Abstract
Invariant NKT (iNKT) cells are a distinctive subtype of CD1d-restricted T cells involved in regulating autoimmunity and capable of producing various T helper type 1 (Th1), Th2 and Th17 cytokines. Activation of iNKT cells by their exogenous ligand α-galactosylceramide (α-GalCer) exerts therapeutic effects in autoimmune diseases such as rheumatoid arthritis (RA). However, the pathophysiological role of iNKT cells in RA, in the absence of exogenous stimulation, is incompletely understood. We investigated the potential pathophysiological effects of iNKT cells in mice with collagen-induced arthritis (CIA), a model of RA. We found that iNKT cells underwent activation only in the early phases of the disease (6 days post-induction). In the liver, but not the spleen or lymph nodes, this early activation led to the release of interleukins -4, -17A and -10 and of interferon-γ; and an increased CD69 expression. Importantly, clinical and histological signs of arthritis were improved by the functional blockade of iNKT cells by a monoclonal antibody to CD1d at the early phase of the disease. This improvement was associated on day 6 post-induction with decreased expression of co-stimulatory molecules (CD80, CD86, CD40) on splenic dendritic cells and macrophages, whereas regulatory T-cell suppressive effects and proportions were not modified. Taken in concert, these findings suggest that iNKT cells are activated early in the course of CIA and contribute to the pathogenesis of arthritis. Therefore, iNKT-cell activation may be a valid treatment target in RA.
Keywords: collagen-induced arthritis, cytokines, inflammation, natural killer T cells, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a multifactorial, polygenic, autoimmune disease characterized by chronic joint inflammation and subsequent destruction of cartilage and bone. The pathophysiology of RA involves three inter-related events: loss of self-tolerance, development of chronic inflammation in several joints, and tissue destruction responsible for additional detrimental effects. Although the trigger is unknown, the cascade of immunological and inflammatory events has been documented in detail.1 Joint destruction results directly from the production of metalloproteinases and free radicals by macrophages, neutrophils, synoviocytes and chondrocytes present within the joint. Activation of these cells results from dysregulation of the cellular balance. According to the classical paradigm, RA is a T helper type 1 (Th1) disease. One of the oldest strategies used in experimental immunotherapy for RA is restoration of the Th1/Th2 balance via induction of the modulating effects of Th2 cells.2–4 Another T-cell population, the Th17 cells has since been shown to participate actively in the inflammatory cascade in RA.5,6 In addition to these T-cell populations, some regulatory cells play a role in the disease process. First, regulatory T (Treg) cells, a heterogeneous lymphocyte population, contribute to peripheral tolerance and to the control of inflammatory responses by exerting suppressive effects in RA.7,8 Then, invariant natural killer T (iNKT) cells also regulate autoimmune diseases such as RA. These cells are characterized by the expression of an invariant Vα14Jα18 antigen receptor, chiefly paired with Vβ8.2 in mice,9 and of the invariant Vα24Jα18/Vβ11 pair in humans.10 These invariant receptors recognize glycolipid antigens in conjunction with major histocompatibility complex (MHC) -like molecules such as CD1d. A distinctive feature of iNKT cells is the ability, depending on the cytokines and cells present in the environment, to rapidly produce massive amounts of interferon-γ (IFN-γ) and interleukin-4 (IL-4),11,12 whose effects are often conflicting. The glycolipid α-galactosylceramide (α-GalCer) is an exogenous ligand for iNKT cells.13 There is also some evidence that isoglobotrihexosylceramide (iGb3) may be an endogenous iNKT-cell ligand,14 although this point remains controversial.15,16
Numerous studies suggest a role for iNKT cells in autoimmune diseases, in which their regulatory effects were first demonstrated. However, improvements in the characterization and detection of iNKT cells have led to new data that create an increasingly complex picture of the role for iNKT cells in the pathophysiology of many autoimmune diseases. In particular, although the role for iNKT cells in diabetes has been extensively studied,17 available data do not prove that iNKT cells specifically exert regulatory effects in this disease. The same is true for models of lupus erythematosus and multiple sclerosis.18,19 Depending on the mode of stimulation (type of ligand, timing and dose), iNKT-cell activation in various RA models can result in protection20–23 or exacerbation22,24 of the disease. In RA in humans, few studies have focused on the status of iNKT cells,25–27 although low iNKT-cell counts have been reported in patients with RA.
The pathophysiological role for iNKT cells in the absence of exogenous stimulation has been studied in arthritis models using Jα18−/− or CD1d−/− mice lacking functional iNKT cells. These mice develop an attenuated form of experimental arthritis,24,28,29 suggesting a deleterious role for iNKT cells in the disease. However, no study has been performed to determine whether these cells play a role at the initiation, development and maintenance phases of arthritis. In addition, the cellular mechanisms by which iNKT cells become activated and exert their deleterious effects are poorly understood.
We conducted this study to investigate the timing and mechanisms of iNKT-cell activation in mice with collagen-induced arthritis (CIA), a model of human RA. We found that iNKT cells exerted deleterious effects early during CIA development. Our results indicate that iNKT-cell activation occurs chiefly in the liver and that iNKT cells can modulate the level of co-stimulatory capacities of antigen-presenting cells. Early iNKT-cell blockade protected the animals from the clinical and histological signs of CIA.
Materials and methods
Mice
Male mice aged 5–9 weeks and belonging to the CIA-susceptible DBA/1 strain were purchased from Harlan Olac (Bicester, UK).
Cytokine assay by multiplex protein analysis
Multiplex suspension bead array immunoassay was performed to identify a panel of cytokines [IL-2, IL-1β, IL-5, IL-6, IL-13, tumour necrosis factor-α (TNF-α), monocyte chemotactic protein 1 (MCP-1), regulated on activation, normal, T-cell expressed and secreted (RANTES) and IL-10] using the Bioplex 200™ system (Bio-Rad, Marnes-la-Coquette, France) and the LincoPlex mouse cytokine 9-Plex assay kit (MCYTO-70K-09; Clinisciences, Montrouge, France), according to the manufacturer’s instructions. This is a multiplexed, particle-based, flow cytometry assay that uses anti-cytokine monoclonal antibodies linked to microspheres incorporating distinct proportions of two fluorescent dyes.
Fifty microlitres of each culture supernatant or cytokine standard diluted in serum diluent was pipetted into the accompanying microtitre plate (MultiScreen 96-well filter plate) containing diluted antibody-coated bead complexes and incubation buffer. Samples were incubated for 2 hr at room temperature in the dark. After filtering and washing with assay diluent (200 μl/well, twice) using a vacuum manifold, 100 μl biotinylated detector antibody was added to all wells, incubated for 1 hr at room temperature in the dark, and washed as described above. Then, 100 μl of streptavidin–phycoerythrin was added to each well and incubated for 30 min at room temperature in the dark. All microtitre wells received a final wash, 100 μl of wash buffer was added to each well, and the wells were analysed. Cytokine concentrations were automatically calculated based on standard curve data using Bio-Plex Manager™ (Bio-Rad, Hercules, CA) software with a detection range of 2–32 000 pg/ml. Calculated concentrations were exported to an Excel spreadsheet for analysis.
Cytokine assays by specific enzyme-linked immunosorbent assays
Levels of IL-4, IL-17A, transforming growth factor-β (TGF-β) and IFN-γ in culture supernatants were measured using commercially available enzyme-linked immunosorbent assays (ELISA) kits (Duoset, R&D Systems, Abingdon, UK), according to the manufacturer’s instructions. The sensitivity of cytokine assays was 20 pg/ml.
Collagen-induced arthritis induction and evaluation
Arthritis was induced with native bovine collagen type II (CII) (Chondrex, Morwell Diagnostics, Zurich, Switzerland) as previously described.30 Male DBA/1 mice were injected subcutaneously at the base of the tail with 100 μg of CII emulsified in complete Freund’s adjuvant (CFA; Difco, Biovalley, Marne la Vallée, France). On day 21, a booster subcutaneous injection of CII in incomplete Freund’s adjuvant was given.
The mice were monitored for evidence of arthritis in the four paws, using a blind procedure. Clinical arthritis severity in each joint (forelimbs: fingers and wrist; hind limbs: toes, tarsus and ankles) was scored as follows: 0, normal; 1, erythema; 2, swelling; 3, deformity; and 4, ankylosis.20 These scores were summed to obtain the arthritis score (maximum = 40). The mean arthritis score on each clinical observation day and in each group was used to evaluate CIA severity. Amax was the maximal clinical score reached during the course of the disease for each individual mouse.
The animals were killed 64 days after CIA induction, and their legs were dissected free and processed for histological studies, as described elsewhere.30,31 At least four serial sections were cut from each paw to ensure extensive evaluation of the arthritic joints. The lesions were evaluated in each joint as previously described using a four-point scale (from 0, normal to 3, severe).20 This global histological score reflects both synovitis (synovial proliferation and inflammatory cell infiltration) and joint destruction (bone and cartilage thickness and irregularity and presence of erosions). We also evaluated joint destruction separately by assessing bone and cartilage damage irrespective of inflammation, on a four-point scale (0, no destruction to 3, subchondral bone erosion).
Liver mononuclear cell culture
Liver mononuclear cells were collected 2 hr after an intraperitoneal injection of 4 μg α-GalCer and placed in 24-well tissue culture plates (Ultra-Low; Costar; Corning, Lowell, MA) at a density of 2 × 106 cells/well, for 16 hr at 37° in RPMI-1640 medium (Gibco, Grand Island, NY) supplemented with 8% heat-inactivated fetal bovine serum, 1% penicillin–streptomycin and 50 μg/ml gentamicin. Cytokine levels were then measured in liver mononuclear cell supernatants using multiplex protein analysis or ELISA.
Cell and tissue preparation
Leucocytes were prepared from spleen and lymph nodes using a homogenizer, and red blood cells were lysed in haemolysis buffer (NH4Cl, KHCO3, ethylenediaminetetraacetic acid). Leucocytes were obtained from afferent and popliteal lymph nodes dissected out of the hind limbs. Liver leucocytes were collected by perfusing the liver with phosphate-buffered saline (PBS) once then pressing it through a mesh. Liver mononuclear cells were separated from parenchymal cells by centrifugation at 50 g for 5 min and were then collected and resuspended in 35% Percoll solution (Amersham Biosciences Europe, Orsay, France) and centrifuged for 25 min at 750 g. They were collected from the pellet, and the red blood cells were lysed as described above. Mononuclear cells were then washed and resuspended in PBS with 5% heat-inactivated fetal calf serum (FCS; Invitrogen, Carlsbad, CA). Blood was collected by heart puncture and peripheral blood mononuclear cells were isolated using a Ficoll gradient (Eurobio, Les Ulis, France). Finally, the knees were dissected and opened, synovial tissue was removed, and synovial tissues from the two knees were pooled for further processing and analysis.
Antibodies and flow cytometry analysis
CD1d-blocking (clone 20H2), non-cell-depleting monoclonal antibodies or control non-isotype-matched whole rat immunoglobulin Gs (Sigma-Aldrich, Saint-Quentin Fallavier, France) were used in vivo. For fluorescence-activated cell sorting, cells were stained using R-phycoerythrin (PE) -labelled anti-MHC II (clone 2G9), PE-labelled anti-CD80 (clone 1G10), PE-labelled anti-CD86 (clone GL1), PE-labelled anti-CD40 (clone 3/23), fluorescein isothiocyanate (FITC) -labelled anti-CD11c (clone HL3), peridinin chlorophyll protein-Cy5.5-labelled anti-CD11b (clone M1/70), FITC-labelled anti-CD25 (clone 3C7) and FITC-labelled anti-CD4 (clone RM 4-5) (all from BD Bioscience, San Jose, CA). Allophycocyanin (APC) -labelled anti-CD14 (clone Sa2-8) and APC-labelled anti-Foxp3 (clone FJK-16s) were purchased from eBioscience (San Diego, CA). The PE-labelled anti-CD69 (clone H1-2F3), PercP-Cy5.5-labelled anti-CD4 (clone L3T4) and FITC-labelled anti-T-cell receptor αβ (clone H57-597) were from BD Bioscience. APC-labelled CD1d tetramers, with or without α-GalCer, were obtained through the National Institutes of Health Tetramer Facility. Cells were stained at 4° in PBS containing 2% heat-inactivated FCS and 0·01 m sodium azide, incubated for 30 min with 2.4G2.3 monoclonal antibody to block the Fcγ receptors, and incubated for 30 min with appropriate dilutions of various monoclonal antibodies coupled to FITC, PE, PerCP-Cy-5.5 or APC. Flow cytometry was performed on a four-colour FACScalibur (Becton Dickinson, Mountain View, CA). Dead cells were excluded on the basis of forward-scatter and side-scatter characteristics. Reported statistical data are based on at least 1000 events gated on the population of interest. Results were analysed using mac cellquest pro software (Becton Dickinson, Mountain View, CA).
Lymphocyte purification
The CD4+ CD25− and CD4+ CD25+ T cells from the spleen were purified using the Regulatory T Cell Isolation Kit according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch-Gladbach, Germany). In brief, CD4+ CD25+ T cells were isolated using a two-step procedure. First, CD4+ T cells were isolated by negative selection using a cocktail of biotin-conjugated antibodies, Anti-Biotin Microbeads, LD column and QuadroMACS (Miltenyi Biotec). Then, CD4+ T cells were directly labelled with a PE-conjugated anti-CD25 antibody and anti-PE MicroBeads. The cell suspension was then loaded onto an MS column, which was placed in the magnetic field of a maagnetic antibody cell sorting (MACS) separator (OctoMACS; Miltenyi Biotech). The flow-through cells were collected and used as CD4+ CD25− cells, whereas the retained cells were eluted from the column and used as CD4+ CD25+ Treg cells. To increase purity, two consecutive column-runs were performed. Flow cytometry showed that purity of the CD4+ CD25− and CD4+ CD25+ cell-enriched fractions was 85–90%.
Assessment of Treg suppressive effect on CD4+ CD25− effector T cells
Spleen CD4+ CD25− T cells (Teff) were pre-labelled with 5 μm carboxyfluorescein succinimidyl ester (CFSE) for 10 min. Then, CFSE-labelled Teff cells (2·5 × 104) were co-cultured in RPMI-1640 with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μm 2-mercaptoethanol, 1m HEPES and soluble anti-CD3 (5 μg/ml) in U-bottomed 96-well plates with Treg cells (2·5 × 104, 1·25 × 104 and 0·62 × 104) corresponding to Teff : Treg ratios of 4 : 4, 4 : 2 and 4 : 1, respectively. Controls were performed with non-CFSE-labelled Teff cells instead of Treg cells (CD4+ CD25−; 2·5 × 104, 1·25 × 104, and 0·62 × 104). Antigen-presenting cells (2·5 × 104) treated with mitomycin were added to the culture medium. The cells were then incubated at 37° in a 5% CO2 atmosphere. After 4 days of culture, Teff-cell proliferation was determined for each Teff : Treg ratio using flow cytometry (FACScalibur) to measure the CFSE dilution. The values were compared to the control, in which Teff cells were cultured without Tregs. The percentage of suppression was calculated as follows: % suppression = [(Teff proliferation without Treg cells – Teff proliferation with Treg cells)/(Teff proliferation without Treg cells)] × 100. Data were analysed using weasel version 2.3 (Walter and Eliza Hall Institute of Medical Research (WEHI), Parkville, Vic., Australia).
Statistical analysis
Results were compared using Student’s t-test or Mann–Whitney U-tests as appropriate. For repeated measures (clinical scores), we used analysis of variance (anova). The χ2 test with Yates’ correction was used to compare qualitative data. All statistical analyses were performed using the statview version 5.0 Software (Abacus Concepts, CA).
Results
Early cytokine secretion by iNKT cells in CIA
To determine whether and when iNKT cells were activated during the course of CIA, we evaluated ex vivo cytokine secretion by iNKT cells from the liver, spleen, lymph nodes and blood at various times after CIA induction (days 6, 22, 42 and 64). DBA/1 mice were immunized with CII/CFA and given an intraperitoneal injection of α-GalCer 2 hr before euthanasia for investigation of cytokine secretion by iNKT cells, as described elsewhere.32 Hepatic MNCs taken 6 days after CII/CFA immunization did not induce any spontaneous (not α-GalCer-induced) cytokine release (data not shown). In iNKT cells from the spleen, lymph nodes and blood, no statistically significant differences between immunized and non-immunized mice were observed for any of the 13 studied cytokines (IL-10, IL-4, IL-17A, IFN-γ, IL-1β, IL-2, IL-13, TGF-β, TNF-α, IL-5, IL-6, MCP-1 and RANTES) at any time (data not shown).
In the liver, no significant changes in concentrations of IL-5, IL-6, RANTES or MCP-1 secreted by iNKT cells were found; TNF-α was released at very low levels at all time-points, whereas TGF-β levels decreased non-significantly on day 6 compared with non-immunized mice, then peaked on day 22 (data not shown).
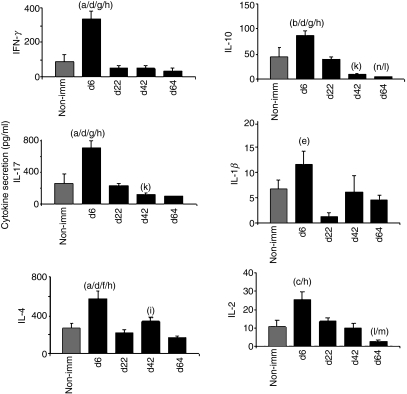
The release of IL-4, IFN-γ and IL-17A in the liver peaked during the early phase of CIA (day 6) in immunized mice compared with non-immunized DBA/1 mice and immunized mice at other time-points after CIA induction (Fig. 1). Similarly, IL-10, IL-2 and IL-1β release increased on day 6, although levels were low for these three cytokines.
Figure 1.
Early cytokine secretion by liver invariant natural killer T (iNKT) cells in collagen-induced arthritis. DBA/1 mice were immunized with collagen II (CII) in complete Freund’s adjuvant (CFA). They were killed 2 hr after an intraperitoneal injection of 4 μg α-galactosylceramide (α-GalCer) on days 6 (n = 10), 22 (n = 10), 42 (n = 10), or 64 (n = 10) post-induction. Liver mononuclear cells were collected and cultured for 16 hr as described in the Material and methods section. Non-immunized (Non-imm) mice (n = 12) were used as controls. Day 6 versus Non-imm: (a) P<0·01; (b) P<0·05; (c) P<0·02. Day 6 versus Day 22: (d) P<0·001; (e) P<0·01. Day 6 versus Day 42: (f) P<0·02; (g) P<0·001. Day 6 versus Day 64: (h) P<0·001. Day 22 versus Day 42: (j) P<0·05; (k) P<0·001. Day 22 versus Day 64: (l) P<0·001. Day 42 versus Day 64: (m) P<0·001 Day 64 versus Non-imm: (n) P<0·05 (Student’s t-test).
Taken together, our results indicate that liver iNKTs released increased amounts of cytokines (most notably IL-4, IFN-γ and IL-17A) early in the development of CIA, i.e. 6 days post-induction.
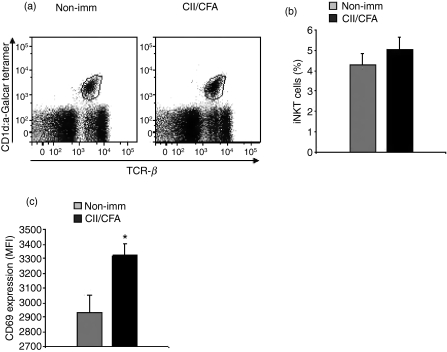
Liver iNKT cell activation in early arthritis
To determine whether the proportions of iNKT cells were modified in early CIA, we examined the proportion of iNKT cells in the liver 6 days after CII immunization. The proportion of iNKT cells was not significantly different between immunized mice and non-immunized mice (Fig. 2a,b). Then, to determine whether iNKT cells were activated in early CIA we then evaluated the expression of the early activation marker CD69 based on mean fluorescence intensity (MFI). Figure 2(c) shows that the CD69 MFI was significantly increased in mice with CIA compared with control mice (P<0·05), indicating activation of liver iNKT cells early in the development of CIA.
Figure 2.
Invariant natural killer T (iNKT) cell counts and CD69 expression in the liver in early arthritis. Liver mononuclear cells from mice immunized with collagen II in complete Freund’s adjuvant (CII/CFA, n = 6) were collected on day 6 post-collagen-induced arthritis induction and labelled with fluorochrome-conjugated anti-T-cell receptor β (TCR-β) and with either CD1d/α-galactosylceramide (α-GalCer) -tetramer or CD1d/vehicle-tetramer then analysed by flow cytometry. Liver mononuclear cells from non-immunized mice (n = 6) were used as controls. (a) The gate of iNKT cells, defined here as TCR-β+ CD1d/α-GalCer-tetramer-positive cells, among liver mononuclear cells is indicated in each dot-plot for one mouse (representative experiment out of 6). (b) The percentages of iNKT cells among liver MNC are given as mean values ± SEM. (c) iNKT cells were labelled with fluorochrome-conjugated anti-CD69 monoclonal antibody. Results are expressed as mean fluorescence intensity (MFI) corrected for non-specific staining. *P<0·05 versus non-immunized mice (Student’s t-test). Data represent one experiment representative of two similar experiments.
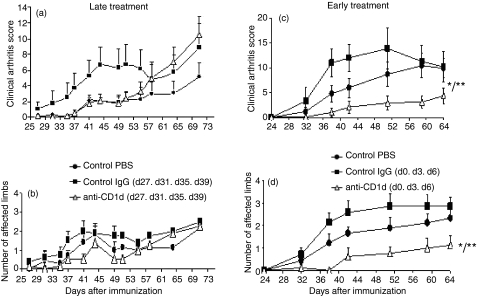
Inhibition of CIA development by early anti-CD1d treatment
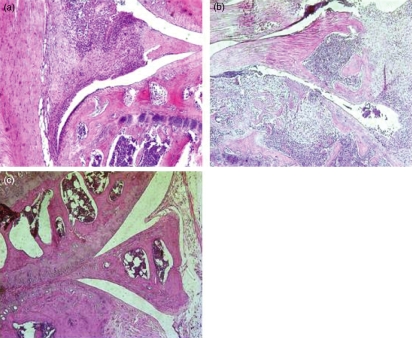
We sought to evaluate the relevance of early iNKT-cell activation to the clinical and histological manifestations of CIA. We hypothesized that iNKT-cell neutralization by anti-CD1d administration would exert therapeutic effects early in the disease, but not at late stages. Figure 3(a,b) shows that late iNKT-cell neutralization (anti-CD1d from days 27 to 39) did not modify the clinical course compared with the control groups given PBS or immunoglobulin. Neither the onset of arthritis nor the incidence of the disease was modified (data not shown). In contrast, early iNKT-cell neutralization by anti-CD1d (days 0, 3 and 6 post-induction) improved the clinical manifestations of arthritis (Fig. 3c,d). Moreover, disease onset was significantly delayed and the mean maximal arthritis score (Amax) was lower in early anti-CD1d-treated mice than in PBS control mice and immunoglobulin control mice (Table 1). Histological evaluation also indicated decreases in joint inflammation and destruction in the anti-CD1d-treated group compared with PBS control mice and immunoglobulin control mice (Table 1 and Fig. 4).
Figure 3.
Effect of functional invariant natural killer T (iNKT) blockade by anti-CD1d in collagen-induced arthritis (CIA): clinical evaluation. All DBA/1 mice were immunized with collagen II in complete Freund’s adjuvant (CII/CFA) on day 0 for arthritis induction, and clinical scores were evaluated in mice treated with anti-CD1d (200 μg at each intraperitoneal injection, n = 8). Controls were mice given the same doses of control immunoglobulin G (IgG; n = 8), or phosphate-buffered saline (PBS; n = 8). Anti-CD1d or control IgG was administered at the late phase of CIA on days 27, 31, 35 and 39 (a, b) or at the early phase of CIA on days 0, 3 and 6 (c, d). (a, c) clinical arthritis score; (b, d) number of affected limbs. Values are means ± SEM. *P<0·0001 anti-CD1d versus control IgG; **P<0·001 anti-CD1d versus PBS (analysis of variance). This experiment is representative of two similar experiments.
Table 1.
Clinical and histological parameters of arthritis in mice treated with anti-CD1d at the early stage of collagen-induced arthritis
| Clinical parameters |
Histological parametersy | ||||
|---|---|---|---|---|---|
| Onset (days) | Incidence | Amax | Inflammation | Destruction | |
| PBS (n = 9) | 45·9 ± 4·2 | 9/9 (100%) | 15·5 ± 3·9 | 1·68 ± 0·36 | 1·67 ± 0·27 |
| Control Ig (n = 7) | 36·3 ± 1·1 | 7/7 (100%) | 12·2 ± 2·3 | 1·43 ± 0·38 | 1·18 ± 0·32 |
| Anti-CD1d (n = 8) | 50·8 ± 3·7** | 5/8 (62%)‡ | 5·3 ± 1·7*† | 0·69 ± 0·35† | 0·50 ± 0·32*† |
The time of arthritis onset is expressed as mean ± SEM. PBS, phosphate-buffered saline; Ig, immunoglobulins; Amax, mean maximum arthritis score.
P< 0·05 versus control immunoglobulin.
P < 0·01 versus control immunoglobulin.
P< 0·05 versus PBS (Mann–Whitney).
P< 0·05 versus PBS (χ2).
Figure 4.
Effect of functional invariant natural killer T (iNKT) cell blockade by anti-CD1d in collagen-induced arthritis: example of histological slide. Knee section (×100) haematoxylin & eosin staining, showing inflammatory synovitis and articular destruction in mice treated with either phosphate-buffered saline (a) or control immunoglobulin G (b). In contrast, mice treated with anti-CD1d (c) showed improvement of histological arthritis.
Early, but not late, iNKT-cell neutralization by anti-CD1d therefore improved the clinical and histological signs of CIA.
iNKT-cell blockade has no effect on the suppressive function of regulatory T cells in early CIA
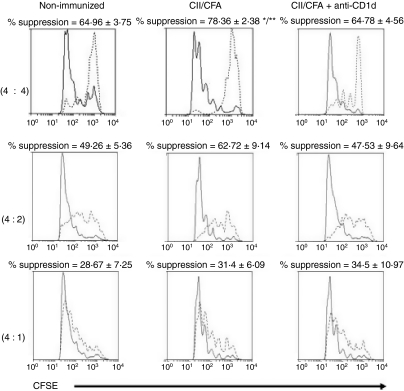
Cross-talk occurs between Treg cells and iNKT cells.33 We therefore studied the effect of early iNKT-cell blockade by anti-CD1d in CIA mice on the percentage of FoxP3+ Treg cells in the spleen and lymph nodes and on the suppressive function of Treg cells from the spleen. Anti-CD1d treatment had no effect on the percentages of Treg cells in the spleen or lymph nodes, compared with immunized mice or non-immunized mice (data not shown). There was also no clear difference in the suppression exerted by Treg cells on Teff-cell proliferation between the three groups (Fig. 5).
Figure 5.
Effect of early invariant natural killer T-cell blockade on regulatory T (Treg) cell suppressive function. Three groups of mice were used: (i) mice immunized on day 0 with collagen II in complete Freund’s adjuvant (CII/CFA) (n = 4), (ii) mice immunized with CII/CFA and given 200 μg of anti-CD1d intraperitoneally on days 0 and 3 (n = 6), and (iii) control group of non-immunized mice (n = 6). CD4+ CD25+ (Treg cells) and CD4+ CD25− (Teff cells) were isolated from the spleens of all mice on day 6. CD4+ CD25− CFSE-labelled T cells were co-cultured with CD4+ CD25+ Treg cells at ratios of Teff : Treg of 4 : 4, 4 : 2 and 4 : 1 for 96 hr, with 5 μg/ml of soluble anti-CD3 and mitomycin-treated antigen-presenting cells. Teff-cell proliferation in presence of Treg cells was determined using fluorescence-activated cell sorting to measure CFSE dilution, and the result was compared with the Teff-cell proliferative response in the absence of Treg cells (see the Material and methods section for computation of the % suppression). Proliferation profiles of Teff cells cultured in the presence (dashed lines) or absence (solid lines) of Treg cells are shown for one representative mouse of each group for the three ratios. Percentages represent the mean ± SEM in each group. *P<0·02 CII/CFA versus CII/CFA+ anti-CD1d; **P<0·05 CII/CFA versus non-immunized (Student’s t-test).
iNKT-cell blockade is associated with decreased co-stimulatory molecule expression on splenic antigen-presenting cells in early CIA
The cross-talk between iNKT cells and dendritic cells34 and the role played by dendritic cells35 and macrophages in arthritis prompted us to investigate whether the harmful effects of iNKT in CIA influenced these two populations of antigen-presenting cells. We studied the counts and maturation of splenic CD11c+ CD11b− and CD11c+ CD11b+ dendritic cells and of macrophages (CD14+) 6 days after CIA induction in mice treated with anti-CD1d and in control immunized and non-immunized mice. Anti-CD1d injection in vivo slightly decreased the percentages of CD11c+ CD11b− and CD11c+ CD11b+ dendritic cells and of macrophages compared with immunized mice given PBS (Table 2).
Table 2.
Effect of invariant natural killer T-cell blockade on the proportion of splenic dendritic cells and macrophages
| CD11c+ CD11b− | CD11c+ CD11b+ | Macrophages (CD14+) | |
|---|---|---|---|
| Non-immunized | 2·03 ± 0·32 | 4·64 ± 0·33 | 0·87 ± 0·14 |
| CII/CFA | 0·72 ± 0·23**** | 2·04 ± 0·36**** | 0·47 ± 0·04*** |
| CII/CFA+ anti-CD1d | 0·64 ± 0·17‡‡‡‡ | 1·82 ± 0·26‡‡‡‡ | 0·41 ± 0·05‡‡‡ |
Three groups of mice were used: mice immunized on day 0 with collagen II in complete Freund’s adjuvant (CII/CFA, n = 6), mice immunized with CII/CFA and given 200 μg of anti-CD1d intraperitoneally on days 0 and 3 (n = 6), and non-immunized mice as controls (n = 6). Spleen cells were collected from all mice (on day 6) and labelled with fluorochrome-conjugated anti-CD11c, anti-CD11b and anti-CD14 for analysis by flow cytometry. The percentage of CD11c+ CD11b− and CD11c+CD11b+ dendritic cells and of CD14+ macrophages are given as mean values ± SEM. Results are expressed as % (mean ± SEM) among all splenocytes and mean fluorescence intensity (MFI ± SEM).
Non-immunized versus CII/CFA:
P< 0·01;
P< 0·001. CII/CFA + anti-CD1d versus non-immunized:
P< 0·01;
P< 0·001. (Student’s t-test).
We then evaluated the expression of co-stimulation molecules. As shown in Table 3, iNKT-cell blockade with anti-CD1d resulted in decreased percentages and decreased MFIs of molecules involved in T-cell co-stimulation (CD40, CD80 and CD86) on CD11c+ CD11b− dendritic cells, compared with immunized mice. Similar results were found for CD40 and CD80 MFIs in CD11c+ CD11b+ dendritic cells. CD40 (% and MFI) and CD80 also decreased in macrophages after anti-CD1d injection compared with immunized mice, although the difference was not significant for CD80. In contrast, expression on dendritic cells or macrophages of the antigen-presenting molecule MHC II was not modified by anti-CD1d treatment compared with immunized mice. These results suggest altered co-stimulatory activities of antigen-presenting cells after early iNKT-cell blockade in CIA mice.
Table 3.
Effect of invariant natural killer T-cell blockade on the phenotype of dentritic cells and macrophages
| MHC II |
CD40 |
CD80 |
CD86 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % | MFI | % | MFI | % | MFI | % | MFI | ||
| (b) | |||||||||
| CD11c+ CD11b− | Non-immunized | 5·6 ± 0·3‡‡‡ | 83·7 ± 19·1**,‡‡‡‡ | 3·34 ± 0·53*** | 132·3 ± 43·7 | 0·7 ± 0·03 | 15·4 ± 1·4 | 3·6 ± 0·5 | 33·5 ± 3·1 |
| CII/CFA | 4·5 ± 0·8 | 190·3 ± 28·5 | 1·07 ± 0·2 | 141·7 ± 32·7 | 0·36 ± 0·1** | 45 ± 11·8* | 1·3 ± 0·1*** | 56 ± 2·1**** | |
| CII/CFA + anti-CD1d | 3·9 ± 0·4 | 226·6 ± 21·7 | 1 ± 0·12‡‡‡ | 66·2 ± 7† | 0·1 ± 0·01‡‡‡‡,† | 18·7 ± 0·7† | 1·2 ± 0·1‡‡‡ | 45·4 ± 3·3†,‡ | |
| CD11c+ CD11b+ | Non-immunized | 13·9 ± 1·4 | 240·5 ± 92·4‡ | 12·9 ± 1·2 | 184·1 ± 34·5 | 1·3 ± 0·1 | 26·1 ± 2·2 | 11·1 ± 0·9 | 31·4 ± 1·5 |
| CII/CFA | 17·6 ± 1·6 | 384·5 ± 27·1 | 10·4 ± 0·9 | 168·9 ± 19·4 | 0·5 ± 0·07**** | 85·3 ± 15·7*** | 6·7 ± 0·3*** | 38·2 ± 2·4* | |
| CII/CFA+ anti-CD1d | 17·3 ± 0·6 | 454·3 ± 29·7 | 10·5 ± 1·03 | 131 ± 6·3 | 1·1 ± 0·2†† | 30·6 ± 1·7††† | 7·7 ± 0·6‡‡ | 40 ± 5 | |
| Macrophages (CD14+) | Non-immunized | 26·9 ± 3·2 | 199·5 ± 80·1**** | 4·9 ± 1·2 | 221·2 ± 54·1 | 0·9 ± 0·1 | 23·1 ± 3·5 | 24·7 ± 1·8 | 28·9 ± 1·2 |
| CII/CFA | 19·5 ± 2 | 830·7 ± 49·7 | 2·07 ± 0·3* | 352·4 ± 85·4 | 0·7 ± 0·3 | 310·4 ± 86*** | 1·1 ± 0·1*** | 82·8 ± 9·8**** | |
| CII/CFA+ anti-CD1d | 21·8 ± 1·1 | 874 ± 36·4‡‡‡‡ | 1 ± 0·04†††,‡‡ | 146·8 ± 22·3† | 0·2 ± 0·05‡‡‡‡ | 186·1 ± 48·7‡‡‡ | 1·1 ± 0·2‡‡‡ | 117·4 ± 24·7†,‡ | |
Three groups of mice were used: mice immunized on day 0 with collagen II in complete Freund’s adjuvant (CII/CFA, n = 6), mice immunized with CII/CFA and given 200 μg of anti-CD1d intraperitoneally on days 0 and 3 (n = 6), and non-immunized mice as controls (n = 6). Spleen cells were collected from all mice (on day 6) and labelled with fluorochrome-conjugated anti-CD11c, anti-CD11b and anti-CD14 for analysis by flow cytometry. CD11c+ CD11b− and CD11c+ CD11b+ dendritic cells, and CD14+ macrophages from the spleen were labelled with fluorochrome-conjugated monoclonal antibodies to major histocompatibility complex class II (MHC II), CD80, CD86 and CD40. Results are expressed as % (mean ± SEM) among all splenocytes and mean fluorescence intensity (MFI ± SEM).
Non-immunized versus CII/CFA:
P< 0·05;
P< 0·02;
P< 0·01;
P< 0·001. CII/CFA versus CII/CFA + anti-CD1d:
P< 0·05;
P< 0·02;
P< 0·01. CII/CFA + anti-CD1d versus non-immunized:
P< 0·05;
P< 0·02;
P< 0·01;
P< 0·001. (Student’s t-test).
Discussion
We found that liver iNKT cells underwent activation (CD69 expression and cytokine secretion) in the early stage of CIA and that early iNKT-cell blockade by anti-CD1d improved the disease. Later in the course of CIA, in contrast, iNKT cells were not activated. Moreover, early iNKT-cell blockade was accompanied by decreased maturation of splenic dendritic cells and macrophages in mice with CIA.
The consequences of absence of iNKT cells has been studied in Jα18−/− and CD1d−/− mice. However, these knockout mice have not been generated in animals with the DBA/1 (H-2q) genetic background, which show the greatest susceptibility to CIA. In Jα18−/− C57BL/6 mice, CIA was attenuated compared with wild-type mice.28 Similarly, CIA was less severe in Jα18−/− or CD1d−/− C57BL/6 mice given K/BxN serum24,28,29 and in CD1d−/− C57BL/6 mice with anti-CII antibody-induced arthritis.28 Taken together, these earlier data and our results suggest a deleterious effect of iNKT cells in the absence of exogenous stimulation in various models of RA. Jα18−/− and CD1d−/− mice do not allow investigations of iNKT cells during the course of arthritis. In contrast, in mice that have iNKT cells, glycolipid recognition by iNKT cells can be blocked by administering an anti-CD1d antibody. Using this method, we showed that iNKT cells were activated only at the early stage of CIA. Thus, anti-CD1d given from days 0 to 6 attenuated the severity of CIA, whereas no such effect was found with anti-CD1d given from days 27 to 39. Interestingly, in an earlier study of DBA/1 mice with CIA, anti-CD1d treatment started on day 21 improved the arthritis.28 This apparent contrast with our findings can be explained by the differences in the timing of treatment because our treatment started later, from days 27 to 39, and did not exert any effect. It is therefore likely that between the boost with CII/incomplete Freund’s adjuvant (day 21) and the onset of clinical arthritis, iNKT-cell activation and function are modified.
The protective effect of iNKT-cell blockade by anti-CD1d early in the course of CIA was similar to that seen after early iNKT-cell stimulation with an exogenous ligand such as α-GalCer (our previous work20) or (2S,3S,4R)-1-O-(alpha-D-galactopyranosyl)-N-tetracosanoyl-2-amino-1,3,4-nonanetriol (OCH).22 One hypothesis is that iNKT cells may elaborate various responses depending on the endogenous or exogenous stimulating antigen. Cytokine secretion by iNKT cells varies with the ligand; for instance, α-GalCer induces strong stimulation with a dramatic Th1 and Th2 cytokine response in vivo, whereas an analogue such as OCH chiefly induces Th2-type cytokine secretion. Activation of iNKT cells after stimulation with their endogenous ligand may therefore differ from the activation obtained using an exogenous synthetic ligand, particularly in terms of cytokine secretion. Although the consequences of endogenous iNKT-cell stimulation in early CIA remain unknown, they probably include an inflammatory response because iNKT blockade with anti-CD1d ameliorates the disease.
Blockade of iNKT cells in early CIA affected the co-stimulatory potential of antigen-presenting cells (dendritic cells and macrophages) in the present study. Dendritic cells are essential partners for iNKT cells. They are crucial for α-GalCer presentation to iNKT cells which, in turn, triggers dendritic cell stimulation,36,37 most notably by inducing CD80 and CD86 up-regulation.38 However, repeated α-GalCer injections can also induce IL-10-secreting dendritic cells exhibiting a regulatory phenotype.39 This fact underscores the considerable diversity of iNKT-cell effects on immune regulation, depending on various parameters linked to the mode of antigen stimulation. In our study, iNKT-cell blockade by anti-CD1d was followed by an inhibition of dendritic cell maturation. Hence, in early CIA, iNKT-cell stimulation by its endogenous ligand via interaction with the T-cell receptor may induce dendritic cell activation, leading to T-cell activation, inflammation and exacerbated arthritis.
Few studies have investigated cross-talk between Treg cells and iNKT cells. It is known that activated iNKT cells can modulate Treg-cell functions through an IL-2-dependent mechanism, whereas Treg cells can suppress iNKT-cell functions via cell–cell contact.33 In experimental models of autoimmune diseases, for instance autoimmune myasthenia, iNKT-cell activation by α-GalCer correlates with Treg-cell expansion and potentiation of Treg-cell suppressive effects.40 In non-obese diabetic (NOD) mice, a model for type I diabetes, protection from the disease by α-GalCer-activated iNKT cells requires the activity of Treg cells and results in modulation of their phenotype.41 In the present study, iNKT-cell blockade in early CIA had no effect on the suppressive effects of Treg cells. Taken together, these findings suggest that activated iNKT cells mainly act through inhibition of antigen-presenting cells.
In summary, our study brings new insights into the pathophysiological role of iNKT cells in arthritis. Early activation of iNKT cells, especially those in the liver, occurs during CIA and participates in the development of the autoimmune inflammatory process. These cells may therefore constitute therapeutic targets in RA. Studies are needed to determine whether they can be effectively blocked with anti-CD1d, for instance, or effectively stimulated with an exogenous ligand.
Acknowledgments
We are grateful to Delphine Lemeiter (EA 4222, Li2P, Paris 13 University) and Monique Etienne and Simone Beranger (Paris 13 University) for their outstanding technical assistance; and to Stephane Chambris (animal facilities, Paris 13 University). This work was supported by the Société Française de Rhumatologie (SFR) and INSERM.
Disclosures
No conflict of interest to declare related to this paper.
References
- 1.Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39–44. doi: 10.1097/01.rhu.0000166673.34461.33. [DOI] [PubMed] [Google Scholar]
- 2.Cottard V, Mulleman D, Bouille P, et al. Adeno-associated virus-mediated delivery of IL-4 prevents collagen-induced arthritis. Gene Ther. 2000;7:1930–9. doi: 10.1038/sj.gt.3301324. [DOI] [PubMed] [Google Scholar]
- 3.Bessis N, Cottard V, Saidenberg-Kermanac’h N, et al. Syngeneic fibroblasts transfected with a plasmid encoding interleukin-4 as non-viral vectors for anti-inflammatory gene therapy in collagen-induced arthritis. J Gene Med. 2002;4:300–7. doi: 10.1002/jgm.275. [DOI] [PubMed] [Google Scholar]
- 4.Bessis N, Boissier MC, Ferrara P, et al. Attenuation of collagen-induced arthritis in mice by treatment with vector cells engineered to secrete interleukin-13. Eur J Immunol. 1996;26:2399–403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- 5.Boissier MC, Assier E, Falgarone G, et al. Shifting the imbalance from Th1/Th2 to Th17/treg: the changing rheumatoid arthritis paradigm. Joint Bone Spine. 2008;75:373–5. doi: 10.1016/j.jbspin.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S, Fox DA. Regulatory T cells in rheumatoid arthritis. Curr Rheumatol Rep. 2008;10:405–12. doi: 10.1007/s11926-008-0065-y. [DOI] [PubMed] [Google Scholar]
- 8.Boissier MC, Assier E, Biton J, et al. Regulatory T cells (Treg) in rheumatoid arthritis. Joint Bone Spine. 2009;76:10–4. doi: 10.1016/j.jbspin.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi M, Harada M, Kojo S, et al. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211–22. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 11.Parekh VV, Wilson MT, Van Kaer L. iNKT-cell responses to glycolipids. Crit Rev Immunol. 2005;25:183–213. doi: 10.1615/critrevimmunol.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Mattner J, Cantu C, III, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. Epub. [DOI] [PubMed] [Google Scholar]
- 15.Speak AO, Salio M, Neville DC, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci USA. 2007;104:5971–6. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porubsky S, Speak AO, Luckow B, et al. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A. 2007;104:5977–82. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak J, Griseri T, Beaudoin L, et al. Regulation of type 1 diabetes by NKT cells. Int Rev Immunol. 2007;26:49–72. doi: 10.1080/08830180601070229. [DOI] [PubMed] [Google Scholar]
- 18.Godo M, Sessler T, Hamar P. Role of invariant natural killer T (iNKT) cells in systemic lupus erythematosus. Curr Med Chem. 2008;15:1778–87. doi: 10.2174/092986708785132988. [DOI] [PubMed] [Google Scholar]
- 19.Jahng AW, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miellot A, Zhu R, Diem S, et al. Activation of invariant NK T cells protects against experimental rheumatoid arthritis by an IL-10-dependent pathway. Eur J Immunol. 2005;35:3704–13. doi: 10.1002/eji.200535235. [DOI] [PubMed] [Google Scholar]
- 21.Kaieda S, Tomi C, Oki S, et al. Activation of invariant natural killer T cells by synthetic glycolipid ligands suppresses autoantibody-induced arthritis. Arthritis Rheum. 2007;56:1836–45. doi: 10.1002/art.22714. [DOI] [PubMed] [Google Scholar]
- 22.Chiba A, Oki S, Miyamoto K, et al. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of α-galactosylceramide. Arthritis Rheum. 2004;50:305–13. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 23.Coppieters K, Van Beneden K, Jacques P, et al. A single early activation of invariant NK T cells confers long-term protection against collagen-induced arthritis in a ligand-specific manner. J Immunol. 2007;179:2300–9. doi: 10.4049/jimmunol.179.4.2300. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Kim HJ, Min HS, et al. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor β1 production. J Exp Med. 2005;201:41–7. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojo S, Adachi Y, Keino H, et al. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44:1127–38. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Kojo S, Tsutsumi A, Goto D, et al. Low expression levels of soluble CD1d gene in patients with rheumatoid arthritis. J Rheumatol. 2003;30:2524–8. [PubMed] [Google Scholar]
- 27.Yanagihara Y, Shiozawa K, Takai M, et al. Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;118:131–6. doi: 10.1046/j.1365-2249.1999.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiba A, Kaieda S, Oki S, et al. The involvement of Vα14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 2005;52:1941–8. doi: 10.1002/art.21056. [DOI] [PubMed] [Google Scholar]
- 29.Kim HY, Kim S, Chung DH. FcγRIII engagement provides activating signals to NKT cells in antibody-induced joint inflammation. J Clin Invest. 2006;116:2484–92. doi: 10.1172/JCI27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saidenberg-Kermanac’h N, Bessis N, Lemeiter D, et al. Interleukin-4 cellular gene therapy and osteoprotegerin decrease inflammation-associated bone resorption in collagen-induced arthritis. J Clin Immunol. 2004;24:370–8. doi: 10.1023/B:JOCI.0000029116.12371.bf. [DOI] [PubMed] [Google Scholar]
- 31.Le Buanec H, Delavallee L, Bessis N, et al. TNFα kinoid vaccination-induced neutralizing antibodies to TNFα protect mice from autologous TNFα-driven chronic and acute inflammation. Proc Natl Acad Sci U S A. 2006;103:19442–7. doi: 10.1073/pnas.0604827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 33.La Cava A, Van Kaer L, Fu Dong S. CD4+ CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–7. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–64. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Lebre MC, Tak PP. Dendritic cells in rheumatoid arthritis: which subset should be used as a tool to induce tolerance? Hum Immunol. 2009;70:321–4. doi: 10.1016/j.humimm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Shimizu K, Hemmi H, et al. Innate Vα14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–98. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 37.Fujii S, Shimizu K, Smith C, et al. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung Y, Chang WS, Kim S, et al. NKT cell ligand α-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–9. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- 39.Kojo S, Seino K, Harada M, et al. Induction of regulatory properties in dendritic cells by Vα14 NKT cells. J Immunol. 2005;175:3648–55. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+ CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 41.Ly D, Mi QS, Hussain S, et al. Protection from type 1 diabetes by invariant NK T cells requires the activity of CD4+ CD25+ regulatory T cells. J Immunol. 2006;177:3695–704. doi: 10.4049/jimmunol.177.6.3695. [DOI] [PubMed] [Google Scholar]