Abstract
Progress toward a highly convergent, asymmetric synthesis of brevenal is reported. Construction of the AB-ring and E-ring cyclic ether fragments was achieved through asymmetric alkylation/ring-closing metathesis strategies. A Horner-Wadsworth-Emmons olefination was used in a key bond-forming step to couple the advanced cyclic fragments and enable rapid access to the AB-E ring system.
Marine polycyclic ether natural products have received much attention over the years due to their unique and highly complex molecular architecture, in addition to their diverse and potent biological activities.1 A number of bioactive polyether natural products have been isolated from the marine dinoflagellate Karenia brevis, the organism responsible for toxic red tides along Florida's Gulf Coast. The most well-known compounds isolated from K. brevis are a family of neurotoxins called the brevetoxins. The brevetoxins are responsible for massive kills of fish and marine animals and can also adversely affect humans; inhaled brevetoxins cause respiratory irritation and breathing difficulties in sensitive populations.2 At high concentrations, ingested brevetoxins lead to a collection of symptoms commonly referred to as neurotoxic shellfish poisoning (NSP).3 Brevenal (1) was isolated in 2004 from K. brevis and has been shown to counteract the toxic effects of the brevetoxins.4 Specifically, brevenal has been shown to competitively displace titrated dihydrobrevetoxin-B from voltage sensitive sodium channels in rat brain synaptosomes in a dose-dependent manner, alleviating the toxic effects of the brevetoxins in vivo.5 More importantly, picomolar concentrations of brevenal were found to increase tracheal mucus velocity to the same degree as that observed with millimolar concentrations of a sodium channel blocker, amiloride, which is used in the treatment of the debilitating lung disorder cystic fibrosis.6 As such, brevenal represents a potential lead for the development of novel therapeutic agents for the treatment of mucociliary dysfunction associated with cystic fibrosis and other lung disorders. To date, two syntheses of brevenal have been reported by the laboratories of Sasaki7 and Yamamoto.8
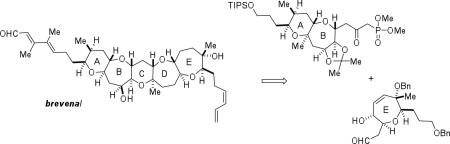
Our retrosynthetic analysis of brevenal includes the installation of the unsaturated side chains at both ends of the molecule late in the synthesis (Scheme 1). The pentacyclic polyether core was envisioned to arise from advanced enone 3 via an acid-catalyzed cyclodehydration to form the D-ring (2) followed by cyclization to afford the C-ring. Enone 3 would be made available through a Horner-Wadsworth-Emmons coupling between AB-ring β-ketophosphonate 4 and E-ring aldehyde 5.
Scheme 1.
Retrosynthetic analysis.
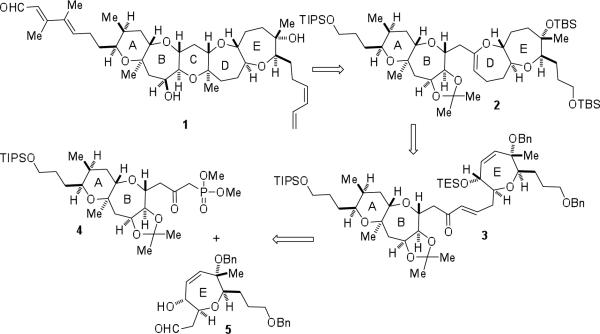
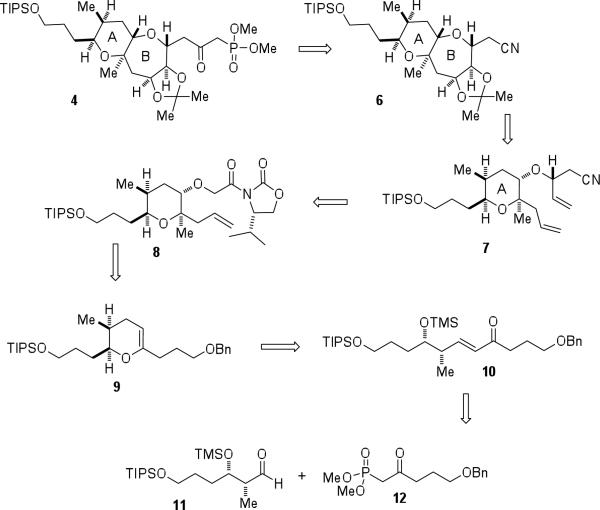
Initial efforts toward the total synthesis of brevenal focused on the construction of AB-ring β-ketophosphonate 4. The retrosynthesis of 4 is centered on a glycolate alkylation/ring-closing metathesis approach, developed in our laboratory, for the enantioselective construction of medium-ring ethers (Scheme 2).9 It was envisioned that the β-ketophosphonate functionality would be installed in three steps from nitrile 6. Access to the 6,7-fused system would hinge on closure of the medium-ring ether via a ring-closing metathesis reaction of diene 7. Stereoselective alkylation of glycolate 8 followed by conversion of the oxazolidinone to the olefin would afford advanced diene 7. The precursor to glycolate 8, enol ether 9, would be accessed from 10 via a cyclodehydration reaction. A Horner-Wadsworth-Emmons coupling of aldehyde 11 and β-ketophosphonate 12 would give rise to 10.
Scheme 2.
Retrosynthetic plan for β-ketophosphonate 4.
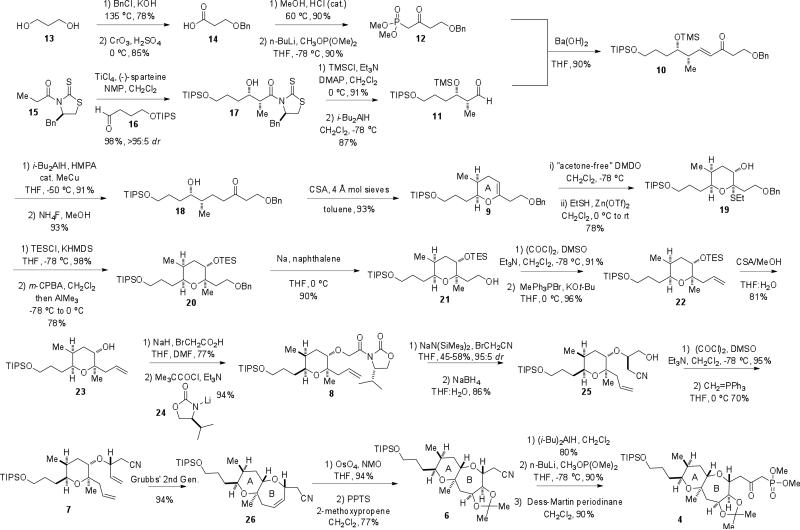
The synthesis commenced with a monobenzylation of commercially available 1,4-butanediol10 (13) followed by a Jones’ oxidation11 to afford carboxylic acid 14 (Scheme 3). Conversion to the methyl ester under acidic conditions followed by displacement with lithiodimethyl methylphosphonate12 then furnished β-ketophosphonate 12 in excellent yield over the four steps. The preparation of aldehyde 11 began with a highly diastereoselective “Evans” syn propionate aldol addition13 between phenylalanine-derived N-propionylthiazolidinethione 15 and aldehyde 16. The resultant aldol adduct 17 was protected as the trimethylsilyl (TMS) ether, and reductive removal of the auxiliary furnished the desired aldehyde 11.
Scheme 3.
Synthesis of AB-ring β-ketophosphonate 4.
With both aldehyde 11 and β-ketophosphonate 12 prepared, efforts were directed toward the completion of the AB-ring fragment. Enone 10 was prepared in 90% yield under mild reaction conditions using a modified Horner-Wadsworth-Emmons olefination.14 1,4-reduction of 1015 followed by deprotection of the TMS group generated hydroxy ketone 18. After screening a variety of cyclodehydration conditions, it was discovered that camphorsulfonic acid (CSA) in the presence of molecular sieves afforded enol ether 9 in excellent yield. Enol ether 9 was then transformed to thioacetal 19 via an epoxide intermediate,16 and the resulting hydroxyl group was protected as the triethylsilyl (TES) ether. The thioethyl group was converted to a methyl group in onepot, using Sasaki's protocol, to give pyran 20.17 It was found that protection of the hydroxyl group was necessary for this reaction to proceed in high yield.
With the necessary A-ring functionality in place, construction of the B-ring then ensued. The benzyl group was cleaved in the presence of sodium naphthalenide18 to generate alcohol 21. Swern oxidation19 of the resultant hydroxyl group, followed by a methylene Wittig reaction,20 afforded olefin 22. The TES ether was then cleaved with CSA and ethanol to give alcohol 23. At this point, our glycolate alkylation/ring-closing metathesis strategy was applied to construct the seven-membered B-ring. Alcohol 23 was transformed to the glycolic acid in the presence of sodium hydride and bromoacetic acid. In one pot, the resultant acid was converted to the mixed pivalic anhydride and treated in situ with the lithium salt of (R)-4-isopropyl-2-oxazolidinone to produce acyl oxazolidinone 8. Treatment of the sodium enolate of 8 with bromoacetonitrile resulted in a highly diastereo-selective glycolate alkylation21 to furnish the nitrile. The modest yield associated with this reaction is attributed to the formation of an unidentifiable byproduct formed during the enolization process. All attempts to optimize this reaction, however, were unsuccessful. The oxazolidinone was then reductively cleaved to reveal alcohol 25. Oxidation of 25 to the aldehyde under Swern conditions occurred in high yield. Initially, the resulting aldehyde was subjected to a methylene Wittig reaction using an ylide generated in situ from potassium t-butoxide and methyltriphenylphosphonium bromide. The presence of the nitrile, however, facilitated an elimination reaction under these rather basic conditions, thus regenerating alcohol 23. To circumvent this undesired elimination, the methylenation reaction was performed using crystallized, salt-free methylene triphenylphosphorane, thereby giving rise to the desired diene 7.22 B-Ring formation was achieved upon exposure of 7 to Grubbs’ second generation catalyst,23 affording bicycle 26 in 94%. Dihydroxylation of the resultant olefin occurred readily to furnish the desired diol as a single diastereomer. Subsequent protection of the diol afforded advanced acetonide 6. Diisobutylaluminum hydride easily reduced the nitrile to the aldehyde. In two steps, 6 was converted to β-ketophosphonate 4 to complete the AB-ring fragment with 27 steps in the longest linear sequence. The stereochemistry was confirmed by nOESY analysis.
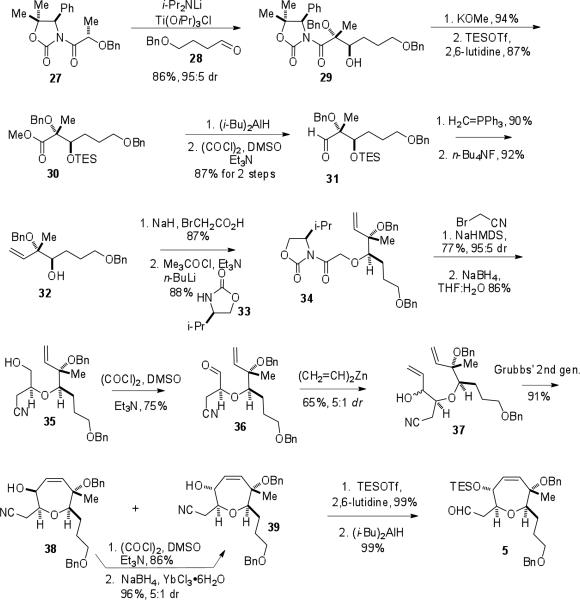
With the AB-ring β-ketophosphonate in hand, efforts then focused on the synthesis of the E-ring aldehyde (5, Scheme 4). An asymmetric aldol addition, originally reported by Kobayashi and coworkers,24 between oxazolidinone 27 and aldehyde 28 was used to prepare aldol adduct 29. The auxiliary was converted to the methyl ester and the free hydroxyl group was protected as the TES ether to give ester 30. Conversion of 30 to aldehyde 31 was carried out using a two step reduction-oxidation sequence. After generation of a terminal olefin from the aldehyde functionality through a Wittig reaction, the TES group was removed to furnish alcohol 32. Alkylation of 32 with bromoacetic acid followed by coupling with a valine-derived oxazolidinone enabled isolation of glycoyl oxazolidinone 34. Generation of the sodium enolate of 34 followed by alkylation with bromoacetonitrile introduced the third stereogenic center with excellent diastereoselectivity (95:5 dr). Reductive removal of the auxiliary then furnished alcohol 35, and the resultant hydroxyl group was easily oxidized to aldehyde 36 under Swern conditions. Treatment of 36 with divinyl zinc installed the second olefin as a mixture of diastereomers (37). Exposure of this diastereomeric mixture to Grubbs’ second generation catalyst enabled the isolation of cyclic ethers 38 and 39. The undesired diastereomer 38 could be converted to 39 via a two step oxidation/reduction sequence. Protection of 39 as a TES ether and reduction of the nitrile generated E-ring aldehyde 5 in 16 steps from 27.
Scheme 4.
Synthesis of aldehyde 5.
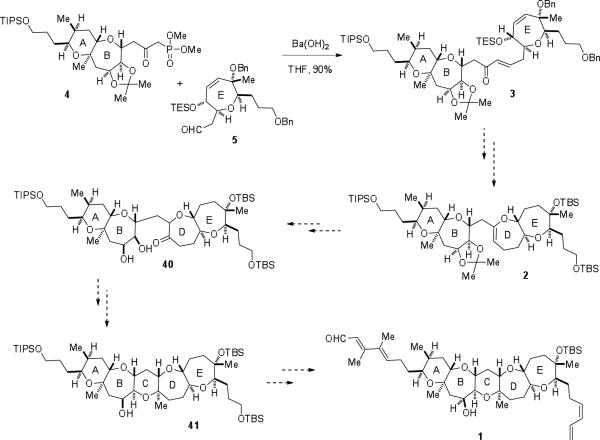
With both the AB-ring β-ketophosphonate and E-ring aldehyde prepared, attention was directed toward coupling of the fragments and completing brevenal (Scheme 5). β-Ketophosphonate 4 and aldehyde 5 were joined through a barium hydroxide-mediated Horner-Wadsworth-Emmons reaction to provide advanced enone 3 in excellent yield. In order to complete the total synthesis of brevenal, we envision the closure of the D-ring via an acid-catalyzed cyclodehydration. Manipulation of the resulting enol ether (2) to give ketone 40 followed by formation of the C-ring via acetalization will give the pentacyclic polyether core (41). Installation of the left- and right-hand side chains will then furnish brevenal (1).
Scheme 5.
Coupling of the fragments and proposed endgame.
In summary, a highly convergent approach toward the total synthesis of brevenal has been reported. Two key cyclic ether fragments have been constructed utilizing our asymmetric glycolate alkylation/ring-closing metathesis approach. Fragment coupling has been carried out in excellent yield and efforts to complete the carbon framework and elaborate the side chains are ongoing.
Supplementary Material
Acknowledgment
Financial support from the National Institute of General Medical Sciences (GM60567) is gratefully acknowledged.
Footnotes
Supporting Information Available: Experimental details and spectral data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Yasumoto T, Murata M. Chem. Rev. 1993;93:1897–1909. [Google Scholar]; b Murata M, Yasumoto T. Nat. Prod. Rep. 2000;17:293–314. doi: 10.1039/a901979k. [DOI] [PubMed] [Google Scholar]; c Nakata T. Chem. Rev. 2005;105:4314–4347. doi: 10.1021/cr040627q. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart GD, Baden DG. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poli MA, Mende TJ, Dickey RW, Elliers PP, Hall S. Toxicon. 2000;38:981–993. doi: 10.1016/s0041-0101(99)00191-9. [DOI] [PubMed] [Google Scholar]
- 4.Bourdelais AJ, Jacocks HM, Wright JLC, Bigwarfe PM, Baden DG. J. Nat. Prod. 2005;68:2–6. doi: 10.1021/np049797o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JLC, Carsi J, Baden DG. Cell. Mol. Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sayer A, Hu Q, Bourdelais AJ, Baden DG, Gibson JE. Arch. Toxicol. 2005;79:683–688. doi: 10.1007/s00204-005-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham WM, Bourdelais AJ, Sabater JR, Ahmed A, Lee TA, Serebriakov I, Baden DG. Am. J. Respir. Crit. Care Med. 2005;171:26–34. doi: 10.1164/rccm.200406-735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a Ebine M, Fuwa H, Sasaki M. Org. Lett. 2008;10:2275–2278. doi: 10.1021/ol800685c. [DOI] [PubMed] [Google Scholar]; b Fuwa H, Ebine M, Bourdelais AJ, Baden DG, Sasaki M. J. Am. Chem. Soc. 2006;128:16989–16999. doi: 10.1021/ja066772y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takamura H, Kikuchi S, Nakamura Y, Yamagami Y, Kishi T, Kadota I, Yamamoto Y. Org. Lett. 2009;11:2531–2534. doi: 10.1021/ol900769d. [DOI] [PubMed] [Google Scholar]
- 9.a Crimmins MT, Emmitte KA. Org. Lett. 1999;1:2029–2032. doi: 10.1021/ol991201e. [DOI] [PubMed] [Google Scholar]; b Crimmins MT, Tabet EA. J. Am. Chem. Soc. 2000;122:5473–5476. [Google Scholar]
- 10.Butler CL, Clapp MJ. J. Am. Chem. Soc. 1938;60:1472–1473. [Google Scholar]
- 11.Davis FA, Kasu PVN, Sundarababu G, Qi HY. J. Org. Chem. 1997;62:7546–7547. [Google Scholar]
- 12.Delamarche I, Mosset P. J. Org. Chem. 1994;59:5453–5457. [Google Scholar]
- 13.Crimmins MT, King BW, Tabet EA, Chaudhary K. J. Org. Chem. 2001;66:894–902. doi: 10.1021/jo001387r. [DOI] [PubMed] [Google Scholar]
- 14.Alvarezibarra C, Arias S, Banon G, Fernandez MJ, Rodriguez M, Sinisterra VJ. J. Chem Soc. Chem. Commun. 1987:1509–1511. [Google Scholar]
- 15.Tsuda T, Hayashi T, Satomi H, Kawamoto T, Saegusa T. J. Org. Chem. 1986;51:537–540. [Google Scholar]
- 16.a Rainier JD, Allwein SP, Cox JM. Org. Lett. 2000;2:231–234. doi: 10.1021/ol991371r. [DOI] [PubMed] [Google Scholar]; b Rainier JD, Allwein SP, Cox JM. J. Org. Chem. 2001;66:1380–1386. doi: 10.1021/jo001514j. [DOI] [PubMed] [Google Scholar]
- 17.Please see reference 7b.
- 18.Philips KD, Horwitz JP. J. Org.. Chem. 1975;40:1856–1858. doi: 10.1021/jo00900a047. [DOI] [PubMed] [Google Scholar]
- 19.Huang SL, Swern D. J. Org. Chem. 1978;43:4537–4538. [Google Scholar]
- 20.Wittig G, Haag W. Chem. Ber. 1955;88:1654–1666. [Google Scholar]
- 21.Crimmins MT, Emmitte KA, Katz JD. Org. Lett. 2000;2:2165–2167. doi: 10.1021/ol006091m. [DOI] [PubMed] [Google Scholar]
- 22.a Schmidba H, Vornberg W, Stuhler H. Chem. Ber. 1972;105:1084. [Google Scholar]; b Larionov OV, Corey EJ. J. Am. Chem. Soc. 2008;130:2954–2955. doi: 10.1021/ja8003705. [DOI] [PubMed] [Google Scholar]
- 23.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 24.Murata Y, Kamino T, Hosokawa S, Kobayashi S. Tetrahedron Lett. 2002;43:8121–8123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.