothelial gap junctions. Arterioscl. Thromb. Vasc. Biol. 2000b;20:1753–62. doi: 10.1161/01.atv.20.7.1753. [DOI] [PubMed] [Google Scholar]
- Yeh HI, Lai YJ, Lee SH, Lee YN, Ko YS, Chen SA, Severs NJ, Tsai CH. Heterogeneity of myocardial sleeve morphology and gap junctions in canine superior vena cava. Circulation. 2001;104:3152–7. doi: 10.1161/hc5001.100836. [DOI] [PubMed] [Google Scholar]
- Yen MR, Saier MH., Jr. Gap junctional proteins of animals: the innexin/pannexin superfamily. Prog. Biophys. Mol. Biol. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilla M, Doyle D, Sawyer JT. Early disulfide bond formation prevents heterotypic aggregation of membrane proteins in a cell-free translation system. J. Cell Biol. 1992;118:245–52. doi: 10.1083/jcb.118.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilinger C, Steffens M, Kolb HA. Length of C-terminus of rCx46 influences oligomerization and hemichannel properties. Biochim. Biophys. Acta. 2005;1720:35–43. doi: 10.1016/j.bbamem.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang W, DeMattia JA, Song H, Couldwell WT. Communication between malignant glioma cells and vascular endothelial cells through gap junctions. J. Neurosurg. 2003;98:846–53. doi: 10.3171/jns.2003.98.4.0846. [DOI] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q, Ghanem A, Kim JS, Kibschull M, Schwarz G, Schwab JO, Nagy J, Winterhager E, Tiemann K, Willecke K. Connexin31 cannot functionally replace connexin43 during cardiac morphogenesis in mice. J. Cell Sci. 2006;119:693–701. doi: 10.1242/jcs.02800. [DOI] [PubMed] [Google Scholar]