Abstract
The kidney plays key roles in extracellular fluid pH homeostasis by reclaiming bicarbonate (HCO3−) filtered at the glomerulus and generating the consumed HCO3− by secreting protons (H+) into the urine (renal acidification). Sodium-proton exchangers (NHEs) are ubiquitous transmembrane proteins mediating the countertransport of Na+ and H+ across lipid bilayers. In mammals, NHEs participate in the regulation of cell pH, volume, and intracellular sodium concentration, as well as in transepithelial ion transport. Five of the 10 isoforms (NHE1-4 and NHE8) are expressed at the plasma membrane of renal epithelial cells. The best-studied isoform for acid-base homeostasis is NHE3, which mediates both HCO3− absorption and H+ excretion in the renal tubule. This article reviews some important aspects of NHEs in the kidney, with special emphasis on the role of renal NHE3 in the maintenance of acid-base balance.
Keywords: sodium/hydrogen exchange, renal acidification, bicarbonate absorption
The free hydrogen ion (H+) concentration in body fluids is regulated exquisitely around 40 nmol/L (pH 7.40) whereas H+ flux through the body greatly exceeds this magnitude. In a 70-kg human being at a basal state, normal metabolic and dietary acid production rate is about 50 to 70 mmoles/d and respiratory volatile acid production at the basal state is around 15,000 mmoles/d, with peak production at maximal exercise reaching 200 mmoles/min. The flux of H+ through the organism over 24 hours is 8 orders of magnitude greater than the total pool of free H+ in total body water (<2 μmoles). This remarkable homeostatic feat is accomplished by concerted efforts of extracellular and intracellular buffers, highly efficient ventilatory responses, metabolic functions of the liver, and renal ammoniagenic and solute transport mechanisms. For excretion of nonvolatile acid and base loads, the kidney assumes the pivotal role.
A filtration-reabsorption nephron bears an exorbitant burden of having to reclaim a vast amount of valuable solutes indiscriminately dispensed in the filtrate; one of which of course is the approximately 4,000 mmoles of bicarbonate (HCO3−) per day. The luminal acid disequilibrium pH implies that the predominant mode of HCO3− absorption involves H+ secretion, although a small concomitant degree of direct HCO3− reabsorption cannot be excluded.1 Two points are noteworthy. First, complete reclamation of the approximately 4,000 mmoles of filtered HCO3− forestalls a physiologic disaster but does not lead to net acid secretion. Further elaboration of H+ into the urine is necessary. Second, whether the organism is catering to the need of excreting a physiologic amount of acid (eg, 50 mmoles of H+ added to the body/d) or base (eg, 50 mmoles OH− added to the body/d), the kidney is always engaged in luminal H+ extrusion because HCO3− reclamation far exceeds physiologic H+ or OH− excretion. Luminal H+ secretion is a quintessential part of renal homeostatic function. Extrusion of H+ into the urinary lumen against its electrochemical gradient is an energetically costly process. Luminal H+ secretion can be coupled directly to adenosine triphosphate (ATP) hydrolysis by the multisubunit V-type ATPase or to the inwardly directed (urine lumen to cell) Na+ gradient because of the low cell [Na+] generated by the Na+/K+-ATPase (Fig 1).
Figure 1.
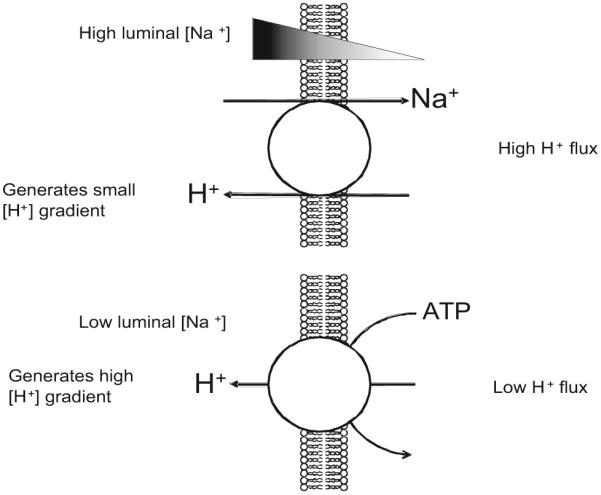
Transport of H+ into the urinary lumen by Na+/H+ exchange or H+-ATPase. In segments where the luminal [Na+] is high (eg, the proximal tubule), the electrochemical driving force can extrude H+ via NHE uphill into the lumen by approximately 1 pH unit. When the luminal [Na+] is relatively low and high luminal [H+] is desired (eg, the collecting duct), H+ ejection into the lumen is accomplished by direct coupling to ATP hydrolysis. The H+ flux (JH+) in proximal segments far exceeds that of distal segments. The turnover rates of these transporters have been estimated indirectly to be in the order of 102 s−1. The relative amount of protein expression in the proximal tubule apical membrane is unknown but the relative JH+ for NHE versus H+-ATPase is about two thirds to one third.
Na+/H+ Exchangers in Mammalian Kidney
In 1949, Pitts et al2 made the observation of an inverse relationship between urine pH and [Na+] (lowest urine pH with highest urinary Na+) and postulated a Na+/H+ exchange process between the renal epithelium and urine. Although the observation is correct, we are now cognizant that the analysis of bladder urine does not possess the resolution to permit conclusion about such transport mechanisms. Pitts et al2 in fact were witnessing the effect of distal Na+ delivery to enhance luminal H+ secretion by the collecting duct, which can be interpreted as a form of Na+/H+ exchange. In 1976, Murer et al3 first showed Na+-driven H+ movement and H+-driven Na+ movement in isolated cortical brush-border membrane vesicles, thereby definitely showing Na+/H+ exchange activity in the kidney. A subsequent report by Kinsella and Aronson4 further characterized this process in more detail and precision. Both of these reports have been revisited as milestone reports by the investigators.5,6 A cornucopia of data on renal Na+/H+ exchange using membrane vesicles and some cultured cells emerged in the 1980s that set the stage for the next level of research.
The era of phenomenologic analysis was supplemented by gene- and protein-specific reagents when the first mammalian NHE was cloned by Sardet et al.7 The relationship between mammalian NHE genetic sequence and those from a multitude of organisms is shown in Fig 2. Na+/H+ exchange across lipid bilayers and proteins that sustain this function are universal in prokaryotic, animal, and plant biology. Genes coding for Na+/H+ exchangers have been cloned from the simplest prokaryote to the most advanced multicellular eukaryotes. A remarkably high degree of conservation exists between NHE gene sequences from different organisms (Fig 2). Although the identification of paralogs and orthologs has been successfully accomplished by nucleotide homology–based cross-hybridization (across genera and species but not across orders and phyla), one common theme in breaking ground in the cloning of complementary DNAs (cDNAs) for Na+/H+ exchangers is the elegant use of functional complementation. One observes remarkable similarity in the approach in cloning NhaA from Escherichia coli by Goldberg et al8 and human (NHE1) by Sardet et al.7 NHE null and NHE over-expressing mutant cells were generated genetically followed by functional selection. Null mutants then were rescued by genomic sequences derived from overexpressing mutants, and functional complementation was used as an index to track down sequences coding for Na+/H+ exchange activity. These pioneering efforts then allowed subsequent cDNA identification by homology cloning of a multitude of cDNAs in vitro and in silico.
Figure 2.
Phylogeny of paralogs and orthologs of Na+/H+ exchangers.
Mammalian NHEs that have been classified functionally to date are all electroneutral transporters with a 1Na+:1H+ stoichiometry. The putative structure of the mammalian protein with a 10- to 12-transmembrane N-terminus and a largely cytoplasmic C-terminus has been discussed in recent reviews.9,10 Currently, the structure of the mammalian NHEs is really unknown. Although a low-resolution version of the prokaryotic protein is available,11 the degree of sequence similarity does not permit homology modeling of the mammalian protein at present. Brett et al12 proposed an elegant categorization of mammalian NHEs into 3 classes based on their sequence and cell biology: (1) primarily plasma membrane-residing, (2) recycling between plasma membrane and endosomes, and (3) intracellular organellar.
A number of mammalian NHEs have been identified in the kidney. The role of intracellular NHEs in the kidney is not known at the moment. Data in model systems of proximal tubule epithelia suggest possible mediation of albumin endocytosis and degradation by endosomal NHE3.13-15 Intracellular [Na+] in renal epithelia is less than 20 mmol/L, whereas basolateral [Na+] ranges from 140 mmol/L in the cortex to as high as 300 mmol/L in the deep medulla. Basolateral NHEs undoubtedly will eject H+ from the cell to the interstitium and hence are unlikely to contribute to luminal acidification. Base-excreting cells (eg, β-intercalated collecting duct cells) use H+-ATPase rather than Na+/H+ exchange for H+ addition into the plasma. Apical NHEs on the other hand are poised strategically to add H+ into the lumen using the inward Na+ chemical gradient. Luminal [Na+] decreases while luminal [H+] increases axially toward the distal nephron to the point at which the ion gradients will no longer support luminal H+ extrusion so the task of luminal H+ extrusion is relegated to the V-ATPase where H+ pumping is energized directly by ATP hydrolysis (Fig 1). Because NH4+ can substitute for H+ as a substrate, NHEs also are important NH4+ transporters. NHEs also perform multiple other transport functions via parallel coupling with other transporters, but these functions are not discussed here. Five Na+/H+ exchanger isoforms (NHE1-4 and NHE8) are expressed at the plasma membrane of renal epithelial cells, with specific distribution within cells and along the nephron (Fig 3).
Figure 3.
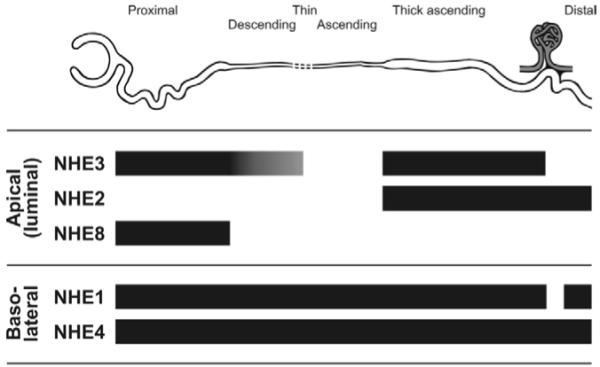
Expression of plasma membrane Na+/H+ exchanger isoforms along the nephron. NHE3 is highly expressed at the apical (luminal) membrane of the proximal tubule and TAL of the loop of Henle, and at lower levels in the thin descending limb. There is no NHE3 at the macula densa. NHE2 is expressed at the luminal side of the TAL and distal nephron, including macula densa. NHE8 is present at the apical membrane of the proximal tubule. NHE1 and NHE4 are both expressed at the basolateral membrane of epithelial cells along the nephron, with the exception of the macula densa and intercalated cells of the cortical collecting duct (not shown), which have no detectable NHE1.
NHE1 is expressed at the plasma membrane of most mammalian cells where it plays multiple roles including in cell pH, sodium and volume homeostasis, cell motility, and provision of a platform for signaling complexes. In the kidney, NHE1 is expressed at the basolateral membrane of all nephron segments (Fig 3), with the exception of the macula densa and cortical collecting duct intercalated cells.16,17 Isolated, microperfused, thick ascending limbs (TALs) from NHE1 knockout mice have decreased HCO3− absorption compared with TALs from wild-type mice.18 This was postulated as regulatory cross-talk between basolateral NHE1 and apical NHE3 because pharmacologic inhibition of basolateral NHE1 downregulates apical NHE3 activity.19,20 The mechanism of this postulated cross-talk is unclear. However, 2 different mouse models lacking NHE1 have no overt disturbance of whole-body acid-base homeostasis.21,22 In addition, NHE1 potentially could mediate basolateral ammonium (NH4+) extrusion in the TAL (Fig 4).
Figure 4.
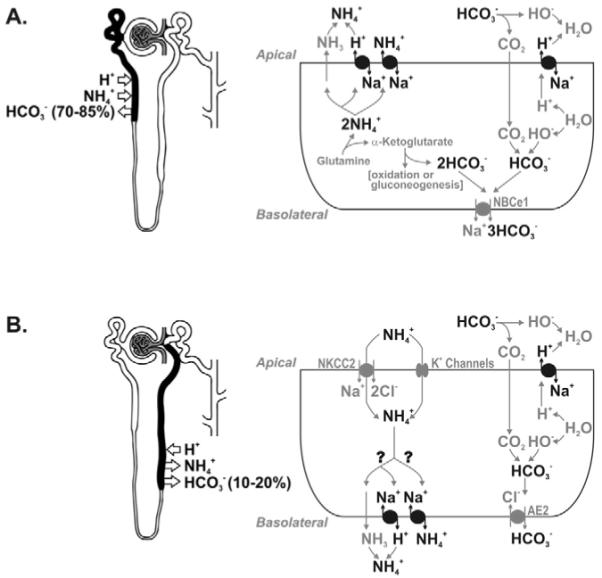
Acid-base homeostasis by renal Na+/H+ exchangers. (A) In the proximal tubule, apical membrane NHE3 mediates ammonium (NH4+) secretion, either by providing a H+ to the ammonia (NH3) that diffuses passively into the tubule, or by catalyzing the counter-transport of NH4+ and Na+. By secreting NH4+ generated from the mitochondrial metabolism of glutamine to α-ketoglutarate, NHE3 is indirectly responsible for the subsequent generation of new bicarbonate. NHE3 also mediates the absorption of most of the filtered bicarbonate in the proximal tubule by providing a H+ that interacts with luminal HCO3− and converts it to CO2. The functions of NHE8 (the other proximal tubule apical Na+/H+ exchanger) may be similar but have not yet been characterized. (B) In the TAL a significant amount of the luminal ammonium is reabsorbed, shunting the distal tubule, and is secreted again in the collecting duct. NHE1 and/or NHE4 may play a role in the transepithelial transport of ammonium by mediating NH4+ extrusion at the basolateral membrane (question marks). Apical membrane NHE3 and NHE2 are responsible for the absorption of most of the remaining luminal bicarbonate by the same mechanism as in the proximal tubule.
NHE2 is expressed at the apical membrane in the TAL along with NHE3. It is the only luminal Na+/H+ exchanger from the macula densa to the distal nephron (Fig 3). In conjunction with NHE3, NHE2 contributes to acidification and bicarbonate absorption in the TAL23 (Fig 4). However, deletion of NHE2 in mice results in no overt acid-base disturbance,24 and double knock-out of NHE2 and 3 does not lead to further worsening of the metabolic acidosis observed in NHE3−/−mice.25 NHE2 thus may have a relatively minor role in the maintenance of acid-base balance compared with NHE3.
NHE3 is expressed at the apical (luminal) membrane of the proximal tubule, some long thin descending limbs, and the TAL of the loop of Henle (Fig 3), where it plays important roles in bicarbonate absorption (Fig 4), salt and volume homeostasis, and in the absorption of other solutes by functional coupling to a variety of other transporters. Mice with targeted disruption of NHE3 have decreased renal absorption of Na+, fluid and HCO3−, metabolic acidosis, hypovolemia, hypotension, and increased mortality.26 This phenotype is ameliorated partially but not abolished by rescue of the intestinal NHE3 defect.27,28 The moderate phenotype of NHE3 knockout mice can be attributed to a number of compensatory mechanisms, including decreased filtered HCO3− load and increased HCO3− absorption in the collecting duct, mediated by the H+-ATPase and H+-K+-ATPase.29 Compensation by the Na+/H+ exchange activity of NHE2 in the TAL and NHE8 in the proximal tubule theoretically is possible, but has not yet been supported by experimental findings.
A large body of literature exists addressing the regulation of NHE3 studied at the level of the intact microperfused tubule, renal cortical slices, enriched proximal tubules in suspension, brush-border membrane vesicles, cultured cells expressing native NHE3 (opposum kidney OK cells and porcine kidney LLC-PK1 cells), various eukaryotic hosts transfected with heterologous NHE3, and as purified recombinant NHE3-derived polypeptides. Table 1 lists some of the acute and chronic regulatory agents.
Table 1.
Summary of Acute (Minutes to a Few Hours) and Chronic (Hours to Days) Regulation of NHE3
| Agonist | Acute Regulation |
Model(s) and Reference(s) |
Chronic Regulation |
Model(s) and Reference(s) |
|---|---|---|---|---|
| α-Adrenergic | ↑ | a, b, c70,71 | NR | |
| Acid | ↑ | d59,72 | ↑ | d, e, f36,47,48,51,73 |
| Adenosine | ↓/↑* | d, g74-76 | NR | |
| Albumin | ↑ | d15 | ↑ | d15 |
| Angiotensin II | ↑ | a, d, f77-81 | ↑ | d82 |
| ATP depletion | ↓ | h, i83,84 | NR | |
| Atrial natriuretic peptide | −/↓† | f85 | NR | |
| Cyclic adenosine monophosphate | ↓ | d, i, j84,86,87 | NR | |
| Dopamine | ↓ | a, d, f88-91 | ↓ | d92 |
| ET-1 | ↑ | a, d, f64,65,67,93-95 | NR | |
| Glucocorticoids | ↑ | a, d, f96-100 | ↑ | d98,101,102 |
| Insulin | ↑ | a, d103-105 | ↑ | d105 |
| Hyperosmolality | ↓ | h106 | ↑ | d107 |
| Ouabain | NR | ↓ | k108 | |
| Parathyroid hormone | ↓ | a, d, e, f, l109-115 | ↓ | e, f116 |
| Phosphatidyl-inositol 3,4,5-trisphosphate | ↑ | d117 | NR |
NOTE. Study models are as follows: (a) isolated perfused proximal tubules; (b) primary cultures of mouse proximal tubule cells; (c) immortalized S1 mouse proximal tubule cells; (d) opossum kidney (OKP) cells expressing native NHE3; (e) in vivo microperfusion; (f) brush-border membrane vesicles; (g) Xenopus laevis A6 cells transfected with NHE3; (h) PS120 fibroblasts transfected with NHE3; (i) NHE-null Chinese hamster ovary cells (AP-1) transfected with NHE3; (j)X laevis oocytes injected with NHE3 cRNA; (k) LLC-PK1 cells expressing native NHE3; and (l) in vivo micropuncture.
Abbreviation: NR, not reported.
Adenosine has inhibitory or stimulatory effects depending on the dosage.75
Atrial natriuretic peptide potentiates the inhibitory effect of dopamine on Na+/H+ exchange in brush border membrane vesicles; atrial natriuretic peptide alone or in addition to parathyroid hormone has no effect in the same system.
NHE4 is expressed ubiquitously at the basolateral membrane of epithelial cells along the nephron, together with NHE1. NHE4 is the only basolateral NHE isoform in the macula densa and intercalated cells of the cortical collecting duct9,30 (Fig 3). Similar to NHE1, NHE4 may mediate NH4+ reabsorption in the TAL (Fig 4). NHE4 knockout mice have no documented overt disease phenotype to date.31
NHE8, the most recently identified renal NHE isoform, is expressed at the luminal membrane of the proximal tubule32,33 (Fig 3). In the neonate, proximal tubule NHE activity is relatively high despite very low NHE3 antigen levels.34 NHE8 expression is higher in young animals and lower in adults, suggesting a potential role for NHE8 during early development.33 The clear presence of apical NHE activity in the double NHE2 and NHE3 knock-out mice may be a reflection of NHE8.35 NHE8 function may be similar to NHE3 (Fig 4), but its precise role in the maintenance of acid-base balance remains to be explored. NHE8 protein expression is not increased in NHE3 knockout mice,33 suggesting that it may not be regulated by acid-base status the way NHE3 is.36
Transcripts of the more ubiquitous intracellular NHE isoforms are present in the kidney but their antigenic localization and functional characterization have not been performed.
Adaptation to Metabolic Acidosis
One remarkable feature of the kidney is to escalate its capacity to excrete net acid in the urine when the organism is confronted with a sustained increment in acid load (or base loss). This adaptation takes the coordinated tripartite form of increased H+ pumping (decreasing urine pH) to trap buffers, increased absolute amount of urinary buffer, and reduction (or elimination) of urinary base, which is primarily bicarbonate and citrate. The immediate response can be a result of kinetic effects such as titration of luminal citrate from the trivalent to divalent form to increase citrate absorption,37 and titration of divalent to monovalent phosphate to decrease phosphate absorption38 in the proximal tubule as a result of a decrease in plasma and filtrate pH. This triggers instantaneous reduction of base equivalents in the urine (hypocitraturia)38 and increased urinary buffer (hyperphosphaturia)39 to carry H+. In addition, acute acid loads in whole animals and in model epithelia can alter H+-ATPase and Cl−/HCO3− exchanger distribution to increase H+ pumping and decrease HCO3− secretion into the distal tubular lumen.40,41 These kinetic and protein trafficking events are quick in onset and rapidly reversible. More sustained acid loads elicit a more permanent adaptation of the tubular epithelium usually involving gene transcription. For example, the suppression of proximal phosphate and enhanced citrate absorption occur by different mechanisms with chronic metabolic acidosis compared with acute decreases of luminal pH.42,43 There are a multitude of genes and gene products that are regulated by chronic systemic metabolic acidosis in the kidney. Table 2 summarizes some of the studies of adaptation of renal metabolism and transport in chronic metabolic acidosis and aims to highlight rather than provide an exhaustive catalogue. The integrated response of increased H+-secretion results in the following: (1) increased absorption and metabolism of potential base in urine such as citrate; (2) increased titration of luminal HCO3− to enhance lumen-to-blood HCO3− flux; (3) increased excretion of low-pK, low-capacity buffers such as phosphate; and (4) increased synthesis and secretion of the high-pK, high-capacity buffer NH3.
Table 2.
Renal Adaptation to Chronic Metabolic Acidosis
| Gene/Protein | Primary Function | Comments |
|---|---|---|
| Glutamine transporter (SN1)118,119 | ↑ glutamine uptake into proximal tubule for ammoniagenesis |
Increased SN1 messenger RNA and protein and insertion into the basolateral membrane; converts apical membrane glutamine uptake from the filtrate to both apical and basolateral glutamine uptake |
| Phosphate-dependent glutaminase120-126 |
↑ deamidation of glutamine to glutamate in the mitochondria to release 1st NH3 |
Primary signal is intracellular acidosis, which increases phosphate-dependent glutaminase messenger RNA stability through a pH responsive element at the 3′-untranslated region |
| Glutamate dehydrogenase127,128 | ↑ deamination of glutamine to α-ketoglutarate in the mitochondria to release 2nd NH3 |
An 8-base AU repeat sequence that destabilizes the transcript; on cell acidification, z-crystall in abundance does not change but its binding affinity to the pH-responsive element increases resulting in prolongation of transcript half-life |
| PEPCK124,129-132 | ↑ conversion from oxaloacetate to phospho-enol-pyruvate in the cytoplasm. The disposal of the carbon skeleton of glutamine requires PEPCK |
Primary signal is intracellular acidosis, which increases PEPCK transcription; intracellular acidification leads to activation of the p38 stress-activated protein kinase and phosphorylation of the transcription factor ATF-2, which binds to the CRE-1 element in the promoter of the PEPCK gene to enhance transcription |
| Na+-phosphate cotransporter133-135 | ↓ proximal phosphate uptake | Increase amount of urinary buffer to furnish H+ carrier (pKa of HPO42−/H2PO4− ~ 6.8) |
| Na+-citrate cotransporter (NaDC-1)42,136,137 |
↑ citrate uptake from filtrate into the proximal tubule cell |
Increased citrate uptake at the apical membrane is part of an integrated response of the proximal tubule to decrease loss of potential base in the urine |
| ATP-citrate lyase138 | ↑ cytoplasmic citrate metabolism |
The increased apical citrate uptake is accompanied by increased citrate metabolism in both the cytoplasm and the mitochondria; in the converse situation of metabolic alkalosis, a decrease in citrate metabolism is sufficient to cause hypercitraturia without adaptation of NaDC1 |
| Aconitase139 | ↑ mitochondrial citrate metabolism |
|
| Na+/H+ exchanger (NHE3)36,51-53,140 |
↑ H+ and NH4+ secretion | Intracellular acidosis as a primary signal activates a complex cascade (Fig 6), resulting in increased H+ excretion and titration of luminal buffer (Fig 5) |
| H+-ATPase73,141,142 | ↑ H+ secretion | Decreases luminal pH to levels not achievable using lumen-to-cell Na+ electrochemical gradients |
| Anion exchanger (AE1)142-144 | ↑ Base addition to plasma | Increase H+ pumping by H+-ATPase in the α-intercalated cell is accompanied by commensurate increased basolateral base exit |
| Na-HCO3− cotransporter (NBC)47,145,146 |
↑ Base addition to plasma | Increased H+ secretion by Na+/H+ exchanger in the proximal nephron is accompanied by commensurate increased basolateral base exit |
| Na+-sulfate cotransporter (NaSi1)147 |
↓ proximal sulfate uptake | The low pK of sulfuric acid precludes its role as a urinary buffer; increased distal SO42− delivery can enhance distal H+ secretion as a nonabsorbable anion |
Abbreviation: PEPCK, phosphoenolpyruvate carboxykinase.
A significant amount of work has been devoted to studying how chronic low-ambient pH regulates renal Na+/H+ exchange. NHE1 has been shown to be stimulated by acid in both animals and cell culture but this is unlikely to be related to transepithelial transport of H+ equivalents.44-46 Chronic adaptation of NHE3 has been described in both the proximal tubule (increased NHE3 activity and protein) and TAL (increased NHE3 protein and transcript) in animals given a sufficient acid load to chronically suppress serum [HCO3−].36,47-53 Presently it is unclear whether the signals and mechanisms responsible for increased NHE3 are the same for the proximal tubule and TAL. The subsequent discussion focuses only on proximal tubule NHE3.
Chronic metabolic acidosis in animals decreases proximal HCO3− transport when measured by free-flow micropuncture,54 but increases maximal proximal tubule HCO3− transport capacity measured by in vivo microperfusion when identical luminal HCO3− loads are presented to the tubule from control or acidotic rats.55 This is an unusual example of adaptive increase in reabsorptive capacity in response to a reduced filtered HCO3− load caused mainly by reduced ultrafilterable [HCO3−] and possibly some reduction in glomerular filtration rate. This finding has been touted by some as a paradox. The teleology of this increased tubular transport capacity and increased NHE3 Vmax and protein in the face of reduced HCO3− load is unclear because logic predicts that capacity should change to accommodate needs. It is difficult to fathom why the kidney in its wisdom would react to a diminished load by escalating capacity. It is conceivable that increased proximal tubule HCO3− reabsorption is to compensate for conditions other than metabolic acidosis. In respiratory acidosis, increased proximal HCO3− absorption serves to counter the high plasma CO2 tension. In potassium deficiency, increased proximal HCO3− absorption is needed to minimize distal delivery of HCO3−, which is kaliuretic. Because both hypercapnia and potassium deficiency may signal via proximal intracellular acidification, increased NHE3 Vmax in reaction to decreased intracellular pH may be hardwired into the proximal tubule cell. Could the response of NHE3 to metabolic acidosis be a mere happenstance because cell pH is decreased?
Having indulged in the earlier-described teleologic reverie, an alternative, and in all likelihood more probable (and desirable) option, is that increased NHE3 Vmax actually is adaptive for metabolic acidosis in addition to respiratory acidosis and potassium deficiency. The potential benefits to acid-base balance are summarized in Fig 5. The purpose of increased NHE3 Vmax can be primarily to accommodate the high reserve of ammonia synthesis56 because a significant portion of the NH3 synthesized reacts with H+ inside the cell and is excreted into the lumen by NHE3 as NH4+.57 Although the filtered HCO3− load is reduced in metabolic acidosis, the increased HCO3− transport capacity may serve to counteract the interstitium-to-lumen HCO3− backleak. In addition, the increased H+ transport by NHE3 does not have to engage HCO3− in the lumen. It can contribute to the increase in citrate absorption and decrease in phosphate absorption as part of the integrated response to acid load (Fig 5). The net result is increased excretion and titration of urinary buffers. Because NHE3 is tethered to numerous scaffold proteins,58 one can speculate that there may be special microlocales where NHE3 may be associated physically with various other transporters to facilitate titration of substrates.
Figure 5.
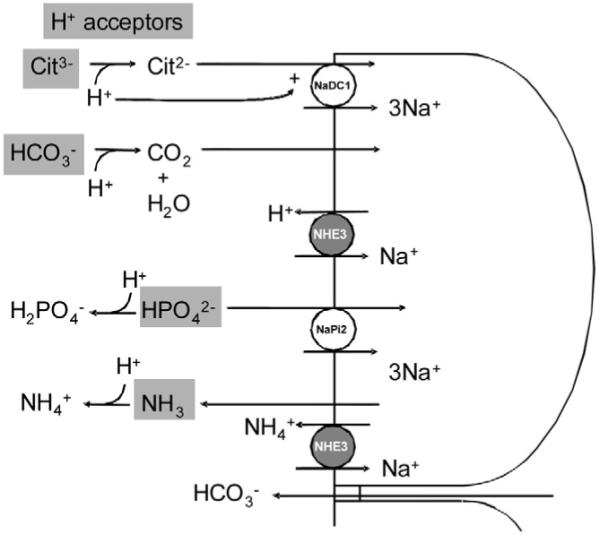
The potential functional significance of adaptation of the proximal tubule NHE3 in metabolic acidosis is to increase excretion and titration of urinary buffers. Apical NHE3 can transport more NH4+ into the urine (bottom NHE3). The increased H+ secreted into the lumen by NHE3 (top NHE3) can titrate a number of H+ acceptors (gray boxes). Citrate absorption can be increased by generation of the transported divalent species (pK = 5.4) or by likely allosteric activation of NaDC-1. Titration of filtered HCO3− will increase net HCO3− absorption to counter paracellular HCO3− backleak. Titration of HPO42− to H2PO4− (pK = 6.8) will decrease phosphate absorption. H+ transported by NHE3 also can contribute to trapping of NH3 that diffused into the lumen as a nonionic solute. NHE3, Na+/H+ exchanger-3; NaPi2, Na+-inorganic phosphate transporters 2; NaDC1, Na+ dicarboxylate cotransporter.
The mechanism by which a low ambient pH and HCO3− concentration increases NHE3 has been studied in detail by Preisig and Alpern48,59-69 using both cell culture and animal models. Figure 6 summarizes the cascade based on the current body of data. It is important to note that this is an evolving model. Either an acid load or frank systemic metabolic acidosis (hypobicarbonatemia) leads to a decrease in proximal tubule cell pH, which activates proline-rich tyrosine kinase 2 (Pyk2, a pH sensor).59 This is a direct specific effect because acidic solutions can activate Pyk2, but not the closely related focal adhesion kinase, in a cell-free system.59 Pyk2 then activates c-Src. Both Pyk2 and c-Src activation are required for acid to activate NHE3 activity.59-62 Downstream from c-Src is endothelin-1 (ET-1) production and secretion by the proximal tubule.53,63 The mechanism by which c-Src stimulates ET-1 secretion is not yet known. ET-1 activates the endothelin receptor type B (ETB), which then activates NHE3 exocytosis into the apical membrane.53,64,65
Figure 6.
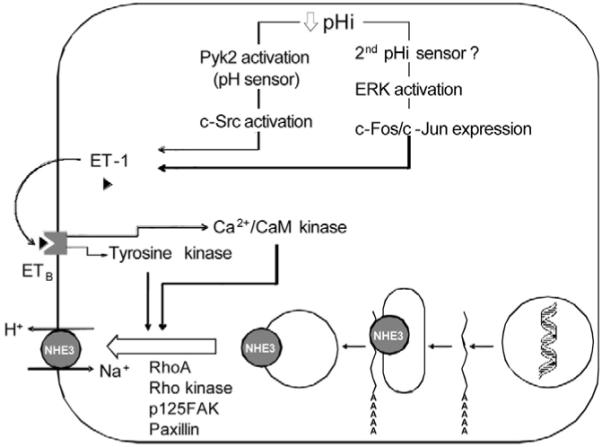
The Preisig and Alpern model of autocrine regulation of NHE3 by acidosis. A decrease in intracellular pH in the renal proximal tubule activates Pyk2, which functions as a pH sensor. Pyk2 then activates c-Src and leads to production and secretion of ET-1. A second parallel signaling cascade leading to ET-1 synthesis and secretion involves activation of extracellular signal–regulated kinase ERK and expression of the immediate early response genes c-Fos/c-Jun, but the identity of the second putative pH sensor is not known. ET-1 has an autocrine effect by binding to the proximal tubule cell ETB receptor, which signals downstream through the activation of tyrosine kinases and calcium- and calmodulin-dependent protein kinases (Ca2+/CaM kinase). ET-1 activation leads to exocytic insertion of NHE3 into the apical membrane, a process dependent on RhoA and Rho kinase activation and associated with stress fiber formation and tyrosine phosphorylation of focal adhesion kinase (p125FAK) and paxillin.
ET-1/ETB mediates acid stimulation of NHE3 involving exocytic insertion of NHE3 into the apical membrane. This process requires an intact cytoskeleton,65 and is associated with NHE3 phosphorylation.64 The functional significance of NHE3 phosphorylation for exocytosis is not known at present. The effect of ET1/ETB on NHE3 required both an increase in cell calcium level and intact tyrosine kinases.66,67 ET-1 stimulation of NHE3 requires an intact C-terminal tail and the consensus sequence KXXXVPKXXXV in the second intracellular loop of the ETB receptor.68 ETA is not involved in ET-1 stimulation of NHE3.64 Acid incubation has been shown to increase NHE3 transcript and total cellular protein in cultured cells. The role of increased NHE3 transcript in the intact kidney is less clear.
In parallel with the Pyk2/c-Src pathway is the acid stimulation of extracellular signal related kinase (ERK) and increased c-Fos/c-Jun expression, which contributes to the increase in ET-1 synthesis via an AP-1 site in the ET-1 promoter.61,69 Although the presence of a second pathway is clear, the identity of this second pH sensor is still elusive.
Conclusion
Renal Na+/H+ exchangers, in particular the NHE3 isoform, are paramount for the maintenance of whole-organism acidbase homeostasis. However, despite the exponentially growing body of data on NHE function and regulation, fundamental questions remain unanswered in areas ranging from molecular structure and detailed mechanism of ion translocation to the integration of different NHE isoforms in the big picture of renal and acid-base physiology. Although the study of NHEs at the cellular and molecular level is undoubtedly of crucial importance, one should not lose sight of the fact that understanding the precise roles of NHEs at the whole-organ and whole-organism level is the ultimate goal of any research in the field.
Acknowledgment
The authors are grateful to Dr. Daniel Fuster for his help in constructing the phylogenetic tree and endless scintillating discussions over the years.
Supported by grants from the National Institutes of Health (DK48482, DK20543 to O.W.M.), the National Kidney Foundation (to I.A.B.), the Charles and Jane Pak Research Fellowship (to I.A.B.), and the Simmons Family Foundation (to O.W.M.).
References
- 1.DuBose TD, Jr, Pucacco LR, Seldin DW, et al. Microelectrode determination of pH and PCO2 in rat proximal tubule after benzolamide: Evidence for hydrogen ion secretion. Kidney Int. 1979;15:624–629. doi: 10.1038/ki.1979.82. [DOI] [PubMed] [Google Scholar]
- 2.Pitts RF, Ayer JL, Schiess WA, et al. The renal regulation of acid-base balance in man. III. The reabsorption and excretion of bicarbonate. J Clin Invest. 1949;28:35–44. [PubMed] [Google Scholar]
- 3.Murer H, Hopfer U, Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976;154:597–604. [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsella JL, Aronson PS. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980;238:F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- 5.Murer H, Hopfer U, Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. J Am Soc Nephrol. 1998;9:143–150. doi: 10.1681/ASN.V91143. 1976. [DOI] [PubMed] [Google Scholar]
- 6.Kinsella JL, Aronson PS. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. J Am Soc Nephrol. 2001;12:1085–1095. doi: 10.1681/ASN.V1251085. 1980. [DOI] [PubMed] [Google Scholar]
- 7.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg EB, Arbel T, Chen J, et al. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:2615–2619. doi: 10.1073/pnas.84.9.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 10.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na(+)/H(+) exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 11.Hunte C, Screpanti E, Venturi M, et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435:1197–1202. doi: 10.1038/nature03692. [DOI] [PubMed] [Google Scholar]
- 12.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 13.Gekle M, Drumm K, Mildenberger S, et al. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol. 1999;520:709–721. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gekle M, Volker K, Mildenberger S, et al. NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am J Physiol. 2004;287:F469–F473. doi: 10.1152/ajprenal.00059.2004. [DOI] [PubMed] [Google Scholar]
- 15.Klisic J, Zhang J, Nief V, et al. Albumin regulates the Na+/H+ exchanger 3 in OKP cells. J Am Soc Nephrol. 2003;14:3008–3016. doi: 10.1097/01.asn.0000098700.70804.d3. [DOI] [PubMed] [Google Scholar]
- 16.Biemesderfer D, Reilly RF, Exner M, et al. Immunocytochemical characterization of Na(+)-H+ exchanger isoform NHE-1 in rabbit kidney. Am J Physiol. 1992;263:F833–F840. doi: 10.1152/ajprenal.1992.263.5.F833. [DOI] [PubMed] [Google Scholar]
- 17.Peti-Peterdi J, Chambrey R, Bebok Z, et al. Macula densa Na(+)/H(+) exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am J Physiol. 2000;278:F452–F463. doi: 10.1152/ajprenal.2000.278.3.F452. [DOI] [PubMed] [Google Scholar]
- 18.Good DW, Watts BA, 3rd, George T, et al. Transepithelial HCO3-absorption is defective in renal thick ascending limbs from Na+/H+ exchanger NHE1 null mutant mice. Am J Physiol. 2004;287:F1244–F1249. doi: 10.1152/ajprenal.00176.2004. [DOI] [PubMed] [Google Scholar]
- 19.Good DW, George T, Watts BA., 3rd Basolateral membrane Na+/H+ exchange enhances HCO3-absorption in rat medullary thick ascending limb evidence for functional coupling between basolateral and apical membrane Na+/H+ exchangers. Proc Natl Acad Sci U S A. 1995;92:12525–12529. doi: 10.1073/pnas.92.26.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts BA, 3rd, George T, Good DW. The basolateral NHE1 Na+/H+ exchanger regulates transepithelial HCO-3 absorption through actin cytoskeleton remodeling in renal thick ascending limb. J Biol Chem. 2005;280:11439–11447. doi: 10.1074/jbc.M410719200. [DOI] [PubMed] [Google Scholar]
- 21.Cox GA, Lutz CM, Yang CL, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–148. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- 22.Bell SM, Schreiner CM, Schultheis PJ, et al. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 23.Bailey MA, Giebisch G, Abbiati T, et al. NHE2-mediated bicarbonate reabsorption in the distal tubule of NHE3 null mice. J Physiol. 2004;561:765–775. doi: 10.1113/jphysiol.2004.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultheis PJ, Clarke LL, Meneton P, et al. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledoussal C, Woo AL, Miller ML, et al. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol. 2001;281:G1385–G1396. doi: 10.1152/ajpgi.2001.281.6.G1385. [DOI] [PubMed] [Google Scholar]
- 26.Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 27.Noonan WT, Woo AL, Nieman ML, et al. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol. 2005;288:R685–R691. doi: 10.1152/ajpregu.00209.2004. [DOI] [PubMed] [Google Scholar]
- 28.Woo AL, Noonan WT, Schultheis PJ, et al. Renal function in NHE3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol. 2003;284:F1190–F1198. doi: 10.1152/ajprenal.00418.2002. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Amlal H, Schultheis PJ, et al. HCO-3 reabsorption in renal collecting duct of NHE-3-deficient mouse: a compensatory response. Am J Physiol. 1999;276:F914–F921. doi: 10.1152/ajprenal.1999.276.6.F914. [DOI] [PubMed] [Google Scholar]
- 30.Chambrey R, St John PL, Eladari D, et al. Localization and functional characterization of Na+/H+ exchanger isoform NHE4 in rat thick ascending limbs. Am J Physiol. 2001;281:F707–F717. doi: 10.1152/ajprenal.2001.281.4.F707. [DOI] [PubMed] [Google Scholar]
- 31.Gawenis LR, Greeb JM, Prasad V, et al. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280:12781–12789. doi: 10.1074/jbc.M414118200. [DOI] [PubMed] [Google Scholar]
- 32.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol. 2003;284:F467–F473. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 33.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol. 2005;288:F530–F538. doi: 10.1152/ajprenal.00229.2004. [DOI] [PubMed] [Google Scholar]
- 34.Baum M, Biemesderfer D, Gentry D, et al. Ontogeny of rabbit renal cortical NHE3 and NHE1: Effect of glucocorticoids. Am J Physiol. 1995;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. [DOI] [PubMed] [Google Scholar]
- 35.Choi JY, Shah M, Lee MG, et al. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambuhl PM, Amemiya M, Danczkay M, et al. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol. 1996;271:F917–F925. doi: 10.1152/ajprenal.1996.271.4.F917. [DOI] [PubMed] [Google Scholar]
- 37.Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol. 1988;255:F301–F306. doi: 10.1152/ajprenal.1988.255.2.F301. [DOI] [PubMed] [Google Scholar]
- 38.Ullrich KJ, Rumrich G, Kloss S. Phosphate transport in the proximal convolution of the rat kidney. III. Effect of extracellular and intracellular pH. Pflugers Arch. 1978;377:33–42. doi: 10.1007/BF00584371. [DOI] [PubMed] [Google Scholar]
- 39.Costa Silva VL, Campiglia SS, de Mello Aires M, et al. Role of luminal buffers in renal tubular acidification. J Membr Biol. 1981;63:13–24. doi: 10.1007/BF01969441. [DOI] [PubMed] [Google Scholar]
- 40.Gluck S, Cannon C, Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982;79:4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature. 1985;318:368–371. doi: 10.1038/318368a0. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: effects of dietary acid and alkali loading. Am J Physiol. 1985;249:F590–F595. doi: 10.1152/ajprenal.1985.249.4.F590. [DOI] [PubMed] [Google Scholar]
- 43.Quamme GA. Effects of metabolic acidosis, alkalosis, and dietary hydrogen ion intake on phosphate transport in the proximal convoluted tubule. Am J Physiol. 1985;249:F769–F779. doi: 10.1152/ajprenal.1985.249.5.F769. [DOI] [PubMed] [Google Scholar]
- 44.Horie S, Moe O, Tejedor A, et al. Preincubation in acid medium increases Na/H antiporter activity in cultured renal proximal tubule cells. Proc Natl Acad Sci U S A. 1990;87:4742–4745. doi: 10.1073/pnas.87.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moe OW, Miller RT, Horie S, et al. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest. 1991;88:1703–1708. doi: 10.1172/JCI115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krapf R, Pearce D, Lynch C, et al. Expression of rat renal Na/H antiporter mRNA levels in response to respiratory and metabolic acidosis. J Clin Invest. 1991;87:747–751. doi: 10.1172/JCI115057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akiba T, Rocco VK, Warnock DG. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987;80:308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988;82:1445–1453. doi: 10.1172/JCI113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soleimani M, Bizal GL, McKinney TD, et al. Effect of in vitro metabolic acidosis on luminal Na+/H+ exchange and basolateral Na+:HCO3-cotransport in rabbit kidney proximal tubules. J Clin Invest. 1992;90:211–218. doi: 10.1172/JCI115838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Good DW, Watts BA., 3rd Functional roles of apical membrane Na+/H+ exchange in rat medullary thick ascending limb. Am J Physiol. 1996;270:F691–F699. doi: 10.1152/ajprenal.1996.270.4.F691. [DOI] [PubMed] [Google Scholar]
- 51.Wu MS, Biemesderfer D, Giebisch G, et al. Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem. 1996;271:32749–32752. doi: 10.1074/jbc.271.51.32749. [DOI] [PubMed] [Google Scholar]
- 52.Laghmani K, Borensztein P, Ambuhl P, et al. Chronic metabolic acidosis enhances NHE-3 protein abundance and transport activity in the rat thick ascending limb by increasing NHE-3 mRNA. J Clin Invest. 1997;99:24–30. doi: 10.1172/JCI119128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laghmani K, Preisig PA, Moe OW, et al. Endothelin-1/endothelin-B receptor-mediated increases in NHE3 activity in chronic metabolic acidosis. J Clin Invest. 2001;107:1563–1569. doi: 10.1172/JCI11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cogan MG, Rector FC., Jr Proximal reabsorption during metabolic acidosis in the rat. Am J Physiol. 1982;242:F499–F507. doi: 10.1152/ajprenal.1982.242.5.F499. [DOI] [PubMed] [Google Scholar]
- 55.Kunau RT, Jr, Hart JI, Walker KA. Effect of metabolic acidosis on proximal tubular total CO2 absorption. Am J Physiol. 1985;249:F62–F68. doi: 10.1152/ajprenal.1985.249.1.F62. [DOI] [PubMed] [Google Scholar]
- 56.Madison LL, Seldin DW. Ammonia excretion and renal enzymatic adaptation in human subjects, as disclosed by administration of precursor amino acids. J Clin Invest. 1958;37:1615–1627. doi: 10.1172/JCI103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest. 1988;81:159–164. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moe OW. Scaffolds: Orchestrating proteins to achieve concerted function. Kidney Int. 2003;64:1916–1917. doi: 10.1046/j.1523-1755.2003.00346.x. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Sato S, Yang X, et al. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest. 2004;114:1782–1789. doi: 10.1172/JCI18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaji Y, Tsuganezawa H, Moe OW, et al. Intracellular acidosis activates c-Src. Am J Physiol. 1997;272:C886–C893. doi: 10.1152/ajpcell.1997.272.3.C886. [DOI] [PubMed] [Google Scholar]
- 61.Tsuganezawa H, Sato S, Yamaji Y, et al. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int. 2002;62:41–50. doi: 10.1046/j.1523-1755.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 62.Yamaji Y, Amemiya M, Cano A, et al. Overexpression of csk inhibits acid-induced activation of NHE-3. Proc Natl Acad Sci U S A. 1995;92:6274–6278. doi: 10.1073/pnas.92.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Licht C, Laghmani K, Yanagisawa M, et al. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004;65:1320–1326. doi: 10.1111/j.1523-1755.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 64.Peng Y, Moe OW, Chu T, et al. ETB receptor activation leads to activation and phosphorylation of NHE3. Am J Physiol. 1999;276:C938–C945. doi: 10.1152/ajpcell.1999.276.4.C938. [DOI] [PubMed] [Google Scholar]
- 65.Peng Y, Amemiya M, Yang X, et al. ET(B) receptor activation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol. 2001;280:F34–F42. doi: 10.1152/ajprenal.2001.280.1.F34. [DOI] [PubMed] [Google Scholar]
- 66.Chu TS, Tsuganezawa H, Peng Y, et al. Role of tyrosine kinase pathways in ETB receptor activation of NHE3. Am J Physiol. 1996;271:C763–C771. doi: 10.1152/ajpcell.1996.271.3.C763. [DOI] [PubMed] [Google Scholar]
- 67.Chu TS, Peng Y, Cano A, et al. Endothelin(B) receptor activates NHE-3 by a Ca2+-dependent pathway in OKP cells. J Clin Invest. 1996;97:1454–1462. doi: 10.1172/JCI118567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laghmani K, Sakamoto A, Yanagisawa M, et al. A consensus sequence in the endothelin B receptor second intracellular loop is required for NHE3 activation by endothelin-1. Am J Physiol. 2005;288:F732–739. doi: 10.1152/ajprenal.00300.2004. [DOI] [PubMed] [Google Scholar]
- 69.Yamaji Y, Moe OW, Miller RT, et al. Acid activation of immediate early genes in renal epithelial cells. J Clin Invest. 1994;94:1297–1303. doi: 10.1172/JCI117448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu F, Gesek FA. alpha(1)-Adrenergic receptors activate NHE1 and NHE3 through distinct signaling pathways in epithelial cells. Am J Physiol. 2001;280:F415–F425. doi: 10.1152/ajprenal.2001.280.3.F415. [DOI] [PubMed] [Google Scholar]
- 71.Nord EP, Howard MJ, Hafezi A, et al. Alpha 2 adrenergic agonists stimulate Na+-H+ antiport activity in the rabbit renal proximal tubule. J Clin Invest. 1987;80:1755–1762. doi: 10.1172/JCI113268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang X, Amemiya M, Peng Y, et al. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol. 2000;279:C410–C419. doi: 10.1152/ajpcell.2000.279.2.C410. [DOI] [PubMed] [Google Scholar]
- 73.Soleimani M, Bookstein C, Singh G, et al. Differential regulation of Na+/H+ exchange and H(+)-ATPase by pH and HCO3– in kidney proximal tubules. J Membr Biol. 1995;144:209–216. doi: 10.1007/BF00236834. [DOI] [PubMed] [Google Scholar]
- 74.Di Sole F, Casavola V, Mastroberardino L, et al. Adenosine inhibits the transfected Na+-H+ exchanger NHE3 in Xenopus laevis renal epithelial cells (A6/C1) J Physiol. 1999;515:829–842. doi: 10.1111/j.1469-7793.1999.829ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Sole F, Cerull R, Petzke S, et al. Bimodal acute effects of A1 adenosine receptor activation on Na+/H+ exchanger 3 in opossum kidney cells. J Am Soc Nephrol. 2003;14:1720–1730. doi: 10.1097/01.asn.0000072743.97583.db. [DOI] [PubMed] [Google Scholar]
- 76.Di Sole F, Cerull R, Babich V, et al. Acute regulation of Na/H exchanger NHE3 by adenosine A(1) receptors is mediated by calcineurin homologous protein. J Biol Chem. 2004;279:2962–2974. doi: 10.1074/jbc.M306838200. [DOI] [PubMed] [Google Scholar]
- 77.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3– cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morduchowicz GA, Sheikh-Hamad D, Dwyer BE, et al. Angiotensin II directly increases rabbit renal brush-border membrane sodium transport: Presence of local signal transduction system. J Membr Biol. 1991;122:43–53. doi: 10.1007/BF01872738. [DOI] [PubMed] [Google Scholar]
- 79.Jourdain M, Amiel C, Friedlander G. Modulation of Na-H exchange activity by angiotensin II in opossum kidney cells. Am J Physiol. 1992;263:C1141–C1146. doi: 10.1152/ajpcell.1992.263.6.C1141. [DOI] [PubMed] [Google Scholar]
- 80.Cano A, Miller RT, Alpern RJ, et al. Angiotensin II stimulation of Na-H antiporter activity is cAMP independent in OKP cells. Am J Physiol. 1994;266:C1603–C1608. doi: 10.1152/ajpcell.1994.266.6.C1603. [DOI] [PubMed] [Google Scholar]
- 81.Poggioli J, Karim Z, Paillard M. Effect of angiotensin ii on Na+/H+ exchangers of the renal tubule. Nephrologie. 1998;19:421–425. [PubMed] [Google Scholar]
- 82.Xu L, Dixit MP, Nullmeyer KD, et al. Regulation of Na(+)/H(+) exchanger-NHE3 by angiotensin-II in OKP cells. Biochim Biophys Acta. 2006;20:20. doi: 10.1016/j.bbamem.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Levine SA, Montrose MH, Tse CM, et al. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem. 1993;268:25527–25535. [PubMed] [Google Scholar]
- 84.Cabado AG, Yu FH, Kapus A, et al. Distinct structural domains confer cAMP sensitivity and ATP dependence to the Na+/H+ exchanger NHE3 isoform. J Biol Chem. 1996;271:3590–3599. doi: 10.1074/jbc.271.7.3590. [DOI] [PubMed] [Google Scholar]
- 85.Winaver J, Burnett JC, Tyce GM, et al. ANP inhibits Na(+)-H+ antiport in proximal tubular brush border membrane: Role of dopamine. Kidney Int. 1990;38:1133–1140. doi: 10.1038/ki.1990.323. [DOI] [PubMed] [Google Scholar]
- 86.Moe OW, Amemiya M, Yamaji Y. Activation of protein kinase A acutely inhibits and phosphorylates Na/H exchanger NHE-3. J Clin Invest. 1995;96:2187–2194. doi: 10.1172/JCI118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamprecht G, Weinman EJ, Yun CH. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem. 1998;273:29972–29978. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- 88.Baum M, Quigley R. Inhibition of proximal convoluted tubule transport by dopamine. Kidney Int. 1998;54:1593–1600. doi: 10.1046/j.1523-1755.1998.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu MC, Fan L, Crowder LA, et al. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: Dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 90.Bacic D, Kaissling B, McLeroy P, et al. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003;64:2133–2141. doi: 10.1046/j.1523-1755.2003.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Felder CC, Campbell T, Albrecht F, et al. Dopamine inhibits Na(+)-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol. 1990;259:F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- 92.Hu MC, Quinones H, Moe OW. Chronic inhibition of NHE3 by dopamine (DA) in OKP cells. J Am Soc Nephrol. 2000;11:5A. abstract issue. [Google Scholar]
- 93.Eiam-Ong S, Hilden SA, King AJ, et al. Endothelin-1 stimulates the Na+/H+ and Na+/HCO3– transporters in rabbit renal cortex. Kidney Int. 1992;42:18–24. doi: 10.1038/ki.1992.255. [DOI] [PubMed] [Google Scholar]
- 94.Garcia NH, Garvin JL. Endothelin’s biphasic effect on fluid absorption in the proximal straight tubule and its inhibitory cascade. J Clin Invest. 1994;93:2572–2577. doi: 10.1172/JCI117268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walter R, Helmle-Kolb C, Forgo J, et al. Stimulation of Na+/H+ exchange activity by endothelin in opossum kidney cells. Pflugers Arch. 1995;430:137–144. doi: 10.1007/BF00373849. [DOI] [PubMed] [Google Scholar]
- 96.Bidet M, Merot J, Tauc M, et al. Na+-H+ exchanger in proximal cells isolated from kidney. II. Short-term regulation by glucocorticoids. Am J Physiol. 1987;253:F945–F951. doi: 10.1152/ajprenal.1987.253.5.F945. [DOI] [PubMed] [Google Scholar]
- 97.Baum M, Quigley R. Glucocorticoids stimulate rabbit proximal convoluted tubule acidification. J Clin Invest. 1993;91:110–114. doi: 10.1172/JCI116158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baum M, Moe OW, Gentry DL, et al. Effect of glucocorticoids on renal cortical NHE-3 and NHE-1 mRNA. Am J Physiol. 1994;267:F437–F42. doi: 10.1152/ajprenal.1994.267.3.F437. [DOI] [PubMed] [Google Scholar]
- 99.Bobulescu IA, Dwarakanath V, Zou L, et al. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol. 2005;289:F685–F691. doi: 10.1152/ajprenal.00447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Freiberg JM, Kinsella J, Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982;79:4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baum M, Cano A, Alpern RJ. Glucocorticoids stimulate Na+/H+ antiporter in OKP cells. Am J Physiol. 1993;264:F1027–F1031. doi: 10.1152/ajprenal.1993.264.6.F1027. [DOI] [PubMed] [Google Scholar]
- 102.Baum M, Amemiya M, Dwarakanath V, et al. Glucocorticoids regulate NHE-3 transcription in OKP cells. Am J Physiol. 1996;270:F164–F169. doi: 10.1152/ajprenal.1996.270.1.F164. [DOI] [PubMed] [Google Scholar]
- 103.Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104–1109. doi: 10.1172/JCI112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gesek FA, Schoolwerth AC. Insulin increases Na(+)-H+ exchange activity in proximal tubules from normotensive and hypertensive rats. Am J Physiol. 1991;260:F695–F703. doi: 10.1152/ajprenal.1991.260.5.F695. [DOI] [PubMed] [Google Scholar]
- 105.Klisic J, Hu MC, Nief V, et al. Insulin activates Na(+)/H(+) exchanger 3: Biphasic response and glucocorticoid dependence. Am J Physiol. 2002;283:F532–F539. doi: 10.1152/ajprenal.00365.2001. [DOI] [PubMed] [Google Scholar]
- 106.Nath SK, Hang CY, Levine SA, et al. Hyperosmolarity inhibits the Na+/H+ exchanger isoforms NHE2 and NHE3: An effect opposite to that on NHE1. Am J Physiol. 1996;270:G431–G441. doi: 10.1152/ajpgi.1996.270.3.G431. [DOI] [PubMed] [Google Scholar]
- 107.Ambuhl P, Amemiya M, Preisig PA, et al. Chronic hyperosmolality increases NHE3 activity in OKP cells. J Clin Invest. 1998;101:170–177. doi: 10.1172/JCI62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oweis S, Wu L, Kiela PR, et al. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol. 2006;290:F997–F1008. doi: 10.1152/ajprenal.00322.2005. [DOI] [PubMed] [Google Scholar]
- 109.Puschett JB, Zurbach P, Sylk D. Acute effects of parathyroid hormone on proximal bicarbonate transport in the dog. Kidney Int. 1976;9:501–510. doi: 10.1038/ki.1976.64. [DOI] [PubMed] [Google Scholar]
- 110.Iino Y, Burg MB. Effect of parathyroid hormone on bicarbonate absorption by proximal tubules in vitro. Am J Physiol. 1979;236:F387–F391. doi: 10.1152/ajprenal.1979.236.4.F387. [DOI] [PubMed] [Google Scholar]
- 111.McKinney TD, Myers P. PTH inhibition of bicarbonate transport by proximal convoluted tubules. Am J Physiol. 1980;239:F127–F134. doi: 10.1152/ajprenal.1980.239.2.F127. [DOI] [PubMed] [Google Scholar]
- 112.Helmle-Kolb C, Montrose MH, Stange G, et al. Regulation of Na+/H+ exchange in opossum kidney cells by parathyroid hormone, cyclic AMP and phorbol esters. Pflugers Arch. 1990;415:461–470. doi: 10.1007/BF00373624. [DOI] [PubMed] [Google Scholar]
- 113.Azarani A, Goltzman D, Orlowski J. Parathyroid hormone and parathyroid hormone-related peptide inhibit the apical Na+/H+ exchanger NHE-3 isoform in renal cells (OK) via a dual signaling cascade involving protein kinase A and C. J Biol Chem. 1995;270:20004–20010. doi: 10.1074/jbc.270.34.20004. [DOI] [PubMed] [Google Scholar]
- 114.Collazo R, Fan L, Hu MC, et al. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;275:31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 115.Kahn AM, Dolson GM, Hise MK, et al. Parathyroid hormone and dibutyryl cAMP inhibit Na+/H+ exchange in renal brush border vesicles. Am J Physiol. 1985;248:F212–F218. doi: 10.1152/ajprenal.1985.248.2.F212. [DOI] [PubMed] [Google Scholar]
- 116.Girardi AC, Titan SM, Malnic G, et al. Chronic effect of parathyroid hormone on NHE3 expression in rat renal proximal tubules. Kidney Int. 2000;58:1623–1631. doi: 10.1046/j.1523-1755.2000.00323.x. [DOI] [PubMed] [Google Scholar]
- 117.Fuster D, Moe OW, Hilgemann DW. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc Natl Acad Sci U S A. 2004;101:10482–10487. doi: 10.1073/pnas.0403930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karinch AM, Lin CM, Wolfgang CL, et al. Regulation of expression of the SN1 transporter during renal adaptation to chronic metabolic acidosis in rats. Am J Physiol. 2002;283:F1011–F1019. doi: 10.1152/ajprenal.00106.2002. [DOI] [PubMed] [Google Scholar]
- 119.Solbu TT, Boulland JL, Zahid W, et al. Induction and targeting of the glutamine transporter SN1 to the basolateral membranes of cortical kidney tubule cells during chronic metabolic acidosis suggest a role in pH regulation. J Am Soc Nephrol. 2005;16:869–877. doi: 10.1681/ASN.2004060433. [DOI] [PubMed] [Google Scholar]
- 120.Curthoys NP, Lowry OH. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973;248:162–168. [PubMed] [Google Scholar]
- 121.Tong J, Harrison G, Curthoys NP. The effect of metabolic acidosis on the synthesis and turnover of rat renal phosphate-dependent glutaminase. Biochem J. 1986;233:139–144. doi: 10.1042/bj2330139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tong J, Shapiro RA, Curthoys NP. Changes in the levels of translatable glutaminase mRNA during onset and recovery from metabolic acidosis. Biochemistry. 1987;26:2773–2777. doi: 10.1021/bi00384a018. [DOI] [PubMed] [Google Scholar]
- 123.Hwang JJ, Perera S, Shapiro RA, et al. Mechanism of altered renal glutaminase gene expression in response to chronic acidosis. Biochemistry. 1991;30:7522–7526. doi: 10.1021/bi00244a022. [DOI] [PubMed] [Google Scholar]
- 124.Hwang JJ, Curthoys NP. Effect of acute alterations in acid-base balance on rat renal glutaminase and phosphoenolpyruvate carboxykinase gene expression. J Biol Chem. 1991;266:9392–9396. [PubMed] [Google Scholar]
- 125.Hansen WR, Barsic-Tress N, Taylor L, et al. The 3′-nontranslated region of rat renal glutaminase mRNA contains a pH-responsive stability element. Am J Physiol. 1996;271:F126–F131. doi: 10.1152/ajprenal.1996.271.1.F126. [DOI] [PubMed] [Google Scholar]
- 126.Laterza OF, Hansen WR, Taylor L, et al. Identification of an mRNA-binding protein and the specific elements that may mediate the pH-responsive induction of renal glutaminase mRNA. J Biol Chem. 1997;272:22481–22488. doi: 10.1074/jbc.272.36.22481. [DOI] [PubMed] [Google Scholar]
- 127.Kaiser S, Hwang JJ, Smith H, et al. Effect of altered acid-base balance and of various agonists on levels of renal glutamate dehydrogenase mRNA. Am J Physiol. 1992;262:F507–F512. doi: 10.1152/ajprenal.1992.262.3.F507. [DOI] [PubMed] [Google Scholar]
- 128.Schroeder JM, Liu W, Curthoys NP. pH-responsive stabilization of glutamate dehydrogenase mRNA in LLC-PK1-F+ cells. Am J Physiol. 2003;285:F258–F265. doi: 10.1152/ajprenal.00422.2002. [DOI] [PubMed] [Google Scholar]
- 129.Parry DM, Brosnan JT. Renal phosphoenolpyruvate carboxykinase during perturbations of acid-base homeostasis in rats. Immunochemical studies. Can J Biochem. 1980;58:1298–1301. doi: 10.1139/o80-174. [DOI] [PubMed] [Google Scholar]
- 130.Mapes RE, Watford M. Effects of metabolic acidosis and diabetes on the abundance of specific renal mRNAs. Int J Biochem. 1989;21:297–305. doi: 10.1016/0020-711x(89)90187-0. [DOI] [PubMed] [Google Scholar]
- 131.Feifel E, Obexer P, Andratsch M, et al. p38 MAPK mediates acidinduced transcription of PEPCK in LLC-PK(1)-FBPase(+) cells. Am J Physiol. 2002;283:F678–F688. doi: 10.1152/ajprenal.00097.2002. [DOI] [PubMed] [Google Scholar]
- 132.O’Hayre M, Taylor L, Andratsch M, et al. Effects of constitutively active and dominant negative MAPK kinase (MKK) 3 and MKK6 on the pH-responsive increase in phosphoenolpyruvate carboxykinase mRNA. J Biol Chem. 2006;281:2982–2988. doi: 10.1074/jbc.M510084200. [DOI] [PubMed] [Google Scholar]
- 133.Jehle AW, Forgo J, Biber J, et al. Acid-induced stimulation of Na-Pi cotransport in OK cells: Molecular characterization and effect of dexamethasone. Am J Physiol. 1997;273:F396–F403. doi: 10.1152/ajprenal.1997.273.3.F396. [DOI] [PubMed] [Google Scholar]
- 134.Jehle AW, Hilfiker H, Pfister MF, et al. Type II Na-Pi cotransport is regulated transcriptionally by ambient bicarbonate/carbon dioxide tension in OK cells. Am J Physiol. 1999;276:F46–F53. doi: 10.1152/ajprenal.1999.276.1.F46. [DOI] [PubMed] [Google Scholar]
- 135.Ambuhl PM, Zajicek HK, Wang H, et al. Regulation of renal phosphate transport by acute and chronic metabolic acidosis in the rat. Kidney Int. 1998;53:1288–1298. doi: 10.1046/j.1523-1755.1998.00901.x. [DOI] [PubMed] [Google Scholar]
- 136.Aruga S, Wehrli S, Kaissling B, et al. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int. 2000;58:206–215. doi: 10.1046/j.1523-1755.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 137.Aruga S, Pajor AM, Nakamura K, et al. OKP cells express the Na-dicarboxylate cotransporter NaDC-1. Am J Physiol. 2004;287:C64–C72. doi: 10.1152/ajpcell.00061.2003. [DOI] [PubMed] [Google Scholar]
- 138.Melnick JZ, Srere PA, Elshourbagy NA, et al. Adenosine triphosphate citrate lyase mediates hypocitraturia in rats. J Clin Invest. 1996;98:2381–2387. doi: 10.1172/JCI119051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melnick JZ, Preisig PA, Moe OW, et al. Renal cortical mitochondrial aconitase is regulated in hypo- and hypercitraturia. Kidney Int. 1998;54:160–165. doi: 10.1046/j.1523-1755.1998.00974.x. [DOI] [PubMed] [Google Scholar]
- 140.Amemiya M, Yamaji Y, Cano A, et al. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am J Physiol. 1995;269:C126–C133. doi: 10.1152/ajpcell.1995.269.1.C126. [DOI] [PubMed] [Google Scholar]
- 141.Amlal H, Wang Z, Soleimani M. Functional upregulation of H+-ATPase by lethal acid stress in cultured inner medullary collecting duct cells. Am J Physiol. 1997;273:C1194–C1205. doi: 10.1152/ajpcell.1997.273.4.C1194. [DOI] [PubMed] [Google Scholar]
- 142.Sabolic I, Brown D, Gluck SL, et al. Regulation of AE1 anion exchanger and H(+)-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int. 1997;51:125–137. doi: 10.1038/ki.1997.16. [DOI] [PubMed] [Google Scholar]
- 143.Fejes-Toth G, Chen WR, Rusvai E, et al. Differential expression of AE1 in renal HCO3-secreting and -reabsorbing intercalated cells. J Biol Chem. 1994;269:26717–26721. [PubMed] [Google Scholar]
- 144.Huber S, Asan E, Jons T, et al. Expression of rat kidney anion exchanger 1 in type A intercalated cells in metabolic acidosis and alkalosis. Am J Physiol. 1999;277:F841–F849. doi: 10.1152/ajprenal.1999.277.6.F841. [DOI] [PubMed] [Google Scholar]
- 145.Amlal H, Chen Q, Greeley T, et al. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001;60:1824–1836. doi: 10.1046/j.1523-1755.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 146.Kwon TH, Fulton C, Wang W, et al. Chronic metabolic acidosis upregulates rat kidney Na-HCO cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol. 2002;282:F341–F351. doi: 10.1152/ajprenal.00104.2001. [DOI] [PubMed] [Google Scholar]
- 147.Puttaparthi K, Markovich D, Halaihel N, et al. Metabolic acidosis regulates rat renal Na-Si cotransport activity. Am J Physiol. 1999;276:C1398–C1404. doi: 10.1152/ajpcell.1999.276.6.C1398. [DOI] [PubMed] [Google Scholar]


