The congenital dyserythropoietic anemias comprise a group of very rare hereditary disorders characterized by ineffective erythropoiesis and by distinct morphological abnormalities of the erythroblasts in the bone marrow. The classification proposed in 19681 is still used today.2, 3 Morphological analysis is the first step in the diagnosis of all types of congenital dyserythropoietic anemia, to be followed by confirmatory tests. The morphological hallmarks of the erythroblasts are not per se specific, but may also be observed as single abnormalities in other disorders of erythropoiesis. We, therefore, attempted to evaluate the quantity of the characteristic morphological abnormalities in patients with CDAI and CDA II.
Smears of peripheral blood and aspirated bone marrow were obtained from the German Registry on Congenital Dyserythropoietic Anemias. All specimens were originally used for diagnostic purposes. Patients were asked for informed consent for their use for scientific research, in agreement with the decision of the ethical committee of the University of Ulm. All patients were analyzed using a unique patient’s number (CDA-UPN) that does not permit recourse of identifying data. Specimens were stained by May-Grünwald Giemsa stain: 1,000 cells were examined for abnormalities. Occurrence of basophilic stippled cells or nucleated erythrocytes in the peripheral blood was listed but not quantified. The relative fraction of erythroblasts was expressed as the ratio of erythroblasts to granulopoietic cells. Macrophages were examined for birefringence under polarized light. The Mann-Whitney –U-test was used to compare cases and controls.
The diagnosis was based on parameters described previously.4,5 Confirmation of the diagnosis of CDA I required a mutation of the CDAN1-gene and/or typical aberrations seen by electron microscopy.2,6 Confirmation of the diagnosis of CDA II required a mutation of the SEC23B-gene or at least one of the following parameters: positive acid serum lysis test with ABO-compatible sera,7 a typical abnormality of band 3 shown by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE),8,9 or a discontinuous double membrane in mature erythroblasts seen by electron microscopy.2 Specimens of 19 (5 males, 14 females) and 36 patients (21 males, 15 females) with CDAI and CDA II, respectively, were available.
Specimens of 10 patients with distinct erythroid hyperplasia were selected as controls (6 with hemolytic anemia, 2 with myeloid dysplastic syndromes, one with thalassemia intermedia, and one with bleeding and iron deficiency).
Peripheral blood showed distinct anisopoikilocytosis in all cases, with grade 2 (25–50% poikilocytes) in 19 (34%), and grade 3 (> 50%) in 36 (66%) patients. There was no difference between types I and II (P=0.34). A few mature erythroblasts were seen in specimens of 31 and basophilic stippled red cells in 36 cases. These changes are highly sensitive but in no way specific. All types of poikilocytes such as ovalocytes, microspherocytes, tear drop cells or irregularly contracted cells are seen. In CDA I, large irregularly shaped macrocytes are seen and may suggest megaloblastic disease. The variegated appearance as described permits the exclusion of hereditary spherocytosis, an erroneous diagnosis that was often made before the diagnosis of CDA I or II was recognized.4,5 Mature erythroblasts may also be found in other types of severe anemia, but rarely in moderate anemias with a hemoglobin concentration of 90–110 g/L characteristic for most patients with congenital dyserythropoietic anemias.2,3
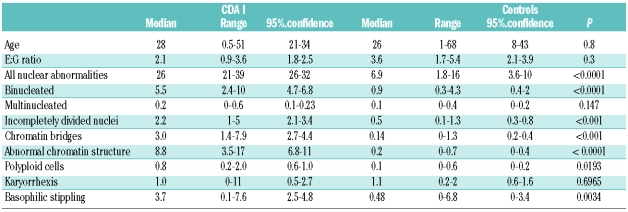
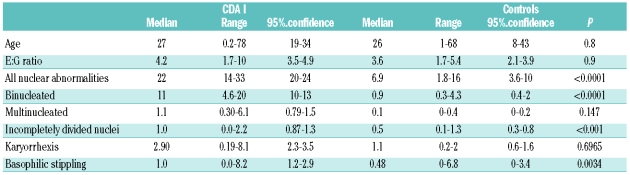
Marrow hypercellularity and distinct erythropoietic hyperplasia were present in all patients. The relative frequency of the pertinent abnormalities of erythroblasts, as compared to controls, is shown in Tables 1 and 2. Highly significant differences were found for binucleated cells, abnormalities of chromatin structures, chromatin bridges between erythroblasts, incompletely divided and large polyploid cells (See Online Supplementary Appendix).
Table 1.
Erythroblasts in specimens of patients with CDA I (n =19) and controls (n = 10). Data except E:G ratio are given in % of all erythroblasts.
Table 2.
Erythroblasts in specimens of patients with CDA II (n =36) and controls (n = 10). Data except E:G ratio are given in % of all erythroblasts.
Pseudo-Gaucher cells containing birefringent needles were seen in 23 (63%) cases with CDA II. Also, in 3 patients with CDA I but not in controls some birefringent material in macrophages was detected.
In clinical practice, evidence of any type of congenital dyserythropoietic anemias is primarily based on the morphology of peripheral blood and bone marrow, although confirmation of the diagnosis of the two most frequent types CDA I and CDA II is based on more refined tests. These tests are expensive and available in only a few specialized laboratories. The correct diagnosis of congenital anemias is often delayed.4,5 Many hematologists or clinical pathologists have never seen a case of CDA and do not recognize the well known morphological abnormalities or because, by misinterpretation of clinical and laboratory findings, a bone marrow biopsy is not performed. On the other hand, the diagnosis of a congenital dyserythropoietic anemia is often erroneously suspected, since the observer overvalues the presence of abnormalities that can be seen in the CDAs but also in other more common red cell disorders.
If an appropriate technique of preparation of bone marrow is used, hypercellularity can be recognized, and is always seen in histobiopsies (data not shown). The relative frequency of red cell precursors in the bone marrow is increased, with a mean E:G ratio of 4 and 8 times the normal in CDA I and CDA II. Previous experience in normal adults showed a range of 0.2 –1.0, corresponding to data on 50 and 67 adults published by Bain10 and den Ottolander,11 respectively. These investigators found a range of 0.2–0.9 ( mean 0.42, 95% confidence limits 0.2–0.9) and a range of 0.24–0.80 (mean 0.46, 95% confidence limits 0.42–1.2), respectively.
The diagnosis of CDA I can be made with high specificity from morphological analysis by light microscopy alone, when aberrations of the erythroblast nuclei as listed in Table 1 are present in more than 20% of these cells. Abnormal chromatin structure and the fine chromatin bridges are the most specific changes. They are not seen in normal bone marrow, but are occasionally observed in single cells in patients with myelodysplastic syndromes or erythroleukemia. In CDA II, the most specific finding is the presence of binucleated cells with equal size of two nuclei. As shown in Table 2, the fraction of such binucleated cells is usually more than 10% of all erythroid precursors. If the fraction of binucleated erythroblasts is related to the compartment of late polychromatic and mature oxyphilic erythroblasts, the 95% confidence interval is 13–16%. No more than 2% of binucleated cells may be found in normal individuals10 or a variety of red cell disorders with erythroid hyperplasia. If more than 10% of typical binucleated erythroblasts are seen, together with more than 2% of cells with karyorrhexis, the diagnosis of CDA II is almost confirmed, and confirmatory tests such as sequencing the SEC23B gene are indicated. Pseudo-Gaucher macrophages containing birefringent needles are found in the majority of cases, but in contrast to previous belief do not permit the differential diagnosis as compared with CDA I.
In conclusion, the diagnosis of CDA I and CDA II can be made with high reliability from diligent analysis of peripheral blood and technically appropriate specimens of aspirated bone marrow. However, the results of this study clearly demonstrate that their specificity for the diagnosis of a congenital dyserythropoietic anemia relies not on their presence but rather on the mosaic of morphological aberrations and the frequency of their occurrence.
Acknowledgments
the authors would like to thank Rosi Leichtle, documentation manager of the German Registry on Congenital Dyserythropoietic Anemias, Sara Öz and Verena Reitmayer for technical support, and the many physicians for contributing data and blood and bone marrow specimens.
Footnotes
Funding: this work was supported by the University of Ulm and the Else Kröner-Fresenius Stiftung Bad Homburg, Germany.
References
- 1.Heimpel H, Wendt F. Congenital dyserythropoietic anemia with karyorrhexis and multinuclearity of erythroblasts. Helv Med Acta. 1968;34(2):103–15. [PubMed] [Google Scholar]
- 2.Wickramasinghe SN. Congenital dyserythropoietic anemias: clinical features, haematological morphology and new biochemical data. Blood Rev. 1998;12(3):178–200. doi: 10.1016/s0268-960x(98)90016-9. [DOI] [PubMed] [Google Scholar]
- 3.Heimpel H, Iolascon A. Congenital dyserythropoietic anemia. In: Beaumont C, Beris Ph, Beuzard Y, Brugnara C, editors. Disorders of homeostasis, erythrocytes, erythropoiesis. 2 ed. Paris: European School of Haematology; 2009. pp. 178–201. [Google Scholar]
- 4.Heimpel H, Anselstetter V, Chrobak L, Denecke J, Einsiedler B, Gallmeier K, et al. Congenital dyserythropoietic anemia type II: epidemiology, clinical appearance, and prognosis based on long-term observation. Blood. 2003;102(13):4576–81. doi: 10.1182/blood-2003-02-0613. [DOI] [PubMed] [Google Scholar]
- 5.Heimpel H, Schwarz K, Ebnöther M, Goede JS, Heydrich D, Kamp T, et al. Congenital dyserythropoietic anemia type I (CDA I): Molecular genetics, clinical appearance and prognosis based on long-term observation. Blood. 2006;107(1):334–40. doi: 10.1182/blood-2005-01-0421. [DOI] [PubMed] [Google Scholar]
- 6.Heimpel H, Forteza-Vila J, Queisser W, Spiertz E. Electron and light microscopic study of the erythropoiesis of patients with congenital dyserythropoietic anemia. Blood. 1971;37(3):299–310. [PubMed] [Google Scholar]
- 7.Crookston JH, Crookston MC, Burnie KL, Francombe WH, Dacie JV, Davis JA, et al. Hereditary erythroblastic multinuclearity associated with a positive acidified-serum test: a type of congenital dyserythropoietic anemia. Br J Haematol. 1969;17(1):11–26. doi: 10.1111/j.1365-2141.1969.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 8.Anselstetter V, Horstmann K, Heimpel H. Congenital dyserythropoetic anaemia, types I and II: Aberrant pattern of erythrocyte membrane proteins in CDA II, as revealed by two-dimensional polyacrylamide gel electrophoresis. Br J Haematol. 1977;35(2):209–15. doi: 10.1111/j.1365-2141.1977.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 9.Delaunay J. Genetic disorders of the red cell membrane. Crit Rev Oncol Hematol. 1995;19(2):79–110. doi: 10.1016/1040-8428(94)00139-k. [DOI] [PubMed] [Google Scholar]
- 10.Bain BJ. The bone marrow aspirate of healthy subjects. Br J Haematol. 1996;94(1):206–9. doi: 10.1046/j.1365-2141.1996.d01-1786.x. [DOI] [PubMed] [Google Scholar]
- 11.Den Ottolander GJ. The bone marrow aspirate of healthy subjects. Br J Haematol. 1996;95(3):574–5. [PubMed] [Google Scholar]