Abstract
Although homeostasis of rapidly renewing tissues like skin epithelia is maintained by stem cells, the committed progeny of stem cells in the basal layer of epidermis retain regenerative potential and are capable of forming epidermis in response to environmental cues. It is not clear, however, at what point within the epidermal lineage keratinocytes lose this regenerative potential. In this study, we examined the extent of tissue formation by post-mitotic differentiated keratinocytes. We show that cultures of mouse keratinocytes that were, by all measures, differentiated were able to reform a self-renewing, hair-bearing skin when transplanted onto suitable sites in vivo. Genetic labeling and lineage-tracing studies in combination with an involucrin-driven Cre/lox reporter system confirmed that transplanted differentiated keratinocytes were indeed the source of the regenerated skin. More importantly, analysis of early stages of skin regeneration showed hallmarks of dedifferentiation of transplanted differentiated keratinocytes. These data indicate that commitment to differentiation does not prohibit cells from re-entering the cell cycle, de-differentiating, and acquiring “stemness”. These findings suggest that epidermis can use different strategies for homeostasis and tissue regeneration.
INTRODUCTION
The epidermis undergoes a continuous process of renewal and loss through cell replication in the basal compartment and desquamation at the surface. As cells exit the proliferative compartment and begin their migration toward the skin surface, they withdraw from cell cycle, commit to terminal differentiation, and eventually slough from the skin surface (Fuchs, 1990). The primary source of cell renewal is through proliferation of stem cells (Potten and Booth, 2002; Blanpain et al., 2007); however, there seems to be some plasticity between stem cells and their early progeny, as most basal keratinocytes maintain their regenerative potential and are capable of regenerating epidermis when activated by appropriate stimuli or transplanted into an in vivo setting (Li et al., 2004). It is unclear, however, at what point the keratinocytes lose their regenerative capacity. Certainly, by the time the nucleus of the cells has begun to disintegrate, the cells have lost the capacity to re-enter the proliferative cycle. The question we pose here is whether keratinocytes in the earlier stages of epidermal differentiation maintain their regenerative capacity.
The model we have chosen to explore involves newborn mouse epidermal keratinocytes that, in cultures containing a low concentration of calcium (Ca), retain their proliferative and tissue-regenerative capacity (Lichti et al., 2008). However, when subjected to high concentrations of Ca in the medium, they undergo differentiation on a culture-wide basis. The nature of this differentiation resembles that which occurs in vivo, namely there is a cessation of cell replication and expression of precursors of the cornified envelope such as involucrin (INV) (Yuspa et al., 1989; Dotto, 1999). In this study, we have explored the nature of this differentiation to determine whether the cells that seem to be differentiated have the capacity to re-enter the proliferative phase and reform epidermis. Our studies show that not only do these cells retain the capacity to re-enter a proliferative phase, but can, when placed in a suitable tissue microenvironment, undergo dedifferentiation and act as stem cells to reform a long term, multilineage skin complete with epidermis, hair follicles, and sebaceous glands. The results confirm the plasticity of early differentiating keratinocytes and extend this further to the point where we can say that keratinocytes in the early stages of epidermal differentiation can return to a stem cell state.
RESULTS
Regeneration of skin epithelia by differentiated epidermal cultures
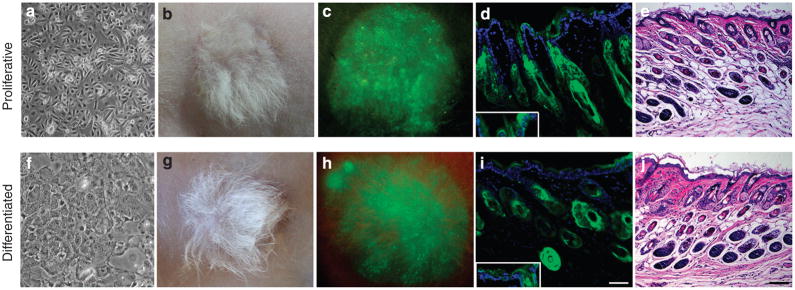
Our basic assay for tissue regeneration involves combining cultures of keratinocytes with newborn dermal fibroblasts and implanting the slurry of cells onto fascia under an enclosed transplantation chamber (Lichti et al., 2008). After 1 week, the overlying chamber is removed exposing the newly formed tissue to the air. To monitor the fate of transplanted keratinocytes, the epidermis of transgenic mice in which all nucleated cells express enhanced green fluorescent protein (GFP) was used as a source of keratinocytes. When cultures of mouse keratinocytes grown in low Ca conditions are used in this assay, a skin containing all three lineages, epidermis, hair follicles, and sebaceous glands is formed (Figure 1a–e)(Lu and Ghazizadeh, 2005). To explore the tissue-regenerating potential of differentiated keratinocytes, confluent epidermal cultures were induced to differentiate by a high concentration of Ca (1.2mM) for 3 days (Figure 1f). When assayed for tissue-forming potential, these differentiated cultures reconstituted hair-bearing skin similar to that formed by proliferating cultures (Figure 1b and g), although new hair appeared with a slight delay. New hair appeared by day 10–11 in the skin reconstituted from proliferating cells and by day 12–14 in the skin reconstituted from differentiated keratinocytes (data not shown).
Figure 1. Regeneration of skin epithelia by keratinocytes grown in low and high Ca conditions.
(a, f) Phase contrast images of epidermal cultures, (b, c) light and (e–h) surface GFP images of skin reconstituted from keratinocytes cultured in low and high Ca at 6 weeks post grafting. (d, i) Fluorescence and (e, j) H&E-stained images of reconstituted skin at 15 weeks post grafting. GFP-expressing cells (green) were present in epidermis, sebaceous glands, and hair follicles derived from keratinocytes cultured in low and high Ca. Higher exposure showing GFP in the basal cells (inset). Sections were counterstained with DAPI (blue nuclear staining). Bar=50 μm (d, i) and 100 μm (e, j).
To ensure persistence of the reconstituted skin as well as the capacity to undergo repeated cycles of hair growth, grafts were observed for 14 weeks at which time the hair was depilated to induce a new hair cycle. At 15 weeks, well-differentiated hair-bearing GFP-expressing skin epithelia were again evident in grafts regenerated from either proliferating or differentiated cultures (Figure 1d and i). When proliferating cultures of GFP-expressing keratinocytes, which were γ-irradiated (5000 rads) to induce irreversible growth arrest, were used for tissue formation, no GFP+ epidermis or hair was formed, and wound bed was invaded by host (non-labeled) epidermis, indicating that keratinocyte proliferation was indeed required for skin regeneration by differentiated cultures (Supplementary Figure S1). These results indicate that differentiated cultures of keratinocytes are capable of regenerating all the three lineages, epidermal, hair follicles, and sebaceous glands.
Characterization of epidermal cultures after Ca-induced differentiation
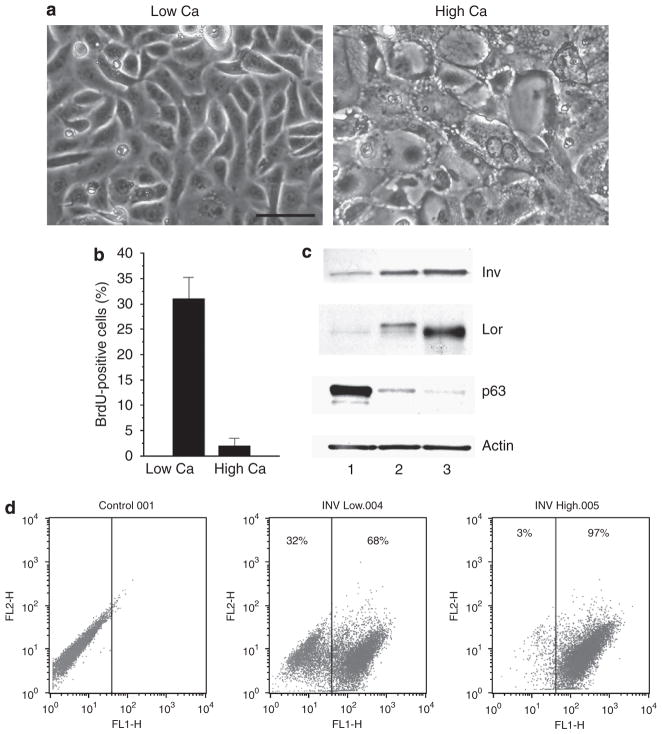
To determine the extent of differentiation in epidermal cultures, cultures were analyzed for expression of proliferation and differentiation markers. As expected, the BrdU-labeling index after 3 days of high Ca exposure decreased from an initial value of more than 35% to less than 2%, confirming growth arrest in the majority of keratinocytes (Figure 2b). Expression of INV and loricrin, precursors of cornified envelopes, increased on exposure to high Ca, whereas levels of p63, a keratinocyte proliferation marker, underwent a marked reduction (Figure 2c). The alterations in the levels of p63 and INV were triggered by confluency alone, but were more pronounced on high Ca switch (Figure 2c). To gain a sense of the proportion of cells undergoing Ca-induced differentiation, INV expression in confluent cultures was assessed by flow cytometry 3 days after a switch to high Ca. The analysis indicated an increase from 68 to 97% (Figure 2d). These experiments show that on exposure to high Ca, almost all cells in the culture withdrew from the cell cycle, and differentiated. However, there were about 2% of cells that resisted Ca-induced growth arrest and may have contributed to tissue formation by differentiated cultures.
Figure 2. Morphology, proliferation rate, and expression of differentiation markers in mouse keratinocytes cultured in high Ca.
(a) Phase contrast photographs of culture in low or high Ca condition, Bar=50 μm. (b) BrdU-labeling index of keratinocytes exposed to low or high Ca after 6 hours pulse. Values are expressed as mean labeled nuclei±SD in a minimum of 10 fields (>100 cells per field) from three independent experiments. (c) Western blot analysis of INV (Inv), loricrin (Lor), p63, and actin in primary mouse keratinocytes grown for 4 days in low Ca to confluence (lane 1), and for additional 3 days either in low Ca (lane 2) or in high Ca (lane 3). (d) Flow cytometric analysis of involucrin expression in confluent cultures of keratinocytes in low Ca (INV Low) or after exposure to high Ca (INV High). The percent involucrin positive keratinocytes in each culture is indicated.
Tissue regeneration by differentiated epidermal cultures cannot be attributed to a small population of differentiation-resistant keratinocytes
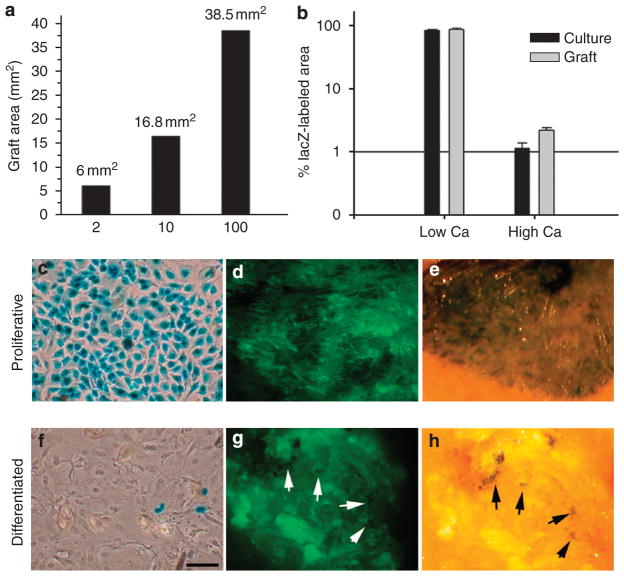
To determine whether this small population of differentiation-resistant cells could have regenerated a skin of the same size as that formed by replicating cells, varying numbers (corresponding to 2, 10, or 100% of total implanted keratinocytes) of GFP-expressing keratinocytes cultured in low Ca conditions were mixed with γ-irradiated non-labeled mouse keratinocytes to a total of 2×106 cells and used to reconstitute skin on the backs of mice. Analysis of surface GFP at 6 weeks post grafting showed a direct correlation between the size of reconstituted skin and the number of implanted, non-irradiated, GFP-expressing keratinocytes (Figure 3a; Supplementary Figure S2), suggesting that a small population of Ca-resistant keratinocytes could not have reconstituted a sizable graft. This confirmed previous reports in which the implantation of large numbers of purified stem cells (more than 100,000 cells) was often required to generate a small graft with modest number of hair follicles (Jaks et al., 2008; Jensen et al., 2008).
Figure 3. Skin regeneration by differentiated cultures is not attributed to a small population of Ca-resistant keratinocytes.
(a) The size of reconstituted skin is directly correlated with the number of implanted proliferating keratinocytes. The calculated area of GFP-expressing skin regenerated from mixtures of GFP-labeled proliferating keratinocytes and γ-irradiated non-labeled keratinocytes at ratios of 2, 10, and 100% at 6 weeks post grafting are shown. (b–h) Retrovirus transduction of differentiated cultures showing a minimal contribution of proliferating cells in differentiated cultures to tissue formation. (c, f) Phase contrast photograph of X-gal-stained cultures of proliferative or differentiated mouse keratinocytes transduced with a retrovirus encoding LacZ and used for fate mapping analysis during skin regeneration (original magnification ×10). (d–f) Surface GFP and X-gal whole mount staining of a representative skin reconstituted from LacZ-transduced cultures are shown. To allow visualization of both X-gal and GFP, whole mounts were examined by an inverted fluorescent microscope. Arrows point to LacZ-positive cells in the graft. Bar=50 μm (c, f) and 700 μm (d–e, g–h). Values in (b) are expressed as mean percentage of labeled cells or area±SD in a log scale (n=3).
To further evaluate the contribution of the Ca-resistant population to the regenerated tissue, we took the advantage of a well-established property of retroviral viruses to transduce only replicating cells (Miller et al., 1990) to selectively label differentiation-resistant cells in high Ca cultures and to follow the fate of these cells during skin regeneration. Cultures grown in either low or high Ca conditions were transduced with a retroviral vector encoding lacZ at a multiplicity of infection of 2 for two consecutive days to transduce the majority of proliferating keratinocytes in the culture (Lu and Ghazizadeh, 2005). Under these conditions, more than 80% of cells in cultures grown in low Ca conditions and about 1% of cells grown in high Ca conditions were transduced, confirming Ca-induced growth arrest in the majority of keratinocytes (Figure 3b,c and f). When these cultures were used in tissue reconstitution assay, the skin derived from low Ca cultures was predominantly LacZ-positive, whereas the skin from the high Ca cultures contained only a few clusters of LacZ-positive cells amongst the GFP-expressing areas of skin (Figure 3e and h). Quantification of labeled areas showed a slight increase in proportion of LacZ-transduced cells from 1 to 1.8% (Figure 2b). This slight increase in fraction of transduced cells could be explained by the overall lower efficiency of tissue formation by differentiated cells when mixed with proliferating keratinocytes before transplantation (Supplementary Figure S3) and may be attributed to the time required for differentiated cells to re-enter the cell cycle. If replicating differentiation-resistant keratinocytes were the sole source of the newly generated tissue, a significant increase in the proportion of LacZ-labeled cells and a prominent labeling (large clusters of blue cells) would have been evident in the reconstituted skin even if not all differentiation-resistant cells were transduced. Overall, these results suggested that differentiated keratinocytes are capable of overcoming cell cycle arrest and regenerating tissue.
Lineage tracing of differentiated cultured keratinocytes during tissue regeneration
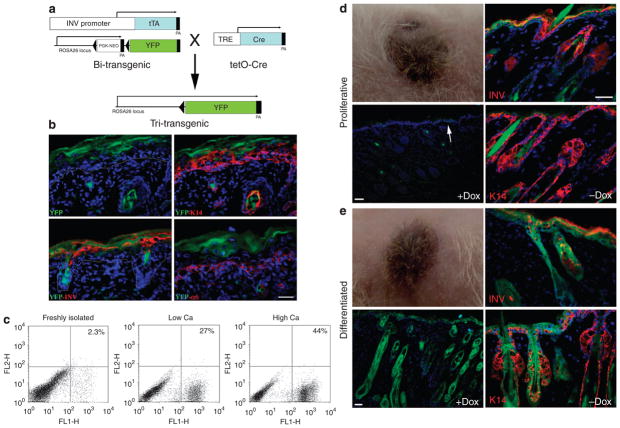
To address, in a more direct manner, the contribution of differentiated keratinocytes to tissue regeneration, we used the INV promoter to activate an inducible Cre/lox-driven recombination in differentiated cells in culture. This recombination event generated a constitutively active reporter gene that could be used to trace the fate of differentiated donor cells in regenerated tissue. The INV promoter has been extensively used to target transgene expression in a tissue- and stratum-specific manner (Carroll et al., 1993). In normal epidermis, INV is expressed in the upper spinous and granular layers, but extends to the lower spinous layers in hyperproliferative conditions (Li et al., 2000). In culture, INV synthesis is correlated with commitment to differentiation as indicated by an increase in cell size and withdrawal from the cell cycle (Watt and Green, 1981; Dover and Watt, 1987). A double-transgenic mouse carrying the RosaR26R-YFP reporter allele and expressing tetracycline-controlled transactivator protein under the control of the INV promoter (Jaubert et al., 2004) was generated, and crossed with tetO-Cre mice in which Cre recombinase expression was controlled by a tetracycline-responsive promoter element (Figure 4a). Expression of YFP in double-transgenic mice was under the control of the cell type-independent ROSA26 promoter but blocked by an upstream loxP-flanked Neor cassette. In triple-transgenic mice in an induced state (no doxycycline (Dox)), INV-driven expression of Cre recombinase was expected to excise the Neor cassette of Rosa26R-YFP, resulting in the labeling of INV-expressing keratinocytes. As YFP expression in these cells was driven by ROSA26 promoter, the progeny of labeled cells could be traced regardless of their INV expression status and independent of Dox. Analysis of YFP expression in cryosections prepared from dorsal skin of newborn triple-transgenic mice in an induced state (no Dox) showed localization of YFP to the upper spinous and granular layers of epidermis, and the inner root sheath of hair follicles, consistent with expression of the endogenous INV protein (Figure 4b).
Figure 4. Genetic labeling and tracing of involucrin-expressing keratinocytes.
(a) A schematic representation of the strategy used to generate triple-transgenic mice allowing lineage tracing of involucrin-expressing keratinocytes. In these mice, activation of involucrin promoter during differentiation induces irreversible Cre-mediated recombination between loxP sites (triangles) resulting in constitutive expression of YFP in all of the daughter cells. (b) Immunofluorescent staining of newborn mouse skin for K14, involucrin (INV), and α6-integrin shows restriction of YFP to suprabasal layers of epidermis. (c) The proportion of YFP-labeled cells in epidermal preparations of newborn triple-transgenic mice in freshly isolated epidermal preparations or in cultures grown in low or high levels of Ca. (d–e) Representative skin reconstituted from keratinocytes isolated from triple-transgenic mice and cultured in low (d) or high Ca conditions (e) at 6 weeks post grafting. Grafted mice were maintained in a repressed state (+Dox) to inhibit de novo expression of YFP after transplantation. Distinct patterns of YFP expression between skin reconstituted from proliferating (d) and differentiated (e) cultures. YFP expression in the latter does not coincide with INV expression and includes K14-expressing keratinocytes. To show differentiation-induced YFP expression in skin reconstituted from proliferating cultures, Dox was removed (− Dox) from drinking water 14 days before analysis. Bars=50 μm.
Epidermal cells isolated from newborn triple-transgenic mice were cultured and grown to confluence before induction of differentiation. Analysis of YFP expression by flow cytometry indicated that in the absence of Dox (induced state), about 27% of keratinocytes grown in low Ca conditions and 44% of those grown in high Ca were induced to express the YFP label (Figure 4c). These percentages were lower than those reported for endogenous INV (Figure 2d), and were likely due to inefficient Cre recombinase expression or Cre-mediated excision, as reported by others (Vasioukhin et al., 1999). Implantation of proliferating or differentiating cultures of keratinocytes in mice that were maintained in a repressed state resulted in the regeneration of hair-bearing pigmented skin (Figure 4d and e). Analysis of tissue sections revealed distinct patterns of YFP expression between the two groups (Figure 4d and e). In skin regenerated from proliferating cultures, almost no labeled keratinocytes were detected when grafted mice were repressed (+Dox, Figure 4d). Although in some sections a column of YFP-positive keratinocytes in the epidermis was observed (Figure 4d, arrow), no YFP-labeled hair follicles were detected. In the induced state (−Dox), YFP expression was mostly restricted to differentiated layers of epidermis and hair follicles consistent with the endogenous INV expression (Figure 4d), although in some areas, YFP expression was extended to the basal layer of epidermis. On the contrary, in skin regenerated from differentiated cultures, YFP was uniformly expressed in all the layers of epidermis, as well as in hair follicles and sebaceous glands whether grafted mice were maintained in Dox-induced or repressed conditions (Figure 4e). This pattern of YFP expression indicated that labeled epidermis, hair follicles, and sebaceous glands were formed by the progeny of differentiated keratinocytes that had once expressed INV in culture.
Differentiated keratinocytes undergo dedifferentiation during early stages of epidermal regeneration
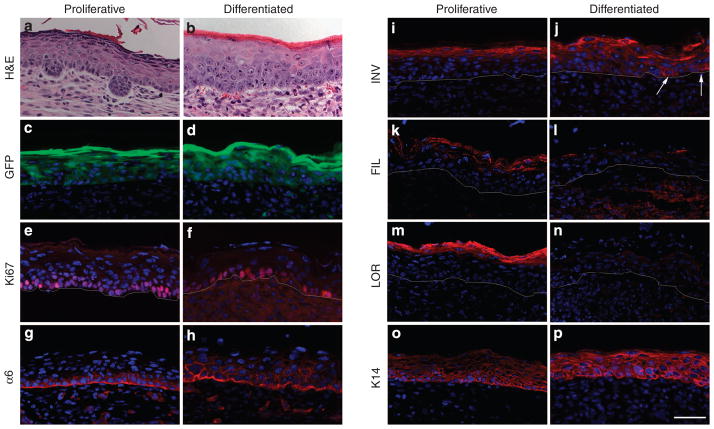
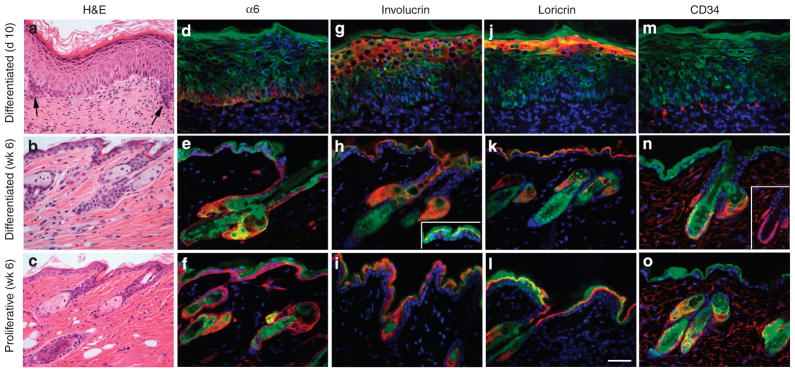
To get a better understanding of what was occurring during the early stages of tissue regeneration, tissues were examined at 1 week post grafting, when the protective chambers were removed. Histological analysis indicated significant morphological differences between tissues regenerated from proliferating and from differentiated keratinocytes (Figure 5a and b). A well-organized and fully differentiated epidermis was formed by proliferating keratinocytes with occasional formation of hair germs, indicating initiation of hair follicle development (Figure 5a). Although the epidermis was hyperproliferative, as indicated by Ki67 staining (Figure 5e), expression of differentiation markers including INV, filaggrin, and loricrin was normalized to the upper spinous and granular layers of epidermis (Figure 5i, k, and m). On the contrary, epidermal tissues regenerated from differentiated keratinocytes were less well organized; the keratinocytes were enlarged and the granular layer was not present, indicating aberrant epidermal differentiation (Figure 5b). Analysis of tissue sections showed expression of INV, aberrantly extending to the lower spinous layers and occasionally in the basal keratinocytes (Figure 5j, arrows), whereas expression of late markers, filaggrin and loricrin, was not evident (Figure 5l and n). There was also a notable difference in distribution of α6-integrin, which is normally expressed in basal keratinocytes at the point of contact with the basement membrane (Sonnenberg et al., 1991), between tissues regenerated from proliferating and differentiated keratinocytes (Figure 5g and h). Despite the aberrant differentiation, tissue formed from the differentiated keratinocytes was highly proliferative (Figure 5f), indicating that grafted keratinocytes had already overcome the cell cycle arrest. Interestingly, however, by 10 days post-grafting, tissue formed from differentiated cultures was normalized and resembled that formed from proliferating keratinocytes at 7 days post grafting (Figure 6a). At this time, hair germs were evident and expression of α6-integrin and differentiation markers including INV and loricrin (Figure 6d, g, and j) was more normalized, indicating that the epidermal differentiation program was not impaired but delayed in tissue formed from differentiated cultures. By 6 weeks post-transplantation, no difference was observed between the skin regenerated from proliferative and differentiated cultures (Figure 6b and c). Expression of epidermal markers was normalized in tissue generated from differentiated cells (Figure 6h, i, k, and l), and expression of CD34, a marker of hair follicle stem cells (Trempus et al., 2003), was evident in the bulge area of reconstituted follicles (Figure 6n and o). These data indicated that differentiated keratinocytes dedifferentiated and participated in the formation of a follicular stem cell niche, and the resulting skin epithelia underwent a normal program of epithelial differentiation and hair growth. The delay in initiation of hair formation and the improper expression of epithelial markers at early stages of epidermal regeneration by differentiated keratinocytes is likely related to the reprogramming required for cells to undergo dedifferentiation.
Figure 5. Dedifferentiation of terminally differentiated keratinocytes during early stages of skin regeneration.
Tissues reconstituted from GFP-expressing keratinocytes cultured in low or high Ca were analyzed at 1 week post grafting. (a–b) H&E staining, (c–d) epifluorescent GFP, and (e–p) immunofluorescent staining for proliferating antigen Ki67, α6-integrin, intermediate differentiation marker involucrin (INV), late differentiation markers filaggrin (FIL) and loricrin (LOR), and to keratin 14 (K14). GFP+ cells appear in green, whereas markers are shown in red. Bar=50 μm.
Figure 6. Normalization of epidermal differentiation in skin epithelia regenerated from differentiated cultures.
(a–c) H&E staining of regenerated tissue showing initiation of hair germ formation (arrows) by day 10 post-grafting. By 6 weeks, no difference is observed between skin regenerated from differentiated and proliferative cultures. (d–o) Immunofluorescent staining of skin grafts harvested at either 10 days or 6 weeks post grafting for markers indicated in the figure. (n) Inset shows CD34 staining in hair follicle below sebaceous gland. GFP+ cells appear in green, whereas markers appear in red. Red staining in sebaceous gland is non-specific. GFP expression is present in the basal layer but at lower levels than cornified layers in the epidermis reconstituted from Nagy-GFP cells (inset in h). Bar=75 μm for a–c and 50 μm for d–o.
DISCUSSION
In the present study, we report an unexpected finding, namely that differentiated cultures of mouse keratinocytes, when placed in a suitable microenvironment, are able to regenerate a multilineage skin. Although the regenerative potential of differentiated keratinocytes has been the subject of speculation (Morasso and Tomic-Canic, 2005; Jones et al., 2007), to our knowledge our study marks the first direct demonstration of multilineage tissue regeneration by post-mitotic keratinocytes that display significant morphological and biochemical changes. As stem cells are best defined by their behavior, that is by long-term tissue regeneration (Blanpain et al., 2007), the long-term reconstitution of skin epithelia from differentiated cultures can be best described by dedifferentiation of differentiated keratinocytes to stem cells.
Under the experimental conditions used in our studies, more than 97% of keratinocytes ceased to proliferate and expressed INV, a precursor of cornified envelope and a well-characterized marker of epidermal differentiation (Watt, 1983; Simon and Green, 1984; Li et al., 2000). Initially, the presence of a small population of cells (about 2–3%) that did not withdraw from the cell cycle, led us to suspect that this population was responsible for the robust regenerative ability of differentiated epidermal cultures. However, our data did not support this hypothesis. First, when the proportion of replicating cells was 2–10% of the total implanted keratinocytes, the grafts were small, indicating that a population of proliferating cells up to 10% of grafted population could not have regenerated a skin graft of the same size formed by differentiated cultures. The architectural and time constraints in the skin reconstitution assay are likely to place a restriction on the minimum number of stem cells required to form a sizable graft. This is supported by several other reports in which either the implantation of more than 100,000 purified hair follicle stem cells was required for regeneration of a small hair-bearing graft (Jaks et al., 2008; Jensen et al., 2008), or a clonogenic stem cell had to be expanded extensively in culture to 0.5–2.5 million cells (depending on the size of the wound bed) to regenerate the skin (Blanpain et al., 2004; Claudinot et al., 2005). Second, our fate analysis of LacZ-marked cells showed no significant expansion of differentiation-resistant keratinocytes during skin regeneration. A comparable percentage of LacZ-labeled cells before and after tissue regeneration by proliferative cultures in this and other studies indicates that retroviral transduction does not alter the tissue-regenerating ability of keratinocytes (Kolodka et al., 1998; Lu and Ghazizadeh, 2005). Therefore, if replicating differentiation-resistant keratinocytes were the sole source of the newly generated tissue, one would have expected to observe large clusters of blue cells in the reconstituted skin, even if not all the differentiation-resistant cells were transduced. A more plausible explanation is that a large fraction of differentiated cells are capable of being reprogrammed to proliferate.
As more than 97% of cells in the differentiated cultures expressed INV and it is virtually impossible to isolate any population to 100% purity, we opted to test the possibility of multilineage skin formation by differentiated keratinocytes by lineage-tracing studies in combination with an INV-driven Cre/lox reporter system. Keratinocytes were labeled in culture by withdrawing Dox from media for 5 days and chased in a repressed state (+Dox) to inhibit de novo expression of YFP after transplantation. Uniform labeling of epidermis, hair follicle, and sebaceous glands in skin reconstituted from differentiated, but not proliferative, cultures provided direct evidence for multilineage tissue formation by the progeny of differentiated keratinocytes that had once expressed INV in culture. Although INV may not be considered a marker of late stages of epidermal differentiation, INV synthesis in culture has been shown to be directly correlated with differentiation, as indicated by an increase in cell size and withdrawal from cell cycle (Watt and Green, 1981; Dover and Watt, 1987). Whether keratinocytes that are in the later stages of differentiation (e.g., expressing loricrin while maintaining their nuclei) could regenerate skin epithelia remains to be investigated.
Further evidence for dedifferentiation of keratinocytes as a mechanism for tissue regeneration by differentiated cultures was provided by the analysis of early stages of tissue formation. Dedifferentiation is characterized by gain of proliferative capacity and a subsequent loss of differentiation markers (Finney et al., 1987). The presence of large numbers of Ki67-positive cells in the basal layer of epidermis regenerated from differentiated cultures, the delay in the initiation of hair formation, and the improper expression of epithelial markers, noted by the opposing expression pattern of INV and loricrin (or filaggrin), at early stages (day 7) followed by a rapid normalization of these markers (day 10), were all highly suggestive of reprogramming required for these cells to undergo dedifferentiation. As almost all the implanted cells expressed INV, the high levels of INV expression in the absence of proper differentiation may be related to the time required to clear INV protein or suppress its promoter activity in keratinocytes undergoing dedifferentiation. Interestingly, the markers of the later stages of differentiation were undetectable during the earlier stages of tissue formation. Although this may be related to the stepwise epidermal differentiation during tissue formation (Fuchs and Byrne, 1994), it is worth noting that despite the high levels of loricrin in the differentiated cultures detected by western blot, only about 30% of keratinocytes stained for loricrin (data not shown). Therefore, it is tempting to speculate that cultured keratinocytes at the later stages of differentiation (when loricrin is expressed) lose their tissue-regenerating capacity or cannot compete with cells at earlier stages of differentiation (Supplementary Figure S3).
Although our study is limited to tissue regeneration by transplanted cultured keratinocytes and we cannot rule out the potential effects of culture conditions or transplantation procedure on enhancement of keratinocytes reprogramming, the ability of differentiated cultured keratinocytes to regenerate skin epithelia reveals that expression of a differentiated state does not prohibit subsequent re-initiation of proliferation and reprogramming. Dedifferentiation is one of the most important principles in tissue regeneration and organ renewal in Drosophila and lower vertebrates, and involves the use of existing differentiated cells rather than stem cells (Kai and Spradling, 2004; Laube et al., 2006; Nakagawa et al., 2007; Guha et al., 2008). Although dedifferentiation in mammals is more controversial, some slowly renewing organs such as liver and pancreas have been shown to maintain themselves without the aid of stem cells. In these tissues, differentiated cells act as “facultative” stem cells and retain a significant proliferative capacity in vivo (Alison et al., 2004; Dor et al., 2004; Rawlins and Hogan, 2006).
Keratinocyte dedifferentiation has biological significance during wound healing when epidermis must rapidly regenerate barrier function. Morphological evaluation of the epidermal migration process during wound healing suggests that primary keratinocytes participating in wound re-epithelialization are suprabasal keratinocytes (Mansbridge and Knapp, 1987; Paladini et al., 1996; Usui et al., 2005). Our data imply that as these differentiated cells assume a basal position, they may receive proper signals from the wound environment to dedifferentiate and acquire biological properties to perform stem cell functions. It remains to be determined whether differentiated keratinocytes in vivo respond the same as cultured keratinocytes to the environmental cues in the wound.
Previous work has emphasized the isolation of keratinocyte stem cells for gene and cell replacement therapies. Our findings encourage a focus on microenvironment in inducing plasticity of non-stem keratinocytes as a more accessible source of progenitors for tissue repair and transplantation.
MATERIALS AND METHODS
Animals
NIH Swiss nu/nu mice were from Taconic Laboratories (Germantown, NY). INV-tTA transgenic mice were described previously (Jaubert et al., 2004); FVB.Tg(tetO-Cre01Jaw/J) (Perl et al., 2002), B6.129-GT(ROSA)26EYFP (Srinivas et al., 2001), FVB-GFPNagy (Hadjantonakis et al., 1998), and FVB-GadGFP (Oliva et al., 2000) transgenic lines were purchased from Jackson Laboratories (Bar Harbor, ME). GFP expression in the latter is limited to hippocampal and cortical GABAergic interneurons in brain (Oliva et al., 2000). As Gad-GFP mice are tolerant to GFP but do not express GFP in their skin, they were used as graft recipients in some studies. Recipient mice were between 7 and 9 weeks of age at the time of skin reconstitution assay. In lineage-tracing studies, graft recipient mice were maintained with or without 1mgml−1 Dox in 5% sucrose in drinking water. All animal studies were performed in accordance with institutional guidelines set forth by the State University of New York.
Cell culture and skin reconstitution
Epidermal cells were prepared from newborn mouse skin using standard procedures (Lichti et al., 2008). All keratinocyte cultures were seeded onto collagen-coated plates in keratinocyte serum-free media (Invitrogen, Grand Island, NY) containing 0.3mM Ca to allow attachment, and 6 hours later media were changed to 0.05mM Ca to allow optimum conditions for cell proliferation. To induce keratinocyte differentiation, confluent cultures were switched to 1.2mM Ca for at least 3 days. For skin reconstitution, a silicon chamber was implanted onto a full-thickness excised site on the back of anesthetized mice, and 2×106 epidermal cells mixed with 4×106 cultured primary newborn dermal fibroblasts (isolated from GadGFP mice) were transferred as a slurry (200 μl volume) through the chamber onto the enclosed fascia. After 1 week, chambers were removed, and wounds were allowed to heal.
Retrovirus-mediated transduction of cultured keratinocytes
Mouse keratinocytes grown under proliferating or differentiating culture conditions were transduced with a retroviral vector encoding LacZ at multiplicity of infection of 2 (Lu and Ghazizadeh, 2005) for two consecutive days starting at 24 hours after high Ca switch. For detection of LacZ-expressing cells, submerged cultures or whole mounts of skin were stained with X-gal. The percentage of LacZ-positive cells in differentiated cultures was determined by counting blue nuclei in the entire culture and calculating the LacZ-labeled:non-labeled ratios. Grafted tissue was examined by a fluorescent inverted microscope rather than a stereoscope to capture both LacZ- and GFP-labeled areas. Image J software (NIH, Bethesda, MD) was used to calculate the area of the graft formed by transduced cells.
Immunofluorescent staining and flow cytometry
For BrdU-labeling index, cultures were pulsed with 10 μM BrdU for 6 hours before immunostaining with anti-BrdU antibodies (1:500, BD Pharmingen, San Jose, CA) and the fraction of BrdU-positive cells in 1000 nuclei was determined by fluorescence microscopy. For tissue staining, reconstituted skin tissues were harvested and fixed in 4% paraformaldehyde for 30 minutes at 4°C before embedding in OCT compound. Frozen sections were stained with antibodies against INV (1:600), loricrin and keratin 14 (1:1000, Covance, Berkeley, CA), integrin α6 (1:4000, Serotec, Raleigh, NC), CD34 (1:100, eBioscience, San Diego, CA), or Ki67 (1:100, Novocastra, New Castle, UK) for 1 hour at RT, followed by biotin-labeled specie-specific antibodies (BioGenex Laboratories, San Ramon, CA). When staining with mouse antibodies, MOM Basic Kits were used (Vector Laboratories, Burlingame, CA). Antibodies were detected using Alexa 594-conjugated streptavidin (Molecular Probes, Eugene, OR). Slides were mounted with Vectashield mounting media containing DAPI (Vector Laboratories) and were examined by a Nikon E800 fluorescent microscope (Nikon Instrument, Melville, NY). For FACS analysis, cultures were trypsinized, fixed in 0.5% paraformaldehyde for 30 minutes at 4°C, and permeablized in 0.1% TritonX-100 before staining with anti-INV antibody (1:100, Covance) for 45 minutes and detected by Phycoerythrin-conjugated anti-rabbit antibody (Molecular Probes).
Detection of GFP-expressing cells in skin
To assess GFP expression in reconstituted skin of live animals, mice were anesthetized and placed under a fluorescent stereoscope (Bio 2M, Zeiss, Thornwood, NY) equipped with mercury lamp and wide band filter set for GFP. Images were captured using a Nikon Coolpix 4500 (Nikon Instrument) and processed using Adobe Photoshop. For detection of YFP or GFP expression in tissue sections, fixed cryosections were dried, rehydrated, mounted in Vectashield mounting medium with DAPI, and analyzed by fluorescent microscopy.
Supplementary Material
Acknowledgments
We are grateful to Drs Lorne Taichman and Marcia Simon for constructive discussion and criticism of the study; and Drs J Segre and S Sinha for providing transgenic INV-tTA mice. This work was supported by grants to SG from NIH (AR050525, AR056013) and NYSDOH (NYSTEM Institutional Development Grant).
Abbreviations
- Ca
calcium
- Dox
doxycycline
- GFP
enhanced green fluorescent protein
- INV
involucrin
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Alison MR, Vig P, Russo F, Bigger BW, Amofah E, Themis M, et al. Hepatic stem cells: from inside and outside the liver? Cell Prolif. 2004;37:1–21. doi: 10.1111/j.1365-2184.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–58. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Carroll JM, Albers KM, Garlick JA, Harrington R, Taichman LB. Tissue- and stratum-specific expression of the human involucrin promoter in transgenic mice. Proc Natl Acad Sci USA. 1993;90:10270–4. doi: 10.1073/pnas.90.21.10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. From the cover: long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA. 2005;102:14677–82. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic [beta]-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Dotto GP. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit Rev Oral Biol Med. 1999;10:442–57. doi: 10.1177/10454411990100040201. [DOI] [PubMed] [Google Scholar]
- Dover R, Watt FM. Measurement of the rate of epidermal terminal differentiation: Expression of involucrin by S-phase keratinocytes in culture and psoriatic plaques. J Invest Dermatol. 1987;89:349–52. doi: 10.1111/1523-1747.ep12471751. [DOI] [PubMed] [Google Scholar]
- Finney R, Ellis M, Langtimm C, Rosen E, Firtel R, Soll DR. Gene regulation during dedifferentiation in Dictyostelium discoideum. Dev Biol. 1987;120:561–76. doi: 10.1016/0012-1606(87)90259-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation. Curr Opin Cell Biol. 1990;2:1028–35. doi: 10.1016/0955-0674(90)90152-5. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet & Dev. 1994;4:725–36. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- Guha A, Lin L, Kornberg TB. Organ renewal and cell divisions by differentiated cells in Drosophila. Proc Natl Acad Sci USA. 2008;105:10832–6. doi: 10.1073/pnas.0805111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–9. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jaubert J, Patel S, Cheng J, Segre JA. Tetracycline-regulated transactivators driven by the involucrin promoter to achieve epidermal conditional gene expression. J Invest Dermatol. 2004;123:313–8. doi: 10.1111/j.0022-202X.2004.23203.x. [DOI] [PubMed] [Google Scholar]
- Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci. 2008;121:609–17. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Simons BD, Watt FM. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell. 2007;1:371–81. doi: 10.1016/j.stem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–9. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc Natl Acad Sci USA. 1998;95:4356–61. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci. 2006;119:4719–29. doi: 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- Li A, Pouliot N, Redvers R, Kaur P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J Clin Invest. 2004;113:390–400. doi: 10.1172/JCI19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ER, Owens DM, Djian P, Watt FM. Expression of involucrin in normal, hyperproliferative and neoplastic mouse keratinocytes. Exp Dermatol. 2000;9:431–8. doi: 10.1034/j.1600-0625.2000.009006431.x. [DOI] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Ghazizadeh S. Host immune responses in ex vivo approaches to cutaneous gene therapy targeted to keratinocytes. Exp Dermatol. 2005;14:727–35. doi: 10.1111/j.1600-0625.2005.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbridge JN, Knapp AM. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987;89:253–63. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–42. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97:173–83. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Yi, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–68. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol. 1996;132:381–97. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888–99. doi: 10.1046/j.1523-1747.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BLM. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–65. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Simon M, Green H. Participation of membrane-associated proteins in the formation of the cross-linked envelope of the keratinocyte. Cell. 1984;34:827–34. doi: 10.1016/0092-8674(84)90032-1. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–17. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William C, Tanabe Y, Jessell T, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–11. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Rep Regen. 2005;13:468–79. doi: 10.1111/j.1067-1927.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–6. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Involucrin and other markers of keratinocyte terminal differentiation. J Invest Dermatol. 1983;81:100s–103s. doi: 10.1111/1523-1747.ep12540786. [DOI] [PubMed] [Google Scholar]
- Watt FM, Green H. Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol. 1981;90:738–42. doi: 10.1083/jcb.90.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–17. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.