Abstract
All organisms, from bacteria to humans, face the daunting task of replicating, packaging and segregating up to two metres (about 6 × 109 base pairs) of DNA when each cell divides. This task is carried out up to a trillion times during the development of a human from a single fertilized cell. The strategy by which DNA is replicated is now well understood. But when it comes to packaging and segregating a genome, the mechanisms are only beginning to be understood and are often as variable as the organisms in which they are studied.
Chromosome segregation is challenging because the cell’s packaging strategy needs to retain the organizational mechanisms that are responsible for delineating gene activity, as well as the higher-order spatial interactions that dictate the propensity of chromosomes to reside in specific domains (or territories). At the same time, chromosome segregation must be executed with high fidelity so that the mother cell and the daughter cell that arise from division receive precisely the same DNA content. The packaging machinery must distinguish sister chromatids from homologous chromosomes, while the segregation machinery must interpret intracellular spatial cues for coordinating chromosome segregation with cell division. Finally, the segregation machinery must function with far greater accuracy than man-made machines and with an exquisitely soft touch to prevent the DNA strands from breaking.
In eukaryotes, the mitotic spindle is responsible for chromosome segregation. This machine comprises dynamic microtubule polymers and forms between the opposite poles of a cell during mitosis. The polymers are constructed from tubulin subunits, which can be added or removed from either end of each polymer. During chromosome segregation, a coupling device, known as the kinetochore, is assembled at the centromere of each sister chromatid (that is, two kinetochores per chromosome), where it is poised to capture the fast-growing end (the plus end) of the microtubules in the mitotic spindle. In addition to this mechanical attachment, a signalling network that ensures the high fidelity of this process is assembled. This signalling network is sensitive to microtubule attachment and to force, presumably in the form of a change in protein structure and/or centromeric chromatin structure. Force can be sensed owing to the special geometry at the kinetochore, which is imparted by the cohesion of the sister chromatids. This geometry results from a DNA-strand-linkage system that is coupled to DNA replication, a system that allows the protein cohesin to link sister chromatids but not non-sister chromatids. When kinetochores form and capture microtubules in the mitotic spindle, the kinetochores of sister chromatids (the sister kinetochores) are attached to opposite spindle poles in the cell, and tension (force) is exerted across the sister chromatids, resulting in separation of the chromatids.
This elaborate machine is in contrast to the streamlined machine used by prokaryotes to facilitate the segregation of small circular DNA molecules known as plasmids. This machine comprises a specialized cis-acting DNA locus (called par), a DNA-binding protein and an actin-like polymer. Growth of the polymer between two plasmids pushes the replicated plasmids apart until the par loci reach the opposite poles of the dividing cell.
Although the design of the segregation machinery differs widely among organisms, it is dictated by the basic physical properties of both the DNA and the protein polymers that drive chromosome and/or plasmid segregation. We therefore begin by discussing the thermodynamics of DNA segregation. We consider the properties of long chain polymers and then look at DNA and RNA polymerases and topology adjusters from a physical perspective. We then discuss the specialized sites for chromosome segregation in bacteria and eukaryotes, and the DNA surrounding these sites in eukaryotes is considered in terms of its spring potential and as an integral structure in the chromosome segregation apparatus. Finally, we review the protein translocation machinery involved in both prokaryotes and eukaryotes.
Physical characteristics of the DNA polymer
To understand the physical problems that segregating a genome presents, consider that the DNA in a cell is orders of magnitude longer than the cell itself. Therefore, central to the problem of segregation is the issue of packaging. Of equal importance is lack of inertia at the size scales within a cell: viscous forces dominate reactions and, without energy input, thermal forces keep chromosomes ‘jiggling’ but do not provide direction (Box 1).
Box 1. Life as seen from the chromosome.
One of the challenges in understanding the mechanical properties of biological materials is realizing that at the size scale of the molecules in question there is essentially no inertia. Thus, biologists must be cautious in letting experience frame their thinking on such small scales. Instead, thermal fluctuations and viscous forces dominate reactions, and the force required to drive a given reaction may only be slightly greater than that of thermal motion. All molecules vibrate in a temperature-dependent manner. This thermal motion is constant and is defined by the Boltzmann constant (kB). The numerical value of this constant in units that are relevant to cellular biophysics at room temperature (295 K) is 4.1 pN nm. This is the energy that every molecule displays.
For long-chain polymers (such as DNA), the principles of polymer physics can be used to derive basic properties, such as the size of the random coil and the magnitude of the spring constant as a coil adopts the most-disordered state. For a randomly coiled polymer, the radius of gyration (Rg) is estimated as (L × lp)0.5, where L is the total contour length of the chain and lp is the persistence length (which describes the polymer’s resistance to thermal fluctuation and is the length scale over which the correlation of the direction of the two ends of a polymer is lost). An entropic spring constant (that is the inward force exerted as a polymer collapses) can be estimated from the Boltzmann constant, the polymer chain length and the polymer persistence length to be ~3kBT/n(2lp)2, where T is absolute temperature and n is the number of segments (as determined by L/lp). The spring constant decreases as chain length increases and thus is exceedingly small for metre-length polymer chains, such as DNA, inside live cells.
The spring constant for a chromosome is much more difficult to estimate and is typically approximated by Hooke’s law, F = k(l − l0), where F is force, k is the spring constant, l is the spring length at a given force, and l0 is the spring length at rest (that is the spring length in the absence of force). This form of the equation is applicable to a slinky (the children’s toy), for which the force and distance can readily be measured and the spring constant can be determined. But is difficult to directly measure force inside a cell. Hooke’s law can be rewritten in terms of the material properties of chromosomes, that is in terms of Young’s modulus (E). Young’s modulus is the stress (pressure)/strain (length change). Length change has no units; therefore, E has units of pressure (Pascals, or N/m2). Pressure multiplied by area (A) yields Nm−1, the form of a spring constant. Therefore, force can be estimated from F = EA(l − l0/l). In this form, it is also clear that force is related to area and is extremely sensitive to the area over which force is generated (that is F goes as distance squared).
Dimensions of DNA random coils
DNA is a very thin polymer (2 nm in diameter for naked DNA), and the total length of DNA in a normal diploid human cell is about 2 m. In the absence of proteins or other cellular material, the polymer adopts a random coil conformation, and the size of this coil is dictated by two factors: the total contour length of the polymer (L), and the persistence length (lp) of the polymer (that is, the length scale over which the polymer is stiff). For the B form of DNA (which is the basis of the Watson–Crick model of DNA), lp is 150 base pairs (bp), which is ~50 nm. The radius of this random coil, denoted Rg for radius of gyration, is (L×l p)0.5. For 1 m of DNA, Rg is ~225 μm. By contrast, the radius of a bacterial cell or a small eukaryotic nucleus is about 0.5 μm, and a large tissue cell has a radius of ~3 μm. Converting these numbers to volumes reveals that the random coil polymer of all the DNA from a human tissue cell would occupy ~4 × 107μm3, but this needs to be compacted into ~100 μm3 in the tissue cell. In the cell, DNA is wrapped around histone proteins to form nucleosomes, and in this way it is compacted by about sevenfold. Higher-order packaging of DNA is poorly understood, but the packaging of arrays of nucleosomes into a chromatin fibre of 30 nm in diameter would involve a further sixfold compaction. It is clear that other processes must be involved, however, because on the basis of simple physical dimensions the chromosome must be compacted by a factor of about 3 × 105. The challenge for the cell is to compact the genome so that the DNA strands do not become entangled or broken during segregation and so that the appropriate genes are accessible to polymerases in the emergent mother and daughter cell. Several diverse protein machines have evolved to carry out these processes.
Entropic springs and swelling forces
The foundation for understanding chromosome packaging begins with studying the behaviour of naked DNA. Long chain polymers such as DNA tend to adopt the most disordered state (that is, they have high entropy), and the recoil from less disorder to greater disorder results in an inward spring force. This inward force is the force vector in the direction that the polymer chain is collapsing. An open circle or straight line has fewer degrees of freedom than a smaller, somewhat unordered circle or crooked line. The more crooked the line, the higher the entropy. The collapse to disorder is entropic in nature because it is driven by the thermal motion of the polymer (Box 1). The entropic spring constant is ~3kBT/n(2lp)2, where kB is the Boltzmann constant, T is absolute temperature, n is the number of persistence-length segments (that is the number of ‘stiff’ units, as determined by L/lp), and kBT= 4.1 pN nm. The spring constant decreases as chain length increases and thus is exceedingly small for metre-length polymer chains inside living cells.
In addition, compressing a random coil into a confined space such as the nucleus or a bacterial cell results in an outward swelling force. That is, entropic pressure is created on compacting a polymer by several orders of magnitude. In the case of entropic swelling, the change in compaction state has been shown to exert force1,2. For the most part, such entropic swelling forces are balanced partly by interactions between histone tails or between non-histone proteins within chromatin3. However, enzymatic modification of these histone-tail interactions can change local states and therefore contribute to local changes in this outward pressure. For instance, the entropic swelling of chromatin has been shown to generate substantial outward force (300 nN), controlling the stability of the nuclear envelope4.
There is also an entropic penalty incurred between entangled polymers, known as polymer repulsion. Jun and Mulder have shown that the penalty due to polymer repulsion is sufficient to drive chains apart in a confined space and may contribute significantly to chromosome segregation mechanisms in bacterial cells5 (Fig. 1a).
Figure 1. Modes of chromosome segregation in prokaryotes and eukaryotes.
a, Polymer repulsion in a confined space. Chromosomal DNA is confined within the boundaries of a cell. DNA is depicted here as tension blobs, which represent the random coil of DNA within the length scale at which thermal forces randomize the conformation of the DNA polymer. Entropy drives fluctuation of each chain (dark and light) to adopt a random coil. Segregation occurs as a result of the entropic repulsion between two chains in a confined space. This mechanism has been proposed to operate in prokayotes5 and might contribute to segregation in eukaryotes that undergo closed mitosis (such as fungi and some protists). b, Polymer growth. The growth of an actin-like polymer (blue) generates force, which propels plasmid DNA (depicted as curved DNA helices) to the opposite sides of a prokaryotic cell. c, Polymer brushes. Multiple polymers can be concentrated on opposite sides of the cell wall, analogous to a brush in which short, flexible bristles are attached to a stiff handle. Polymer physicists use this type of strategy to switch force rapidly from attraction to repulsion. In the bacterium Caulobacter crescentus, polymers of PopZ form a network attached to opposite sides of the bacterial cell wall and anchor the two circular DNA chromosomes to effect chromosome segregation. d, Mitotic spindle. Antiparallel arrays of dynamic microtubules (green rods) extend from microtubule-organizing centres (green ovals) at opposite poles of the eukaryotic cell. These microtubules attach to chromosomes (red) (which are wrapped around histone proteins to form nucleosomes, green) by way of the kinetochore (blue). Ring-like complexes (pink) hold replicated sister chromatids together. The spindle microtubules provide an outward force (towards the spindle pole) that acts against the inward force emanating from the linkage between sister chromatids and drives segregation.
The most striking example of polymer-confinement pressure comes from bacteriophages, which expend considerable amounts of energy from the host cell to package their DNA into capsids6. This energy is then put to use for injecting the polymer through the cell wall of a new host. For chromosome segregation, entropic forces are useful only in certain cases (for example in the bacterial nucleoprotein particles called nucleoids). Active mechanisms are usually necessary (for example in larger eukaryotes).
Force exerted through DNA-processing machines
The compaction state of chromatin is controlled by various types of DNA-processing enzyme. DNA-binding proteins (such as the lac repressor7) can mediate DNA looping, whereas helicases can split the DNA double strand, generating greater than 20 pN of force in the process8. Nucleic-acid-processing enzymes that move along the helix (such as DNA polymerases and RNA polymerases) are extremely powerful motors, generating forces of up to 40 pN (for Escherichia coli RNA polymerase9,10), and it has been proposed that this force may contribute to genome segregation in bacterial cells11. By contrast, the microtubule-associated molecular motors (which belong to the kinesin and dynein families) and the actin-filament-associated motors (which belong to the myosin family), typically generate less than 10 pN of force. RNA polymerase is thought to generate a larger force as a result of its shorter step size (in other words, it has a low gear). The force generated during protein binding is roughly the ratio of the energy released on binding to the change in length of the polymer. For example, a 0.5 nm length change as a result of a 5 kcal mol−1 protein–DNA interaction can be expected to generate a force of 5 kcal mol−1/0.5 nm = 10 kcal mol−1nm−1= 60 pN (given that 1 kcal mol−1nm−1 = 6 pN). These forces are in the same range as external machines such as microtubule-associated motors and mitotic spindles. These and other mechanochemical enzymes (including chromatin-remodelling ATPases, condensin and DNA topoisomerases) influence the higher-order packaging of DNA by changing the local conformation of chromatin. From a thermodynamic perspective, it is not simply the position of these proteins that dictates their functions but how their actions change the polymeric state of the DNA.
Chromatin compaction by ‘topology adjusters’
Histone proteins provide the first level of compaction of DNA. The DNA polymer is wound 1.65 times around an octamer of histone proteins, to form the nucleosome, resulting in sevenfold compaction of the DNA polymer. This array of nucleosomes is further compacted into thicker fibres that are irregular in organization and refractory to simple modelling of their structure. At the nuclear level, however, chromosomal domains such as heterochromatin and euchromatin can be readily distinguished, and by using live cell microscopy and techniques to label specific chromosomal loci, specific chromosomal domains can be discerned. In addition, replication of the DNA duplex results in two entangled double-stranded molecules. These entanglements must be resolved before the chromosomes can be segregated. Chromosomal domains are organized as loops emanating from an axial core (Fig. 2), so entanglements can be topologically confined, giving rise to catenated DNA (that is, to interlocked loops of DNA that arise from replication of a helical polymer). The enzymes that adjust the topology and/or decatenate DNA are called topoisomerases, and they are crucial to the process of chromosome segregation12.
Figure 2. Chromosomal loops.
Regions of a chromosome can be unravelled, forming a loop, either to allow specialized functions (such as cell division) or in specific cell types or at specific developmental stages. Examples of DNA extrusion from the axis of the chromosome are shown. a, DNA looping was first observed in squash preparations of salamander eggs under the light microscope, by the embryologist Oskar Hertwig in the early 1900s91. A single egg is shown here. Scale bar, 50 μm. b, Paulson and Laemmli found DNA loops when examining chromosome spreads in isolated mammalian cells22. In the electron micrograph of this cell in metaphase, loops emanate from the protein-rich chromosome scaffold (the darker stained, X-shaped structure towards the bottom of the image); the inset shows a whole metaphase chromosome, highlighting its similarity to the isolated chromosome scaffold. Scale bars, 2 μm. (Image reproduced, with permission, from ref. 22.) c, DNA loops were also found on a single meiotic bivalent chromosome from the oocyte of the newt Triturus viridescens, by using light microscopy21. These are sites of intense transcriptional activity. Each lateral loop represents a single DNA duplex. Scale bar, 15 μm. (Image reproduced, with permission, from ref. 21.)d, And loops were observed in DNA undergoing transcription when heat-shock loci in the fly Chironomus tentans were examined by using light microscopy. The depiction of the findings shows chromosomal DNA (red) as a strand that forms loops (centre) but elsewhere is wrapped tightly in bundles (green; proposed by the authors to be nucleosomes)20. e, DNA looping was first proposed to occur at the kinetochore in mammals92. Plates of microtubule-binding segments are tandemly repeated and interspersed with linker segments. On microtubule attachment, microtubule-binding segments loop out from the chromosome axis, forming a cluster of repeats on the surface of the chromosome, as depicted for one loop of centromeric DNA in f. f, DNA looping was later proposed to occur in Saccharomyces cerevisiae also17. The single pericentromeric loop is comparable to multiple loops of linker segments and microtubule-binding segments (e) that form on microtubule attachment in mammalian cells. The kinetochore (not shown here) forms at the junction between microtubules (green rods) and the unique nucleosome (blue) at the apex of the pericentromeric loop.
One of the exciting prospects in this field is the convergence of experimental studies with statistical modelling of the mechanics of DNA13,14. Such studies consider the physical properties of the chromatin fibre, as well as the active processes that regulate chromatin compaction and decompaction. The integrity of the chromosome is provided mainly by a crosslinked DNA network rather than by a protein scaffold15. Therefore, the consequences of strand intertwining and crosslinking from the perspective of polymer repulsion must be integrated into models of higher-order chromosome structures. From this perspective, it is interesting that two of the three major classes of protein that have essential functions in chromosome architecture can catenate or decatenate DNA. These are the SMC (structural maintenance of chromosomes) proteins, which form part of several protein complexes in eukaryotes (including cohesin and condensin), and DNA topoisomerase II. The SMC proteins are characterized by intertwined coiled-coil domains that have hinges at both ends (Fig. 3a), enabling them to embrace a DNA strand(s) dynamically. The structure of condensin (which contains SMC2 and SMC4) is a ring with a narrow (5–10 nm) diameter16. Cohesin (which contains SMC1 and SMC3) also has a ring structure, with an inner diameter of ~30–40 nm, a crucial feature of models proposing that one ring encircles sister chromatids. In addition, condensin has an ATPase-dependent supercoiling activity that can result in the positively supercoiling of DNA. However, it remains unclear how this supercoiling activity relates to the ability of condensin to shape chromosome structure.
Figure 3. SMC-protein-containing rings and the distribution of force.
a, SMC proteins assemble into complexes that adopt a ring-like conformation. The backbone of the ring is formed by the SMC proteins themselves (MukB in bacteria; Smc2 and Smc4 in Saccharomyces cerevisiae condensin, and Smc1 and Smc3 in S. cerevisiae cohesin). In eukaryotes, the SMC monomer is folded in an antiparallel coiled coil. At one end (top of molecule as depicted), the two monomers associate to form a hinge, and at the other end is an ATP-binding head domain. Closure of the ring at the head domain is carried out by proteins known as kleisins, including Scc1 (also known as Mcd1) and Brn1. Each dimer is associated with additional proteins (for example Ysc4, Ycg1, Scc3 (also known as Irr1), Rad61 and Pds5) at the head domain to form a functional complex in vivo. In bacteria, the SMC coiled coils are bound by ScpA and ScpB. b, Cohesin rings (pink) and condensin rings (blue) are depicted linking chromatids into a network (that is creating links within a chromatid, such as those that allow DNA looping) and, for cohesin, holding sister chromatids. c, A model for how cohesin and condensin rings may contribute directly to the elastic properties of the centromere and/or chromosome. Protein rings that function as slip rings (or molecular pulleys) provide a mechanism to distribute tension from one location to the entire network, and cohesin (pink) and condensin (blue) have the physical attributes to function as slip rings and regulate centromere elasticity.
Cohesin and condensin are enriched in the region surrounding the centromere of eukaryotes (known as the pericentromeric or pericentric region) during mitosis. The finding that cohesin is enriched at centromeres raised a paradox: how does a protein that holds sister chromatids together become enriched at sites of separated DNA? The discovery that the pericentromeric region adopts an intramolecular loop structure17 invivo provided a solution to this paradox. So, instead of holding sister chromatids together, cohesin may contribute to the mechanisms that promote centromeric DNA looping (Figs 2e, 2f and 3b) and architectural features responsible for the exposure of centromeric chromatin on the outer surface of the chromosome. Alternatively, the cohesin and condensin rings may contribute directly to the elastic properties of the centromere and/or chromosome. When tension is applied to a crosslinked network, the heterogeneous distribution of crosslinks can lead to local regions of high stress. Slip rings (or molecular pulleys) provide a mechanism to distribute tension from one location to the entire network (Fig. 3c). Such a topological distribution of stress was originally proposed in the Edwards–de Gennes reptation tube model18 and was demonstrated in a recent study of the chemistry of polyrotaxanes19. Cohesin and condensin have the physical attributes to function as slip rings and to provide the chemistry for regulating elasticity through topology in living cells.
The major topology-adjusting enzyme in the chromosome is DNA topoisomerase II. DNA topoisomerase II is enriched along an axial core that runs the length of condensed chromosomes. However, as discussed earlier and in ref. 15, it is unlikely that this enzyme contributes to the mechanical properties of the chromosome. So in what way does it affect chromosome structure? One of the most conserved features of chromosomes in all organisms is the extrusion of DNA loops from an axial core (Fig. 2). DNA looping was initially observed in the early 1900s and gained serious attention in pioneering studies of heat-shock loci in the fly Chironomus tentans20, meiotic bivalent chromosomes from the oocyte of the newt Trituris viridescens (also known as Notophthalmus viridescens)21 and chromosome spreads22. The lesson from these studies is that particular regions of a chromosome can be spooled out for specialized functions in specific cell types or developmental stages. Perhaps a similar strategy is used at the centromere to allow exposure of the centromere to cytoskeletal components, be it actin-related proteins in prokaryotes or microtubules in eukaryotes. The increased concentration of SMC proteins and DNA topoisomerase may reflect two activities, one that affects topology and another that controls the extension stiffness of centromeric loops.
Coordinating the processes required for segregation
A key finding about the variety of SMC-protein-containing complexes is how their biochemical activity varies across the cell cycle. Just as cohesin deposition is coupled to the DNA replication cycle, the timing of condensin-mediated compaction of DNA in bacteria seems to be closely linked to the chromosome segregation cycle23. This late stage of compaction (relative to segregation) may reflect a strategy to harness SMC-mediated compaction forces for segregation. By contrast, in eukaryotes, separated chromosomes have been observed, by using phase-contrast microscopy, long before they are segregated to opposite spindle poles. The biochemical pathway regulating this process has been described and is denoted the prophase pathway24,25. Thus, the bulk of chromosome compaction precedes resolution of sister DNA chromatids in a typical plant or animal cell. This presumably reflects a division of labour that allows streamlining of chromosome segregation when the cell enters anaphase. With the advent of labelling strategies to visualize chromosomes in bacteria and small eukaryotes (for example yeast, including Saccharomyces cerevisiae, Schizosaccharomyces pombe and Candida albicans), it is possible to deduce the relationship between compaction and segregation in unicellular eukaryotes, in which chromosomes are not readily discernable by light microscopy. Chromosome compaction precedes segregation in the budding yeast S. cerevisiae26, as is typical of plant and mammalian cells, whereas compaction and segregation are temporally linked in bacterial cells. Thus, at a comparable size scale (bacteria have a diameter of ~1 μm and the yeast nucleus is ~1.5 μm), prokaryotes and eukaryotes use a different timing mechanism. The timing of compaction relative to segregation may be related to the mode of segregation (mitotic spindle versus simple polymer partitioning system) rather than a consequence of scale.
Even though the individual chromatids seem to be separate in eukaryotes before segregation, they remain mechanically linked26–28. What is purpose of retaining mechanical connectivity between separated strands? Force exerted at a specialized DNA locus (for example a centromere) will be resisted by persistent mechanical linkages. On movement of the specialized DNA locus to a spindle pole or attachment site, the strain between linked strands will increase, leading to a potential source of stored energy. In biophysical studies of stretched polymers, a freely relaxing polymer ‘remembers’ how it was straightened29. Force stored in linked strands will promote recoil on cleavage of the remaining linkages and will enhance segregation and compaction of the recoiling arms. The entropic recoil of chromosome arms to daughter cells may be facilitated by the increased strain exerted during active partitioning. The linear memory in the polymer may also contribute to mechanisms responsible for the inheritance of chromosome territories30.
Specialized sites
Accurate chromosome segregation is achieved through protein machines that interact with specific sites on chromosomes. In bacteria, these sites are known as partitioning (par) loci (Table 1). In eukaryotes, the interaction site is the centromere.
Table 1.
Chromosome segregation strategies and components in prokaryotes and eukaryotes
| Organism | DNA locus | DNA-binding protein | Molecular motor or polymer | Anchor protein |
|---|---|---|---|---|
| Type Ia ATPase (Escherichia coli) | ||||
| P1 plasmid | parS | ParB | ParA | NA |
| F plasmid | sopC | SopB | SopA | NA |
| Type Ib ATPase (Salmonella spp.) | ||||
| TP288 plasmid | parH | ParG | ParF | NA |
| Type II ATPase | ||||
| RI plasmid | parC | ParR | ParM | NA |
| Caulobacter crescentus genome | Adjacent to oriC | ParB | ParA | PopZ |
| Eukaryotes | ||||
| 2 micron plasmid (Saccharomyces cerevisiae)88 | stb | Rep proteins and histone core (H4 and H3 variant) | Piggyback onto the mitotic spindle | NA |
| Genome | Centromere | Histone core (H4 and H3 variant (CENP-A)) | Mitotic spindle | NA |
| Synthetic89,90 | lac operator | Lac repressor fusion to Ask1 | Microtubules | NA |
NA, not applicable.
Bacterial par loci
The par loci were described in the early 1980s by Austin, Hiraga and colleagues31,32. In each bacterium, these loci include a DNA cis-acting centromere-like site and loci that encode two trans-acting proteins (a DNA-binding protein and an actin-like polymer). One example is the bacterial plasmid R1, whose cis-acting site, parC, is characterized by two sets of 11-bp repeats separated by a small promoter region (~40 bp). As is the case for eukaryotes (discussed in the section ‘Eukaryotic centromeres’), the cis-acting elements in bacteria are extremely varied. The common theme in bacteria is the occurrence of multiple repeat elements (containing repeats of 6–11 bp): for example, the centromere of the F plasmid, sopC, contains 12 tandem repeats of a 43-bp element; and parS in Agrobacterium contains 13 repeats separated by an integral number of turns of the DNA (see ref. 33 for a review) (Table 1).
The common feature among the various cis-acting elements and the DNA-binding proteins is that the repeated centromeric DNA is wrapped around a supramolecular core of oligomerized proteins. ParR contains a ribbon–helix–helix domain and an oligomerization domain. The parC DNA is wrapped in a large superhelical turn (with a pitch of 24 nm around six ParR protein dimers), leading to a nucleoprotein complex of about 18 nm in diameter. This is almost twice the diameter of the canonical eukaryotic nucleosome. The F plasmid centromere sopC is wrapped in a right-handed superhelix around a multimeric SopB core34. In both cases the cis-acting elements are bent, a feature that is enhanced by the binding proteins. The bending and right-handed nucleosome wrapping are recapitulated in eukaryotic centromeres (see the next subsection). The bending of DNA reduces its persistence length and increases its flexibility. This may be an important mechanical feature of the par loci.
Eukaryotic centromeres
Also in the early 1980s, the first centromeric DNA of a eukaryote (S. cerevisiae) was isolated and defined at the molecular level35. The centromere is present in a short (125 bp) DNA sequence that contains a 25-bp conserved partial palindrome (called CDEIII) flanked on one side by ~80 bp of DNA with more than 90% (A+T) content (called CDEII) and a smaller (8 bp) conserved element (called CDEI). This DNA is wrapped in a right-handed superhelix36 around a highly conserved variant of the histone protein H3, Cse4 (known as CENP-A in mammalian cells) and is the site of deposition of DNA-sequence-specific-binding proteins and the kinetochore. In the fission yeast S. pombe and multicellular eukaryotes, the centromeres are expansive: ~30–65 kilobases in S. pombe and of the order of megabases in humans. These regions are characterized by hierarchical arrays of simple sequence (such as the 171 bp repeats of alphoid DNA in mammalian cells). The sequence-specific centromeres of S. cerevisiae have been denoted ‘point’ centromeres, whereas the expansive centromeres of S. pombe and multicellular eukaryotes, which do not seem to contain a specific DNA sequence, have been denoted ‘regional’ centromeres.
Nucleosomes received much attention after a post-translational histone code was uncovered37. Canonical nucleosomes in eukaryotes are surprisingly invariant in their subunit structure and composition: 146 bp of DNA is wrapped 1.65 times in a left-handed superhelix around an octamer of histones (comprising two subunits of each of H2A, H2B, H3 and H4). A distinctive feature of all eukaryotic centromeres is that H3 is replaced with the H3 variant CENP-A. Recent studies indicate that the function of CENP-A may be to direct the DNA into a right-handed superhelix36 and destabilize the nucleosome into a tetrameric structure37–39.
The wrapping of DNA around core histones in a left-handed direction results in negative supercoiled plasmids when DNA is isolated from eukaryotic cells. In plasmid DNA, negative superhelical turns are in equilibrium with regions of single-stranded DNA in what is otherwise a double helix (10.4 bp, or one turn of the helix, is in thermal equilibrium with one negative superhelical turn). The wrapping of DNA in a right-handed or left-handed direction around a nucleosome has significant and different outcomes with respect to the number of base pairs per helical turn upon histone gain or loss. Recent microscopy experiments using photobleaching demonstrate that histones are exchangeable, providing evidence to support the idea that nucleosomes have dynamic structures. On loss of a histone octamer, the negatively supercoiled helix naturally fluctuates between wound and underwound (that is double-stranded and single-stranded) configurations. These fluctuations are likely to assist DNA metabolic enzymes in melting the helix for most transactions that take place in the nucleus, such as replication, repair and transcription. Centromeric DNA, by contrast, is the landing pad for kinetochore proteins and the site of attachment of microtubules, both of which interact with the DNA in the cytoplasm after the nuclear membrane breaks down. The wrapping of DNA in the opposite direction around a variant nucleosome introduces a positive superhelical turn. So any dynamics in the binding to core histones at the centromere results in centromeric DNA fluctuating between wound and overwound structures, providing a strong topological basis for preventing the unwinding of centromeric DNA.
From the perspective of nuclear transactions, the centromere must be protected from ‘rogue’ polymerases40. When a strong transcriptional promoter is introduced adjacent to the centromere, centromere function is compromised40. Interestingly, the active RNA polymerase does not transcribe through the centromere40 and several kinetochore proteins remain bound despite transcriptional inactivation41. Is the unique structure of the nucleosomes at the centromere responsible for blocking polymerase passage or for the bound kinetochore proteins? If the latter is the case, then the binding energy of kinetochore proteins must exceed that of a transcribing RNA polymerase (that is, it must be >8kBT per turn of the helix). DNA wrapping around the histone may impart a topological block to transcription. In this model, nucleosome chirality at the centromere, as well as the path of DNA as it enters and exits the nucleosome, may have evolved to inhibit transcribing polymerases from inactivating the centromere, which would otherwise lead to chromosome loss.
Recent biophysical experiments provide additional insight into the functional consequences of the opposite chirality at the centromere. It has been known for more than a decade that a tetramer of H3 and H4 can flip from the left-handed chiral conformation to the right-handed one42. It has now been shown, by Bancaud and colleagues, that this chiral transition can be induced by positive torsion43. In this study, nucleosomes were reconstituted, and one end of the chromatin was attached to a glass surface and the other to a magnetic bead43. Using a pair of magnets above the molecule, torsion and extension could be controlled. When positive turns were introduced to the DNA, there was a strong hysteretic response. These positive turns were trapped in chromatin, reflecting a chiral transition from left-handed nucleosomes to right-handed tetrasomes. This finding has important implications for our understanding of the mechanism of transcription. As RNA polymerase travels along the DNA, a leading wave of positive supercoiling breaks nucleosomes into tetramers facilitating transcriptional elongation. From a mechanical perspective, encoding a right-handed turn at the centromere may be the equivalent of introducing a ‘crack’ in an otherwise ordered arrangement of nucleosomes, and this may favour the capture of a growing microtubule. An alternative explanation is that the change in chirality may prevent centromeric nucleosomes from being densely packaged, thereby contributing to their exposure on the outer surface of the chromosome.
Positively supercoiled DNA wrapped around a tetramer of histones is in thermodynamic equilibrium with negatively supercoiled DNA42. To determine the torsional rigidity of positive supercoils compared with negative supercoils, Selvin and colleagues used the anisotropic decay of the fluorescence of ethidium bromide intercalated into DNA as a readout for the flexibility of topoisomers of a plasmid (ref. 44). They found that positively supercoiled nucleosomes showed greater torsional flexibility than negatively supercoiled ones44. None of the existing information on segregation mechanisms in any organism provides insight into the potential function of a flexible nucleosome at the centromere. From a topological perspective, the change in chirality may expose adjacent DNA to kinetochore-binding proteins, leading to increased nuclease sensitivity of flanking DNA45. From a polymer perspective, torsional flexibility might be used to coordinate the elastic response time of the centromere with the gain and loss of subunits from the microtubule plus end, which is embedded in the kinetochore attachment complex (see the section ‘Coupling devices’).
Pericentromeric heterochromatin
The main constriction that is visible in condensed mitotic chromosomes reflects the proportion of the genome devoted to chromosome packaging versus microtubule attachment. This packaged chromatin is known as pericentromeric heterochromatin, and its role in kinetochore function is now becoming clear. Heterochromatin and RNA interference mechanisms are required to establish the bolus of CENP-A-containing chromatin at centromeres in S. pombe and multicellular eukaryotes46.
Studies in S. cerevisiae have also uncovered important functions for pericentromeric chromatin. Cohesin and condensin are enriched threefold in the pericentromeric regions in S. cerevisiae47,48. And Eckert and colleagues have shown that the centromere or kinetochore creates an epigenetic domain that is favourable to cohesin recruitment49. Similarly, in S. pombe, heterochromatin is responsible for recruiting cohesin.
When pericentromeric cohesin was directly observed in S. cerevisiae undergoing mitosis, the DNA was arranged in an unexpected manner. Specifically, the pericentromeric cohesin was organized into a cylindrical array surrounding the mitotic spindle17. This cylindrical organization of cohesin reflects the arrangement of intramolecularly paired loops of pericentromeric DNA from each of the 16 chromosomes of the S. cerevisiae genome17 (Fig. 2f). Given that the 16 kinetochores are structurally analogous to the organization of DNA in 1 mammalian kinetochore, the distinction between organisms with point centromeres and those with regional centromeres might reflect the mode of CENP-A deposition more than the organization of the centromeric DNA and kinetochore. As indicated above, in both S. cerevisiae and S. pombe, cohesin is enriched in the pericentromeric regions, which are heterochromatic. One of the mechanisms for restricting the spreading of heterochromatin is the presence of transfer RNA genes, which function as barriers to heterochromatin formation in S. pombe50. When barriers to cohesin distribution are introduced artificially, they result in a high frequency of chromosome loss in S. cerevisiae49, indicating that the ability to create a specialized region of heterochromatin is paramount to mechanisms of chromosome segregation fidelity.
Springs in the spindle
Several lines of evidence reveal that pericentromeric chromatin functions as a spring between sister kinetochores in mitosis51–53. These studies are based on the dynamics of centromere stretching in live cells and indicate that chromatin packaging is an integral biophysical component of the mitotic apparatus. In mammalian cells, the stretch of the centromere has been visualized by using light microscopy54 or, more recently, by staining the cells with antibodies specific for kinetochore proteins or by introducing fluorescently labelled fusion proteins (such as green fluorescent protein attached to a particular kinetochore protein)53. The pericentromeric chromatin (including the centromeric DNA) is considerably more compliant than the kinetochore55,56. These studies show that intra-kinetochore stretch, although small in magnitude relative to that of the centromere, is monitored by the surveillance mechanisms responsible for ensuring that chromosomes are oriented in a bipolar manner on the spindle before anaphase onset. In addition, these studies indicate that the linkage between sister chromatids (including pericentromeric chromatin, the centromere and the kinetochore) is characterized by a force response that is probably more complex than the simple linear force extension of Hookean springs in series. A Hookean spring describes an elastic material whose extension is in direct proportion to the applied force (Box 1). Centromeres and pericentromeric chromatin show spring-like properties in metaphase. However, the physical source of the spring (whether the proteins that link sister chromatids, the pericentromeric chromatin or the DNA) has not been elucidated.
In S. cerevisiae centromeres, chromatin compaction can be controlled through the repression of histone synthesis. The repression of new histone (H3 or H4) synthesis resulted in an increase in spindle length in metaphase, reflecting a twofold increase in sister centromere separation57. Deletion of the genes encoding outward force generators, the kinesins Cin8 and Kip1, restored ‘wild-type’ spindle length in cells with reduced histone protein synthesis. The increase in spindle length that occurred on repression of histone synthesis and the restoration of ‘wild-type’ spindle length after the loss of plus-end-directed motors suggest that, during metaphase, centromere separation and spindle length are controlled in part by the stretching of pericentromeric chromatin, indicative of the fact that chromatin is an elastic component of the spindle.
Does a DNA spring function in the force range that is pertinent to protein machines? This question was experimentally addressed recently58. From a theoretical viewpoint, the energy introduced by protein binding per unit length of bent DNA is approximately 20kBT (ref. 58). The thermodynamic stability of a protein is likewise approximately 10–20kBT. Several research groups have shown that DNA springs can induce conformational changes in proteins (see ref. 58 for a review). These experiments used protein–DNA chimaeras and demonstrated that the stiff DNA duplex can disrupt the tertiary structure of a ribozyme and inactivate its activity59. Thus, the energy scales of protein conformational changes are in a range indicating that DNA springs may regulate protein machines. Although DNA is not covalently linked to spindle microtubules or motor proteins, it may act as a spring in its capacity to absorb force and therefore prevent molecular motors from travelling too fast.
The best-defined pericentromeric chromatin structure is that of S. cerevisiae. From the principles discussed above for naked DNA (in the subsection ‘Dimensions of DNA random coils’), there is a strong expectation that pericentromeric chromatin will show behaviour resembling that of a molecular spring. The evidence that the centromere is elastic is not surprising when considering the entropic properties of DNA. The next question is what kind of spring is pericentromeric chromatin. Hooke’s law — F=k(l − l0), where F is force, k is the spring constant, l is the spring length at a given force, and l0 is the spring length at rest (that is the spring length in the absence of force) — is often invoked as the simplest of all assumptions. But, even for a simple coil spring, Hooke’s law is only valid over a limited linear range of the force extension curve. It is probable that proteins such as topology adjusters (that is SMC proteins and DNA topoisomerase II) contribute to the physical spring behaviour. In addition, post-translationally regulated processes such as loss and re-establishment of cohesin binding60 and ATP-dependent condensation and decondensation61,62, may contribute to the elastic properties of the chromatin. Thus, alternatives are needed to the hypothesis that pericentromeric chromatin behaves like a linear Hookean spring. The chromatin spring strain may increase linearly with stress over a range of force (as is the case for a coil spring), or it may be relatively constant over a range of extension (as is the case for a common tape measure). Only when technology has advanced to the point at which forces can be measured in vivo63 will we be able to address the actual physical behaviour of the chromatin spring. There is not an obvious equivalent to pericentromeric chromatin in bacteria, raising the possibility that the organization of this region might reflect a eukaryote-specific solution to the problem of tension.
Specialized machines
One of the more startling outcomes arising from the ability to label cells with fluorescent proteins was the discovery of the bacterial cytoskeleton64. Polymer systems related to eukaryotic actin and tubulin abound in bacteria and function in basic mechanical processes such as chromosome segregation and cytokinesis (the late stage of mitosis in which the cytoplasm is divided into two). There are three main types of machine for DNA segregation: simple protein polymers, DNA translocation pumps and mitotic spindles.
Simple protein polymers
One mode of plasmid separation is driven by polymerization of the protein encoded by mreB and other proteins. This is a classic thermal ratchet mechanism65 and has been elegantly reconstituted in vitro66. In biology, thermal ratchets refer to a class of general mechanisms that rectify the random diffusive movement of a molecule by presenting an asymmetrical binding potential at the expense of chemical energy. In the reconstituted par system, a protein–DNA complex stabilizes a growing MreB filament. Thermal motion allows intermittent addition of MreB subunits at both ends of the MreB filament, thereby lengthening the filament and, in the process, pushing the two oriC loci to opposite ends of the dividing cell (Fig. 1b).
A different way of organizing polymers is to anchor many chains to a substrate (Fig. 1c). In the field of polymer physics, this is an important strategy for regulating forces between polymers (for example in polymer brushes) and the environment, and for creating methods to switch rapidly from attraction to repulsion. One type of brush is a Velcro-like structure, in which a highly oligomerized protein is attached to a subcellular site. This kind of interaction has recently been discovered to facilitate chromosome segregation in Caulobacter crescentus (also known as Caulobacter vibrioides)67–69. Polymers of the C. crescentus protein PopZ assemble into a higher-order filamentous network that functions as an anchor for chromosome capture (Table 1).
DNA translocation machines
Unlike bacterial growth, when Bacillus subtilis forms spores, the DNA is segregated after cytokinesis. The mechanism for this involves SpoIIIE and FtsK, which are AAA ATPases that promote intercompartmental chromosome translocation in bacteria. SpoIIIE pumps approximately three-quarters of the chromosome (>3 megabases) into the spore. During this process, it strips proteins from the DNA strand, introducing naked DNA into the spore70,71.
Mitotic spindles
In eukaryotes, as discussed earlier, there is a highly organized microtubule-based system for segregating DNA (Fig. 1d). During mitosis, microtubules emanate from two microtubule-organizing centres at opposite poles of the cell, forming a mitotic spindle, which is a robust macromolecular machine (see ref. 72 for recent review). Microtubules are self-assembling polymers and are especially dynamic at the plus end (the end growing away from the pole). The motors associated with microtubules function as crosslinkers that regulate the length of the spindle and stabilize its bipolar organization. The mitotic spindle in S. cerevisiae is highly streamlined and requires only two motors: the plus-end-directed motor Cin8 (a homologue of kinesin-family member 5), and either Kip3 (a homologue of kinesin-family member 8) or the minus-end-directed motor Kar3 (a homologue of kinesin-family member 14)73.
Coupling devices
The kinetochore is a complex coupling device that links the plus ends of microtubules to centromeric DNA. It is known to comprise more than 70 proteins, as determined by a variety of genetic, biochemical and genomic approaches74. It has a mass about three times greater than the ribosome but is significantly smaller than a nuclear pore. Recent quantitative approaches, including the counting of protein numbers and super-resolution microscopy, have made it possible to describe the structure of the kinetochore in live cells at the nanometre scale75,76 (Fig. 4).
Figure 4. Protein architecture of the Saccharomyces cerevisiae kinetochore.
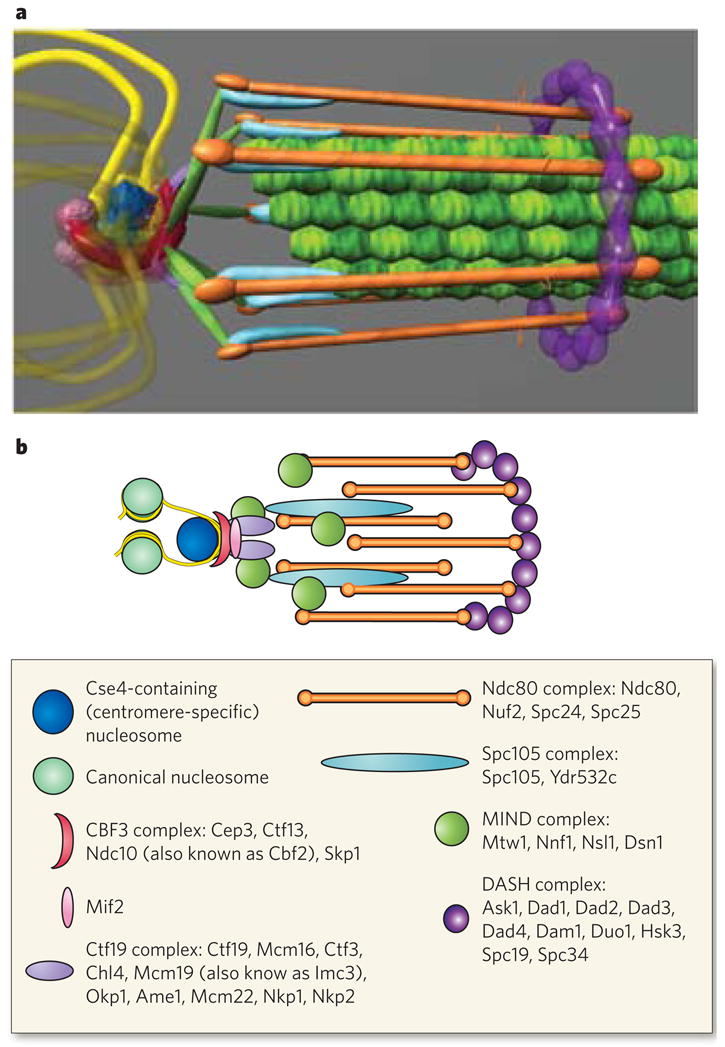
The structure of the kinetochore is depicted in two ways. a, This structure reflects the average positions of kinetochore proteins within the S. cerevisiae kinetochore measured by invivo super-resolution microscopy75. The positions of microtubule-binding proteins with respect to the microtubule plus end are key to understanding the mechanism of force generation at the kinetochore. The interface between the microtubule-binding side of the kinetochore and the chromatin-binding side in S. cerevisiae, as well as in multicellular eukaryotes, remains poorly characterized93. From the left, the DNA (yellow strands) is wrapped in a positive supercoil around a histone core that contains the centromere-specific H3 variant (dark blue). The DNA-binding region of the kinetochore is formed by the CBF3 complex (dark pink). An additional DNA-binding protein, Mif2, is shown proximal to centromeric DNA (light pink sphere). From the right, the microtubules (green filaments) are surrounded by the DASH complex (purple ring), whose constituents are abundant enough to form a ring. The main microtubule-binding sites within the kinetochore are in the Ndc80 complex (orange rods). There are several linker complexes: the Spc105 complex is depicted in light blue, and the MIND complex in green; the Ctf19 complex is shown as small purple linkers between the CBF3 and MIND complexes. b, This illustration is a schematic representation of the structure in a. Data are based on the hydrodynamic properties, protein number and spatial position of the members of each protein complex. The structure assumes a symmetrical arrangement of kinetochore protein complexes around the microtubule lattice (green in a). The known components of the various protein complexes are listed in the box.
The architecture of the kinetochore at a single microtubule attachment site is a cylindrical structure of 40 × 80 nm2 in both yeast and mammals. The kinetochore has a microtubule-binding domain at one end, and this is linked to a DNA-binding domain by way of several linkers. The quantitative analysis shows that a complex of eight rods surrounds a microtubule and that a dimer of protein complexes is present at the end that binds to centromere-specific nucleosomes. The microtubule-binding proteins are the key devices for coupling force from depolymerizing microtubules to power for chromosome movement.
The mechanism of force generation
The directed movement of chromosomes requires active forces on the chromosomes. Furthermore, the process of chromosome segregation must occur in conjunction with the overall division of the cell. The mitotic spindle supplies force, as well as positional cues, to the chromosome so that chromosome movements are consistent with the geometry of the dividing cell. Reliance on this microtubule-based spindle dictates the types of mechanism that can be used to generate force. Although almost all microtubule-based movement in a cell is driven by motor proteins, chromosomes deploy the kinetochore for segregation. The kinetochore engages with microtubules in a unique geometry by attaching specifically at the plus end. Unlike tip-tracking proteins (for example EB1) in the cell, microtubule-binding kinetochore proteins do not turn over at the plus end but, instead, have a persistent attachment to the same plus end while it is polymerizing or depolymerizing. Thus, the kinetochore can generate bidirectional movements spanning a few micrometres during metaphase and then unidirectional movements (coupled to microtubule depolymerization) in anaphase.
These features of microtubule binding limit the force-generating mechanisms to two categories (Fig. 5): biased diffusion and forced walk. The first category includes mechanisms that involve the thermal diffusion of multiple kinetochore proteins to the same microtubule plus end. Directionality, or bias, to these movements is provided by growth or shortening at the microtubule tip. The first such mechanism to be proposed was specifically for depolymerization-coupled movement and was put forward by Hill in 1985 (ref. 77). Hill modelled the kinetochore as a sleeve (based on electron-microscopy ultrastructural data available at the time) with an array of regularly spaced microtubule-binding sites on its inner surface. In this model, a microtubule plus end inserted into such a sleeve forms many weak interactions with the sleeve. In the absence of any forces on this system and with a stable microtubule plus end, maximum insertion of the microtubule plus end into the sleeve is energetically favoured. If the microtubule plus end starts to lose subunits, then such a sleeve would in effect follow the receding plus end. The Hill sleeve can sustain depolymerization-coupled movement even with opposing forces of up to 10 pN acting on the system. However, it is clear that binding sites within the Hill sleeve may not be evenly distributed throughout the length of the sleeve. Microtubule-binding sites may be clustered, reminiscent of pop rivets, at either end or throughout the Hill sleeve. The principle of biased diffusion, however, may still be at work at the kinetochore. The two relevant characteristics are the magnitude of force that can be generated and the persistence of attachment with a microtubule plus end.
Figure 5. Proposed mechanisms for microtubule-depolymerization coupled force generation at the kinetochore.
Two types of mechanism have been proposed: biased diffusion (a) and forced walk (b, c). a, Microtubules can be persistently attached to lattice-binding proteins such as the Ndc80 complex (Fig. 4) through many diffusive couplers (for example, flexible, positively charged amino acids at the amino terminus of the Ndc80 subunit of the Ndc80 complex94,95) attached at different distances from the plus end. Thermal diffusion is depicted as random fluctuations of the microtubule lattice (double-headed arrows). Depolymerization at the microtubule plus end provides a bias to the diffusive movements while attachment can be maintained. b, The discovery of DASH rings and subsequent in vitro studies led to the idea that ring couplers (such as the DASH complex) are involved in force generation. Energy from microtubule depolymerization drives the rings towards the microtubule minus end (curved arrows). c, Subsequently, ultrastructural study of the vertebrate kinetochore uncovered the presence of fibrillar couplers (pink) that attach to peeling microtubule protofilaments at specific locations. This discovery led to the idea that fibrillar couplers can themselves be force generators. The mechanisms proposed in a, b and c are all remain valid. d, Finally, when the plus end of a microtubule polymerizes against a rigid barrier, such as the cell wall, this can also generate forces, albeit smaller forces, but these forces are not thought to have a significant role in kinetochore motility.
The second category of model incorporates the forced-walk mechanism, which harvests the strain energy stored in the microtubule lattice to generate movement78. Experiments, as well as theoretical considerations, show that a large proportion of the energy generated by GTP hydrolysis is stored as strain in the microtubule lattice, resulting in bending of the tubulin protofilaments during microtubule depolymerization. A kinetochore-based coupler that resists the free bending of the proto-filaments can use the strain energy in the lattice to drive chromosome movement. The recently discovered DASH complex (also known as the Dam1 complex)79,80, which forms rings around the microtubule lattice in vitro, is one example of such a forced-walk coupler. Theoretical studies show that the dimensions of the DASH-complex ring are optimal for generating maximum force while maintaining persistent movement81–83. Another type of coupler was recently proposed based on ultrastructural analysis of microtubule protofilaments within the kinetochore84. This study found that the curling of the protofilaments of a depolymerizing microtubule is restrained within the kinetochore compared with depolymerizing microtubules elsewhere in the cell. Furthermore, averaging analysis of many protofilaments revealed fibrillar structures that are about 30–100 nm in length extending from the kinetochore structure and attaching over a specific region of these curling protofilaments. Theoretical modelling of this geometry of attachment shows that such fibrillar couplers can also generate depolymerization-coupled forces. These models also predicted that at least two such fibrillar connections per protofilament are necessary for generating persistent movement. Theoretical work, as well as experimental data, shows that these mechanisms can generate large forces (up to 60–70 pN), converting a large proportion of the strain energy into work. Several microtubule-binding proteins present in the kinetochore or its fibrillar structures (Ndc80, KNL-1, CENP-E, CENP-F and XMAP215) have been proposed84 as candidates for this coupler within the kinetochore.
The unanswered question is which of these two categories of mechanism is responsible for chromosome motility in vivo. DASH and Ndc80 complexes are the two principle microtubule-binding protein complexes. Of these, the DASH complex has only been discovered in yeast (S. cerevisiae and S. pombe), although functional homologues may be present in multicellular eukaryotes. In vitro studies of these complexes show that they can generate movement through either of the two mechanisms discussed above82,85,86. Thus, characterizing the relative contributions of these two mechanisms at the kinetochore will be an important advance in understanding kinetochore behaviour.
Looking forward
The world of molecular biology and genome science has flourished since the elucidation of the structure of DNA in the 1950s. Although remarkable strides have been made in cataloguing chromosome-packaging proteins, topology adjusters and segregation machines, major gaps remain in understanding how the DNA polymer is organized inside a cell. On the one hand, many features of chromatin may be explained from first principles learned in polymer physics. On the other hand, there are many ATPases that act on chromatin. Both perspectives must be incorporated in refining our current understanding.
Another major challenge is relating single-molecule experiments and force measurements carried out in vitro to the invivo situation. How much force is needed to segregate a chromosome? It is difficult to replicate the cellular viscosity and molecular crowding conditions that impinge on a segregating chromosome. Classic micromanipulation experiments on grasshopper spermatocytes showed that the mitotic spindle is an extremely weak (nine orders of magnitude weaker than the bacterial flagellar motor) but accurate machine87. One of the next goals is to determine the material properties of the chromosome and the surrounding environment so that the magnitude of forces can be deduced in living cells. Hooke’s law is our first approximation for modelling chromatin springs in cells. This law (F=k(l − l0)) can be rewritten as F=EA(l − l0/l), where E is Young’s modulus, A is the cross-sectional area of the kinetochore, l is the length between sister kinetochores at a given force and l0 is this length at rest (Box 1). The relationship between force and the material property of the spring (Young’s modulus) illustrates that Young’s modulus can be used to calculate force at a given strain (l − l0). In this form, it is clear that force is related to area (A) and is thus extremely sensitive to the area of force generation (that is the kinetochore). In the case of yeast, similar estimates of the force per microtubule are obtained whether the diameter used is that of the single kinetochore attachment (40 nm) or of the composite 16 chromosomes (250 nm) (Table 2). The area of the composite approximates the information available for grasshopper kinetochores87 and it shows that forces at the yeast kinetochore are within an order of magnitude of those of more complex kinetochores. When it becomes possible to make estimates of the material properties of structures and measure force inside cells, we will understand how these remarkable machines function with such exquisite accuracy.
Table 2.
Estimates of force at the kinetochore per microtubule-attachment site
| Chromosome elasticity (Young’s modulus (E), N m−2) | Dimensions of kinetochore (diameter (d), μm)* | Estimation of force (pN) EA(l − l0/l), where A = d2π/4 | Number of kinetochore microtubules | Force per microtubule (pN) |
|---|---|---|---|---|
| Yeast (Saccharomyces cerevisiae) | ||||
| 400 | 0.040 | 400(0.04 × 10−6)2 × π/4 × 1.0 = 0.5 | 1 | 0.5 |
| 400 | 0.250 | 400(0.25 × 10−6)2 × π/4 × 1.0 = 19.6 | 16 | 1.2 |
| Mammals | ||||
| 400 | ~1 | 400(1 × 10−6)2 × π/4 × 0.5 = 157 | ~30 | ~5 |
The diameter of a single kinetochore attachment site is 0.04 μm, whereas the composite of all 16 chromosomes is 0.25 μm. A, cross-sectional area of the kinetochore; l, length between sister kinetochores at a given force; l0, length between sister kinetochores at rest.
Acknowledgments
We thank E. Yeh, J. Haase and J. Verdaasdonk (Department of Biology, University of North Carolina at Chapel Hill) for comments on the manuscript, and L. Vicci and R. M. Taylor III (Department of Computer Science, University of North Carolina at Chapel Hill) and M. Rubinstein (Department of Chemistry, University of North Carolina at Chapel Hill) for discussions concerning the mechanical properties of biological molecules.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:R721–R733. doi: 10.1016/j.cub.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 2.Wolgemuth CW, Mogilner A, Oster G. The hydration dynamics of polyelectrolyte gels with applications to cell motility and drug delivery. Eur Biophys J. 2004;33:146–158. doi: 10.1007/s00249-003-0344-5. [DOI] [PubMed] [Google Scholar]
- 3.Mazumder A, Shivashankar GV. Gold-nanoparticle-assisted laser perturbation of chromatin assembly reveals unusual aspects of nuclear architecture within living cells. Biophys J. 2007;93:2209–2216. doi: 10.1529/biophysj.106.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazumder A, Roopa T, Basu A, Mahadevan L, Shivashankar GV. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys J. 2008;95:3028–3035. doi: 10.1529/biophysj.108.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci USA. 2006;103:12388–12393. doi: 10.1073/pnas.0605305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. doi: 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- 7.Finzi L, Gelles J. Measurement of lactose repressor-mediated loop formation and breakdown in single DNA molecules. Science. 1995;267:378–380. doi: 10.1126/science.7824935. [DOI] [PubMed] [Google Scholar]
- 8.Bianco PR, et al. Processive translocation and DNA unwinding by individual RecBCD enzyme molecules. Nature. 2001;409:374–378. doi: 10.1038/35053131. [DOI] [PubMed] [Google Scholar]
- 9.Wang MD, et al. Force and velocity measured for single molecules of RNA polymerase. Science. 1998;282:902–907. doi: 10.1126/science.282.5390.902. [DOI] [PubMed] [Google Scholar]
- 10.Yin H, et al. Transcription against an applied force. Science. 1995;270:1653–1657. doi: 10.1126/science.270.5242.1653. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin J, Losick R. Does RNA polymerase help drive chromosome segregation in bacteria? Proc Natl Acad Sci USA. 2002;99:14089–14094. doi: 10.1073/pnas.182539899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm C, Goto T, Wang JC, Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- 13.Emanuel M, Radja NH, Henriksson A, Schiessel H. The physics behind the larger scale organization of DNA in eukaryotes. Phys Biol. 2009;6:25008. doi: 10.1088/1478-3975/6/2/025008. [DOI] [PubMed] [Google Scholar]
- 14.Towles KB, Beausang JF, Garcia HG, Phillips R, Nelson PC. First-principles calculation of DNA looping in tethered particle experiments. Phys Biol. 2009;6:25001. doi: 10.1088/1478-3975/6/2/025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pope LH, Xiong C, Marko JF. Proteolysis of mitotic chromosomes induces gradual and anisotropic decondensation correlated with a reduction of elastic modulus and structural sensitivity to rarely cutting restriction enzymes. Mol Biol Cell. 2006;17:104–113. doi: 10.1091/mbc.E05-04-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano T. At the heart of the chromosome: SMC proteins in action. Nature Rev Mol Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 17.Yeh E, et al. Pericentric chromatin is organized into an intramolecular loop in mitosis. Curr Biol. 2008;18:81–90. doi: 10.1016/j.cub.2007.12.019. Pericentromeric chromatin adopts an intramolecular loop that is stretched between sister centromeres in mitosis. It is proposed that these loops function as DNA springs in the mitotic spindle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi M, Edwards S. The Theory of Polymer Dynamics. Oxford Univ. Press; 1992. [Google Scholar]
- 19.Okumura Y, Ito K. The Polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv Mater. 2001;13:485–487. [Google Scholar]
- 20.Daneholt B, Anderson K, Fagerlind M. Large-sized polysomes in Chironomus tentans salivary glands and their relation to Balbiani ring 75S. RNA J Cell Biol. 1977;73:149–160. doi: 10.1083/jcb.73.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gall JG. In: Methods in Cell Physiology. Prescott DM, editor. II. Academic; 1966. pp. 37–60. [Google Scholar]
- 22.Paulson JR, Laemmli UK. The structure of histone-depleted metaphase chromosomes. Cell. 1977;12:817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan NL, Marquis KA, Rudner DZ. Recruitment of SMC by ParB–ParS organizes the origin region and promotes efficient chromosome segregation. Cell. 2009;137:697–707. doi: 10.1016/j.cell.2009.04.044. This study showed that SMC protein components localize to the origin of replication in Bacillus subtilis and demonstrated that chromosome condensation coupled to replication is a major mechanism of chromosome segregation in bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM. Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol. 2000;151:749–762. doi: 10.1083/jcb.151.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 26.Harrison BD, Hoang ML, Bloom K. Persistent mechanical linkage between sister chromatids throughout anaphase. Chromosoma. 2009;118:633–645. doi: 10.1007/s00412-009-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paliulis LV, Nicklas RB. Micromanipulation of chromosomes reveals that cohesion release during cell division is gradual and does not require tension. Curr Biol. 2004;14:2124–2129. doi: 10.1016/j.cub.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 28.Storlazzi A, et al. Coupling meiotic chromosome axis integrity to recombination. Genes Dev. 2008;22:796–809. doi: 10.1101/gad.459308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermayer B, Mobius W, Hallatschek O, Frey E, Kroy K. Freely relaxing polymers remember how they were straightened. Phys Rev E. 2009;79:021804. doi: 10.1103/PhysRevE.79.021804. [DOI] [PubMed] [Google Scholar]
- 30.Meaburn KJ, Misteli T. Chromosome territories. Nature. 2007;445:379–381. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- 31.Austin S, Abeles A. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J Mol Biol. 1983;169:373–387. doi: 10.1016/s0022-2836(83)80056-4. [DOI] [PubMed] [Google Scholar]
- 32.Ogura T, Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983;32:351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher MA. Structural biology of plasmid partition: uncovering the molecular mechanisms of DNA segregation. Biochem J. 2008;412:1–18. doi: 10.1042/BJ20080359. [DOI] [PubMed] [Google Scholar]
- 34.Lynch AS, Wang JC. Use of an inducible site-specific recombinase to probe the structure of protein–DNA complexes involved in F plasmid partition in Escherichia coli. J Mol Biol. 1994;236:679–684. doi: 10.1006/jmbi.1994.1179. [DOI] [PubMed] [Google Scholar]
- 35.Clarke L, Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980;287:504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- 36.Furuyama T, Henikoff S. Centromeric nucleosomes induce positive DNA supercoils. Cell. 2009;138:104–113. doi: 10.1016/j.cell.2009.04.049. This paper reports that the direction of DNA coiling around histone complexes containing the centromere-specific histone variant CENP-A is opposite to the canonical negative supercoils around histones. This is one of several features that distinguish centromeric chromatin from the rest of the genome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 38.Dalal Y, Furuyama T, Vermaak D, Henikoff S. Structure, dynamics, and evolution of centromeric nucleosomes. Proc Natl Acad Sci USA. 2007;104:15974–15981. doi: 10.1073/pnas.0707648104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalal Y, Wang H, Lindsay S, Henikoff S. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 2007;5:e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill A, Bloom K. Genetic manipulation of centromere function. Mol Cell Biol. 1987;7:2397–2405. doi: 10.1128/mcb.7.7.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins KA, Castillo AR, Tatsutani SY, Biggins S. De novo kinetochore assembly requires the centromeric histone H3 variant. Mol Biol Cell. 2005;16:5649–5660. doi: 10.1091/mbc.E05-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamiche A, et al. Interaction of the histone (H3-H4)2 tetramer of the nucleosome with positively supercoiled DNA minicircles: potential flipping of the protein from a left- to a right-handed superhelical form. Proc Natl Acad Sci USA. 1996;93:7588–7593. doi: 10.1073/pnas.93.15.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bancaud A, et al. Nucleosome chiral transition under positive torsional stress in single chromatin fibers. Mol Cell. 2007;27:135–147. doi: 10.1016/j.molcel.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Selvin PR, et al. Torsional rigidity of positively and negatively supercoiled DNA. Science. 1992;255:82–85. doi: 10.1126/science.1553534. [DOI] [PubMed] [Google Scholar]
- 45.Bloom KS, Carbon J. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell. 1982;29:305–317. doi: 10.1016/0092-8674(82)90147-7. [DOI] [PubMed] [Google Scholar]
- 46.Folco HD, Pidoux AL, Urano T, Allshire RC. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science. 2008;319:94–97. doi: 10.1126/science.1150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blat Y, Kleckner N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell. 1999;98:249–259. doi: 10.1016/s0092-8674(00)81019-3. [DOI] [PubMed] [Google Scholar]
- 48.Weber SA, et al. The kinetochore is an enhancer of pericentric cohesin binding. PLoS Biol. 2004;2:e260. doi: 10.1371/journal.pbio.0020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckert CA, Gravdahl DJ, Megee PC. The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev. 2007;21:278–291. doi: 10.1101/gad.1498707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haldar D, Kamakaka R. T tRNA genes as chromatin barriers. Nature Struct Mol Biol. 2006;13:192–193. doi: 10.1038/nsmb0306-192. [DOI] [PubMed] [Google Scholar]
- 51.He X, Asthana S, Sorger PK. Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell. 2000;101:763–775. doi: 10.1016/s0092-8674(00)80888-0. [DOI] [PubMed] [Google Scholar]
- 52.Pearson CG, Maddox PS, Salmon ED, Bloom K. Budding yeast chromosome structure and dynamics during mitosis. J Cell Biol. 2001;152:1255–1266. doi: 10.1083/jcb.152.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribeiro SA, et al. Condensin regulates the stiffness of vertebrate centromeres. Mol Biol Cell. 2009;20:2371–2380. doi: 10.1091/mbc.E08-11-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push–pull mechanism. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchida KS, et al. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouck DC, Bloom K. Pericentric chromatin is an elastic component of the mitotic spindle. Curr Biol. 2007;17:741–748. doi: 10.1016/j.cub.2007.03.033. This paper shows that the level of nucleosomal DNA compaction regulates the length of the mitotic spindle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zocchi G. Controlling proteins through molecular springs. Annu Rev Biophys. 2009;38:75–88. doi: 10.1146/annurev.biophys.050708.133637. This review discusses how the elastic energy of the DNA spring can induce a conformational change in a protein through the construction of a novel protein–DNA chimaera. [DOI] [PubMed] [Google Scholar]
- 59.Zelin E, Silverman SK. Allosteric control of ribozyme catalysis by using DNA constraints. Chembiochem. 2007;8:1907–1911. doi: 10.1002/cbic.200700437. [DOI] [PubMed] [Google Scholar]
- 60.Ocampo-Hafalla MT, Katou Y, Shirahige K, Uhlmann F. Displacement and re-accumulation of centromeric cohesin during transient pre-anaphase centromere splitting. Chromosoma. 2007;116:531–544. doi: 10.1007/s00412-007-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerlich D, Hirota T, Koch B, Peters JM, Ellenberg J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr Biol. 2006;16:333–344. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira RA, Coelho PA, Sunkel CE. The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis. Mol Cell Biol. 2005;25:8971–8984. doi: 10.1128/MCB.25.20.8971-8984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher JK, et al. DNA relaxation dynamics as a probe for the intracellular environment. Proc Natl Acad Sci USA. 2009;106:9250–9255. doi: 10.1073/pnas.0812723106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callaway E. Bacteria’s new bones. Nature. 2008;451:124–126. doi: 10.1038/451124a. [DOI] [PubMed] [Google Scholar]
- 65.Cordova NJ, Ermentrout B, Oster GF. Dynamics of single-motor molecules: the thermal ratchet model. Proc Natl Acad Sci USA. 1992;89:339–343. doi: 10.1073/pnas.89.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garner EC, Campbell CS, Weibel DB, Mullins RD. Reconstitution of DNA segregation driven by assembly of a prokaryotic actin homolog. Science. 2007;315:1270–1274. doi: 10.1126/science.1138527. This paper shows that prokaryotic DNA segregation can be reconstituted in vitro with defined components. The mechanism of segregation is polymer growth at the sites of contact with the DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramamurthi KS, Losick R. Grasping at origins. Cell. 2008;134:916–918. doi: 10.1016/j.cell.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Bowman GR, et al. A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell. 2008;134:945–955. doi: 10.1016/j.cell.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebersbach G, Briegel A, Jensen GJ, Jacobs-Wagner C. A self-associating protein critical for chromosome attachment, division, and polar organization in Caulobacter. Cell. 2008;134:956–968. doi: 10.1016/j.cell.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marquis KA, et al. SpoIIIE strips proteins off the DNA during chromosome translocation. Genes Dev. 2008;22:1786–1795. doi: 10.1101/gad.1684008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ptacin JL, et al. Sequence-directed DNA export guides chromosome translocation during sporulation in Bacillus subtilis. Nature Struct Mol Biol. 2008;15:485–493. doi: 10.1038/nsmb.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int Rev Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- 73.Cottingham FR, Gheber L, Miller DL, Hoyt MA. Novel roles for Saccharomyces cerevisiae mitotic spindle motors. J Cell Biol. 1999;147:335–350. doi: 10.1083/jcb.147.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Welburn JP, Cheeseman IM. Toward a molecular structure of the eukaryotic kinetochore. Dev Cell. 2008;15:645–655. doi: 10.1016/j.devcel.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Joglekar AP, Bloom K, Salmon ED. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr Biol. 2009;19:694–699. doi: 10.1016/j.cub.2009.02.056. This paper shows the protein architecture of the S. cerevisiae kinetochore in metaphase and anaphase, by using live-cell super-resolution microscopy of fluorescently labelled kinetochore proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan X, et al. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. This study used two-colour super-resolution microscopy to visualize fixed HeLa cells stained with antibodies, allowing the spatial position of proteins in the mammalian kinetochore to be determined with nanometre accuracy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill TL. Theoretical problems related to the attachment of microtubules to kinetochores. Proc Natl Acad Sci USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 79.Miranda JJ, De Wulf P, Sorger PK, Harrison SC. The yeast DASH complex forms closed rings on microtubules. Nature Struct Mol Biol. 2005;12:138–143. doi: 10.1038/nsmb896. [DOI] [PubMed] [Google Scholar]
- 80.Westermann S, et al. Formation of a dynamic kinetochore–microtubule interface through assembly of the Dam1 ring complex. Mol Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 81.Efremov A, Grishchuk EL, McIntosh JR, Ataullakhanov FI. In search of an optimal ring to couple microtubule depolymerization to processive chromosome motions. Proc Natl Acad Sci USA. 2007;104:19017–19022. doi: 10.1073/pnas.0709524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grishchuk EL, et al. The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc Natl Acad Sci USA. 2008;105:15423–15428. doi: 10.1073/pnas.0807859105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grishchuk EL, et al. Different assemblies of the DAM1 complex follow shortening microtubules by distinct mechanisms. Proc Natl Acad Sci USA. 2008;105:6918–6923. doi: 10.1073/pnas.0801811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McIntosh JR, et al. Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell. 2008;135:322–333. doi: 10.1016/j.cell.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asbury CL, Gestaut DR, Powers AF, Franck AD, Davis TN. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc Natl Acad Sci USA. 2006;103:9873–9878. doi: 10.1073/pnas.0602249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Powers AF, et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicklas RB. The forces that move chromosomes in mitosis. Annu Rev Biophys Biophys Chem. 1988;17:431–449. doi: 10.1146/annurev.bb.17.060188.002243. [DOI] [PubMed] [Google Scholar]
- 88.Mehta S, et al. The 2 micron plasmid purloins the yeast cohesin complex: a mechanism for coupling plasmid partitioning and chromosome segregation? J Cell Biol. 2002;158:625–637. doi: 10.1083/jcb.200204136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiermaier E, Woehrer S, Peng Y, Mechtler K, Westermann SA. Dam1-based artificial kinetochore is sufficient to promote chromosome segregation in budding yeast. Nature Cell Biol. 2009;11:1109–1115. doi: 10.1038/ncb1924. [DOI] [PubMed] [Google Scholar]
- 90.Lacefield S, Lau DT, Murray AW. Recruiting a microtubule-binding complex to DNA directs chromosome segregation in budding yeast. Nature Cell Biol. 2009;11:1116–1120. doi: 10.1038/ncb1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hertwig O. Lehrbuch der Entwicklungsgeschichte des Menschen und der Wirbeltiere. Verlag von Gustav Fischer; 1906. [Google Scholar]
- 92.Zinkowski RP, Meyne J, Brinkley BR. The centromere–kinetochore complex: a repeat subunit model. J Cell Biol. 1991;113:1091–1110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hori T, et al. CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008;135:1039–1052. doi: 10.1016/j.cell.2008.10.019. This paper identified kinetochore components that interact with nucleosomal DNA that does not contain the centromere-specific histone. This is a key finding for understanding how chromatin is organized within a mammalian centromere. [DOI] [PubMed] [Google Scholar]
- 94.Miller SA, Johnson ML, Stukenberg PT. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80 (Hec1) Curr Biol. 2008;18:1785–1791. doi: 10.1016/j.cub.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guimaraes GJ, Dong Y, McEwen BF, Deluca JG. Kinetochore–microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]






