Abstract
Quantum Mechanical/Molecular Mechanical (QM/MM) geometry optimizations of the X-ray crystal structures of PDE10-AMP (PDB code 2OUN) and PDE10-GMP (PDB code 2OUQ) complexes have been performed to characterize the state of the AMP and GMP products, respectively. Results show that only one phosphate oxygen atom (O1) is protonated for both AMP and GMP product complexes. In addition, the QM/MM calculations have resolved the orientation of the amide group of Gln726 in PDE10-GMP which was in conflict with the assignment of the guanine group of GMP in the X-ray crystal structure. Calculations reveal that the amide oxygen and nitrogen atom of Gln726 are rotated 180° resulting in two strong hydrogen bonds formed between the amide group of Gln726 and guanine group of GMP. Binding free energy calculations for both QM/MM optimized structures confirm the new conformational assignment of Gln726 in PDE10-GMP. The calculated binding free energy of the rotated structure is ~22 kcal/mol lower than the X-ray crystal assignment. The lower energy is mainly derived from the formation of two hydrogen bonds between the amide group of Gln726 and guanine group of GMP. This implies that the orientation of the amide oxygen and nitrogen atoms in PDE10-AMP is different from PDE10-GMP. Finally, our results help to understand why PDE10 can hydrolyze both cAMP and cGMP.
Introduction
Cyclic nucleotide phosphodiesterases (PDE’s) regulate physiological processes by degrading intracellular secondary messengers, cyclic adenosine 3´,5´-monophosphate (cAMP) or cyclic guanosine 3´,5´-monophosphate (cGMP) to 5´-nucleotide monophosphates AMP or GMP through PDE-catalyzed hydrolysis.1,2,3,4,5,6,7,8,9 PDEs constitute a large superfamily (with at least 11 different gene families, i.e. PDE1 to PDE11) of structurally related, functionally distinct, and highly regulated enzymes.10 There are three catagories related to the substrate specificity of PDE’s: cAMP-specific enzymes (PDE4, PDE7, and PDE8); cGMP-specific enzymes (PDE5, PDE6, and PDE9); and the dual specificity (PDE1, PDE2, PDE3, PDE10, and PDE11). Most cells contain representatives of multiple PDE gene families, but in different amounts, proportions, and subcellular locations. For example, PDE3A is highly expressed in cardiac muscle, vascular and visceral smooth muscle, and platelets, whereas PDE3B, the product of a distinct gene, is expressed in adipocytes, hepatocytes, spermatocytes, and renal collecting duct epithelium.11 PDE4 is mainly expressed in inflammatory cells and central neurons. PDE5 isomers are relatively abundant in vascular smooth muscle, including the pulmonary vasculature and corpus cavernosum of the penis.12 PDE10 was first reported by three groups in 1999.13,14,15 This enzyme is highly expressed in the central nervous cystem (CNS), making it an important target for therapeutic approaches for the treatment of psychoses.16,17,18 Consequently, different families of PDE’s are potential drug targets for the treatments of different diseases.
Crystal structures for the catalytic domains of nine different PDE families have been solved over the past few years. In 2007, Ke et al.19 first published eight X-ray crystal structures of the catalytic domain of the wild-type and mutated PDE10A2 including complexes with products AMP and GMP. The binding modes of AMP and GMP in the active site of PDE10 are very similar except for the hydrogen bonding mode between the amide group of Gln726 and adenine or guanine group of products AMP and GMP, respectively.
In the present computational study, we mainly focused on the crystal structures of PDE10 with its two products (AMP and GMP) and examined the detailed binding interactions of the enzyme-product complexes. As the structures of the products AMP and GMP are similar to those the substrates cAMP and cGMP, respectively, a detailed structural understanding of the enzyme-product complexes should be valuable for understanding the detailed structures of the enzyme-substrate complexes and the catalytic mechanisms of PDE10.
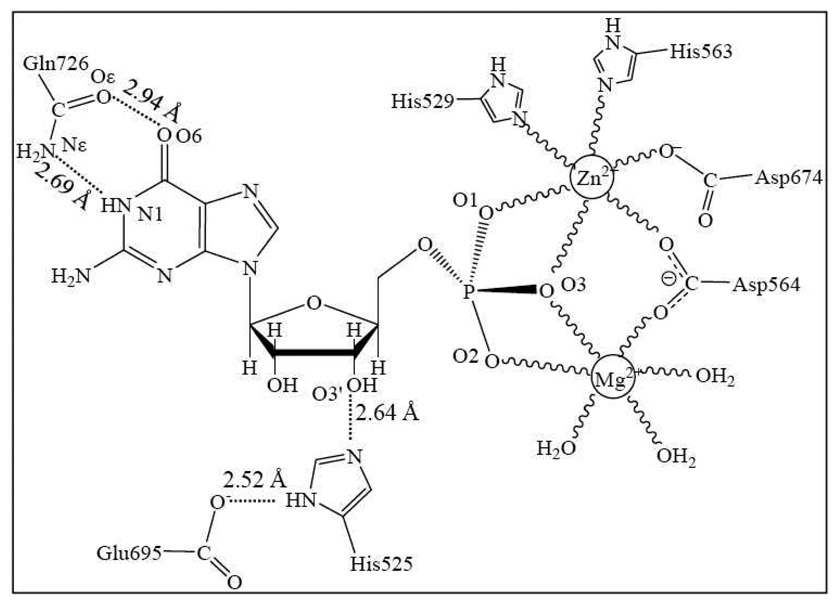
The catalytic domain of PDE10 adopts a compact structure consisting of 15 α-helices without β-sheets. The crystal structures show that the overall topology is very similar with other known structures of PDE families. The two metal ions in the active site were assigned as Zn(II) and Mg(II). In both PDE10-AMP and PDE10-GMP complexes, Zn(II) ion was coordinated to His529, His563, Asp564, Asp674, and two phosphate oxygen atoms (O1 and O3). Mg(II) was coordinated to Asp564, two phosphate oxygen atoms (O2 and O3), and three water molecules. O3 acts as the bridging atom connecting two metal ions and forms one strong hydrogen bond with Oδ atom of Asp674. Therefore, there should be one hydrogen atom between O3 and Oδ. However it is not clear whether this hydrogen atom is on O3 or Oδ since hydrogen atoms cannot be determined by X-ray diffraction. The distance between O3´ and Nδ atoms of His525 is approximately 2.7 Å, thus there should be a strong hydrogen bond between them. The hydrogen bond donor is the hydrogen atom on O3´ which is the hydrolyzed oxygen, and Nε atom of His525 forms a strong hydrogen bond with Glu695 as well. Therefore in the following calculations, His525 will be assigned as HID (See Chart 1).
Chart 1.
The active site structure of PDE10-GMP complex:19 Zn(II) is coordinated to His529, His563, Asp564, Asp674, and two phosphate oxygen atoms (O1 and O3); Mg(II) is coordinated to Asp564, two phosphate oxygen atoms (O2 and O3), and three water molecules.  refers to a coordination bond. There are unfavorable interactions between the GMP guanine group and Gln726 amide group as these two groups are nearly coplanar.
refers to a coordination bond. There are unfavorable interactions between the GMP guanine group and Gln726 amide group as these two groups are nearly coplanar.
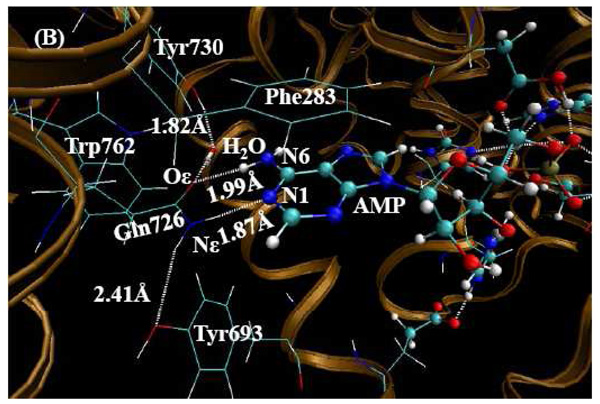
Two hydrogen bonds have been assigned between N1, N6 of AMP and amide group of Gln726 in PDE10-AMP complex. N1, as the hydrogen bond acceptor, forms a hydrogen bond with the hydrogen atom on amide nitrogen of Gln726, and N6 as the hydrogen bond donor forms a hydrogen bond with Oε in Gln726. Gln726 forms a hydrogen bond with Tyr693 and a water molecule as well. However, the orientation of the amide group of Gln726 in PDE10-GMP is the same as PDE10-AMP complex. Comparing the adenine group of AMP and guanine group of GMP, the hydrogen bond character appears to be reversed. Based on this assignment, the hydrogen bond donor and acceptor of Gln726 amide group suggest severe torsional strain with guanine group of GMP. Ke et al.19 reported only one hydrogen bond between Gln726 and GMP guanine group. In the X-ray crystal structure of PDE10-GMP complex, the distance between Nε atom of Gln726 and N1 of GMP is 2.69 Å, implying a strong hydrogen bond. However, there are two hydrogen atoms on Nε and one hydrogen atom on N1, thus, the hydrogen atoms on both nitrogen atoms exhibit steric hindrance with each other. The distance between Oε atom of Gln726 and O6 of GMP is 2.94 Å, which is too close for two oxygen atoms without forming a hydrogen bond between them. However, if we flip the positions of Oε and Nε atoms of Gln726, the Oε forms a hydrogen bond with the hydrogen atom on N1, and the hydrogen atom on Nε forms a hydrogen bond with O6. Notably, this rotation does not affect the hydrogen bond between Gln726 and Tyr693.
This glutamine residue (Gln726) is absolutely conserved in all PDEs families. In the X-ray crystal structures of PDE4-AMP (PDB code 1TB7) and PDE5-GMP (PDB code 1T9S) complexes, the amide oxygen and nitrogen atoms of this conserved glutamine are reversed. As a result, Zhang et al.20 proposed the “glutamine switch” mechanism for nucleotide selectivity by phosphodiesterase, although this “glutamine switch” mechanism has been questioned by other investigators who performed mutagenesis experiments. In cGMP-specific PDE5, this glutamine (Gln817) is stabilized by Gln775 and forms two hydrogen bonds with the GMP product. When Gln817 was mutated to alanine, the mutation only weakened PDE5-cGMP binding, but did not significantly affect the PDE5-cAMP binding.21 As a result, Ke at al.19 proposed a “substrate specificity pocket" or S-pocket mechanism, in which the amino acids in the active site of PDEs and the conformation of substrates cAMP and cGMP impact substrate specificity. It is our contention that this conserved glutamine is the key residue in determining specificity in some PDE enzymes, but it is not the key element for other PDE families.
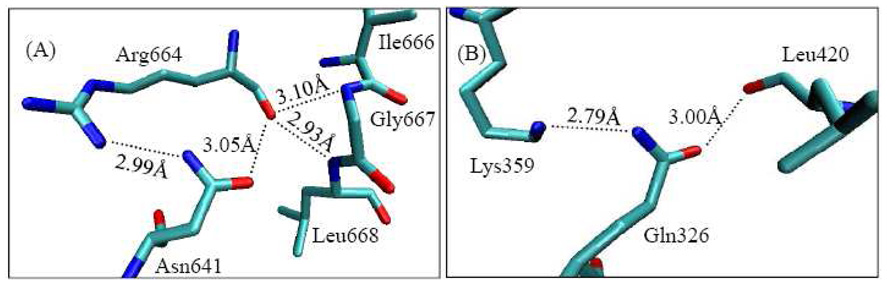
The amide groups in the side chains of asparagine (Asn) and glutamine (Gln) mostly act as hydrogen bond donors and acceptors in the X-ray crystal structures. It is difficult to distinguish the amide nitrogen and oxygen atoms as the electron density only traces the shape of the molecules, but does not clearly identify the exact atom except at an extremely high resolution. Also, the amide group of Asn and Gln may be flipped by 180° with essentially no affect on the electron density. Therefore the positions of the amide nitrogen and oxygen atoms should be assigned based on their chemical environments, i.e. the hydrogen bonding network around them. A number of studies over the past decade 22 , 23 , 24 , 25 , 26 have addressed the Asn and Gln amide assignment controversy. Approximately 20% of the current protein structure database24,27,28 contains incorrect Asn and Gln amide rotamer assignments. In the X-ray crystal structures of known PDE families, there are many Asn/Gln amide rotamers with sterically unfavorable interactions (see Figure 1). In order to confirm the correct Gln726 amide orientation, we used two web-based services to detect incorrect Asn/Gln rotamers of the PDE10-GMP complex. First, we used Lovell’s version to evaluate the X-ray crystal structure of PDE10-GMP complex by minimizing steric hindrances after adding hydrogen atoms to the enzyme system (http://molprobity.biochem.duke.edu/). The results indicated the Gln276 amide group should be flipped. In 2006, Sippl et al. 27,28,29 created NQ-Flipper which is a web service to automatically detect and correct erroneous Asn/Gln amide rotamers (https://flipper.services.came.sbg.ac.at/). According to the scores obtained by NQ-Flipper, Gln726 is rated as “ambiguous” as the scores are almost identical regardless of whether or not the amide group is flipped. NQ-Flipper does not consider ligands and non-standard groups, and scores of residues in the vicinity of non-protein groups are unreliable. We performed various high level QM/MM calculations in order to evaluate the Gln725 amide group and estimate the energy difference between the flipped and non-flipped rotamers.
Figure 1.
H-bonding interactions of amide group of Asn/Gln in X-ray crystal structures of PDEs. (A) The amide oxygen and nitrogen atoms of Asn641 have sterically unfavorable interactions with the main chain oxygen of Arg664 which has two H-bonds with backbone nitrogen atoms of Gly667 and Leu668 in 2OUQ (PDE10, resolution 1.9Å).19 (B) The amide oxygen and nitrogen atoms of Gln326 have sterically unfavorable interactions with the main chain oxygen of Leu420 and the ammonium ion of the side chain of Lys359 in PDB code 1ZXL (PDE7, resolution 1.67Å).30
Computational Methods
QM/MM calculations
All QM/MM calculations were performed using pseudobond QM/MM method.31,32 The pseudobond QM/MM method was initially implemented in revised Gaussian03 and Tinker programs.31,32 In this study, we used a newly revised version33,34,35 of Gaussian03 and Amber8 programs to perform the QM/MM calculations.
The initial PDE10-AMP and PDE10-GMP structures used in the paper were directly extracted from the corresponding X-ray crystal structure deposited in the Protein Data Bank (PDB code: 2OUN and 2OUQ).19 All hydrogen atoms were added to the enzyme using AMBER8.0 program,36 the standard protonation states at physiological condition (pH ≈ 7.4) were set to all ionizable residues. Then the structure with all added hydrogen atoms was minimized with all the heavy atoms fixed in about 3000 steps. This minimized structure was used to prepare the input file for both the quantum mechanical and molecular mechanics portions. The quantum part was optimized by employing density functional theory (DFT) using Becke’s three-parameter hybrid exchange functional and the Lee-Yang-Parr correlation functional (B3LYP)37,38,39 with the 6–31G(d) basis set40 in Gaussian03 suite.41 The molecular mechanics portion was calculated with all other atoms fixed 20 Å from the phosphorus atom of AMP or GMP using AMBER8.0 program. The quantum portion contains the ligand, two metal ions, the residues coordinated to the metal ions, an additional histidine (His525), and an additional glutamate (Glu695) (see Figures 2 to 4 where all QM atoms are shown as balls).
Figure 2.
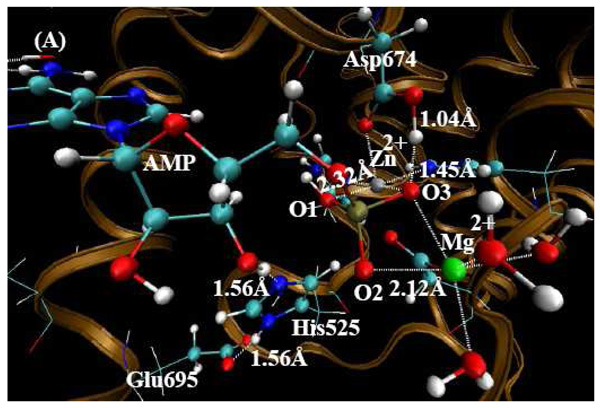
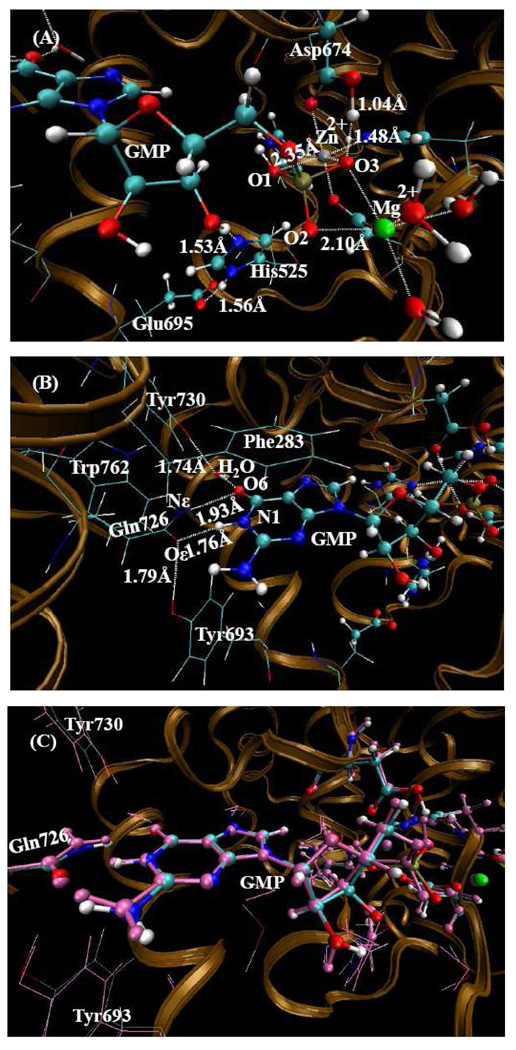
QM/MM-optimized PDE10-AMP structure (the charge on AMP is -1) at the B3LYP/6-31G*:Amber level. The QM atoms in the PDE10 active site are represented by balls. The lines and all the other atoms represent MM atoms. (A) From this orientation, one can clearly see the metal sites and the state of AMP. (B) From this orientation, one can clearly see the hydrogen bonded network around Gln726 residue.
Figure 4.
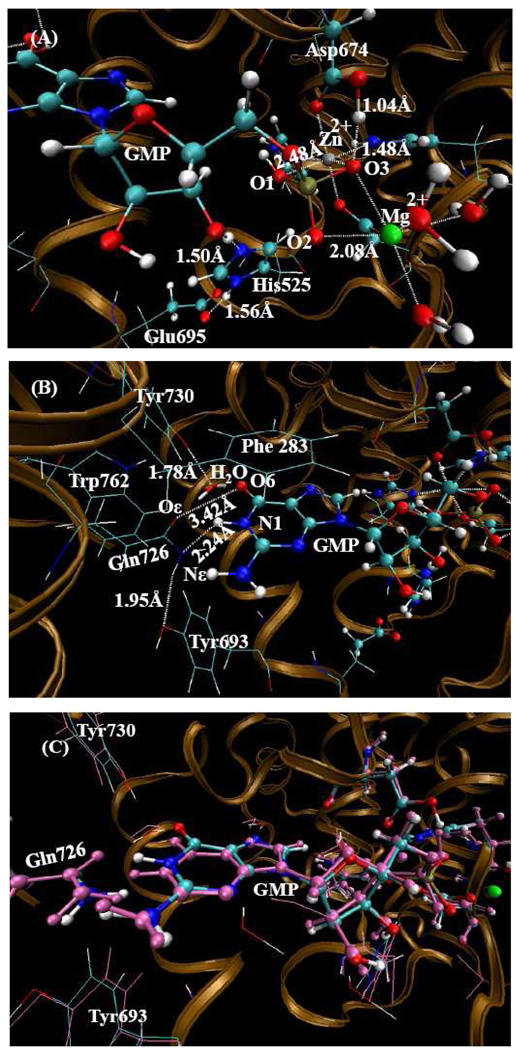
Geometry of the PDE10-GMP structure (the charge of GMP is −1) optimized by the QM/MM method at the B3LYP/6-31G*: Amber level. The QM atoms in the PDE10 active site are represented by balls. The lines and all the other atoms represent MM atoms. (A) In this orientation, one can see the metal sites and the state of GMP. (B) In this orientation, one can see the hydrogen bonding network around Gln726 residue after interchange of side chain atoms and the formation of two hydrogen bonds between Gln726 and guanine group of GMP. (C) The comparison between the QM/MM-optimized structure and the X-ray crystal structure in which the amide orientation of Gln726 is changed. The pink atoms represents the X-ray crystal structure added H-atoms, the atoms shown as the atom type represents the QM/MM-optimized structure.
Binding Free Energy Calculations
The binding free energies between PDE10 and the ligands were calculated with quantum mechanics/molecular mechanics (QM/MM) and Poisson-Boltzmann surface area model (PBSA). We have labeled the method as QM/MM-PBSA.
In the QM/MM-PBSA method, the free energy of a ligand binding with a protein, ΔGbind, is calculated from the difference between the free energy of the receptor-ligand complex (Gcpx) and the sum of the free energies of the unbound receptor (Grec) and ligand (Glig) as follows:
The binding free energy ΔGbind was evaluated as a sum of the changes in the QM/MM gas-phase binding energy (ΔEQM/MM), solvation free energy (ΔGsolv), and entropy contribution (−TΔS).
The QM/MM gas-phase binding energy ΔEQM/MM is partitioned into three terms: EQM/MM(com), E QM/MM(rec), and EQM(lig). EQM/MM(com) is calculated at the B3LYP/6-31G*:Amber level using the revised Gaussian03 and Amber8 software developed in our lab.33,34 EQM/MM(rec) was calculated at the same level using the QM/MM method as well. The receptor was optimized again after the ligand was deleted from the optimized complex structure. All quantum mechanical calculations contain the same atoms except the ligand atoms. For the ligand, we always used the lowest-energy geometry optimized at the B3LYP/6-31G* level and the corresponding energy.
The sum of ΔEQM/MM and ΔGsolv is denoted by ΔEbind. The solvation free energy is the sum of the electrostatic solvation free energy (ΔGPB) and the nonpolar solvation energy (ΔGnp). ΔGPB was calculated from the finite-difference solution to the Poisson-Boltzmann (PB) equation implemented in the Delphi program42,43 by using the same RESP charges as used in the MD simulations for the receptor and the quantum mechanically calculated ESP charges for the ligands. The dielectric constants used for the solute and solvent-water were 1 and 80, respectively. The MSMS program44 was used to calculate solvent-accessible surface area (SASA) from which the nonpolar solvation energy is determined, using parameter values, γ = 0.00542 kcal/Å2 and β = 0.92 kcal/mol. The standard van der Waals radii built into the Amber program for solvation calculations were also used.
The entropy contribution to the binding free energy (−TΔS) was obtained by using a local program developed in our laboratory. In this method, the entropy contribution is attributed to two terms, solvation free entropy (ΔSsolv) and conformational free entropy (ΔSconf).45
Results and Discussion
Depicted in Figure 2 is the QM/MM-optimized geometry of PDE10-AMP complex with O1 protonated. In Table 1, several important geometric parameters of PDE10-AMP complex involving metal ions are included for comparison with the corresponding distances in the X-ray crystal structure.
Table 1.
a Key internuclear distances (Å) involving the metal ions of the QM/MM-optimized geometries of PDE10-AMP complex in comparison with the corresponding distances in the X-ray crystal structure (PDB code: 2OUN).19
| Internuclear distances | PDE10-AMP (O3H) b | PDE10-AMP (O1H,O3H)c | PDE10-AMP (O2H,O3H)d | Expt. |
|---|---|---|---|---|
| Zn(II)-O (OP-bridging) | 2.718 | 2.526 | 2.663 | 2.457 |
| Zn(II)-O (Asp-bridging) | 2.088 | 2.000 | 2.075 | 2.050 |
| Zn(II)-O (Asp-Zn) | 2.042 | 2.148 | 2.133 | 2.074 |
| Zn(II)-N (His1) | 2.022 | 2.031 | 2.024 | 2.096 |
| Zn(II)-N (His2) | 2.099 | 2.044 | 2.071 | 2.103 |
| Zn(II)-O (PO4) | 2.084 | 2.319 | 2.085 | 2.371 |
| Mg(II)-O (OP-bridging) | 2.200 | 2.219 | 2.140 | 2.299 |
| Mg(II)-O (Asp-briding) | 2.068 | 2.064 | 2.085 | 2.080 |
| Mg(II)-O (W1) | 2.116 | 2.064 | 2.085 | 2.190 |
| Mg(II)-O (W2) | 2.079 | 2.053 | 2.090 | 2.121 |
| Mg(II)-O (W3) | 2.067 | 2.061 | 2.095 | 2.084 |
| Mg(II)-O (PO4) | 2.016 | 2.124 | 2.137 | 2.249 |
| Zn(II)-Mg(II) | 4.243 | 4.129 | 4.146 | 4.000 |
| O3'-OP(Mg(II)) | 3.378 | 2.963 | 2.735 | 2.989 |
| O3'-OP(Zn(II)) | 3.618 | 3.277 | 3.643 | 3.332 |
| N6-OE1(5GP-GLN726) | 2.934 | 3.003 | 2.986 | 3.039 |
| N1-NE2(5GP-GLN726) | 2.888 | 2.884 | 2.892 | 2.996 |
| MUE (Å) e | 0.14 | 0.07 | 0.12 |
The geometries were fully optimized by performing QM/MM calculations at the B3LYP/6-31G*:Amber level.
The charge on AMP = −1 with O3 protonated in the original file. This proton still resides on O3 atom after the QM/MM geometry optimization.
The charge on AMP = 0 with O1 and O3 protonated in the original file. The proton on O3 atom migrated to Oδ atom of Asp674 after the QM/MM geometry optimization. So O1 was only protonated after the geometry optimization.
The charge on AMP = 0 with O2 and O3 protonated in the original file. The proton on O3 atom migrated to Oδ atom of Asp674, the proton on O2 transferred to O3´, the proton on O3´ transferred to His525 after the QM/MM geometry optimization. Therefore, no phosphate oxygen atoms are protonated, and His525 is protonated after the geometry optimization.
The mean unsigned error between the calculated and experimental distances.
The protonated state of AMP and GMP
The products AMP and GMP in both PDE10-AMP and PDE10-GMP complexes exhibit similar interactions with the two metal ions in the active site. Phosphate oxygen O1 coordinates only with Zn(II) ion and O2 coordinates with Mg(II) ion. Phosphate oxygen O3 acts as a bridging atom connecting two metal ions and forms one strong hydrogen bond with Oδ of Asp674. This bonding arrangement implies that there must be a hydrogen atom between O3 and Oδ of Asp674. Therefore in preparing the QM/MM calculation a hydrogen atom on O3 was added as there is no protonated aspartic acid parameter in Amber8. There are two protonated states for free AMP or GMP molecules. In state one, only one phosphate oxygen is protonated, whereas in state two, two phosphate oxygen atoms are protonated. Thus there are three distinct QM/MM calculations according to the protonated state of AMP or GMP - (1) only O3 is protonated; (2) both O1 and O3 are protonated; and (3) O2 and O3 are protonated.
In Table 1, several important internuclear distances (Å) are reported comparing metal ions in the QM/MM-optimized geometries of PDE10-AMP complex with the corresponding distances in the X-ray crystal structures. When O3 is protonated (the charge on AMP is -1), the mean unsigned error (i.e. the average of the absolute values of the distance errors) is 0.14 Å between the calculated distances and X-ray crystal data. Second, when O1 and O3 are both protonated, the proton on O3 migrates to the Oδ atom of Asp674, so the charge of AMP of -1 is conserved. The optimized structure is much closer to the X-ray crystal structure. The mean unsigned error is only 0.07 Å compared to the experimental data. However, when O2 and O3 are both protonated, the proton on O3 again migrates to the Oδ atom on Asp674, while the proton on the hydrolyzed O3' atom is transferred to the Nε atom of His525 making His525 protonated and the proton on O2 is transferred to the hydrolyzed O3' atom. In this scenario, the charge of AMP is -2 with His525 protonated in the optimized structure. The calculated Zn(II)-O1 and Zn(II)-O3 distances are 0.2~0.3 Å longer than the corresponding experimental distances. The mean unsigned error is 0.12 Å in comparison to the X-ray crystal structure. After comparing all the optimized results, the assigned charge on AMP is -1 with O1 protonated in PDE10- AMP complex (see Figure 2). When and only when O1 is protonated, the calculated Zn(II)-O3 and Zn(II)-O1 distances are reasonably consistent with the corresponding experimental distances in the X-ray crystal structure.
List in Table 2 are important internuclear distances with the Gln amide group flipped (and without the Gln amide group flipped) involving the metal ions in the QM/MM-optimized geometries of PDE10-GMP complex along with the corresponding distances in the X-ray crystal structure. When O3 is protonated with a −1 charge on GMP, the proton on O3 migrates to the Oδ atom on Asp674, resulting in the charge on GMP increasing to −2. The distance between Zn(II) ion and O3 is 2.83 Å which is 0.6 Å longer than the experimental distance. And the distance between Zn(II) ion and O1 is 2.05 Å which is 0.48 Å longer than the experimental distance. When O1 and O3 are both protonated, the proton on O3 migrates to the Oδ atom of Asp674, so the charge on GMP remains at −1. The optimized structure is very close to the X-ray crystal structure in terms of the key internuclear distances, i.e. the Zn(II)-O3 and Zn(II)-O1 distances. The calculated mean unsigned error is only 0.13 Å between the calculated distances and X-ray crystal data. However, when O2 and O3 are both protonated, the proton on O3 again migrated to the Oδ atom of Asp674; and the proton on hydrolyzed O3' atom transfers to the Nε atom of His525 which makes His525 protonated; and the proton on O2 transfers to the hydrolyzed O3' atom. As a result, the charge of GMP increases to −2 with His525 protonated in the optimized structure. After comparing all the optimized results, the charge on GMP should be −1 with O1 protonated in PDE10-GMP complex. When O1 and O3 (or O2 and O3) are protonated, the proton on O3 also transfers to the Oδ atom of Asp674. The calculated mean unsigned error is 0.24 (0.23), 0.13 (0.22), and 0.21 (0.25) Å, respectively, for those three optimized structures in comparison with experimental data. Thus, the optimized structure mirrors most closely the X-ray crystal structure, particularly for the Zn(II)-O3 and Zn(II)-O1 distances, when and only when O1 is protonated (see Figures 3 and 4).
Table 2.
a Several important internuclear distances (Å) involving the metal ions in the QM/MM-optimized geometries of PDE10-GMP complex in comparison with the corresponding distances in the X-ray crystal structure (PDB code 2OUQ). 19
| Internuclear distances | PDE10-GMP (O3H) b | PDE10-GMP (O1H,O3H) c | PDE10-GMP (O2H,O3H) d | Expt. |
|---|---|---|---|---|
| Zn(II)-O (OP-bridging) | 2.830 (2.821) | 2.310 (2.399) | 2.799 (2.819) | 2.227 |
| Zn(II)-O (Asp-bridging) | 2.061 (2.062) | 2.005 (2.006) | 2.066 (2.089) | 2.052 |
| Zn(II)-O (Asp-Zn(II)) | 2.120 (2.133) | 2.156 (2.142) | 2.130 (2.139) | 2.072 |
| Zn(II)-N (His1) | 2.034 (2.023) | 2.045 (2.035) | 2.020 (2.013) | 2.108 |
| Zn(II)-N (His2) | 2.101 (2.096) | 2.036 (2.037) | 2.078 (2.065) | 2.108 |
| Zn(II)-O (PO4) | 2.051 (2.049) | 2.502 (2.479) | 2.092 (2.065) | 2.532 |
| Mg(II)-O (OP-bridging) | 2.156 (2.150) | 2.267 (2.245) | 2.123 (2.140) | 2.356 |
| Mg(II)-O (Asp-briding) | 2.098 (2.098) | 2.042 (2.042) | 2.071 (2.092) | 2.080 |
| Mg(II)-O (W1) | 2.086 (2.083) | 2.084 (2.125) | 2.128 (2.132) | 2.241 |
| Mg(II)-O (W2) | 2.091 (2.090) | 2.084 (2.063) | 2.085 (2.090) | 2.271 |
| Mg(II)-O (W3) | 2.068 (2.071) | 2.072 (2.079) | 2.092 (2.103) | 2.299 |
| Mg(II)-O (PO4) | 2.043 (2.044) | 2.099 (2.084) | 2.135 (2.111) | 2.451 |
| Zn(II)-Mg(II) | 4.177 (4.155) | 4.023 (4.064) | 4.201 (4.180) | 3.870 |
| O3'-OP(Mg(II)) | 3.162 (3.141) | 2.964 (2.901) | 2.792 (2.688) | 2.612 |
| O3'-OP(Zn(II)) | 3.525 (3.562) | 3.088 (3.088) | 3.461 (3.666) | 2.867 |
| O6-NE2(5GP-GLN726) | 2.893 (3.402) | 2.938 (3.419) | 2.919 (3.416) | 2.939 |
| N1-OE1(5GP-GLN726) | 2.798 (2.971) | 2.778 (2.992) | 2.793 (3.054) | 2.687 |
| MUE (Å) e | 0.24 (0.23) | 0.13 (0.22) | 0.21 (0.25) |
The geometries were fully optimized by performing QM/MM calculations at the B3LYP/6-31G*:Amber level. The values outside the parentheses represent flipped amide group of Gln726, and the values inside the parentheses represent the calculated distances when maintaining the original amide orientation of Gln726.
The charge on GMP = −1 with O3 protonated in the original file. This proton on O3 migrated to Oδ atom of Asp674 after the QM/MM geometry optimization. Also there were no phosphate oxygens protonated after the QM/MM geometry optimization.
The charge on GMP = 0 with O1 and O3 protonated in the original file. The proton on O3 atom migrated to Oδ atom of Asp674 after the QM/MM geometry optimization. Also only O1 was protonated after the QM/MM geometry optimization.
The charge on GMP = 0 with O2 and O3 protonated in the original file. The proton on O3 atom migrated to Oδ atom of Asp674; the proton on O2 migrated to O3´; the proton on O3´ migrated to His525 after the QM/MM geometry optimization. Also no phosphate oxygens were protonated, and His525 was protonated after the QM/MM geometry optimization.
The mean unsigned error between the calculated and experimental distances.
Figure 3.
QM/MM-optimized PDE10-GMP structure (the charge on GMP is −1) at the B3LYP/6-31G*:Amber level. The QM atoms in the PDE10 active site are represented by balls. All MM atoms are represented as lines. (A) From this orientation, one can see the metal sites and the state of GMP. (B) From this orientation, one can see the hydrogen bonding network around Gln726 residue and one weak hydrogen bond between Gln726 and guanine group of GMP. (C) The comparison between the QM/MM-optimized structure and the X-ray crystal structure while maintaining the original amide position of Gln726. The pink atoms represent the X-ray crystal structure added H-atoms, and the remaining atom-types represent the QM/MM-optimized structure.
The amide oxygen and nitrogen orientation in PDE10-GMP complex
When the original position of the X-ray crystal structure of amide oxygen and nitrogen atoms of Gln726 residue for PDE10-GMP complex were maintained, the guanine group moved up and the amide of Gln726 moved down following QM/MM optimization. As a result, the hydrogen on the amide nitrogen and N1 atom of GMP interfere strongly with each other, and the distance between Oε atom of Gln726 and O6 of GMP is 2.94 Å which is too close for two oxygen atoms without an intervening hydrogen bond. These two steric repulsions force the amide group of Gln726 and guanine group of GMP to move away from each other, resulting in a change in the distance between Oε and O6 to approximately 3.4 Å relative to the original 2.94 Å distance. The distance between Nε and N1 changed to 2.99 Å compared to the original 2.69 Å distance. Figure 3C shows the comparison between the QM/MM optimized structure and the X-ray crystal structure when the original amide position of Gln726 is maintained. One can clearly see that the amide group of Gln726 moved down and guanine group of GMP moved up. This result implies that these four atoms could not possibly be this close to each other if the assignments from the X-ray crystal structure are maintained.
When the positions of the amide oxygen and nitrogen atoms of Gln726 residue are interchanged and following the QM/MM geometry optimization, the positions of Gln726 and guanine group of GMP are nearly identical to the original experimental positions. In this atomic arrangement, two strong hydrogen bonds between Gln726 and guanine group are formed, and there is one hydrogen bond between Gln726 and Tyr693. Figure 4C shows the comparison between the QM/MM-optimized structure after interchange of the amide oxygen and nitrogen atoms of Gln726 with the X-ray crystal structure. One can see the formation of two strong hydrogen bonds between the amide group of Gln726 and guanine group of GMP molecule. In addition, the rest of the hydrogen bonding network looks quite reasonable.
Binding free energy calculations were also performed on both the QM/MM optimized structure while maintaining the original Gln726 position and the interchanged version for PDE10-GMP system. The calculated binding free energy for the interchanged structure is ~22 kcal/mol lower than the original one, as seen in Table 3. The significant energy decrease is mainly derived from two hydrogen bonds formed between the amide group of Gln726 and guanine group of GMP. This confirms that the amide oxygen and nitrogen atoms in PDE10-AMP should be different from PDE10-GMP. As a result, PDE10 can hydrolyze both cAMP and cGMP primarily because Gln726 can flip the amide group and bind to both cyclic nucleotides.
Table 3.
Energetic considerations (kcal/mol) obtained from the QM/MM-PBSA calculations at T = 298.15 K and P = 1 atm for the GMP binding with PDE10.
| GMP (O1H,O3H)-flip-Glu726 a | GMP (O1H,O3H) b | |
|---|---|---|
| ΔEQM/MM(gas) | −206.6 | −196.8 |
| ΔGsolv | 109 | 122.2 |
| ΔE (bind) or ΔH | −97.6 | −74.6 |
| −TΔS | 24.9 | 23.9 |
| ΔGbind(aqueous) | −72.7 | −50.7 |
| ΔGbindrela(aqueous) | 0.0 | 22.0 |
The charge on GMP = 0 with O1 and O3 protonated in the original file. The proton on O3 atom migrated to Oδ atom of Asp674 after the QM/MM geometry optimization. O1 was protonated only after the geometry optimization. The amide orientation of Glu726 was interchanged relative to the X-ray crystal structure.
The charge on GMP = 0 with O1 and O3 protonated in the original file. The proton on O3 atom migrated to Oδ atom of Asp674 after the QM/MM geometry optimization. O1 was protonated only after the geometry optimization. The original-ray crystal structure amide orientation of Glu726 was maintained.
Conclusion
Quantum Mechanical/Molecular Mechanical (QM/MM) geometry optimizations starting from the X-ray crystal structure have been performed to characterize the PDE10 structure and the state of the products AMP and GMP in the PDE10-AMP and PDE10-GMP complexes. All results suggest that there is only one phosphate oxygen atom (O1) protonated for both AMP and GMP molecules in the product complexes.
The conflict between the amide group of Gln726 and guanine group of GMP in the X-ray crystal structure has been resolved by flipping the amide group of Gln726. As a result of the interchange of the amide nitrogen and oxygen atoms, two strong hydrogen bonds between the amide group of Gln726 and guanine group of GMP are formed. Binding free energies were calculated for the QM/MM-optimized structures where in one case the amide position of Gln726 in the X-ray crystal structure was maintained and in the second case where the amide group is flipped in the PDE10-GMP system. The calculated binding free energy in the flipped case is ~22 kcal/mol lower than the X-ray crystal structure. The significant energy decrease is derived mainly from the formation of two hydrogen bonds between the amide group of Gln726 and guanine group of GMP. This implies that the amide oxygen and nitrogen atoms in PDE10-AMP are different from PDE10-GMP. Therefore, PDE10 can hydrolyze both cAMP and cGMP. Furthermore, our study concludes that the conformation of the amide oxygen and nitrogen atoms in PDE10-AMP is different from that in PDE10-GMP.
Acknowledgments
The research was supported by the NIH (grant RC1MH088480). The authors also acknowledge the Center for Computational Sciences (CCS) at University of Kentucky for supercomputing time on IBM X-series Cluster with 340 nodes or 1,360 processors.
References
- 1.Callahan SM, Cornell NW, Dunlap PV. J. Biol. Chem. 1995;270:17627–17632. doi: 10.1074/jbc.270.29.17627. [DOI] [PubMed] [Google Scholar]
- 2.Conti M, Jin SLC, Monaco L, Repaske DR, Swinnen JV. Endocr. Rev. 1991;12:218. doi: 10.1210/edrv-12-3-218. [DOI] [PubMed] [Google Scholar]
- 3.Houslay MD. Semin. Cell Dev. Biol. 1998;9:161–167. doi: 10.1006/scdb.1997.0221. [DOI] [PubMed] [Google Scholar]
- 4.Conti M, Jin SLC. Prog. Nucleic. Acid. Res. 2002;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- 5.Mehats C, Andersen CB, Filopanti M, Jin SLC, Conti M. Trends Endocrin. Met. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 6.Park JY, Richard F, Chun SY, Park JH, Law E, Horner K, Jin SLC, Conti M. Mol. Endocrinol. 2003;17:1117–1130. doi: 10.1210/me.2002-0435. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HT, Steketee JD, Jin SLC, Conti M, O'Donnell JM. FASEB J. 2003;17:A206–A207. [Google Scholar]
- 8.Houslay MD, Adams DR. Biochem. J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamato S, Shoda R, Akiyama J, Uemura N, Tokuhara M, Shimizu T, Matsueda K. Gastroenterology. 2003;124:A579–A579. [Google Scholar]
- 10.Francis SH, Turko IV, Corbin JD. Prog. Nucleic. Acid. Res. Mol. Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 11.Karnam SM, Huiping Z, Gabriel MM. Am. J. Physiol. Cell Physiol. 2001;282:C508–C517. [Google Scholar]
- 12.Manganiello V. Mol. Pharmacol. 2003;63:1209–1211. doi: 10.1124/mol.63.6.1209. [DOI] [PubMed] [Google Scholar]
- 13.Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K. J. Biol. Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- 14.Soderling SH, Bayuga SJ, Beavo JA. Proc. Natl. Acad. Sci. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loughney K, Snyder PB, Uher L, Rosman GJ, Ferguson K, Florio VA. Gene. 1999;234:109–117. doi: 10.1016/s0378-1119(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 16.Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, Shrikhande A, Wong S, Menniti FS, Schmidt CJ. Neuropharmacology. 2006;51:386–396. doi: 10.1016/j.neuropharm.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Siuciak JA, McCarthy SA, Chapin DS, Fujiwara RA, James LC, Williams RD, Stock JL, McNeish JD, Strick CA, Menniti FS, Schmidt CJ. Neuropharmacology. 2006;51:374–385. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Rodefer JS, Murphy ER, Baxter MG. Eur. J. Neurosci. 2005;21:1070–1076. doi: 10.1111/j.1460-9568.2005.03937.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Liu Y, Hou J, Zheng M, Robinson H, Ke H. Prog. Nucleic. Acid. Res. 2007;104:5782–5787. doi: 10.1073/pnas.0700279104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang KY, Card GL, Suzuki Y, Artis DR, Fong D, Gillette S, et al. Mol. Cell. 2004;15:279–286. doi: 10.1016/j.molcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Zoraghi R, Corbin JD, Francis SH. J. Biol. Chem. 2006;281:5553–5558. doi: 10.1074/jbc.M510372200. [DOI] [PubMed] [Google Scholar]
- 22.McDonald IK, Thornton JM. Protein Eng. 1995;8:217–224. doi: 10.1093/protein/8.3.217. [DOI] [PubMed] [Google Scholar]
- 23.Hooft RW, Sander C, Vriend G. Proteins. 1996;26:363–376. doi: 10.1002/(SICI)1097-0134(199612)26:4<363::AID-PROT1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Word JM, Lovell SC, Richardson JS, Richardson DC. J. Mol. Biol. 1999;285:1735–1747. doi: 10.1006/jmbi.1998.2401. [DOI] [PubMed] [Google Scholar]
- 25.Lovell SC, Davis IW, Arendall WB., III Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 26.Higman VA, Boyd J, Smith LJ, Redfield C. J. Biomol. NMR. 2004;30:327–346. doi: 10.1007/s10858-004-3218-y. [DOI] [PubMed] [Google Scholar]
- 27.Weichenberger CX, Sippl MJ. Structure. 2006;14:967–972. doi: 10.1016/j.str.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Weichenberger CX, Sippl MJ. Bioinformatics. 2006;22:1397–1398. doi: 10.1093/bioinformatics/btl128. [DOI] [PubMed] [Google Scholar]
- 29.Weichenberger CX, Sippl MJ. Nucleic Acids Res. 2007;35:W403–W406. doi: 10.1093/nar/gkm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Liu Y, Hou J, Chen Y, Robinson H, Ke H. J. Biol.Chem. 2005;280:30949–30955. doi: 10.1074/jbc.M504398200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Lee T, Yang WJ. Chem. Phys. 1999;110:46–54. [Google Scholar]
- 32.Zhang YJ. Chem. Phys. 2005;122:024114. doi: 10.1063/1.1834899. [DOI] [PubMed] [Google Scholar]
- 33.Zheng F, Yang W, Ko M-C, Liu J, Cho H, Gao D, Tong M, Tai H-H, Woods JH, Zhan C-G. J. Am. Chem. Soc. 2008;130:12148. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Hamza A, Zhan C-G. J. Am. Chem. Soc. 2009;131:11964. doi: 10.1021/ja903990p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Y, Liu J, Yang G, Zhan C-G. J. Comput. Chem. 2009 doi: 10.1002/jcc.21356. in press (online version available) [DOI] [PubMed] [Google Scholar]
- 36.Case DA, Darden TA, Cheatham TE, I, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Wang B, Pearlman DA, Crowley M, Brozell S, Tsui V, Gohlke H, Mongan J, Hornak V, Cui G, Beroza P, Schafmeister C, Caldwell JW, Ross WS, Kollman PA. AMBER 8. San Francisco: University of California; 2004. [Google Scholar]
- 37.Becke AD. J. Chem. Phys. 1993;98:5648. [Google Scholar]
- 38.Lee C, Yang W, Parr RG. Phys. ReV. B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 39.Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J. Phys. Chem. 1994;98:11623. [Google Scholar]
- 40.Hehre WJ, Radom L, Schleyer PR, Pople JA. Ab Initio Molecular Orbital Theory. New York: John Wiley & Sons; 1987. [Google Scholar]
- 41.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03,revision A.1. Pittsburgh, PA: Gaussian, Inc.; 2003. [Google Scholar]
- 42.Gilson MK, Sharp KA, Honig BH. J. Comput. Chem. 1988;9:327. [Google Scholar]
- 43.Jayaram B, Sharp KA, Honig BH. Biopolymers. 1989;28:975. doi: 10.1002/bip.360280506. [DOI] [PubMed] [Google Scholar]
- 44.Sanner MF, Olson AJ, Spehner JC. Biopolymers. 1996;38:305. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C305::AID-BIP4%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Pan Y, Gao D, Zhan C-G. J. Am. Chem. Soc. 2008;130:5140. doi: 10.1021/ja077972s. [DOI] [PMC free article] [PubMed] [Google Scholar]