Abstract
We have previously shown the transcription factor SOX9 to be required for the maintenance of multipotential pancreatic progenitor cells in the early embryonic pancreas. However, the association of pancreatic endocrine defects with the Sox9-haploinsufficiency syndrome campomelic dysplasia (CD) implies additional later roles for Sox9 in endocrine development. Using short-term lineage tracing in mice, we demonstrate here that SOX9 marks a pool of multipotential pancreatic progenitors throughout the window of major cell differentiation. During mid-pancreogenesis, both endocrine and exocrine cells simultaneously arise from the SOX9+ epithelial cords. Our analysis of mice with 50%-reduced Sox9 gene dosage in pancreatic progenitors reveals endocrine-specific defects phenocopying CD. By birth, these mice display a specific reduction in endocrine cell mass, while their exocrine compartment and total organ size is normal. The decrease in endocrine cells is caused by reduced generation of endocrine progenitors from the SOX9+ epithelium. Conversely, formation of exocrine progenitors is insensitive to reduced Sox9 gene dosage, thus explaining the normal organ size at birth. Our results show that not only is SOX9 required for the maintenance of early pancreatic progenitors, but also governs their adoption of an endocrine fate. Our findings therefore suggest that defective endocrine specification might underlie the pancreatic phenotype of individuals with CD.
Keywords: Sox9, Ngn3, pancreas, development, progenitor cell, haploinsufficient, gene dosage, islet, heterozygous, endocrine, differentiation, lineage
Introduction
During embryonic development, all differentiated cells in the vertebrate pancreas are generated from a common pool of multipotential pancreatic progenitor cells. Lineage tracing studies in mice have shown that progenitors marked by the transcription factors PDX1 and PTF1a give rise to the acinar and ductal cells of the exocrine pancreas as well as to the endocrine lineages of the islets of Langerhans, including the insulin-producing β-cells, the glucagons-producing α-cells, the somatostatin-producing δ-cells, and the pancreatic polypeptide (PP)-producing cells (Gu et al., 2002; Kawaguchi et al., 2002). With the initiation of the secondary transition, which marks the onset of major endocrine and exocrine cell differentiation around embryonic day (e) 13.5, PDX1 and PTF1a become markers of mature pancreatic cell types. PDX1 expression becomes restricted to insulin+ cells and newly differentiated acinar cells (Guz et al., 1995), while PTF1a becomes compartmentalized within the acini (Krapp et al., 1996). Because neither PDX1 nor PTF1a are exclusive progenitor cell markers throughout the duration of pancreas development, it is still unclear whether multipotential progenitor cells persist in the pancreas throughout its development. Endocrine cell differentiation is initiated by the transcription factor NGN3, which marks a transient population of specified endocrine progenitors throughout embryogenesis. Studies in mice have shown that Ngn3 is both required and sufficient to induce endocrine differentiation (Gradwohl et al., 2000; Gu et al., 2002; Jensen et al., 2000; Schwitzgebel et al., 2000). While endocrine committed progenitors can be found as early as e10.5, exocrine commitment does not occur until around e14. PTF1a and carboxypeptidase A (CPA) expressing cells are initially multipotential, but later in development exclusively mark committed exocrine progenitors that reside in the tips of the branching pancreatic epithelium (Zhou et al., 2007).
We have recently shown that the HMG box transcription factor SOX9 is co-expressed with PDX1 in multipotential progenitors of the undifferentiated pancreatic epithelium between e9 and e12.5. However, in contrast to PDX1, SOX9 is excluded from lineage-committed progenitors and differentiated cells at the beginning of the secondary transition (Seymour et al., 2007). During this time, SOX9 becomes exclusively localized to the epithelial cords, which have been suggested to harbor uncommitted progenitor cells (Fujitani et al., 2006). Notably, in the epithelial cords, NGN3+ cells exist in an intercalated arrangement amongst the SOX9+ cells, suggesting that endocrine progenitors may arise from the SOX9+ cell population. Postnatally, SOX9 expression becomes restricted to the ductal and centroacinar cell compartment (Seymour et al., 2007), which has been suggested to harbor endocrine-differentiation-competent progenitors (Bonner-Weir et al., 2000; Sharma et al., 1999; Xu et al., 2008). This raises the question how long SOX9+ cells normally contribute to cell neogenesis duration of pancreas development.
Through pancreas-specific Pdx1-Cre-mediated inactivation of Sox9, we demonstrated that SOX9 controls the maintenance of pancreatic progenitors by stimulating their proliferation, survival, and persistence in an undifferentiated state (Seymour et al., 2007). However, because the Pdx1-Cre transgene results in efficient early deletion of Sox9 and severe pancreatic hypoplasia by e11.5, our analysis of SOX9-deficient pancreata precluded the dissection of possible additional roles of SOX9 during pancreatic cell differentiation at later stages of development. Several pancreatic transcription factors have been found to play distinct early and later roles in pancreogenesis. For example, PDX1 initially promotes outgrowth of the pancreatic anlagen (Jonsson et al., 1994), but subsequently plays a role in maintaining proper β-cell function (Ahlgren et al., 1998). Furthermore, Pdx1-haploinsufficiency is associated with increased islet apoptosis in adult mice (Johnson et al., 2003), suggesting that correct Pdx1 gene dosage is required for endocrine cell maintenance. Likewise, Sox9 is a known haploinsufficient gene: in humans, heterozygosity for loss-of-function mutations in Sox9 is associated with the semi-lethal skeletal malformation syndrome campomelic dysplasia (CD) (Foster et al., 1994; Wagner et al., 1994). Neonates with CD have recently been shown to display pancreatic islet defects, including islet hypoplasia and decreased expression of hormones and β-cell maturity markers (Piper et al., 2002). Though the findings in individuals with CD strongly suggest a role for Sox9 in islet cell development, the mechanism underlying islet hypoplasia as a result of Sox9-haploinsufficiency has yet to be identified.
In this study, we have examined whether the SOX9+ epithelial cords serve as a reservoir of multipotential progenitors throughout pancreatic development and whether Sox9 function is required to initiate cell differentiation from this epithelium. Utilizing Sox9-eGFP mice for short-term in vivo lineage tracing of SOX9+ cells, we show that during mid-pancreogenesis the SOX9+ epithelium simultaneously gives rise to NGN3+ endocrine progenitors, the different endocrine cell types and exocrine acinar cells. Our analysis is the first to demonstrate that a compartment of multipotential progenitors persists after the secondary transition of pancreas development. To further test whether Sox9 plays a role in pancreatic cell differentiation, we analyzed pancreatic development in mice in which Pdx1-Cre mediates deletion of a single Sox9flox allele. Similar to individuals with CD, such Sox9+/Δpan mice display a 50% reduction in islet cell mass, while their pancreatic exocrine compartment is unaffected. We show that the reduction in endocrine cells results from a defect in the generation of sufficient endocrine progenitors, thus revealing a dosagesensitive requirement for SOX9 in initiating endocrine fate.
Materials and Methods
Mouse Strains
Sox9-eGFP (Tg(Sox9-EGFP)209Gsat/Mmcd) mice (Gong et al., 2003) were obtained from the MMRRC and were maintained on a CD1 genetic background. Sox9+/Δpan embryos and Cre− littermate controls were generated by crossing heterozygous males of the Pdx1-Cre line (Gu et al., 2002) with homozygous females of the Sox9-flox line (Kist et al., 2002). The Sox9-flox line was maintained on a mixed 129P2/OlaHsd ×C57Bl/6J genetic background. Mice were maintained on a 12h light-dark cycle and all protocols were approved by the UC Irvine Institutional Animal Care and Use Committee. Embryos were harvested from timed matings in which noon of the day of vaginal plug appearance was considered as e0.5. For BrdU labeling, pregnant females were injected i.p. with 50 µg/g body-weight of BrdU (Sigma) and embryos were harvested 1h after injection.
Histological Analysis
Staining was performed on sections of whole embryos (e12.5), dissected guts (e15.5), or isolated pancreata (e18.5). Length of fixation in 4% paraformaldehyde in PBS at 4°C was determined empirically. For frozen sections, tissues were cryoprotected in 30% sucrose in PBS, embedded in O.C.T. (Sakura Finetek) and sections cut at 10µm. For paraffin sections, tissue was dehydrated in an increasing ethanol series, cleared in xylene and embedded in Paraplast (Kendall): sections were cut at 7µm. H&E staining and immunofluorescence analysis of proteins was performed as described previously (Sander et al., 1997). When required, antigen retrieval was performed in pH 6.0 citrate buffer followed by additional permeabilization in 0.15 % Triton X-100 in PBS. For detection of BrdU, DNA was denatured with 2 M HCl at 37°C for 1h and a M.O.M. Kit (Vector Labs) was used. For triple staining, the M.O.M. Kit was used in conjunction with AMCA Avidin D (Vector Labs). DBA was stained for using biotinylated DBA (1:200) and visualized with Texas Red Avidin (Vector Labs). TUNEL staining was performed using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon). When necessary, nuclei were counterstained with DAPI (Sigma) at 0.1µg/ml.
The following primary antibodies were used at the given dilutions: rabbit anti-SOX9 (Stolt et al., 2003), 1:2000; rabbit anti-SOX9 (Chemicon), 1:1000; guinea-pig anti-PDX1 (kindly provided by C. V. E. Wright, Vanderbilt University, Nashville, TN), 1:10,000; rabbit anti-PDX1, (kindly provided by H. Edlund, Umeå University, Sweden), 1:3000; rabbit anti-PTF1a (kindly provided by H. Edlund), 1:1000; guinea-pig anti-NGN3 (Henseleit et al., 2005), 1:1000; guinea-pig anti-NKX6.1 (Henseleit et al., 2005), 1:1000; rabbit anti-NKX6.1 (kindly provided by P. Serup, Hagedorn Research Institute, Gentofte, Denmark), 1:10,000; rabbit anti-MAFA (Bethyl Labs), 1:1500; guinea-pig anti-ISL1 (kindly provided by J. Ericson, Karolinska Institute, Stockholm, Sweden), 1:5000; rabbit anti-HB9 (kindly provided by J. H. Kehrl, NIH), 1:8000; rat anti-E-cadherin/uvomorulin (Sigma), 1:1000; guinea-pig anti-insulin (DAKO), 1:5000; mouse anti-insulin (Sigma), 1:5000; mouse anti-glucagon (Sigma), 1:5000; goat anti-ghrelin (Santa Cruz), 1:1000; mouse anti-somatostatin (P. Serup), 1:2000; rabbit anti-pancreatic polypeptide (DAKO), 1:2000; rabbit anti-amylase (Sigma), 1:500; rabbit anti-carboxypeptidase A (Biotrend), 1:1000; rabbit anti-GLUT2 (Alpha Diagnostics), 1:200; rabbit anti-PC1/3 (kindly provided by D. F. Steiner, University of Chicago, IL), 1:2000; rabbit anti-IAPP/amylin (Peninsula), 1:2000; mouse anti-GAPDH (Ambion), 1:100,000; and mouse anti-BrdU (Chemicon), 1:50.
For immunofluorescence detection, the following goat-raised secondary antibodies were used (all at 1:2000 dilution): Cy3-conjugated anti-rabbit, anti-mouse, anti-guinea-pig or anti-goat, Cy5-conjugated anti-rabbit or anti-mouse (all Jackson Labs, raised in donkey); Alexa- (488 nm) conjugated anti-rabbit, anti-mouse, or anti-guinea-pig (all Molecular Probes) and Alexa- (555 nm) conjugated anti-mouse (Molecular Probes).
Specimens were viewed on a Zeiss Axioplan 2 microscope and 24-bit TIFF images were acquired with a Zeiss AxioCam digital camera driven by Zeiss AxioVision v. 3.1 software. Colocalization studies for GFP, SOX9 and NGN3 in Sox9-eGFP mice were performed with a Zeiss LSM510META laser scanning confocal microscope. Images were processed with Adobe Photoshop 6.0. Cell counting and/or morphometry was performed on every fifth section through the early embryonic pancreas from a minimum of three mutants and three wild-type somite-matched littermates. Morphometry was conducted using Image-Pro Plus v. 5.0.1 (Media Cybernetics). Relative pancreatic areas of β-cells, α-cells and acini were calculated and cell mass determined as described previously (Garofano et al., 1998).
Islet Isolation and Quantitative RT-PCR
Islets were isolated by intra-ductal injection of Liberase (0.5 mg/ml), purified on a Ficoll gradient and hand-picked under a microscope. RNA was isolated with the RNeasy Mini and Micro kit (Qiagen) and treated with DNAse. Synthesis of cDNA was performed with the Superscript III cDNA kit (Invitrogen). Q-PCR reactions were performed in triplicate using the Sybr Green kit (Applied Biosystems). Listed 5’ to 3’, primer sequences were as follows: Glut2 forward: TCAGAAGACAAGATCACCGGA and reverse: GCTGGTGTGACTGTAAGTGGG; MafA forward: AGGAGGAGGTCATCCGACTGAT and reverse: CTTCTCGCTCTCCAGAATGTG; beta-actin forward: AACCCTAAGGCCAACCGTGAAAAG and reverse: CAGGATGGCGTGAGGGAGAGC; GABA-A receptor intron 5 forward: AACACACACTGGCAGGACTGGCTAGC and reverse: CAATGGTAGGCTCACTCTGGGAGATGATA. Primers for the Neurog3 promoter were designed as described (Lynn et al., 2007). The core PCR program was 94°C for 10 sec, 60°C for 10 sec and 72°C for 10 sec for 39 cycles. Analysis of the data was performed using the comparative ΔCt method (Livak and Schmittgen, 2001).
Chromatin Immunoprecipitation (ChIP)
e15.5 pancreata (n=25) were dissected and fixed at room temperature with 1% formaldehyde for 10 min then were quenched with 125mM glycine. Fixed pancreata were subsequently homogenized using a pestle with cell lysis buffer and were stored in liquid nitrogen until the day of use. The ChIP assay was performed as described (Lynn et al., 2007). For each assay, 5µg of rabbit anti- SOX9 antibody (Chemicon) or rabbit IgG (Santa Cruz) was used. Pulled-down DNA was purified by phenol/chloroform/isoamyl alcohol-extraction and the concentration was determined using a Nano-Drop spectrophotometer.
Insulin and Glucagon Assays
Total protein was isolated from freshly-dissected e18.5 pancreata via overnight acid-ethanol extraction at 4°C following sonication. Total protein content of the supernatant was measured by the Bradford assay (Bradford, 1976). Insulin and glucagon content were assayed in supernatant and diluted to be in the linear range of detection using Mercodia Ultrasensitive Mouse Insulin ELISA (Alpco Diagnostics) and Rat Glucagon ELISA (Wako) kits, respectively.
Western Blots
Total protein was isolated from freshly-dissected e15.5 pancreata (three of each genotype) into protein lysis buffer (10mM Tris-HCl pH7.8; 150mM NaCl; 1mM EDTA; 1% Nonidet P40 and protease inhibitor cocktail tablet [Roche]), sonicated for 3 ×15sec, and then incubated on ice for 30 min. 1mg of protein was loaded on an SDS-Page gel and proteins subsequently transferred onto a nitrocellulose membrane in transfer buffer (500mM Glycine, 50mM Tris-HCl, 0.01% SDS and 20% methanol) at 200V for 1h. Membranes were: washed in PBST (1 × PBS, 0.1% Tween pH 7.4); blocked with 3% non-fat milk for 1h; incubated in primary antibody diluted in 1% BSA in PBST O/N at 4°C; washed with PBST and incubated with HRP-conjugated secondary antibody for 1h at RT. After washing, signal was visualized using the ECL™ Western Blotting Analysis System (Amersham/GE Healthcare). Protein expression levels were quantified with ImageQuant v. 5.0.
Statistical Analysis
Statistical significance was determined by Student’s t-test using Minitab v. 15.
Results
SOX9-Expressing Progenitors Are Multipotent Giving Rise to All Pancreatic Cell Lineages
In previous studies we have shown that in the embryonic pancreas SOX9 is exclusively expressed in undifferentiated cells and absent from endocrine progenitors as well as differentiated endocrine and acinar cells (Seymour et al., 2007). To investigate whether Sox9-expressing cells represent a population of multipotential progenitor cells throughout embryogenesis, we conducted short-term lineage tracing studies based on the expression of green fluorescent protein (GFP).
To follow the fate of SOX9+ cells, we analyzed pancreata of Sox9-eGFP (Tg(Sox9-EGFP)209Gsat/Mmcd) mice (Gong et al., 2003) at e10.5, e15.5 and e18.5 as well as postnatally. In these transgenic mice, expression of eGFP is controlled by a BAC containing 80 kb of 5’ and 140 kb of 3’ Sox9 regulatory sequences. Due to the protein stability of GFP, cells will continue to express GFP for > 24 hours after the Sox9 promoter is no longer active (Li et al., 1998), thus allowing for short-term tracing of progeny that have arisen from SOX9+ cells. To confirm that the half-life of GFP indeed exceeds that of SOX9 protein in the developing pancreas, we cultured embryonic pancreatic anlagen from Sox9-eGFP mice in the presence of the protein synthesis inhibitor cycloheximide. After 12 hours of culture, SOX9 protein was no longer detected in the explant, while GFP was readily detectable (Suppl. Fig. 1A–C). This validates that Sox9-eGFP mice can be used for short-term lineage tracing. To verify that the BAC transgene contains requisite regulatory elements to target the Sox9-expressing domain, we analyzed multiple tissues from Sox9-eGFP mice for co-localization of SOX9 and GFP. In adult pancreas, which is characterized by low cell turnover, the domains of SOX9 and GFP expression were identical (Suppl. Fig. 1D). Likewise, a complete overlap of SOX9 and GFP expression was observed in the adult cerebellum (Suppl. Fig. 1E). These data show that the BAC transgene directs expression of GFP exclusively to Sox9-expressing cells. The finding that the BAC transgene faithfully recapitulates the domain of endogenous SOX9 expression is consistent with previous studies which have demonstrated that the BAC transgene size renders them more resistant to position effects than smaller transgenes (Giraldo and Montoliu, 2001; Gong et al., 2003).
In contrast to the adult CNS, where cells are largely postmitotic, the embryonic CNS is characterized by rapid neurogenesis from progenitors, which are localized in the so-called ventricular zone in the medial aspect of the neural tube. In Sox9-eGFP embryos, SOX9 protein was exclusively detected in the ventricular zone, while GFP expression was also seen in differentiated neurons of the adjacent mantle zone (Suppl. Fig. 1F). The intensity of GFP signal in the neural tube decreased from medial to lateral. The pattern of GFP intensity mirrors the time that elapsed since the neuron has been generated, because neurons arise from the progenitors in the ventricular zone and subsequently begin migrating laterally into the mantle zone. The pattern of GFP distribution in the neural tube of Sox9-eGFP mice therefore validates the idea that the stability of GFP protein in cells that no longer express Sox9 can be used for short-term lineage tracing.
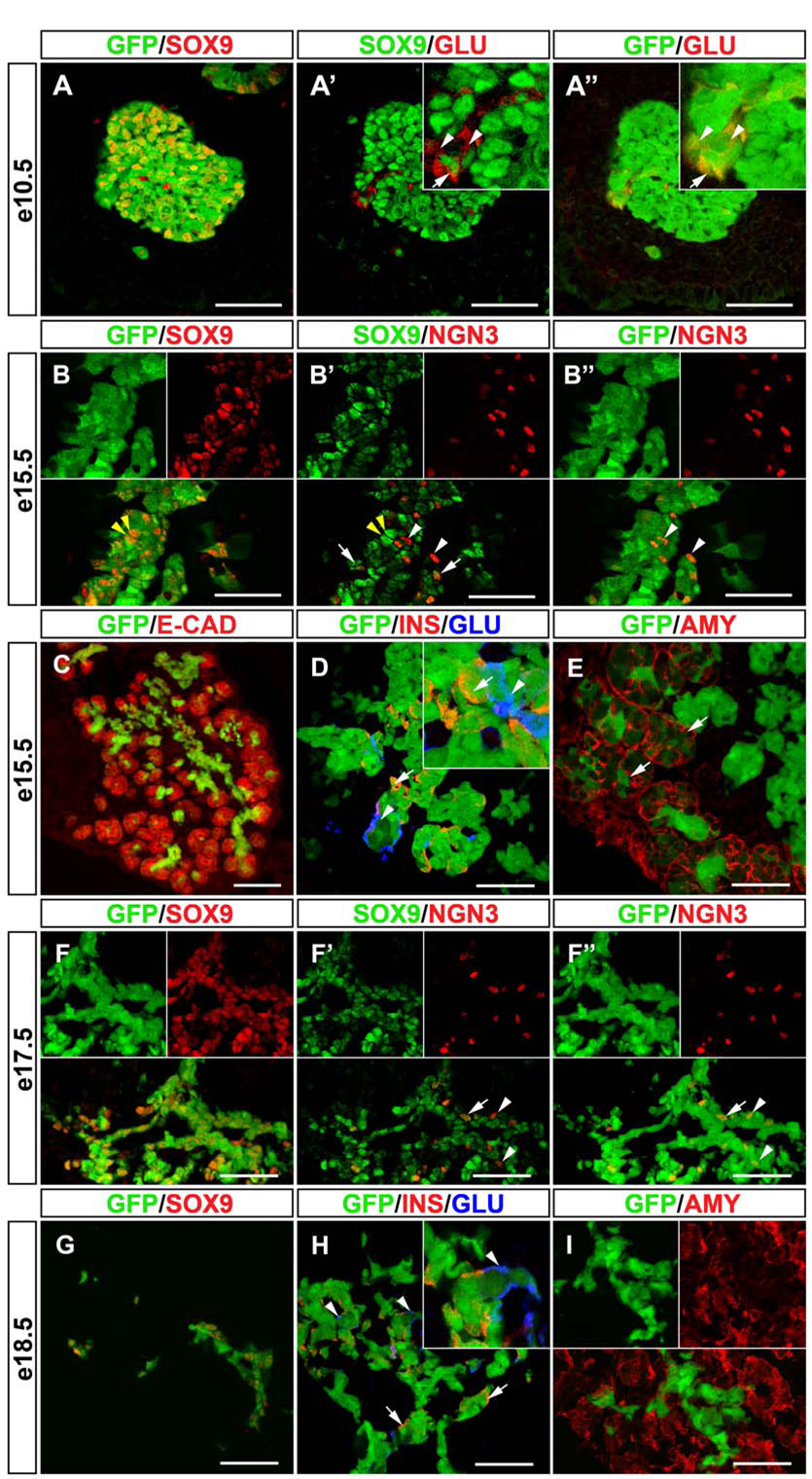
As expected, virtually all SOX9+ cells in the pancreas also expressed GFP throughout embryonic development (Fig. 1A,B,F,G). At e10.5, when most pancreatic epithelial cells are still undifferentiated progenitors, we observed almost complete overlap between the GFP+ and SOX9+ domain (Fig. 1A-A”). Occasionally, cells expressing GFP but not SOX9 were found (Fig. 1A’,A”). As these cells were also glucagon+, it is likely that they represent early-differentiated cells that arose from a SOX9+ progenitor. Notably, a small subpopulation of glucagon+ cells also expressed SOX9 (Fig. 1A’). At e15.5, the pancreatic epithelium comprises undifferentiated progenitors, as well as differentiated endocrine and exocrine cells. At this stage, the GFP+ area was confined to the central part of the developing organ and absent from the developing acini (Fig. 1C). SOX9+/GFP+ cells at e15.5 marked the epithelial cords (Fig. 1B), which have been speculated to be a compartment for uncommitted progenitors (Fujitani et al., 2006). Using confocal imaging of sections co-stained for SOX9 and NGN3, we detected three different populations of cells: SOX9+/NGN3−, SOX9+/NGN3+ and SOX9−/NGN3+ cells (Fig. 1B’). While previous studies by others and us have suggested that only a small subset of NGN3+ cells expresses SOX9 (Lynn et al., 2007; Seymour et al., 2007), our confocal analysis revealed a significant fraction of NGN3+ cells with weak SOX9 immunoreactivity. Careful quantification demonstrated that 74% of NGN3+ cells also express low levels of SOX9 (Table 1). This suggests that during endocrine differentiation cells might undergo the following transition: SOX9+/NGN3− −> SOX9+/NGN3+ −> SOX9−/NGN3+ −> SOX9−/NGN3−/hormone+. Indeed, the observation that SOX9−/NGN3+/GFP+ cells are found in Sox9-eGFP mice (Fig. 1B’,B”) indicates that NGN3+ cells originate from SOX9+ progenitors. The finding that such a cell population could still be detected at e17.5 (Fig. 1F’,F”) further suggests that NGN3+ cells continue to arise from SOX9+ cells later in embryogenesis. To determine whether SOX9+ cells give rise to the different endocrine lineages, we next assayed for co-expression of insulin and glucagon together with GFP in Sox9-eGFP embryos. In contrast to SOX9, which is not co-expressed with endocrine hormones (Seymour et al., 2007), we detected GFP in a large number of glucagon+ and insulin+ cells at both e15.5 (Fig. 1D) and e18.5 (Fig. 1H). At e15.5, ~90% of all insulin+ cells also expressed GFP, while ~65% insulin+ cells were found to be GFP+ at e18.5. These findings imply that endocrine cells continuously arise from SOX9+ progenitors between ~e14 and e18. To fully assess the developmental potential of SOX9+ progenitors at different stages of development, we also determined whether GFP colocalizes with the exocrine marker amylase. At e15.5, GFP was detected in acini that are budding from the tips of the epithelial cords (Fig. 1E). At e18.5, however, no colocalization between GFP and amylase was observed (Fig. 1I), therefore suggesting that acinar cells arise from SOX9+ progenitors around ~e14, but not in late embryogenesis. Together, these results show that SOX9+ cells are multipotential and give rise to the endocrine and exocrine lineages of the pancreas.
Figure 1. Endocrine and Exocrine Cells Originate from the SOX9+ Epithelial Cords.
(A-A”) Simultaneous detection of SOX9, glucagon and GFP in Sox9-eGFP mice and subsequent confocal analysis shows almost complete overlap of GFP and SOX9 expression in the dorsal pancreatic bud at e10.5. While most glucagon+ cells do not express SOX9 (A’, arrowheads in inset), occasional SOX9/glucagon coexpressing cells are detected (A’, arrow in inset). All glucagon+ cells express GFP (A”, arrow and arrowheads). Staining of sections from Sox9-eGFP mice for E-cadherin reveals that the GFP signal is restricted to the epithelial progenitor cords at e15.5 (C). (B-B”) Simultaneous detection of SOX9, NGN3 and GFP at e15.5 shows that the majority of the SOX9+/GFP+ cells do not express NGN3 (B,B’, yellow arrowheads), while a small proportion of the SOX9+ cells also express NGN3 (B’, white arrows). Notably, a subset of NGN3+ cells shows no SOX9 expression but retains GFP label (B’,B”, white arrowheads), suggesting that NGN3+ cells arise from the SOX9+ domain. Also, at e15.5, GFP co-localizes with insulin (D, arrows) and glucagon (D, arrowheads) as well as amylase (D, arrows). Since SOX9 protein is absent from endocrine and exocrine cells at e15.5, this indicates that both lineages originate from SOX9+ cells. (F-F”) Simultaneous detection of SOX9, NGN3 and GFP at e17.5 shows that NGN3+ cells continue to arise from SOX9+ progenitors late in embryogenesis. Note the NGN3+/SOX9− cells that are GFP+ (F’,F”, arrowheads). The arrow (F’,F”) points to a cell that expresses SOX9, NGN3 and GFP. At e18.5, GFP co-localizes with SOX9 in the pancreatic ducts (G). GFP label continues to be seen in a proportion of insulin+ (H, arrows) and glucagon+ cells (H, arrowheads). However, amylase+ cells no longer retain GFP at e18.5 (I), suggesting that endocrine, but not exocrine cells, continue to arise from SOX9+ progenitors until birth. Note that the SOX9 signal was digitally color-converted in A-A’, B-B” and F-F”. INS, insulin; GLU, glucagon; AMY, amylase; E-CAD, E-cadherin. Scale bar = 50µm in A–B and D–I; 100µm in C.
Table 1.
Quantification of NGN3+ Endocrine Progenitor Cells Expressing SOX9 Protein.
| SOX9 Colocalization Amongst NGN3+ Population | ||
|---|---|---|
| (% of Total NGN3+ Cells Counted) | ||
| SOX9Hi | SOX9Lo | SOX9− |
| 13.1±3.59 | 74.1±3.07 | 12.8±5.83 |
The average number of NGN3+ cells expressing high (Hi) levels, low (Lo) levels, or no detectable SOX9 protein was determined on sections from e15.5 embryos after coimmunofluorescence detection of SOX9 and NGN3 and confocal microscopy (n=107 NGN3+ cells). The numbers shown represent the mean number ± standard error of the mean (SEM).
Gross Pancreatic Morphology is Normal in Sox9+/Δpan Mice
Next, we sought to determine whether SOX9 is important for the differentiation of specific cell lineages in the pancreas. Studies in mice and humans have shown that Sox9 is a dosage-sensitive gene. A reduction in gene dosage by mutation of one Sox9 allele causes Campomelic Dysplasia (CD) (Foster et al., 1994), a human syndrome characterized by skeletal malformations, variable sex reversal, and abnormalities of the pancreatic islets (Piper et al., 2002). Since the severe early developmental defects of Sox9-deficient pancreata precluded an analysis of later roles for SOX9 in pancreatic cell differentiation, we analyzed pancreatic development in mice in which Pdx1-Cre-mediated recombination inactivated one Sox9flox allele early during pancreas development (Sox9+/Δpan mice).
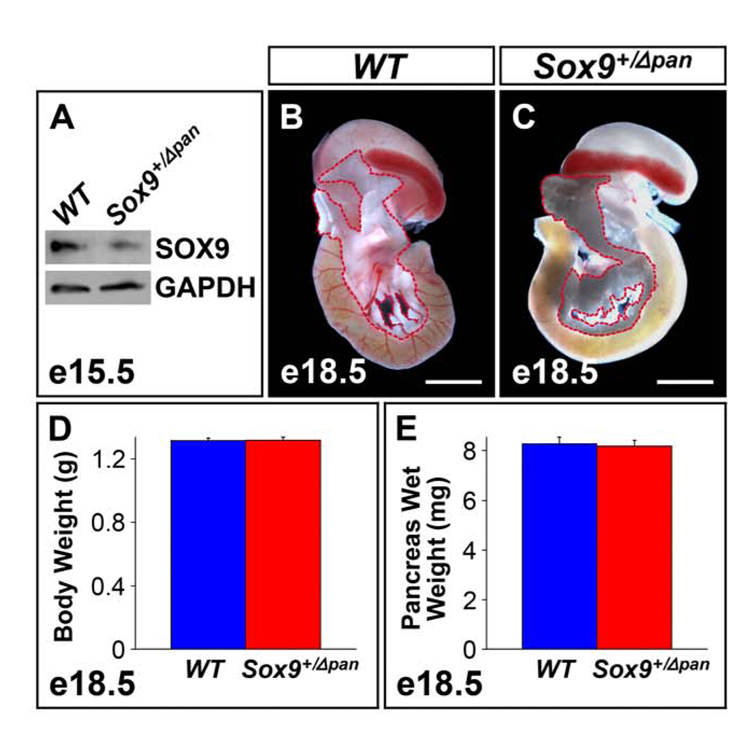
Using quantitative Western blot analysis of protein extracts obtained from whole pancreata of Sox9+/Δpan and wild-type littermates, we determined that conditional deletion of one Sox9 allele resulted in a 46% reduction (n=3) of SOX9 protein in Sox9 heterozygous mutant pancreata (Fig. 2A).
Figure 2. A Two-Fold Reduction in Sox9 Gene Dosage Has No Effect on Organ Size.
(A) Western blot of protein extracts from e15.5 pancreata (n=3) shows a 46% decrease in SOX9 in Sox9 heterozygous pancreatic mutants (Sox9+/Δpan) vs. wild-type (WT) littermates. At e18.5, gross anatomy of the pancreas (demarcated by a red dashed line) in Sox9+/Δpan mice (C) is undistinguishable from that of wild-type littermates (B). In concordance, body weight (D) and pancreas wet weight (E) are equivalent in Sox9+/Δpan (n=19) and wild-type (n=31) embryos at e18.5. (D,E) The values shown represent mean values ± SEM (standard error of the mean). (B,C) Scale bar = 200µm.
Sox9+/Δpan mice were fully viable and fertile and their appearance was indistinguishable from wild-type littermates. In contrast to homozygous Sox9Δpan/Δpan mice, the overall pancreatic size and morphology appeared normal in Sox9+/Δpan mice (Fig. 2B,C). Accordingly, body weight (Fig. 2D) and pancreas weight (Fig. 2E) of Sox9+/Δpan embryos at e18.5 were no different from those of wild-type littermates. Therefore, unlike Sox9 null mutations in the pancreas, a 50% reduction of Sox9 gene dosage does not affect gross pancreas morphogenesis.
Islet Size is Reduced in Sox9+/Δpan Mice
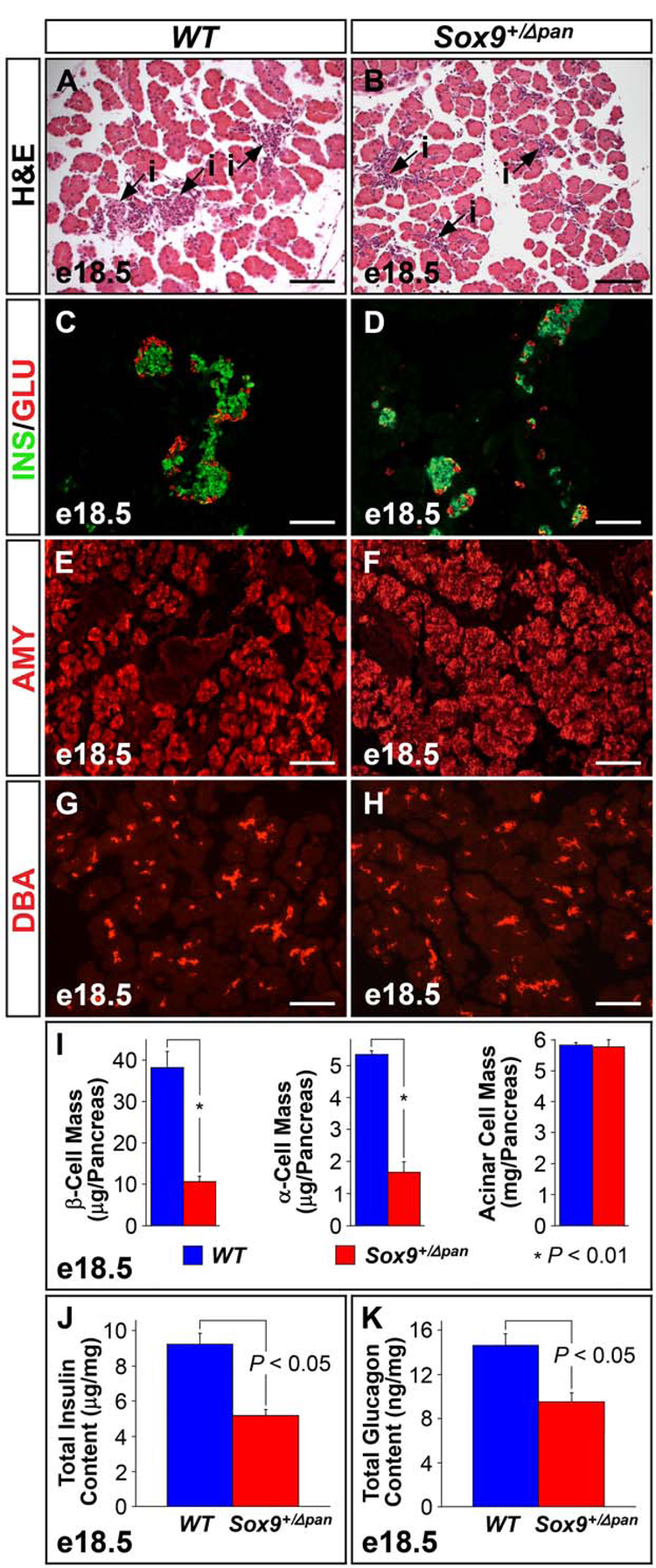
To determine whether reduced Sox9 gene dosage affects the development of the different pancreatic cell types, we examined the histology of Sox9+/Δpan pancreata. In H&E-stained pancreatic sections from e18.5 embryos, the highly eosinophilic exocrine compartment exhibited a similar appearance and size in both Sox9+/Δpan and Sox9+/+ embryos (Fig. 3A,B). By contrast, the endocrine islets in Sox9+/Δpan mice (purple, heavily nucleated cells in Fig. 3B) were reduced in size compared with those of wild-type littermates (Fig. 3A). These findings suggest that a 50% reduction of SOX9 levels selectively affects the development of the endocrine compartment.
Figure 3. Sox9-Haploinsufficiency Leads to Islet-Specific Hypoplasia.
Hematoxylin and eosin (H&E) staining reveals significant islet hypoplasia in Sox9 heterozygous mutant (Sox9+/Δpan) (B) compared to wild-type (WT) pancreas (A). Numbers of insulin+ β-cells and glucagon+ α-cells are equally reduced in Sox9+/Δpan islets (C,D), while the amylase+ acinar (E,F) and DBA+ ductal compartments (G,H) of the exocrine pancreas, are indistinguishable between Sox9+/Δpan and wild-type pancreas. (I) Morphometry confirms significant reductions in both α- and β-cell mass in Sox9+/Δpan vs. control pancreata at e18.5, while the acinar cell mass, as assessed by amylase+ tissue area, is unchanged (n=3). ELISAs verify significant decreases in both total pancreatic insulin (J) and glucagon (K) in Sox9+/Δpan compared to wild-type pancreas (n=5). The values shown represent mean values ± SEM (standard error of the mean). INS, insulin; GLU, glucagon; AMY, amylase; DBA, Dolichos biflorus agglutinin, i, islet. (A–H) Scale bar = 100µm.
To examine whether hormone-expressing cells form in Sox9 heterozygous mutant pancreata, we performed immunostaining for insulin and glucagon as markers of β- and α-cells, respectively. The islets of Sox9+/Δpan mice showed normal islet architecture with a core of insulin+ cells surrounded by the three other endocrine cell types (Fig. 3D, data not shown). However, the overall numbers of endocrine cells appeared to be reduced in Sox9 heterozygous mutant pancreata (Fig. 3C,D). These findings were further supported by morphometric analyses, which revealed β- and α-cell mass to be 57% and 55% reduced, respectively (Fig. 3I). Likewise, δ- and PP-cells, marked by somatostatin and pancreatic polypeptide staining, respectively, and ghrelin+ cells showed a similar decrease in numbers in Sox9+/Δpan pancreata at e18.5 (Suppl. Fig. 2).
To assess the extent of overall reduction in pancreatic insulin and glucagon levels in Sox9+/Δpan mice, total pancreatic insulin and glucagon content were assayed by ELISA from extracts of whole e18.5 pancreata. In close concordance with the morphometric data, total pancreatic insulin was reduced by 44% in Sox9+/Δpan mice relative to wild-type littermates (mean total pancreatic insulin = Sox9+/Δpan: 5.17 ± 0.342 µg/mg total protein vs. Sox9+/flox: 9.23 ± 0.623 µg/mg total protein; n = 5; P = 0.001) (Fig. 3J), while total pancreatic glucagon was decreased by 34.9% (mean total pancreatic glucagon = Sox9+/Δpan: 9.51 ± 0.832 ng/mg total protein vs. Sox9+/flox: 14.6 ± 1.06 ng/mg total protein; n = 5; P = 0.007) (Fig. 3K).
In contrast to the endocrine compartment, acinar morphology and proportional area representation (on the basis of amylase staining) was normal in Sox9+/Δpan embryos at e18.5 (Fig. 3E,F,I). Likewise, Dolichos biflorus agglutinin (DBA) staining revealed no difference in the numbers and distribution of DBA+ cells between Sox9+/Δpan and control pancreata (Fig. 3G,H). Notably, identical phenotypic changes in the pancreas were observed in a mouse model of heterozygous germline deletion for Sox9 as well as in mice, in which wild-type Sox9 mRNA levels are reduced as a consequence of aberrant splicing into the neo cassette in intron 1 of Sox9 (unpublished mouse model, Ralf Kist and Gerd Scherer) (Suppl. Fig. 3).
This analysis reveals a dosage-dependent requirement for SOX9 in pancreatic development, specifically in the formation of the endocrine lineages. The similar reduction of endocrine cell numbers and hormone content suggests that each endocrine cell produces normal amounts of hormone. To gain further insight into whether Sox9-haploinsufficiency affects the terminal differentiation of endocrine cells, we performed a comprehensive immunohistochemical analysis for various islet markers.
Islets in Sox9+/Δpan Embryos Show Normal Expression of β-cell Markers
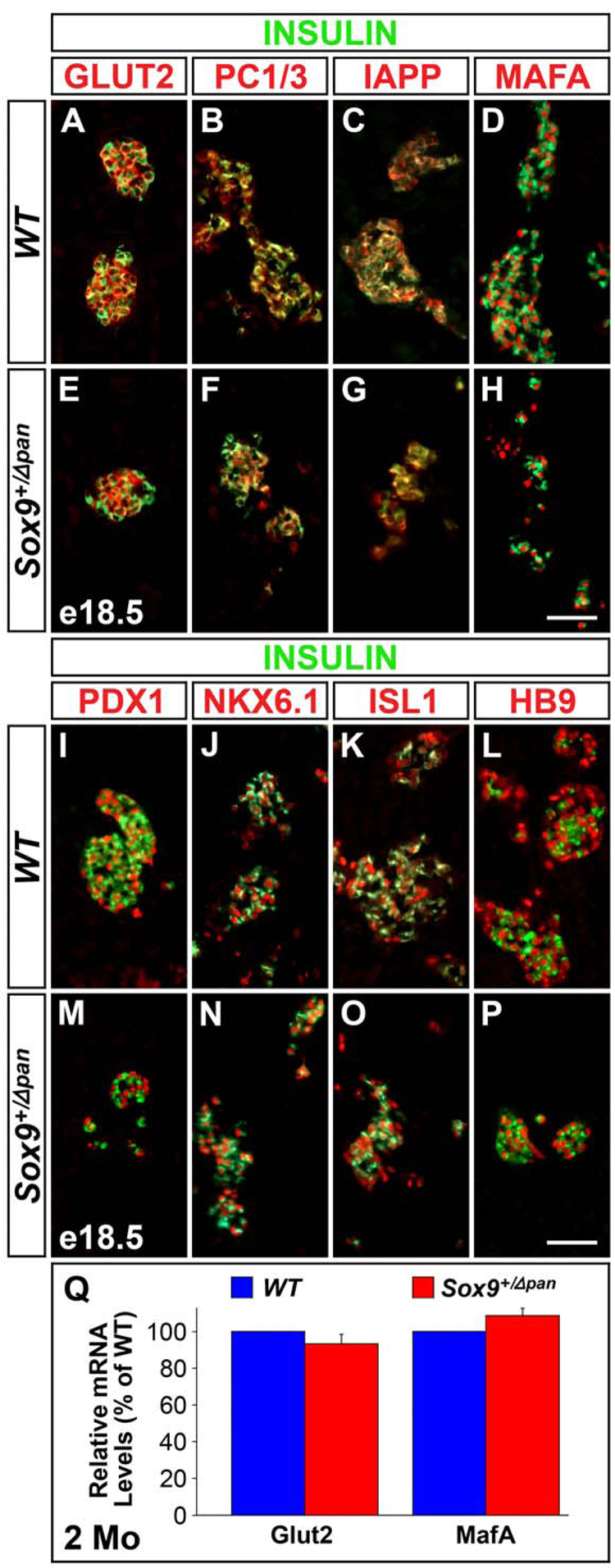
Proper glucose sensing and insulin processing in β-cells requires the expression of the glucose transporter GLUT2 and the insulin processing enzyme prohormone convertase (PC), respectively. To determine whether β-cells in Sox9-haploinsufficient pancreata express these functionally important proteins, we performed coimmunofluorescence analysis for GLUT2 and PC1/3 in conjunction with insulin. Expression of both GLUT2 (Fig. 4A,E) and PC1/3 (Fig. 4B,F) was, however, maintained in Sox9+/Δpan islets. Likewise, β-cells in Sox9+/Δpan mice expressed islet amyloid polypeptide (IAPP) (Fig. 4C,G), a protein that is co-secreted with insulin.
Figure 4. Sox9-Haploinsufficiency Does Not Result in Impaired Terminal Differentiation of Endocrine Cells.
Immunofluorescence staining for markers of differentiated endocrine cells, including GLUT2, PC1/3, IAPP, MAFA, PDX1, NKX6.1, ISL1 and HB9 reveals all markers to be equally expressed in insulin+ cells of Sox9 heterozygous mutant (Sox9+/Δpan) (E–H; M–P) and wild-type (WT) (A–D; I–L) pancreas at e18.5. qRT-PCRs performed on pooled mRNA from 200 isolated islets per genotype, confirm that islet Glut2 and MafA mRNA levels are similar in two 2-month-old Sox9+/Δpan and WT mice. The values shown represent mean values ± SEM (standard error of the mean) (n=3) (Q). Scale bar = 50µm.
Since proper β-cell differentiation has been shown to depend upon the expression of numerous islet transcription factors, we next assayed for their expression in β-cells of Sox9+/Δpan mice. Similar to wild-type mice, islets from Sox9-haploinsufficient mice expressed the transcription factors MAFA (Fig. 4D,H), PDX1 (Fig. 4I,M) NKX6.1 (Fig. 4J,N), ISL1 (Fig. 4K,O), and HB9 (Fig. 4L,P). Quantification of mRNA levels for Glut2 and Mafa in isolated islets and Western Blots for GLUT2 in embryonic pancreas further confirmed that β-cells express normal amounts of these factors (Fig. 4Q, data not shown).
Taken together, these findings suggest that decreased Sox9 gene dosage has no impact on the terminal differentiation of pancreatic β-cells. This implies that Sox9 may have an earlier role in endocrine cell genesis possibly in the release of sufficient numbers of endocrine progenitors from the SOX9+ multipotential progenitor cell compartment.
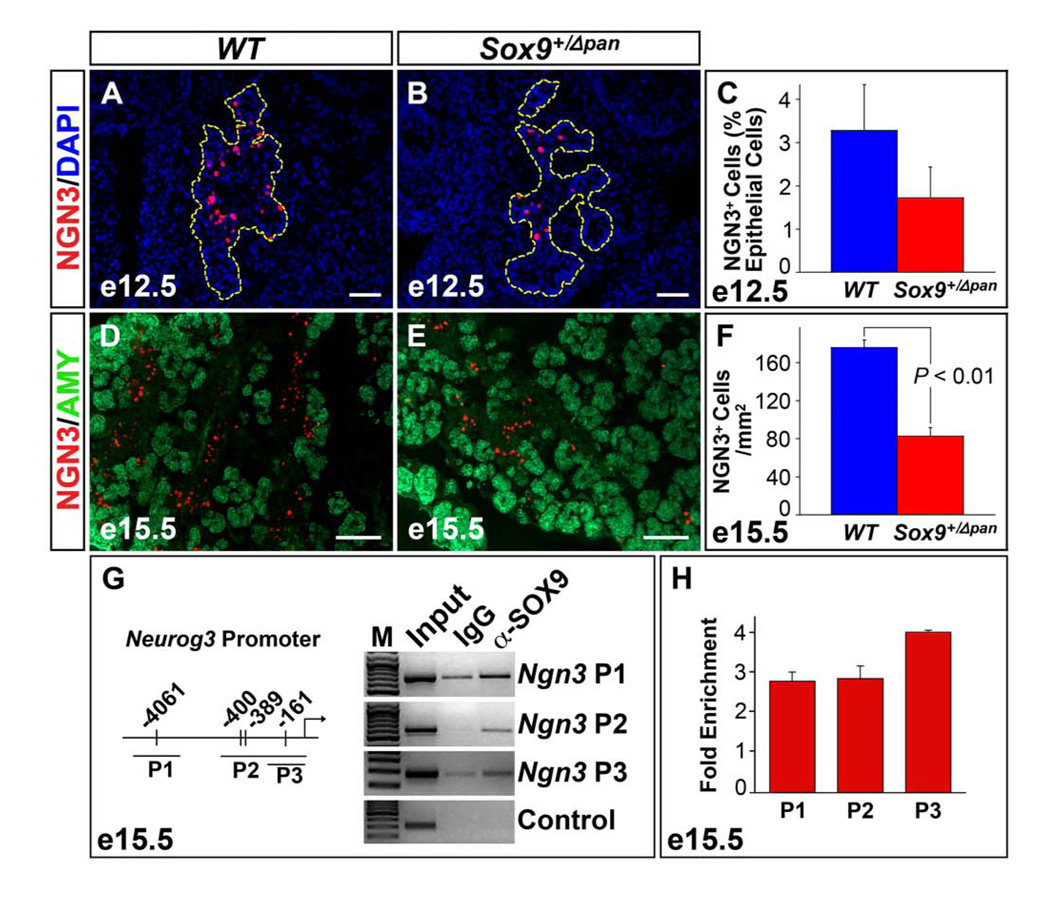
Sox9+/Δpan Embryos Exhibit a Two-Fold Reduction in the Numbers of Endocrine Progenitors
If SOX9 is indeed required for the specification of endocrine progenitors, the expression of the endocrine progenitor cell marker NGN3 should be reduced in Sox9+/Δpan pancreata. Consistent with this notion, immunofluorescence analysis for NGN3 and subsequent morphometric quantification of NGN3+ cells in pancreata from Sox9+/Δpan embryos and wild-type littermates revealed a ~50% reduction in the numbers of NGN3+ cells relative to the pancreatic epithelial cells at e12.5 (Fig. 5A–C) and e15.5 (Fig. 5D–F). Since a recent study suggested that Neurog3 regulatory sequences are directly occupied by SOX9 in the mPAC ductal cell line (Lynn et al., 2007), we directly tested whether SOX9 binds to the Neurog3 promoter in embryonic pancreas. Combining CHIP and qRT-PCR analysis in primary embryonic pancreas tissue, we found that SOX9 was indeed bound to three of the previously identified regions in the Neurog3 promoter (Fig. 5G,H).
Figure 5. Endocrine Progenitors are Two-Fold Reduced in Sox9 Heterozygous Mutant Pancreata.
Numbers of NGN3+ endocrine progenitor cells are 50% reduced in the Sox9 heterozygous mutant (Sox9+/Δpan) compared to wild-type (WT) pancreas (n=3) both as a proportion of total (DAPI+) pancreatic epithelial cells at e12.5 (A–C; pancreatic epithelium highlighted by yellow dashed line in A,B) and per square millimeter of pancreas tissue at e15.5 (D–F). Chromatin immunoprecipitation studies of cross-linked chromatin from dissected pancreata at e15.5 with anti-SOX9 antiserum show that SOX9 occupies sequences in the Neurog3 promoter. Three fragments, as previously described by Lynn et al. 2007, were amplified by PCR from either input DNA, DNA precipitated with IgG or anti-SOX9 antiserum. Sequences from the GABA-A receptor intron 5 were amplified in the control reaction (G). qRT-PCR analysis of DNA precipitated with anti-SOX9 antiserum shows a 3–4-fold enrichment over DNA precipitated with IgG (n=4) (H). The values shown represent mean values ± SEM (standard error of the mean). AMY, amylase. (A,B,D,E) Scale bar = 50µm.
These data show that the two-fold reduction in the numbers of differentiated endocrine cells observed at e18.5 is preceded by a similar, two-fold decrease in the numbers of endocrine progenitors, therefore suggesting that the neonatal islet cell defect in Sox9-haploinsufficient mice is the result of impaired islet cell neogenesis.
Progenitor Cell Proliferation is Sox9 Dosage-Sensitive
Our previous analysis of Sox9-deficient pancreata suggested that relative to the total progenitor cell pool, an excess number of early endocrine cells differentiate in the absence of SOX9 activity (Seymour et al., 2007). Therefore, we considered the possibility that the reduction in NGN3+ cells in Sox9 heterozygous mutant pancreata is a consequence of precocious depletion of the progenitor cell pool due to accelerated differentiation at an early developmental time-point.
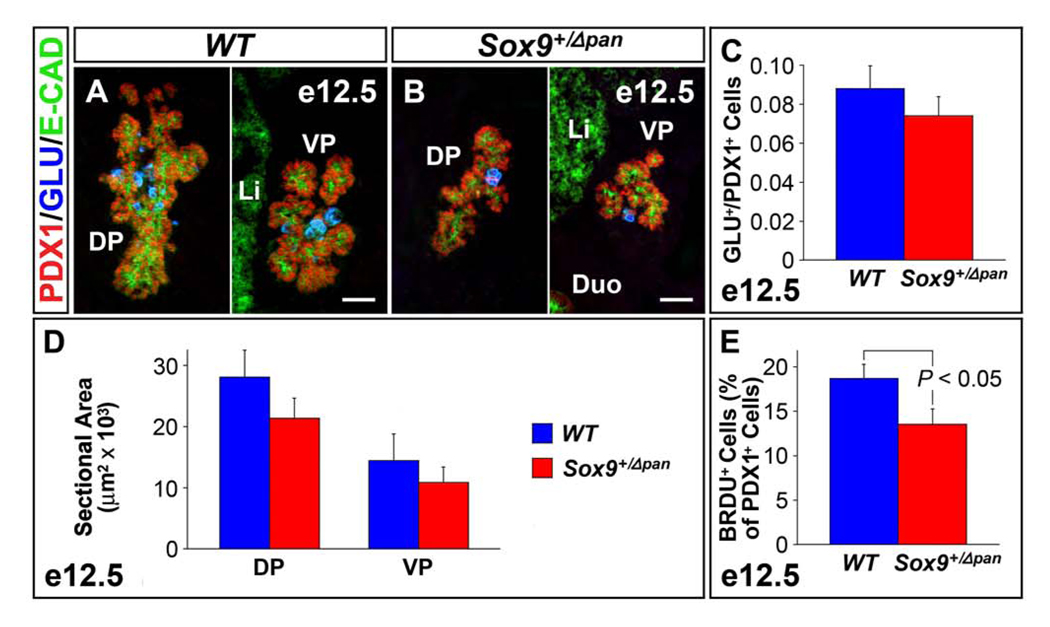
To assay for premature differentiation in Sox9+/Δpan mice, we stained pancreata from e12.5 embryos for PDX1 and glucagon, as glucagon+ cells are the first cell type to differentiate in the developing pancreas (Jensen, 2004). In contrast to SOX9-deficient pancreata, no difference was seen in the ratio of glucagon+ cells to undifferentiated PDX1+ progenitors between Sox9-haploinsufficient and wild-type embryos (Fig. 6A–C). These findings suggest that the reduction in NGN3+ endocrine progenitors in Sox9+/Δpan mice is not caused by premature differentiation of the multipotent progenitor cell pool. Consistent with this idea, the mRNA levels for Hes1 were unchanged in e12.5 pancreatic anlagen from Sox9+/Δpan compared to wild-type embryos (data not shown). Therefore, unlike homozygous Sox9 deletion (Seymour et al., 2007), Sox9-haploinsufficiency does not affect expression of the key Notch effector Hes1.
Figure 6. Sox9-Haploinsufficiency Causes a Mild Proliferation Defect of Pancreatic Progenitors.
The ratio of glucagon+ endocrine cells to PDX1+ pancreatic progenitors is not significantly different between Sox9 heterozygous mutant (Sox9+/Δpan) and wild-type (WT) pancreas (n=3) at e12.5 (A–C). By contrast, the proliferation of PDX1+ progenitors (as measured by percentage BrdU incorporation of PDX1+ cells) is 25% reduced in Sox9+/Δpan pancreas (n=3) at e12.5 (E). Consequently, mean sectional area of both dorsal and ventral Sox9+/Δpan pancreas is 25% reduced compared with those of wild-type littermates at e12.5 (D). The values shown represent mean values ± SEM (standard error of the mean). E-CAD, E-cadherin; GLU, glucagon; DP, dorsal pancreas; VP, ventral pancreas; Duo, duodenum; Li, liver. (A,B) Scale bar = 50µm.
To determine whether the requirement for SOX9 in progenitor cell proliferation is dosage-dependent, we examined proliferation within the multipotential progenitor cell pool via co-immunofluorescence analysis for BrdU and PDX1 at e12.5. This analysis revealed mild reduction of BrdU incorporation by PDX1+ pancreatic progenitors in Sox9+/Δpan embryos compared with wild-type littermates (Sox9+/Δpan: 13.5 ± 1.76% vs. Sox9+/flox: 18.7 ± 1.58%, n = 3; P < 0.05) (Fig. 6E). However, this reduction in progenitor cell proliferation did not result in an overt size reduction of the pancreatic buds (Fig. 6A,B). Subsequent morphometric analysis of the E-cadherin+ epithelial cell area revealed a slight, but insignificant reduction in size of both dorsal and ventral pancreas in Sox9+/Δpan mice (for dorsal bud, mean sectional area = Sox9+/Δpan: 21,396 ± 3230 µm2 vs. Sox9+/flox: 28,129 ± 4358 µm2, n = 3; for ventral bud, mean sectional area = Sox9+/Δpan: 10,906 ± 2504 µm2 vs. Sox9+/flox: 14,452 ± 4390 µm2, n = 3) (Fig. 6D). Unlike cell proliferation, cell survival was not affected by reduced Sox9 gene dosage, as TUNEL analysis showed no increase in cell death in Sox9+/Δpan pancreata (data not shown).
In summary, our data show that not all early functions of SOX9 in multipotent progenitors require full Sox9 gene dosage. While progenitor cell proliferation was decreased in Sox9+/Δpan embryos, no increased cell death or relative increase of early endocrine cells was observed. These results suggest that the reduction in endocrine progenitors in Sox9+/Δpan embryos is caused by insufficient generation of NGN3+ cells from the SOX9+ progenitor cell pool, therefore implying a dosage-sensitive requirement for SOX9 in initiating cell differentiation. Since SOX9+ progenitors give rise to both endocrine and exocrine cells, this raises the question of whether reduced SOX9 levels also affect exocrine differentiation.
Exocrine Differentiation is Unaffected by Reduced Sox9 Levels
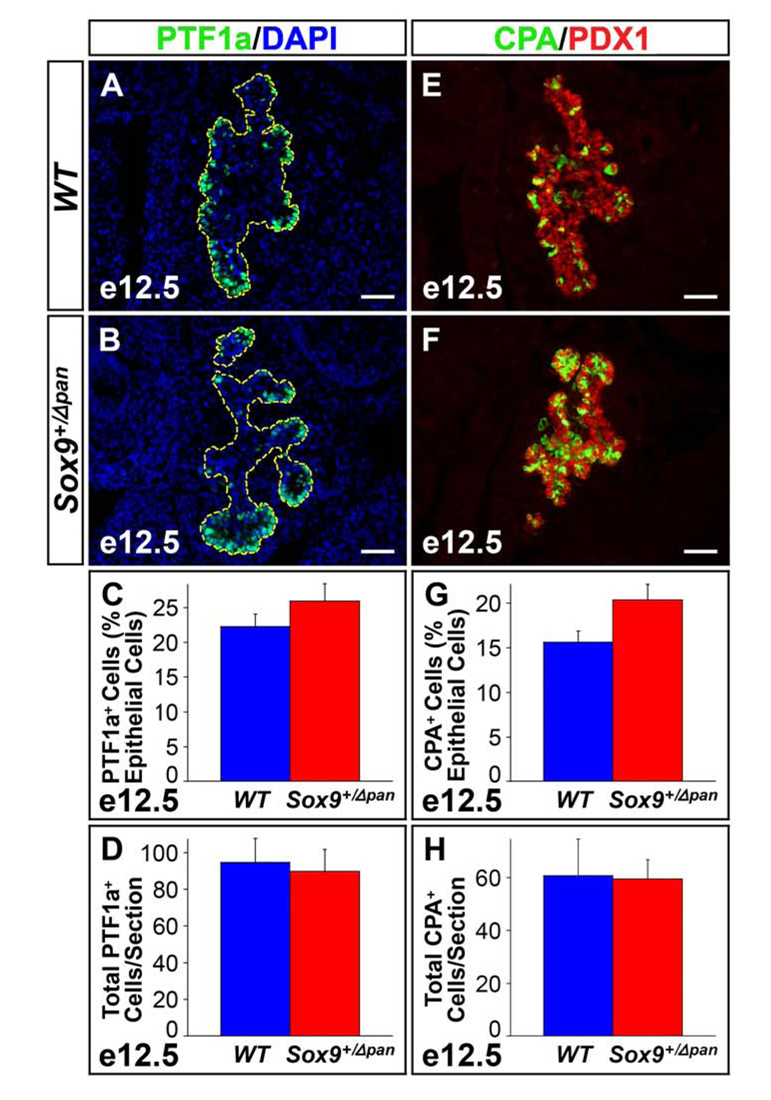
To investigate whether exocrine differentiation was affected in Sox9-haploinsufficient embryos, we examined the abundance of exocrine progenitors in Sox9+/Δpan and wild-type embryos at e12.5. We performed immunofluorescence analysis for PTF1a (Fig. 7A,B) and carboxypeptidase A (CPA) (Fig. 7E,F), both of which become restricted to the distal tips of the branching epithelium around the time when tip cells commit to an exocrine fate (Zhou et al., 2007). Compared to wild type littermates, we observed a small, but insignificant increase in the proportion of the CPA+ and PTF1a+ cells relative to the epithelial area in Sox9+/Δpan embryos at e12.5 (Fig. 7C,G). This suggests that reduction in Sox9 dosage in multipotential progenitors favors adoption of an exocrine fate at the expense of an endocrine fate. Notably, when expressed as absolute CPA+ or PTF1a+ cell numbers to account for the decreased size of the pancreatic epithelium in Sox9+/Δpan embryos, no difference was seen between Sox9+/Δpan embryos and wild-type controls (Fig. 7D,H). Consistent with the observed normal absolute numbers of exocrine-fated progenitors at e12.5, the size of the exocrine compartment and total organ size was indistinguishable between Sox9+/Δpan and wild-type pancreata at e15.5 (Suppl. Fig. 4). This shows that the development of the exocrine compartment proceeds normally in Sox9+/Δpan embryos and explains the overall normal organ size at birth despite the initial reduction of the progenitor cell pool at the pancreatic bud stage. In summary, our findings demonstrate a role for SOX9 in pancreatic endocrine cell differentiation and suggest that SOX9 levels may dictate fate choices of multipotential pancreatic progenitors.
Figure 7. Reduced Sox9 Gene Dosage Does Not Affect the Formation of Exocrine-Fated Cells.
Relative numbers of “proto-acinar” cells, as marked by expression of PTF1a (A–D) or carboxypeptidase A (CPA) (E–H), are mildly increased in e12.5 Sox9 heterozygous mutant (Sox9+/Δpan) pancreas compared to wild-type (WT) littermates (n=3). To take into account the epithelial hypoplasia of Sox9+/Δpan pancreata, the PTF1a+ and CPA+ cell numbers were normalized to the total DAPI+ pancreatic epithelial cell area (C,G). However, absolute numbers of PTF1a+ (D) or CPA+ (H) cells per section are unchanged between Sox9+/Δpan and wild-type pancreas. (A,B) Pancreatic epithelium is highlighted by yellow dashed line. The values shown represent mean values ± SEM (standard error of the mean). (A,B,E,F) Scale bar = 50µm.
Discussion
While our previous analysis of mice deficient for pancreatic SOX9 revealed a requirement for SOX9 in the maintenance of early pancreatic progenitor cells (Seymour et al., 2007), the severe early pancreatic growth arrest precluded an analysis of possible roles for SOX9 in pancreatic cell differentiation. Here, we demonstrate that SOX9+ cells are multipotent and give rise to both endocrine and exocrine cells throughout much of the window of pancreatic differentiation. By analyzing mice with a 50% reduction in pancreatic SOX9 protein levels, we further show that SOX9 is required for the generation of sufficient endocrine progenitors and consequently, endocrine cells.
SOX9 Marks a Multipotential Pancreatic Progenitor Cell Compartment
Lineage-tracing experiments have proven an informative tool to determine the developmental potential of different progenitor cell populations. Cre recombinase-mediated lineage-tracing studies have shown that all pancreatic cell types, comprising endocrine, acinar and ductal cells, arise from a common PDX1+/SOX9+ cell pool of the early pancreatic buds (Akiyama et al., 2005b; Gu et al., 2002). While these experiments have demonstrated the existence of a multipotential progenitor cell pool at the earliest stages of pancreas development, it has remained unclear whether such a cell population persists at later developmental time-points. Our previous finding that SOX9+ cells are devoid of pro-endocrine, endocrine and exocrine markers throughout embryogenesis suggested that SOX9 might serve as a marker for a multipotential progenitor cell compartment. Exploiting the protein stability of GFP, our short-term lineage tracing study in Sox9-eGFP mice is the first to demonstrate the persistence of a multipotential pancreatic progenitor cell pool until late in gestation. The presence of GFP in the α- and β-cell lineages at both e15.5 and e18.5 implies that endocrine cells arise by a neogenic mechanism from a SOX9+ progenitor cell pool from the onset of the secondary transition until birth. Notably, our observation that a portion of NGN3+ cells retained strong GFP signal, but no SOX9 protein further suggests that committed endocrine progenitors arise from the SOX9+ epithelial cords. The presence of GFP in both endocrine cells and amylase+ acinar cells is especially notable. This finding suggests that both endocrine and acinar cells simultaneously delaminate from the SOX9+ cord/ductal epithelium, thus demonstrating that the SOX9+ compartment constitutes a pool of multipotential pancreatic progenitor cells. Furthermore, our observation of weaker GFP expression in acinar cells than endocrine cells is consistent with GFP dilution in acinar cells due to cell division while endocrine cells in contrast are largely postmitotic. The most striking difference we observed between the endocrine and exocrine compartment was the retention of GFP in endocrine, but not in exocrine cells until birth. This implies that different mechanisms account for expansion of the acinar cell compartment during early and late embryogenesis. While our data indicate that acinar cells delaminate from SOX9+ progenitors immediately after the secondary transition at around e14, the absence of GFP in exocrine cells in e18.5 embryos suggests that this mechanism no longer accounts for acinar cell formation in late embryogenesis. Given the high proliferation rate of acinar cells in late embryogenesis, it is possible that only a first wave of acinar cells is generated by neogenesis, while cell proliferation ensures subsequent expansion of the acinar cell compartment.
Pancreatic Haploinsufficiency for Sox9 in Mice Phenocopies Pancreatic Defects Associated with Campomelic Dysplasia
In the present study, we found that ablation of a single Sox9 gene copy manifests in islet-specific hypoplasia by birth while pancreatic exocrine development is unperturbed. Sox9, therefore, governs pancreatic morphogenesis in a dosage-dependent manner. This is consistent with a previous report of islet defects associated with human CD (Piper et al., 2002). Pancreata from neonates diagnosed with CD were found to exhibit hypoplastic islets with variable expression of endocrine hormones and reduced expression of the mature β-cell markers PC1/3 and IAPP (Piper et al., 2002). Although islet hypoplasia is common to both murine and human Sox9 haploinsufficiency, we observed no loss of mature β-cell marker expression in mice with heterozygous Sox9 deletion in the pancreas. This is consistent with normal terminal differentiation of β-cells in Sox9-haploinsufficient mouse pancreas, suggesting that the defect responsible for endocrine hypoplasia occurs upstream of endocrine terminal differentiation, at the initiation of endocrine development. It is conceivable that such inconsistencies in marker expression might reflect minor differences in programs of pancreatic differentiation between mouse and human or alternatively, different histological methodologies between the two studies. Given the highly autolytic nature of the pancreas, and more challenging nature of human tissue collection at autopsy, it is also possible that discrepancies between our findings and those of Piper et al. (2002) might be attributable to differences in tissue preservation.
Interestingly, not all phenotypic aspects of the CD syndrome are observed in Sox9-haploinsufficient mice. Although for example, the skeletal malformations characteristic of CD are phenocopied in Sox9 heterozygous mutant mice (Bi et al., 2001), the XY sex reversal associated with the disorder, is not. Instead, complete sex reversal in mice requires the inactivation of both Sox9 alleles (Barrionuevo et al., 2006). Thus, sensitivity of organ development to Sox9 gene dosage appears to differ for certain organs between mice and humans. However, despite the minor difference in β-cell marker expression, the Sox9-haploinsufficient pancreatic phenotype in mouse and humans is strikingly similar. This, in conjunction with the highly conserved pattern of Sox9 expression in the embryonic mouse and human pancreas (Piper et al., 2002; Seymour et al., 2007) strongly indicates a functionally equivalent role for SOX9 in pancreas development in humans and mice. Therefore, insights gained in mice into how SOX9 regulates the pancreatic differentiation program should be applicable in the development of cell-based therapies for type 1 diabetes in humans.
Formation of Endocrine Progenitors is Sensitive to Sox9 Gene Dosage
Despite an initial 25% reduction in size at e12.5, the Sox9-haploinsufficient pancreas attains wild-type-equivalent organ size by birth. Reflecting the normal organ size, the exocrine compartment was unaffected in Sox9+/Δpan mice, while islet size was significantly reduced at the end of gestation. One possible explanation for the reduced islet size in neonatal mice is that it is a direct consequence of the diminished progenitor cell pool at the early pancreatic bud stage. However, such depletion of early progenitors will initially not only affect endocrine cell mass, but also the size of the exocrine compartment. In contrast to this prediction, we found absolute numbers of exocrine-fated cells to be normal in Sox9-haploinsufficient embryos despite the size reduction of the pancreatic buds. Furthermore, at e15.5, when the exocrine compartment has just begun to form, overall acinar cell mass was similar in Sox9+/Δpan and wild-type embryos. These findings argue that the islet-specific defect in Sox9+/Δpan embryos is not caused by a general reduction in pancreatic progenitors, but that Sox9 plays a specific role in initiating endocrine cell neogenesis. Consistent with this notion, we found that the reduction of all major endocrine cell types in Sox9+/Δpan mice was preceded by a 50% reduction in NGN3+ cells throughout the window of major cell differentiation. Thus, our analysis of Sox9-haploinsufficient embryos reveals a novel role for Sox9 in initiating endocrine cell differentiation from an undifferentiated multipotential progenitor cell epithelium. Whether SOX9 directly regulates Neurog3 gene expression is currently still unclear. While this and another study (Lynn et al., 2007) suggest that Neurog3 regulatory sequences are occupied by SOX9, activation of endogenous Neurog3 transcription by SOX9 remains to be demonstrated.
This raises the question of whether SOX9 is specifically required for the specification of the endocrine lineage or whether it also plays a role in exocrine cell development. In support of the idea that Sox9 dosage determines cell fate assignment in multipotential progenitors, we observed a modest increase in exocrine progenitors relative to the overall diminished progenitor cell pool in Sox9-haploinsufficient embryos. As a net result, the absolute numbers of exocrine progenitors were similar in Sox9+/Δpan and control embryos. Since exocrine cells account for the majority of the pancreatic mass at later developmental time-points, the normal size of the exocrine progenitor cell pool explains the normal organ size at birth. Our findings suggest that a 50% reduction of SOX9 levels in pancreatic progenitor cells slightly favors their differentiation towards an exocrine fate. Temporally controlled deletion of Sox9 during the time window of exocrine cell fate specification will be required to fully address the role of Sox9 in exocrine cell development.
Our finding that reduced SOX9 levels affect pancreatic cell fate specification illustrates the necessity to tightly control transcription factor levels during development. Notably, intracellular SOX9 protein levels are not only controlled by gene dosage, but are also post-translationally regulated by the ubiquitin-proteasome proteolytic system (Akiyama et al., 2005a). The paradigm that transcription factors regulate commitment to specific cell fates at distinct protein levels extends beyond SOX9 and has also been demonstrated for other regulators of stem cell maintenance, such as OCT3/4, Nanog and SOX2 (Kopp et al., 2008; Mitsui et al., 2003; Niwa et al., 2000; Taranova et al., 2006). For example, high levels of OCT3/4 have been shown to induce differentiation of mesoderm and endoderm from embryonic stem cells, while low concentrations induce trophectoderm (Niwa et al., 2000). If applied to the embryonic pancreas, it is possible that SOX9 activates different target genes at distinct protein thresholds. Presumably, a 50% reduction in SOX9 protein levels activates Ngn3 expression in a sub-optimal manner, thereby reducing the probability that multipotential progenitors will adopt an endocrine fate. At a molecular level, activation of different target genes at distinct protein thresholds could be explained by a differential requirement of target gene promoters for monomeric or dimeric SOX9 binding. For example, SOX9 dimerization is required for target gene activation in chondrogenesis but not in sex determination (Bernard et al., 2003). Since SOX9 monomers and dimers form at distinct protein thresholds (Sock et al., 2003), a reduction in protein levels will exert differential effects on the expression of individual target genes. This molecular mechanism may contribute to the wide spectrum of phenotypic alterations seen in different CD individuals and may also prove relevant for differential target gene activation during pancreas development.
In summary, this study provides the first evidence that the SOX9+ epithelial cords in the developing pancreas represent a compartment of multipotential pancreatic progenitors that gives rise to endocrine and exocrine cell lineages. Moreover, our analysis of heterozygous Sox9-deficient mice uncovers a novel role for Sox9 in the initiation of endocrine development. Future studies will address whether Sox9 has a specific role in endocrine cell specification or whether it specifies different fates at distinct protein thresholds.
Supplementary Material
In explants of e11.5 Sox9-eGFP dorsal pancreatic buds grown for 24h in culture, the domains of GFP and SOX9 expression coincide completely (A), demonstrating that the BAC transgene directs the expression of GFP exclusively to Sox9-expressing cells. After a further 12h in vitro, expression of SOX9 and so, GFP, is still maintained throughout the pancreatic epithelium in control (CTL) explants (B). However, after 12h in the presence of the de novo protein synthesis inhibitor cycloheximide (CHX), SOX9 protein is no longer detectable while GFP clearly persists (C) due to superior protein stability over endogenous SOX9 protein. In slowly dividing tissues, such as the postnatal pancreas (D-D”) and central nervous system (CNS) (E-E”), the domains of GFP and SOX9 expression overlap completely in Sox9-eGFP mice as above, confirming that the expression of GFP is restricted exclusively to Sox9-expressing cells. In rapidly dividing tissues such as the embryonic neural tube (F-F”), the perdurance of GFP compared with endogenous SOX9 protein results in retention of GFP label in SOX9− descendants (F”, arrows) of GFP+/SOX9+ neural progenitor cells, which are located proximal to the midline. Together, this shows that GFP can be used to trace the immediate progeny of SOX9+ cells. Scale bar = (A–C) lower panels, 100µm; insets in (A–C) and (D–F), 50µm.
Similar to the reduction in insulin+ and glucagon+ cell numbers seen in Sox9 heterozygous mutant (Sox9+/Δpan) pancreata (Fig. 3C,D), numbers of somatostatin+, pancreatic polypeptide+ (B) and ghrelin+ (D) endocrine cells are reduced in e18.5 Sox9+/Δpan pancreata in comparison to wild-type (WT) littermate controls (A,C). SOM, somatostatin; PP, pancreatic polypeptide; INS, insulin; GHR, ghrelin. Scale bar = 100µm.
At e18.5, gross anatomy of the pancreas (demarcated by a red dashed line) in mice in which one allele of Sox9 is deleted in the male germline (Sox9+/−) (B) is undistinguishable from that of wild-type littermates (A). In concordance, body weight (C) and pancreas wet weight (D) are equivalent in Sox9+/− and wild-type embryos (n=8) at e18.5. Immunofluorescence analysis reveals that at e18.5, numbers of insulin+ β-cells and glucagon+ α-cells are equivalently reduced in Sox9+/− islets (E,F) while the amylase+ acinar compartment is undistinguishable between Sox9+/− and wild-type pancreas. Morphometry confirms 53% and 76% reductions in β-cell mass (G) and α-cell mass (H) respectively in Sox9+/− vs. control pancreata at e18.5 (n=3). Similarly, in comparison to wild-type (WT) littermates, islet hypoplasia is evident in e16.5 mice homozygous for a mutation in which wild-type Sox9 mRNA levels are reduced as a consequence of aberrant splicing into the neo cassette in intron 1 of Sox9 (Sox9neo/neo); total pancreas size however, remains unchanged (I–J’). Immunofluorescence analysis reveals numbers of insulin+ and glucagon+ cells to be equivalently reduced in Sox9neo/neo (L) vs. WT littermate control pancreata (K). The values shown represent mean values ± SEM (standard error of the mean). AMY, amylase; INS, insulin; GLU, glucagon; p, pancreas; i, pancreatic islet. Scale bar = (A,B) 2mm; (E,F,I’–L) 100µm; (I,J) 200µm.
(A) Pancreatic size, as assessed by total pancreatic epithelial cell area based on E-cadherin staining, is similar in Sox9 heterozygous mutant (Sox9+/Δpan) and wild-type (WT) embryos at e15.5 (n=3). (B) Likewise, Sox9+/Δpan and control embryos show no difference in the ratio of amylase+ cells per square millimeter of E-cadherin+ pancreas tissue at e15.5. The values shown represent mean values ± SEM (standard error of the mean).
Acknowledgements
We are grateful to G. Scherer and R. Kist for providing mice and for sharing unpublished data. We would like to thank D. Melton, M. Wegner, C. V. E. Wright, H. Edlund, P. Serup, J. Ericson, J. H. Kehrl and D. F. Steiner for their generous gifts of mice and antibodies. We also thank F. Zaldivar of the GCRC, UC Irvine for performing ELISAs, K. Kamdar, M. Tran and D. Panlasigui for assistance in phenotypic analysis, S. Krauss for Western blot quantification, and members of the Sander laboratory for critical reading of the manuscript. This work was supported by grants from the NIH/NIDDK (RO1 DK078803-01 and RO1 DK68471-01) to M.S., by postdoctoral fellowships from the JDRF to P.A.S. and CIRM to K.K.F., and a graduate student fellowship from the CIRM to C.L.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlgren U, et al. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, et al. The transcription factor Sox9 is degraded by the ubiquitin-proteasome system and stabilized by a mutation in a ubiquitintarget site. Matrix Biol. 2005a;23:499–505. doi: 10.1016/j.matbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Akiyama H, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005b;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, et al. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Bernard P, et al. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- Bi W, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Foster JW, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, et al. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–266. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofano A, et al. Beta-cell mass and proliferation following late fetal and early postnatal malnutrition in the rat. Diabetologia. 1998;41:1114–1120. doi: 10.1007/s001250051038. [DOI] [PubMed] [Google Scholar]
- Giraldo P, Montoliu L. Size matters: use of YACs, BACs and PACs in transgenic animals. Transgenic Res. 2001;10:83–103. doi: 10.1023/a:1008918913249. [DOI] [PubMed] [Google Scholar]
- Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, et al. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, et al. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guz Y, et al. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Henseleit KD, et al. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development. 2005;132:3139–3149. doi: 10.1242/dev.01875. [DOI] [PubMed] [Google Scholar]
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- Jensen J, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Johnson JD, et al. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest. 2003;111:1147–1160. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson J, et al. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kist R, et al. Conditional inactivation of Sox9: a mouse model for campomelic dysplasia. Genesis. 2002;32:121–123. doi: 10.1002/gene.10050. [DOI] [PubMed] [Google Scholar]
- Kopp JL, et al. Small Increases in the Level of Sox2 Trigger the Differentiation of Mouse Embryonic Stem Cells. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Krapp A, et al. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. Embo J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynn FC, et al. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Niwa H, et al. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Piper K, et al. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. 2002;116:223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Sander M, et al. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Seymour PA, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- Sock E, et al. Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum Mol Genet. 2003;12:1439–1447. doi: 10.1093/hmg/ddg158. [DOI] [PubMed] [Google Scholar]
- Stolt CC, et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranova OV, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou Q, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In explants of e11.5 Sox9-eGFP dorsal pancreatic buds grown for 24h in culture, the domains of GFP and SOX9 expression coincide completely (A), demonstrating that the BAC transgene directs the expression of GFP exclusively to Sox9-expressing cells. After a further 12h in vitro, expression of SOX9 and so, GFP, is still maintained throughout the pancreatic epithelium in control (CTL) explants (B). However, after 12h in the presence of the de novo protein synthesis inhibitor cycloheximide (CHX), SOX9 protein is no longer detectable while GFP clearly persists (C) due to superior protein stability over endogenous SOX9 protein. In slowly dividing tissues, such as the postnatal pancreas (D-D”) and central nervous system (CNS) (E-E”), the domains of GFP and SOX9 expression overlap completely in Sox9-eGFP mice as above, confirming that the expression of GFP is restricted exclusively to Sox9-expressing cells. In rapidly dividing tissues such as the embryonic neural tube (F-F”), the perdurance of GFP compared with endogenous SOX9 protein results in retention of GFP label in SOX9− descendants (F”, arrows) of GFP+/SOX9+ neural progenitor cells, which are located proximal to the midline. Together, this shows that GFP can be used to trace the immediate progeny of SOX9+ cells. Scale bar = (A–C) lower panels, 100µm; insets in (A–C) and (D–F), 50µm.
Similar to the reduction in insulin+ and glucagon+ cell numbers seen in Sox9 heterozygous mutant (Sox9+/Δpan) pancreata (Fig. 3C,D), numbers of somatostatin+, pancreatic polypeptide+ (B) and ghrelin+ (D) endocrine cells are reduced in e18.5 Sox9+/Δpan pancreata in comparison to wild-type (WT) littermate controls (A,C). SOM, somatostatin; PP, pancreatic polypeptide; INS, insulin; GHR, ghrelin. Scale bar = 100µm.
At e18.5, gross anatomy of the pancreas (demarcated by a red dashed line) in mice in which one allele of Sox9 is deleted in the male germline (Sox9+/−) (B) is undistinguishable from that of wild-type littermates (A). In concordance, body weight (C) and pancreas wet weight (D) are equivalent in Sox9+/− and wild-type embryos (n=8) at e18.5. Immunofluorescence analysis reveals that at e18.5, numbers of insulin+ β-cells and glucagon+ α-cells are equivalently reduced in Sox9+/− islets (E,F) while the amylase+ acinar compartment is undistinguishable between Sox9+/− and wild-type pancreas. Morphometry confirms 53% and 76% reductions in β-cell mass (G) and α-cell mass (H) respectively in Sox9+/− vs. control pancreata at e18.5 (n=3). Similarly, in comparison to wild-type (WT) littermates, islet hypoplasia is evident in e16.5 mice homozygous for a mutation in which wild-type Sox9 mRNA levels are reduced as a consequence of aberrant splicing into the neo cassette in intron 1 of Sox9 (Sox9neo/neo); total pancreas size however, remains unchanged (I–J’). Immunofluorescence analysis reveals numbers of insulin+ and glucagon+ cells to be equivalently reduced in Sox9neo/neo (L) vs. WT littermate control pancreata (K). The values shown represent mean values ± SEM (standard error of the mean). AMY, amylase; INS, insulin; GLU, glucagon; p, pancreas; i, pancreatic islet. Scale bar = (A,B) 2mm; (E,F,I’–L) 100µm; (I,J) 200µm.
(A) Pancreatic size, as assessed by total pancreatic epithelial cell area based on E-cadherin staining, is similar in Sox9 heterozygous mutant (Sox9+/Δpan) and wild-type (WT) embryos at e15.5 (n=3). (B) Likewise, Sox9+/Δpan and control embryos show no difference in the ratio of amylase+ cells per square millimeter of E-cadherin+ pancreas tissue at e15.5. The values shown represent mean values ± SEM (standard error of the mean).