Abstract
Transforming growth factor β1 (TGFβ1) expression is elevated by tumor promoters in the mouse skin, but its role in tumor promotion has not been well defined. To investigate this, we have compared TGFβ1+/+ and +/− mice in a two-stage skin chemical carcinogenesis protocol. Surprisingly, TGFβ1+/− mice had fewer number and incidence of benign papillomas, reduced epidermal and tumor cell proliferation and reduced epidermal TGFβ1 and nuclear p-Smad2 localization in response to the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) compared with TGFβ1+/+ mice. Maximal TPA activation of protein kinase C (PKCα) as measured by activity assays and activation of target genes and induction of cornified envelopes correlated with TGFβ1 gene dosage in keratinocytes and addition of exogenous TGFβ1 restored the cornification defect in TGFβ1+/− keratinocytes. Similarly, inhibition of ALK5-suppressed TPA-mediated PKCα activation suggesting that physiological levels of TGFβ1 are required for maximal activation of PKC-dependent mitogenic responses. Paradoxically, the TPA-induced inflammatory response was greater in TGFβ1+/− skin, but TGFβ1+/+ papillomas had more tumor infiltrating myeloperoxidase-positive cells and pro-inflammatory gene expression was elevated in v-rasHa-transduced TGFβ1+/+ but not TGFβ1+/− keratinocytes. Thus, ras activation switches TGFβ1 to a pro-inflammatory cytokine. Despite this differential proliferative and inflammatory response to TPA and enhanced papilloma formation in the TGFβ1+/+ mice, the frequency of malignant conversion was reduced compared with TGFβ1+/− mice. Therefore, TGFβ1 promotes benign tumors by modifying tumor promoter-induced cell proliferation and inflammation but retains a suppressive function for malignant conversion.
Introduction
Transforming growth factor β1 (TGFβ1) is a regulatory cytokine that has stage-specific stimulatory and suppressive actions in cancer development (1). In the two-stage mouse skin carcinogenesis model, benign epidermal papillomas are caused by topical application of the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) and repeated promotion with 12-O-tetradecanoylphorbol 13-acetate (TPA). Inhibition of TGFβ1 signaling in this model accelerates malignancy and overexpression of TGFβ1 suppresses benign tumor formation (1) but causes outgrowth of highly malignant spindle cell cancers (2) with increased tumor cell metastases (3). TPA and other tumor promoters cause epidermal hyperplasia, dermal inflammation (4) and induce TGFβ1 expression in the epidermis (5). Although in mammary carcinogenesis, reduced TGFβ1 levels in the TGFβ1+/− mouse enhance tumorigenesis (6), it is not known what role TGFβ1 has in tumor promotion. The surprising resistance of Smad3-null mice to skin tumor formation (7) suggests that in the epidermis, TGFβ1 signaling may not simply act as a negative feedback pathway. To examine the role of TGFβ1 in skin tumor promotion, we compared the response of TGFβ1+/+ and +/− mice to acute and chronic treatment with TPA and evaluated tumor development in a two-stage skin carcinogenesis assay. Our studies show that TGFβ1 enhances tumor promotion through effects on PKC, but the benign tumors that form have a low frequency of premalignant progression to squamous cell carcinoma (SCC).
Materials and methods
Animal studies
Seven- to eight-week-old TGFβ1+/+ and TGFβ1+/− mice (8) backcrossed onto a Balb/c background were used for all in vivo studies. Adult TGFβ1−/− mice were not used due to post-natal lethality (8). TGFβ1+/+ and TGFβ1+/− mice were given topically with a single dose of 50 μg of DMBA (Sigma, St Louis, MO) and 5 or 10 μg of TPA (Calbiochem, La Jolla, CA) in 200 μl of acetone twice a week for 25 weeks and tumors >2 mm recorded weekly. Short-term tumor induction was done similarly for 10 weeks. For acute TPA, mice were treated once with 5 μg of TPA/200 μl acetone or acetone alone and dorsal skins were harvested as indicated. For chronic promotion, mice were treated with TPA (5 μg) twice weekly for 5 weeks, and tissues were isolated after 72 h. Double transgenic mice with conditional expression of active TGFβ1 (9) were given doxycycline (2 mg) intraperitoneally 24 h prior to TPA treatment. All animals were housed and treated according to approved Institutional animal protocols.
Tissue analysis
Analysis of tumors and measurement of epidermal thickness was done on hematoxylin- and eosin-stained sections of neutral buffered formalin-fixed tissues. Epidermal layers were quantitated at every 20 basal cell for each section, and five sections averaged per treatment group. Specific antibodies to Smad2 (Santa Cruz Biotechnology, Santa Cruz, CA) and phospho-Smad2 (Millipore, Billerica, MA) (10–12) were used to detect these proteins in frozen and ethanol-fixed sections, respectively, by indirect immunofluorescence. Stained sections were imaged using an Olympus FV300 Laser Scanning Confocal Microscope. A Smad2-specific blocking peptide (Santa Cruz Biotechnology) was used to demonstrate specificity of Smad2 cytoplasmic and nuclear staining. Cell proliferation was measured using anti-bromodeoxyuridine immunohistochemistry as described previously (9) and apoptotic cells identified using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay (TUNEL) and expressed as a percentage of total basal cells per section. Inflammatory cells were detected using anti-myeloperoxidase (MPO) antibodies (Dako, Carpinteria, CA) and the average of positive cells per section was scored. Quantitation of epidermal layers and immunostaining was done blindly. Photomicrographs of tissue sections were made using an Olympus BX61Epi-Fluorescence Microscope.
Cell culture
Primary keratinocytes or dermal fibroblasts obtained from crosses of TGFβ1+/− adults were genotyped and cultured as described previously (13) and treated with TGFβ1 (R&D Systems, Minneapolis, MN) and TPA as indicated. Where indicated, SB431542 (Sigma, St Louis, MO) was added 15 min before TPA. Keratinocytes were infected with the ψ2 v-rasHa retrovirus as described (13). Keratinocytes were transfected with 0.23 μg activator protein 1 (AP-1)-luciferase reporter (Stratagene, Cedar Creek, TX) and a renilla-luciferase control plasmid were treated with 5 ng/ml TPA, and luciferase activity was determined using a Promega 20/20 luminometer. PKC activity was measured in 0.3% Triton X-100 extracts of TPA-treated (25 ng/ml) TGFβ1+/+ and +/− primary keratinocytes as described (14) with PKC [Ser25] (19-31) peptide (AnaSpec, Fremont, CA). Cornified envelopes were measured 36 h after TPA treatment as described elsewhere (14,15).
Analysis of protein and RNA
Keratinocytes or whole skin were homogenized in a 1% Triton X-100 lysis buffer with protease and phosphatase inhibitors, and specific proteins were detected by immunoblotting and enhanced chemiluminescence (Pierce, Rockford, IL) using antibodies directed against Smad 2/3, p-Smad2, p-Erk, Erk, p-stress-activated protein kinase/jun N-terminal kinase, p-c-jun or c-jun (Cell Signaling Technology, Danvers, MA). Densitometric analysis of c-jun and p-c-jun blots was done using the Kodak Gel Logic Imaging System and Molecular Imaging Software. Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), and quantitative reverse transcription–polymerase chain reaction done for the indicated genes using the MyIQ system (BioRad Laboratories, Hercules, CA). All values were normalized to β-actin. Primer sequences were obtained from published studies or using Primer 3 (16) software with GeneBank sequence information.
Statistical analysis
Values are expressed as the mean ± SE. Student's t-test was used to compare the indicated groups, and the significance of the difference was described. P values of <0.05 were regarded as indicating a significant difference. Difference in papilloma per mouse for each genotype was measured using an unpaired t-test.
Results
Papilloma formation but not malignant conversion is suppressed in TGFβ1 +/− mice
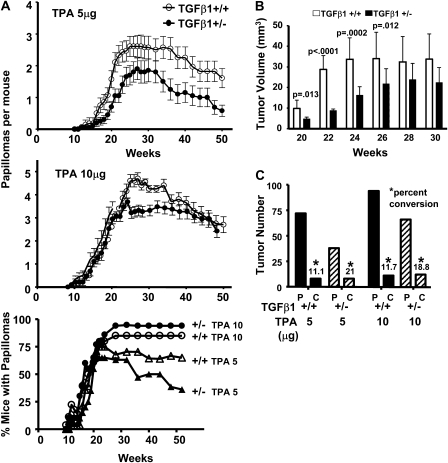
To determine if TGFβ1+/+ and TGFβ1+/− mice differ in the induction of epidermal squamous tumors, 7- to 8-week-old TGFβ1+/+ and TGFβ1+/− mice were treated topically with DMBA and promoted with 5 or 10 μg TPA twice a week for 25 weeks. At both doses of TPA, the papilloma frequency was significantly reduced in TGFβ1+/− mice compared with TGFβ1+/+ mice, with maximum frequency of 1.8 and 2.6 (5 μg) papillomas per mouse and 3.6 and 4.7 (10 μg) papillomas per mouse, respectively (Figure 1A). As expected the papillomas frequency declined after cessation of TPA promotion due to regression and removal of mice with malignancies. The percentage of mice developing tumors was lower in the TGFβ1+/− mice at 5 μg TPA but there was little difference between genotypes at the higher dose (Figure 1A). Similar results were obtained in a pilot study with fewer mice per group. Additionally, at early time points measured between weeks 20–26, papillomas in the TGFβ1+/+ mice were significantly larger than in the TGFβ1+/− animals, although this was not significant at later time points (Figure 1B). Despite larger numbers of papillomas in the TGFβ1+/+ mice at both TPA doses, similar numbers of SCC formed in both genotypes (Figure 1C), indicating a 2-fold increase in frequency of malignant conversion in the TGFβ1+/− mice. Thus, the additional TGFβ1+/+ papillomas are not at high risk for malignant conversion, suggesting that TGFβ1 acts as a suppressor of malignant conversion but enhances benign tumor formation.
Fig. 1.
Papilloma frequency and size are greater in TGFβ1+/+ mice, but conversion to SCC is reduced. TGFβ1+/+ and +/− mice were initiated with DMBA and promoted with TPA twice per week for 25 weeks. The number of papillomas >2 mm was determined on a weekly basis. (A) Papillomas per mouse from mice promoted with 5 μg (top) and 10 μg (middle) TPA and percent of mice with papillomas (bottom). The papilloma frequency was significantly higher in TGFβ1+/+ mice promoted with 5μg TPA (P = 0.0013) and 10 μg TPA (P = 0.01). At each dose, 21/28 and 20/24 TGFβ1+/+ and 13/20 and 19/20 TGFβ1+/− mice developed tumors. (B) Tumor volumes between weeks 20–30 were measured using a digital micrometer. Average volumes were determined from measurements of length × width × height, and statistical significance determined using a T-test to compare genotypes at each time point. (C) The total papilloma and SCC yield and percent conversion for each genotype. Percent conversion at each TPA dose was determined by dividing the total number of SCC that formed during the course of the study by the maximum number of papillomas; P, papilloma; C, carcinoma.
TPA-induced proliferation is reduced in epidermis and papillomas of TGFβ1+/− mice
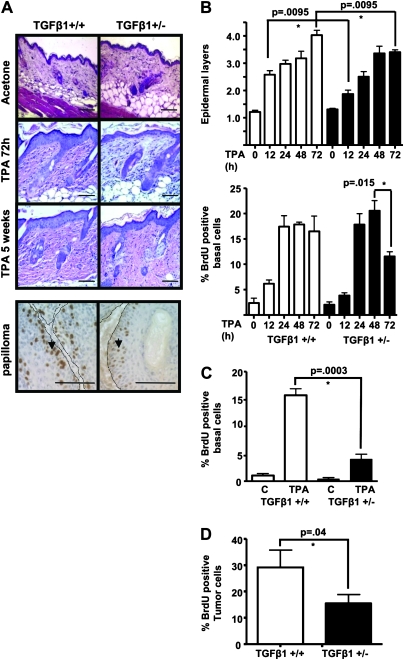
To test if TGFβ1 levels altered the epidermal response to TPA, TGFβ1+/+ and TGFβ1+/− mice were treated with TPA alone once or biweekly for 5 weeks. TPA-induced epidermal hyperplasia was significantly greater in the TGFβ1+/+ mice after 72 h of treatment (4.03 ± 0.17 versus 3.40 ± 0.08) (Figure 2A and B), and although the initial increase in epidermal proliferation was similar between the two genotypes, by 72 h, post-TPA epidermal proliferation had decreased significantly in the TGFβ1+/− mice (Figure 2B, bottom). Similarly, after chronic TPA treatment, there was a greater number of epidermal layers (Figure 2A) and higher epidermal labeling index (Figure 2C) in the TGFβ1+/+ mice compared with TGFβ1+/− mice, but no significant difference in TPA-induced hyperkeratosis. Papillomas generated in TGFβ1+/+ mice after DMBA and 10 week TPA promotion also had a significantly higher percentage of bromodeoxyuridine-positive tumor cells (29.1 ± 6.6) compared with TGFβ1+/− papillomas (15.4 ± 3.4) (Figure 2A and D). There was no difference in expression of the known TGFβ1 target gene p21waf1 (17) in response to TPA (data not shown). There was also no significant difference in TUNEL-positive epidermal keratinocytes between genotypes after acute (TGFβ1+/+ 2.55 ± 0.3% versus TGFβ1+/− 1.73 ± 0.3% at 72 h; P = 0.1) or chronic TPA treatment (TGFβ1+/+ 3.04 ± 0.1% versus TGFβ1+/− 2.25 ± 0.3%; P = 0.1).
Fig. 2.
TGFβ1 enhances TPA-induced proliferation in normal epidermis and tumors. (A) Top: representative hematoxylin-and eosin-stained sections of acetone or TPA-treated skin at 72 h post treatment and after 5 weeks chronic TPA treatment, magnification ×200. Bottom: detection of proliferating tumor cells (arrows) with anti-bromodeoxyuridine (BrdU) immunohistochemistry in TGFβ1+/+ and TGFβ1+/− 10 week papillomas. Magnification ×400. Tumor basement membrane indicated by dashed line. Scale bars represent 20 μm for all images. (B) Quantitation of epidermal hyperplasia (top) and proliferation (bottom) in TPA-treated TGFβ1+/+ mice compared with TGFβ1+/− mice. The number of cell layers in hematoxylin- and eosin-stained sections was determined every 20 basal cells along a section and averaged from 5 mice per group. BrdU-positive cells were quantitated from anti-BrdU-stained sections and averaged from 5 mice per time point for each genotype. (C) Quantitation of epidermal proliferation in TGFβ1+/+ and TGFβ1+/− skin after chronic TPA treatment. BrdU-positive epidermal keratinocytes were quantitated from mice treated twice per week with TPA or acetone (c) for 5 weeks (N = 5 mice per group). (D) Quantitation of tumor cell proliferation. BrdU-positive tumor cells were quantitated from papillomas generated in each genotype with DMBA and 10 weeks TPA promotion (N = 5 tumors per group). Papillomas were isolated 72 h after last TPA treatment. Asterisk represents significantly different from indicated group.
TGFβ1 response to TPA is reduced in TGFβ1+/− skin and keratinocytes
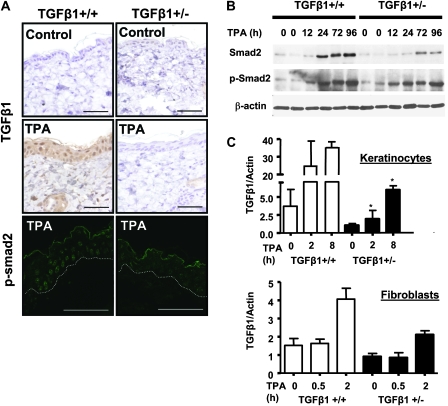
Since, surprisingly, a wild-type TGFβ1 genotype was associated with enhanced proliferative responses to TPA, we examined TGFβ1 induction, a well-characterized response to tumor promoters in the mouse epidermis (5,18). Twelve hours after TPA treatment, TGFβ1 protein levels were increased in all the layers of the TGFβ1+/+ epidermis consistent with published reports (19), but there was little detectable change in the TGFβ1+/− epidermis (Figure 3A). Similarly, levels of nuclear p-Smad2 and total Smad2 as detected by indirect immunofluorescence with two well-characterized antibodies (10–12) were reduced in TPA-treated TGFβ1+/− epidermal keratinocytes compared with TGFβ1+/+ keratinocytes (Figure 3A, bottom and Figure S1 is available at Carcinogenesis Online). In agreement, immunoblot analysis of total skin protein extracts in TGFβ1+/+ skin showed an increase in total Smad2 24 h post-TPA that was sustained through 96 h, whereas in the TGFβ1+/− skin, the increase in total Smad2 was reduced and delayed until 72 h (Figure 3B). Phosphorylation of Smad2 was seen 12 h after topical TPA treatment in both genotypes. However, p-Smad2 levels in the TGFβ1+/+ skin after 24 h and through 96 h were consistently higher than in the TGFβ1+/− skin, particularly at the later time points (Figure 3B). Although there was an induction of p-Smad2 from 12 to 96 h in the TPA-treated TGFβ1+/− skin (Figure 3B), it is possible that this is due to TGFβ1 pathway activation in non-epidermal cells since phospho-Smad2-positive cells were detected in the dermis of TPA-treated skin (Figures S1A and S2A are available at Carcinogenesis Online). Figure 3C shows that as expected for a hemizygous state, TGFβ1+/− keratinocytes had roughly 50% basal expression of TGFβ1 messenger RNA (mRNA) compared with TGFβ1+/+ keratinocytes. However, TPA treatment caused a rapid 9-fold increase in TGFβ1 mRNA in the TGFβ1+/+ keratinocytes but a slower 5-fold induction by 8 h in the TGFβ1+/− keratinocytes. In contrast, TPA caused a similar fold induction of TGFβ1 mRNA in primary dermal fibroblasts even though the absolute level was half in the heterozygote fibroblasts (Figure 3C). Keratinocytes from both genotypes responded similarly to exogenous TGFβ1 indicating that TGFβ1+/− keratinocytes do not have a general defect in ability to activate the TGFβ1 pathway (data not shown).
Fig. 3.
TPA induction of TGFβ1 and p-Smad2 nuclear localization is reduced in TGFβ1+/− epidermis. (A) Top and middle: immunohistochemical detection of TGFβ1 protein in skin 12 h after acetone or TPA treatment, (magnification ×400). Bottom: detection of phospho-Smad2 by indirect immunofluorescence with an anti-pSmad2 ser 465/467 antibody in tissue 24 h after TPA treatment. Magnification ×1000 and scale bars represent 20 μm. Exposure times for TGFβ1+/+ and TGFβ1+/− skins were identical. Figure S1A available at Carcinogenesis Online shows individual and merged images with TO-PRO 3 nuclear counterstaining. Location of the basement membrane is indicated by a dashed line. (B) Immunodetection of total Smad2 and p-Smad2 in whole skin protein extracts isolated at the indicated time points after TPA treatment using specific anti-Smad2/3 and p-Smad2 antibodies and β-actin as a loading control. (C) Quantitative reverse transcription–polymerase chain reaction analysis of TGFβ1 mRNA induction by TPA (25 ng/ml) in primary keratinocytes (top) and fibroblasts (bottom) of the indicated genotype. Results are the average of three independent experiments. Asterisk represents significantly different from TGFβ1+/+ (P < 0.05).
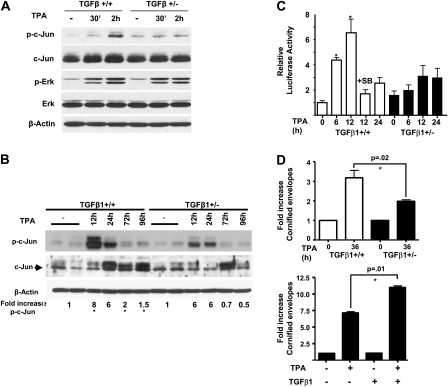
Activation of PKC and AP-1 pathway is reduced in TGFβ1+/− keratinocytes
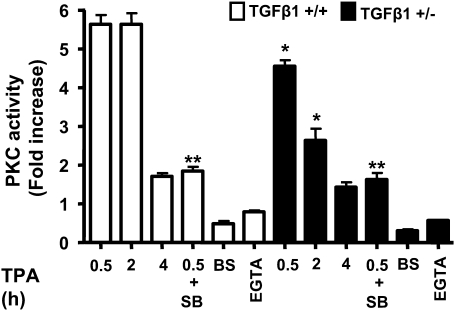
Since TPA effects are mediated primarily through PKC, we investigated if TGFβ1 levels influenced PKC activation in primary keratinocytes from each genotype. Within 30 min after TPA treatment, there was a 5.5-fold increase in PKC activity in the TGFβ1+/+ keratinocytes relative to basal levels that was sustained through 2 h post treatment but declined to near baseline by 4 h (Figure 4). In contrast, the maximal level of TPA-induced PKC activity was less and was not sustained in the TGFβ1+/− keratinocytes, suggesting more rapid downregulation of enzyme activity. Furthermore, pretreatment of keratinocytes of either genotype with the TGFβ1 type 1 receptor (ALK5) inhibitor SB431542 reduced PKC activity (Figure 4), supporting the idea that maximal PKC activity in keratinocytes is dependent on activation of TGFβ1 signaling. As expected, inclusion of the PKC inhibitor bisindolylmaleimide I (25 mM) and 2.5 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid blocked enzyme activity indicating that PKCα the major Ca2+ dependent PKC isoform in keratinocytes (20) was responsible (Figure 4). To examine the dependence of PKC signaling on TGFβ1 levels further, we analyzed signaling pathways downstream of PKC. There were no differences in TPA-induced phosphorylation of extracellular signal-regulated kinase 1/2 (Figure 5A), stress-activated protein kinase/c-jun N-terminal kinase or p-AKT levels between genotypes (data not shown). However, the TPA induction of c-jun phosphorylation, a known target of PKCα (20), was reduced in the TGFβ1+/− keratinocytes (Figure 5A) but there was no change in total c-jun levels. Similarly, TPA caused a rapid 8-fold induction of p-c-jun in the skin of the TGFβ1+/+ mice but the fold increase in p-c-jun was less and reduced to baseline levels more quickly in the TGFβ1+/− skin (Figure 5B). Since induction of TGFβ1 mRNA by TPA is mediated in part through activation of AP-1 (21,22), we tested whether a generic AP-1 target was similarly affected by reduced TGFβ1 levels. TPA induction of a transfected AP1-luciferase reporter was significantly reduced in TGFβ1+/− keratinocytes compared with TGFβ1+/+ keratinocytes (Figure 5C). No significant differences were found between treatments within the TGFβ1+/− cells or between untreated control cells (P > 0.5). This response was blocked by SB431542 (Figure 5C), supporting the involvement of the TGFβ1 signaling pathway. Interestingly, this difference between genotypes was not observed at higher TPA doses (data not shown), correlating with diminution of TGFβ1 genotype effects on tumor development at higher TPA doses. Finally, the induction of cornified envelopes, the end product of epidermal differentiation and a well-characterized response of keratinocytes to TPA dependent on AP-1 activity (23,24), was significantly higher in the TGFβ1+/+ keratinocytes (Figure 5D). In TGFβ1+/− keratinocytes, addition of exogenous TGFβ1 enhanced the induction of cornified envelopes by TPA (Figure 5D, P < 0.01) indicating a direct relationship between reduced TGFβ1 induction and altered differentiation in response to TPA in this genotype. Taken together these results indicate that maximal biological and biochemical responses to TPA in keratinocytes require physiological levels of TGFβ1.
Fig. 4.
TGFβ1 modulates PKC activation by TPA. PKC enzyme activity was measured at the indicated times after TPA treatment (25 ng/ml) in triplicate cultures of primary keratinocytes of each genotype and expressed as fold increase over untreated control (1751.5 ± 256 c.p.m. for TGFβ+/+ and 1642.2 ± 171.19 c.p.m. for TGFβ+/− cells). TGFβ1+/+ keratinocytes were also pretreated for 15 min with the small molecule ALK5 inhibitor SB431542 (0.5 μM) before addition of TPA, and PKC activity was measured after 30 min. Similar results were obtained in three independent experiments. Bisindolylmaleimide I (BS) and ethyleneglycol-bis(aminoethylether)-tetraacetic acid (EGTA) were included in the activity assay to inhibit total and calcium-dependent PKC activity, respectively. Asterisk represents significantly different from indicated group.
Fig. 5.
TGFβ1 regulates AP-1-mediated effects of TPA. (A) Reduced induction of c-jun phosphorylation in TGFβ1+/− keratinocytes. Primary keratinocytes of each genotype were treated with 25 ng/ml TPA and cell lysates immunoblotted for the indicated proteins using total and phospho-specific antibodies, and β-actin as a loading control. (B) Reduced induction of c-jun phosphorylation in TPA-treated TGFβ1+/− skin. TGFβ1+/+ and +/− mice were treated with 5 μg TPA or acetone and whole skin protein extracts were immunoblotted with anti-p-c-jun and total c-jun antibodies, and β-actin as a loading control. Detection of c-jun was confirmed using a positive control from primary keratinocytes (data not shown). The fold increase in c-Jun phosphorylation was averaged from densitometric measurement of band intensity from four different samples at each time point. Asterisk represents significantly different from TGFβ1+/− at corresponding time points P < 0.05. (C) Reduced AP-1 transactivation in TGFβ1+/− keratinocytes. Keratinocytes of each genotype were transfected with an AP-1 luciferase-reporter, treated with TPA (5 ng/ml) and 0.5 μM SB431542 (SB) where indicated and firefly luciferase activity normalized to that of renilla luciferase control. Data are representative of three independent experiments and each histogram is the average of five replicate transfections. Asterisk represents significantly different from other genotype. (D) Top: reduced TPA-induced terminal differentiation in TGFβ1+/− keratinocytes. Keratinocytes of each genotype were treated with TPA and cornified envelopes counted with a hemocytometer. Bottom, TGFβ1+/− were treated with TPA with or without 0.05 ng/ml TGFβ1 and cornified envelopes were quantitated. Average of three experiments is shown.
TGFβ1 reduces the TPA-induced skin inflammatory response
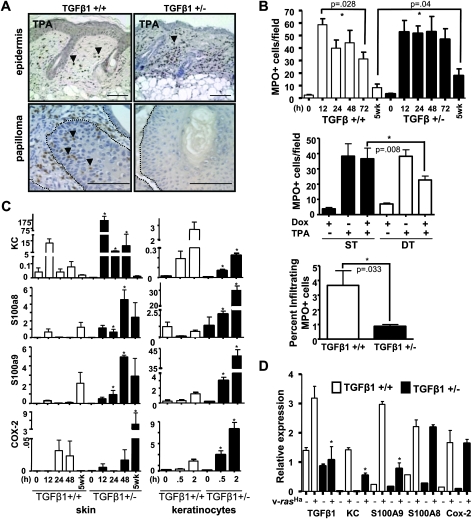
In addition to proliferation, inflammation is a critical component of the response to TPA. Dermal inflammation was detected as early as 12 h post-TPA in both TGFβ1+/+ and TGFβ1+/− mice and was mainly composed of MPO-positive cells (Figure 6A). In contrast to the proliferative response, there was a decrease in MPO+ cells by 72 h post-TPA in the TGFβ1+/+ skin that did not occur in the TGFβ1+/− mice (Figure 6A and B top), and there were twice as many dermal inflammatory cells in chronically TPA-treated TGFβ1+/− skin compared with TGFβ1+/+ skin (Figure 6B top). Additionally, to test if TGFβ1 expression could block TPA-induced cutaneous inflammation, we used a bitransgenic K5/rTA X tetOTGFβ1 mouse line in which the expression of a constitutively active form of TGFβ1 can be regulated by doxycycline with an epidermally targeted reverse tet transactivator (9). When these transgenic mice were dosed with doxycycline to induce TGFβ1 expression in the epidermis (2 mg intraperitoneally 24 h prior to topical TPA treatment), the number of skin infiltrating MPO+ cells was decreased by ∼50% compared with mice treated with TPA alone (Figure 6B middle) indicating that TGFβ1 could directly inhibit TPA-induced inflammation.
Fig. 6.
TGFβ1 suppresses inflammation in normal skin but enhances inflammation in tumors. (A) Detection of infiltrating inflammatory cells using anti-MPO immunohistochemistry in 72 h TPA-treated skin (top), magnification ×200 or 10 week papillomas (bottom), magnification ×400. Tumor basement membrane is indicated by dashed lines, arrows indicate tumor infiltrating MPO+ cells. Scale bars represent 20 μm. (B) Quantitation of cutaneous MPO+ cells after acute TPA treatment (top), after acute overexpression of TGFβ1 in K5/rTa X tetOTGFβ1 double transgenic (DT) or K5/rTa single transgenic (ST) mice (middle) and in TGFβ1+/+ and +/− 10 week papillomas (bottom). Histograms in top and middle panels represent the average MPO+ cells per field from five mice per group. The percentage of tumor infiltrating MPO+ cells was determined from five papillomas of each genotype relative to tumor cells. (C) Quantitative reverse transcription–polymerase chain reaction analysis of KC, S100a8, S100a9 and COX-2 gene expression in TPA-treated whole skin and primary keratinocytes. Average of three independent experiments is shown. (D) Quantitative reverse transcription–PCR analysis of TGFβ1, KC, S100a8, S100a9 and COX-2 gene expression in TGFβ1+/+ and +/− control and v-rasHa oncogene expressing primary keratinocytes. Average relative expression values of three independent experiments are shown. Gene expression in C and D was normalized to β-actin. Asterisk represents significantly different from TGFβ1+/+ P < 0.05.
The difference in cutaneous inflammation between genotypes was mirrored by expression of pro-inflammatory genes. We analyzed the expression of pro-inflammatory and neutrophil chemotactic molecules such as S100a8, S100a9 and KC (Cxcl1) (25,26) and COX-2 that regulates inflammatory responses through prostaglandin and thromboxane metabolism (27). Although transcripts for the KC, S100a8 and S100a9 were induced in both genotypes, the levels were sustained and significantly higher in the TGFβ1+/− skin for at least 48 h post-TPA, but these decayed by 24 h in the TGFβ1+/+ skin (Figure 6C). No change in another S100 family member, S100a11 was seen in either genotype after TPA treatment (data not shown). With chronic TPA treatment, the expression of COX-2 and S100a8 was significantly higher in the TGFβ1+/−-treated skin but there was no significant difference in KC or S100a9 between genotypes (Figure 6C). Similarly, in primary TGFβ1+/− keratinocytes, the absolute level and fold induction of COX-2, S100a8 and S100a9 was higher compared with TGFβ1+/+ keratinocytes (Figure 6C). Thus, reduced levels of TGFβ1 directly enhanced TPA-induced pro-inflammatory gene expression in keratinocytes. Although the absolute expression levels were lower in isolated fibroblasts, the TPA-driven induction of COX-2, S100a8 and S100a9 expression was transient in TGFβ1+/+ fibroblasts but sustained in TGFβ1+/− fibroblasts, resembling the difference in expression pattern between keratinocytes of each genotype (Figure S2B is available at Carcinogenesis Online). Although TPA induced KC expression in TGFβ1+/− keratinocytes, the pattern of expression between the genotypes did not match that observed in whole TPA-treated skin (Figure 6C). However, the expression pattern of KC in TPA-treated fibroblasts was similar to that in whole skin (Figure S2B is available at Carcinogenesis Online), suggesting that responses from fibroblasts could be responsible for the higher induction of this chemokine in whole TGFβ1+/− skin.
Elevated inflammatory response in TGFβ1+/+ tumors
In contrast to TPA-treated normal skin, papillomas that developed after 10 weeks of promotion had approximately four times as many tumor infiltrating MPO+ cells in the TGFβ1+/+ papillomas (3.7 ± 1 MPO+ cells per 100 tumor cells) compared with the TGFβ1+/− papillomas (0.86 ± 0.13 MPO+ cells per 100 tumor cells) (Figure 6A and B, bottom). In the two-stage skin chemical carcinogenesis model with DMBA as the initiating carcinogen, mutations at codon 61 in the c-rasHa gene occur at an extremely high frequency in papillomas and carcinomas (28,29). Similarly, introduction of oncogenic v-ras into primary keratinocytes with a replication-defective retrovirus generates a benign tumor cell phenotype in vitro and in vivo following skin grafting of transduced keratinocytes (30,31). Thus, to determine if ras activation altered effects of TGFβ1 on the inflammatory response, we examined pro-inflammatory cytokine gene expression in primary keratinocytes of both genotypes transduced with the v-rasHa retrovirus. As expected TGFβ1 expression is higher in normal and v-rasHa expressing TGFβ1+/+ keratinocytes (Figure 6D). While there was little difference in expression of S100a9 or KC between primary keratinocytes of each genotype, there were 5-fold higher level of S100a9 and 3-fold higher level of KC in v-rasHa expressing TGFβ1+/+ keratinocytes. There was no effect of v-rasHa on the expression of S100a8 and COX-2 between genotypes (Figure 6D). Thus, in the context of ras oncogene activation and developing papillomas, TGFβ1 acts as a pro-inflammatory cytokine.
Discussion
In the two-stage skin carcinogenesis model, repeated application of the phorbol ester tumor promoter TPA drives expansion of initiated clones of keratinocytes by enhancing proliferation and creating a chronic inflammatory environment (32). Many studies detailing the growth inhibitory and tumor suppressive actions of TGFβ1 support the idea that the induction of TGFβ1 by tumor promoters in keratinocytes is a negative feedback pathway to re-establish tissue homeostasis (13,33). Indeed, several skin-targeted TGFβ1 transgenic models show that overexpression of this growth factor can inhibit tumor promotion and epidermal proliferation (2,34) as well as enhance cutaneous inflammation (7,9). However, using a model of TGFβ1 haploinsufficiency, we find that TGFβ1+/− mice developed fewer benign tumors with reduced incidence and size compared with TGFβ1+/+ mice. Furthermore, we observed that the proliferative response to the tumor promoter TPA was less in the epidermis and papillomas that formed in TGFβ1+/− mice, consistent with the resistance of Smad3 null mice to skin carcinogenesis (7). In contrast, TPA-induced inflammation was reduced in TGFβ1+/+ skin but enhanced in TGFβ1+/+ papillomas. Taken together these results suggest that physiological levels of TGFβ1 play an important positive role in tumor promotion by paradoxically enhancing epidermal proliferation and limiting cutaneous inflammatory responses to a tumor promoting stimulus in normal skin, but stimulating inflammation within a developing tumor. Since the difference in tumor number and incidence between genotypes was reduced at higher TPA doses, tissue levels of TGFβ1 are probably to play a determinative role in tumor development at suboptimal promoter doses and could positively impact human tumor development under conditions of weak or intermittent chronic promoting stimuli.
In keratinocytes, PKCα is one of the major targets for TPA during tumor promotion (30,35). Our data showing transient activation of PKC, transient phosphorylation of downstream targets such as c-jun, reduced induction of TGFβ1 an AP-1 target gene (21,36) and AP-1 luciferase activity in TGFβ1+/− keratinocytes as well as suppression of PKC activation and AP-1 luciferase activity by the ALK5 inhibitor SB431542 in wild-type keratinocytes suggest that TGFβ1 directly influences the extent of PKC activation in response to TPA. These data also show that TGFβ1 does not influence initial activation of PKC but rather pathways regulating sustained activity or downregulation, such that the signal strength from PKC activation is diminished. Previous reports have linked PKCα and PKCδ activation by TGFβ1 to p21waf1 induction, collagen I expression and phosphatase and tensin homolog transcriptional downregulation (37–39) but we did not observe altered expression of p21 between genotypes. Our results suggest that regulation of AP-1 activity is a key PKC target that is modulated by TGFβ levels. Previous studies have shown that induction of TGFβ1 mRNA and protein is a rapid response of the epidermis to TPA (5,19). Our results suggest that the induction of TGFβ1 in response to TPA may be important for the cornification response as this was reduced in TGFβ1+/− keratinocytes and enhanced in these cells with addition of exogenous TGFβ1. Although we did not observe a significant difference in hyperkeratosis in response to TPA between the genotypes, the reduced formation of cornified envelopes in vitro in the TPA-treated TGFβ1+/− keratinocytes suggests that the observed differences in proliferation and hyperplasia may be indirectly linked to TPA-induced terminal differentiation and epidermal turnover, which is mediated in part in keratinocytes by AP-1 (40,41). Further studies will be required to explore this possibility in more detail.
TGFβ1 is known to have both pro-inflammatory and anti-inflammatory properties in the skin and other tissues (42). In contrast to the reduced proliferative response in the TGFβ1+/− papillomas and epidermis, the TPA-induced inflammatory response in normal skin, as measured by total inflammatory infiltrate, MPO+ cells number and expression of pro-inflammatory cytokines in vivo and in primary keratinocytes in vitro, was greater and sustained over a longer time period in TGFβ1+/− mice compared with TGFβ1+/+ mice. Thus, in the normal epidermis, TGFβ1 acts to suppress inflammation and this is probably to be a direct effect since overexpression of TGFβ1 reduced TPA-associated inflammation. Since AP-1 can repress transcription of S100a8 and S100a9 genes in keratinocytes (43), it is possible that the rapid and increased induction of these pro-inflammatory mediators by TPA in TGFβ1+/− keratinocytes is due to reduced AP-1 activity. However, we observed that TGFβ1+/+ early papillomas had abundant intraepithelial MPO+ cells, whereas there were virtually none in the TGFβ1+/− lesions. Thus, in the context of a developing papilloma with probably ras oncogene activation elevated TGFβ1 production by keratinocytes contributes to a pro-inflammatory environment. Supporting this pro-inflammatory switch, we found that v-rasHa-transduced TGFβ1+/+ keratinocytes expressed elevated levels of the proinflammatory genes S100a9 and KC (25) as well as TGFβ1, relative to v-rasHa-transduced TGFβ1+/− keratinocytes. The neutrophil chemotactic properties of TGFβ1 (44,45) as well as these additional pro-inflammatory cytokines could contribute to the pro-inflammatory switch in developing tumors, although additional factors must also allow infiltration of inflammatory cells into the epithelial component of the papilloma, as this was never observed in TPA-treated normal skin of either genotype. While the data presented here provide strong evidence that the observed differential proliferative and inflammatory responses are due in part to reduced TGFβ1 expression in keratinocytes, we cannot rule out the influence of reduced TGFβ1 levels in fibroblasts and inflammatory cells as contributing to the observed responses in the intact animal. Tissue specific knockout of TGFβ1 in keratinocytes and other cutaneous cell types will be needed to directly test their role in proliferative and inflammatory responses associated with tumor promotion. Nevertheless, these data point to TGFβ1-mediated compartmentalization of inflammatory responses between normal skin and expanding clones of initiated keratinocytes as important factors for tumor promotion.
Despite the increased number of benign tumors, there was no increase in SCC in the TGFβ1+/+ compared with the TGFβ1+/− mice at either promoter dose. Thus, the additional papillomas that developed in the TGFβ1+/+ mice are at low risk for malignant conversion. Studies in SENCAR mice show that the majority of papillomas do not progress to SCC, whereas a much smaller subpopulation is the precursors to SCC (46). These high-risk papillomas are characterized by reduced or absent expression of TGFβ1 relative to low-risk papillomas (47) and a reduced inflammatory gene expression signature similar to SCC (48). While the TGFβ1-associated increase in tumor cell proliferation and inflammation is linked to tumor outgrowth, the latter may suppress premalignant progression. In contrast, reduced levels of tumor-associated TGFβ1 may prevent inflammatory responses but enhance genetic instability (47,49,50) leading to more rapid malignant progression. Therefore, even at the earliest stages, physiological levels of TGFβ1 play a paradoxical role in cancer by enhancing tumor promotion and tumor outgrowth, but inhibiting premalignant progression.
Supplementary material
Supplementary Figures S1 and S2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (RO1 CA117957, CA122109) to A.B.G; intramural program of the National Cancer Institute.
Supplementary Material
Acknowledgments
We thank Dr Mitchell Denning, Loyola University Chicago School of Medicine, and Dr Luowei Li, National Cancer Institute, for helpful suggestions with the PKC assay and Matthew Licata for technical assistance. Digital and Confocal microscopy was done at the Cytometry Facility at the Huck Institutes of the Life Sciences, Penn State University.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AP-1
Activator protein 1
- DMBA
7,12-dimethylbenz[a]anthracene
- MPO
myeloperoxidase
- mRNA
messenger RNA
- PKC
protein kinase C
- SCC
squamous cell carcinoma
- TGFβ1
transforming growth factor β1
- TPA
12-O-tetradecanoylphorbol 13-acetate
References
- 1.Glick AB. TGFbeta1, back to the future: revisiting its role as a transforming growth factor. Cancer Biol. Ther. 2004;3:276–283. doi: 10.4161/cbt.3.3.849. [DOI] [PubMed] [Google Scholar]
- 2.Cui W, et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 3.Weeks BH, et al. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001;61:7435–7443. [PubMed] [Google Scholar]
- 4.Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. Eur. J. Cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Akhurst RJ, et al. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988;331:363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- 6.Tang B, et al. Transforming growth factor-β is a new form of tumor suppressor with true haploid insufficiency. Nat. Med. 1998;4:802–807. doi: 10.1038/nm0798-802. [DOI] [PubMed] [Google Scholar]
- 7.Li AG, et al. Smad3 knockout mice exhibit a resistance to skin chemical carcinogenesis. Cancer Res. 2004;64:7836–7845. doi: 10.1158/0008-5472.CAN-04-1331. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni AB, et al. Transforming growth factor-β1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA. 1992;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, et al. Conditional epidermal expression of TGFbeta 1 blocks neonatal lethality but causes a reversible hyperplasia and alopecia. Proc. Natl Acad. Sci. USA. 2001;98:9139–9144. doi: 10.1073/pnas.161016098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banas MC, et al. Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney. J. Histochem. Cytochem. 2007;55:275–285. doi: 10.1369/jhc.6A7083.2006. [DOI] [PubMed] [Google Scholar]
- 11.Li J, et al. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cells. 2007;25:697–706. doi: 10.1634/stemcells.2006-0133. [DOI] [PubMed] [Google Scholar]
- 12.Tait LR, et al. Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts. Int. J. Cancer. 2007;120:2127–2134. doi: 10.1002/ijc.22572. [DOI] [PubMed] [Google Scholar]
- 13.Glick AB, et al. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 1994;8:2429–2440. doi: 10.1101/gad.8.20.2429. [DOI] [PubMed] [Google Scholar]
- 14.Nakadate T, et al. Comparison of protein kinase C functional assays to clarify mechanisms of inhibitor action. Biochem. Pharmacol. 1988;37:1541–1545. doi: 10.1016/0006-2952(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 15.Rice RH, et al. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977;11:417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- 16.Rozen S, et al. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 17.Datto MB, et al. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patamalai B, et al. Altered expression of transforming growth factor-beta 1 mRNA and protein in mouse skin carcinogenesis. Mol. Carcinog. 1994;9:220–229. doi: 10.1002/mc.2940090406. [DOI] [PubMed] [Google Scholar]
- 19.Escherick JS, et al. Transforming growth factor β1 induction is associated with transforming growth factors β2 and β3 down-modulation in 12- O-tetradecanoylphorbol-13-acetate-induced skin hyperplasia. Cancer Res. 1993;53:5517–5522. [PubMed] [Google Scholar]
- 20.Mellor H, et al. The extended protein kinase C superfamily. Biochem. J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SJ, et al. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J. Biol. Chem. 1989;264:19373–19378. [PubMed] [Google Scholar]
- 22.Tsubouchi Y, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem. Biophys. Res. Commun. 2000;270:400–405. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
- 23.Hawley-Nelson P, et al. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp. Cell Res. 1982;137:155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- 24.Yuspa SH, et al. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982;42:2344–2349. [PubMed] [Google Scholar]
- 25.Ryckman C, et al. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 26.Tessier PA, et al. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 27.Williams CS, et al. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 28.Balmain A, et al. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307:658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- 29.Harper JR, et al. Analysis of the rasHa oncogene and its p21 product in chemically induced skin tumors and tumor-derived cell lines. Carcinogenesis. 1987;8:1821–1825. doi: 10.1093/carcin/8.12.1821. [DOI] [PubMed] [Google Scholar]
- 30.Dlugosz AA, et al. Alterations in murine keratinocyte differentiation induced by activated rasHa genes are mediated by protein kinase C-alpha. Cancer Res. 1994;54:6413–6420. [PubMed] [Google Scholar]
- 31.Roop DR, et al. An activated Harvey ras oncogene produces benign tumours on mouse epidermal tissue. Nature. 1986;323:822–824. doi: 10.1038/323822a0. [DOI] [PubMed] [Google Scholar]
- 32.Lewis JG, et al. Early inflammatory changes in the skin of SENCAR and C57BL/6 mice following exposure to 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1987;8:889–898. doi: 10.1093/carcin/8.7.889. [DOI] [PubMed] [Google Scholar]
- 33.Go C, et al. Blocking transforming growth factor β signaling in transgenic epidermis accelerates chemical carcinogenesis: a mechanism associated with increased angiogenesis. Cancer Res. 1999;59:2861–2868. [PubMed] [Google Scholar]
- 34.Blessing M, et al. Chemical skin carcinogenesis is prevented in mice by the induced expression of a TGF-β related transgene. Teratog. Carcinog. Mutagen. 1995;15:11–21. doi: 10.1002/tcm.1770150103. [DOI] [PubMed] [Google Scholar]
- 35.Breitkreutz D, et al. Protein kinase C family: on the crossroads of cell signaling in skin and tumor epithelium. J. Cancer Res. Clin. Oncol. 2007;133:793–808. doi: 10.1007/s00432-007-0280-3. [DOI] [PubMed] [Google Scholar]
- 36.Tsunobuchi H, et al. Expressions of inhibitory Smads, Smad6 and Smad7, are differentially regulated by TPA in human lung fibroblast cells. Biochem. Biophys. Res. Commun. 2004;316:712–719. doi: 10.1016/j.bbrc.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 37.Runyan CE, et al. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am. J. Physiol. Renal Physiol. 2003;285:F413–F422. doi: 10.1152/ajprenal.00082.2003. [DOI] [PubMed] [Google Scholar]
- 38.Kim YK. TGF-beta1 induction of p21WAF1/cip1 requires smad-independent protein kinase C signaling pathway. Arch. Pharm. Res. 2007;30:739–742. doi: 10.1007/BF02977636. [DOI] [PubMed] [Google Scholar]
- 39.Chow JY, et al. TGF-beta mediates PTEN suppression and cell motility through calcium-dependent PKC-alpha activation in pancreatic cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G899–G905. doi: 10.1152/ajpgi.00411.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutberg SE, et al. Differentiation of mouse keratinocytes is accompanied by PKC-dependent changes in AP-1 proteins. Oncogene. 1996;13:167–176. [PubMed] [Google Scholar]
- 41.Rutberg SE, et al. Opposing activities of c-Fos and Fra-2 on AP-1 regulated transcriptional activity in mouse keratinocytes induced to differentiate by calcium and phorbol esters. Oncogene. 1997;15:1337–1346. doi: 10.1038/sj.onc.1201293. [DOI] [PubMed] [Google Scholar]
- 42.Glick A, et al. Context dependent regulation of cutaneous immunological responses by TGF{beta}1 and its role in skin carcinogenesis. Carcinogenesis. 2007;29:9–14. doi: 10.1093/carcin/bgm215. [DOI] [PubMed] [Google Scholar]
- 43.Gebhardt C, et al. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene. 2002;21:4266–4276. doi: 10.1038/sj.onc.1205521. [DOI] [PubMed] [Google Scholar]
- 44.Ashcroft GS, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response [see comments] Nat. Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 45.Parekh T, et al. Neutrophil chemotaxis in response to TGF-beta isoforms (TGF-beta 1, TGF-beta 2, TGF-beta 3) is mediated by fibronectin 20. J. Immunol. 1994;152:2456–2466. [PubMed] [Google Scholar]
- 46.Glick A, et al. The high-risk benign tumor: evidence from the two-stage skin cancer model and relevance for human cancer. Mol. Carcinog. 2007;46:605–610. doi: 10.1002/mc.20345. [DOI] [PubMed] [Google Scholar]
- 47.Glick A, et al. Defects in transforming growth factor-β signaling cooperate with a ras oncogene to cause rapid aneuploidy and malignant transformation of mouse keratinocytes. Proc. Natl Acad. Sci. USA. 1999;96:14949–14954. doi: 10.1073/pnas.96.26.14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darwiche N, et al. Expression profile of skin papillomas with high cancer risk displays a unique genetic signature that clusters with squamous cell carcinomas and predicts risk for malignant conversion. Oncogene. 2007;26:6885–6895. doi: 10.1038/sj.onc.1210491. [DOI] [PubMed] [Google Scholar]
- 49.Glick AB, et al. Transforming growth factor-β1 suppresses genomic instability independent of a G1 arrest, p53 and Rb. Cancer Res. 1996;56:3645–3650. [PubMed] [Google Scholar]
- 50.Kirshner J, et al. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 2006;66:10861–10869. doi: 10.1158/0008-5472.CAN-06-2565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.