Abstract
Purpose
We have previously reported that MMP-2, MMP-9 and the complex MMP-9/NGAL can be detected in urine of patients with a variety of cancers including prostate and bladder carcinoma. In addition, we also detected several unidentified urinary gelatinase activities with molecular weights >125kDa. The objective of the current study was to identify these high molecular weight (HMW) species, determine their potential as predictors of disease status and ask whether a tumor-specific pattern existed based on urinary MMP (uMMP) analysis.
Experimental Design
Chromatography, zymography and mass spectrometry was used to identify HMW gelatinase species of ∼140, 190 and >220kDa in urine of cancer patients. To determine whether a tumor-specific pattern of appearance existed among the MMPs detected, we analyzed the urine of 189 patients with prostate or bladder cancer and controls.
Results
The ∼140, >220kDa and ∼190 HMW gelatinase species were identified as MMP-9/TIMP-1 complex, MMP-9 dimer and ADAMTS-7 respectively. The frequency of detection of any MMP species was significantly higher in urine from prostate and bladder cancer groups than controls. MMP-9 dimer and MMP-9 were independent predictors for distinguishing between patients with prostate or bladder cancer (P<0.001 for each) by multivariable analysis.
Conclusions
This study is the first to identify a tumor-specific uMMP fingerprint that may noninvasively facilitate identification of cancer presence and type. This information may be of diagnostic and prognostic value in the detection and/or clinical monitoring of disease progression and therapeutic efficacy in patients with bladder or prostate cancer.
Introduction
Matrix metalloproteases (MMPs) comprise a family of proteolytic enzymes that have been implicated in tumor growth, invasion and metastasis in experimental cancer models and in human tumors (1-8). Two members of this family in particular, MMP-2 and MMP-9, degrade typeIV collagen, fibronectin and laminin, major components of the basement membrane and are commonly used as markers of the malignant phenotype. MMP activity is regulated by a group of four distinct tissue inhibitors of metalloproteases (TIMPs) (1,5,9).
Overexpression of MMPs in tumor tissue and stroma can result in increased levels of MMP activity in various body fluids. Increased presence of MMP-2 and MMP-9 has been detected in the serum and plasma of tumor bearing rats and in humans with malignant tumors (10-15). An increase in MMP-9 levels has been reported in the tissue of animals bearing prostate and bladder tumors (16,17). We have previously reported that MMPs can be detected in urine from patients with a variety of cancers and are independent predictor of disease status (18-22). The MMP species detected in urine from cancer patients include MMP-2 (∼72kDa), MMP-9 (∼92kDa)(18), a complex of MMP-9 with human neutrophil gelatinase associated lipocalin (NGAL) (∼125kDa)(19,20) and several unidentified high molecular weight (HMW) gelatinase species. Since our original report, other groups have confirmed our findings of elevated levels of MMP-2 and MMP-9 in urine from prostate and bladder cancer patients (23-30). Despite the potential importance of HMW MMPs in predicting the presence of disease, the identity of several of these HMW MMPs has remained unknown.
The objective of the current study was to identify and characterize these as yet unidentified HMW gelatinase activities in urine from cancer patients and to determine whether their presence might be relevant to disease status. Using a combination of mass spectrometry, substrate gel electrophoresis and fractionation, we have now identified three HMW gelatinase species in urine from cancer patients. These include ∼140kDa gelatinase identified as a complex of MMP-9 and its endogenous inhibitor TIMP-1 and a >220kDa gelatinase species identified as MMP-9 dimer. In addition, a novel ∼190kDa gelatinase band was identified as disintegrin and metalloproteinase with thrombospondin motifs-7 (ADAMTS-7).
Our study also identifies, for the first time, a tumor-specific fingerprinting pattern based on the detection of MMPs in urine of patients with prostate or bladder tumors. Four MMP species were reproducibly detected in the urine of cancer patients: MMP-2, MMP-9, MMP-9/NGAL complex and MMP-9 dimer. A tumor-specific urinary MMP (uMMP) fingerprint was found by comparing samples from prostate and bladder cancer patients. While MMP-2 and MMP-9/NGAL complex were detected with comparable frequency in the urine of these patients, MMP-9 (∼92kDa) was detected with significantly higher frequency in the urine of patients with prostate cancer compared to those with bladder cancer. Frequency of positive expression of MMP-9 dimer (>220kDa) was significantly higher in patients with bladder compared to prostate cancer.
Materials and Methods
Urine collection and processing
One hundred and eighty-nine samples were analyzed in this study, including samples from patients diagnosed with organ-confined prostate (n=103) and bladder (n=41) cancer and controls (n=45). Cancer groups and controls were comparable with respect to age both in terms of the mean and the range. Urine was collected according to the institutional bioethical guidelines pertaining to discarded clinical material (18). Specimens were obtained prior to surgical or other therapeutic intervention. Samples were collected in sterile containers and immediately frozen at −20°C. Urine was tested for presence of blood and leukocytes using Multistix 9 Urinalysis Strips (Bayer, Elkhart IN) and samples containing blood or leukocytes were excluded. Protein concentration of urine was determined by the Bradford method using bovine serum albumin as the standard (18,31).
Substrate gel electrophoresis
Gelatinases in urine were detected using gelatin zymography as described previously (18). Briefly, urine (40μl) from controls or cancer patients and pure (2ng) MMP-2, MMP-9, MMP-9 dimer or MMP-9/TIMP-1 complex were mixed with sample buffer and resolved via electrophoresis. Substrate digestion was conducted as previously described (18). Gels were stained with Coomassie and imaged using Biorad Imager. Bands of enzyme activity were detected as zones of clearance on a background of uniform blue staining. To correlate gelatin-degrading activities in urine, purified MMP and MMP-complexes were loaded onto each gel for co-migration studies. Zymograms were evaluated independently in a binary fashion (presence or absence) by two investigators in a blinded manner.
Immunoblot Analysis
Immunoblotting was used to verify the presence of MMP-9 and the various MMP complexes in urine. Equal amounts of proteins (20μg) were separated by SDS-PAGE under non-reducing conditions and treated as previously described (31). Monoclonal antibodies against human MMP-9 (MAB13415, Millipore) and TIMP-1 (Ab-1, Millipore) were used. For detection of ADAMTS-7, urine (100μg) was incubated with 50μl gelatin sepharose beads for 16h at 4°C. Subsequently, the sample was spun down, supernatant discarded and sample buffer added. After 1h incubation at room temperature samples were analyzed as described above using anti-ADAMTS-7 antibodies (CL1ADAMTS-7, Cedarlane Labs, Burlington NC and ab28596, Abcam, Cambridge MA) at 1μg/ml each.
Immunoprecipitation
Urine (50μl) containing MMP-9 dimer activity was mixed with equal volume of RIPA buffer (0.1M Hepes, pH 8.0, 0.15M NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) and MMP-9 antibody (44236, EMD Chemicals, Gibbstown NJ) or a non-immune serum control and treated as described previously (19). Antibody-antigen complexes were pelleted by centrifugation at 10,000 × g and the supernatant subjected to zymography to detect any remaining gelatinase activity.
Identification of urinary ADAMTS-7
Urine (25ml) was concentrated (Amicon YM-5, Millipore), diluted with 1 vol of 50mM Tris, pH 7.5 (buffer A) and applied to a HiTrap Q Sepharose column (GE Healthcare, Piscataway NJ). A gradient of 0−500mM NaCl in buffer A was applied. Fractions containing ∼190kDa gelatinase activity were pooled, the NaCl concentration adjusted to 200mM and loaded onto Gelatin Sepharose column (GE Healthcare) pre-equilibrated with buffer A containing 200mM NaCl (buffer B). Elution was performed using a stepwise gradient of 5, 10, and 20% DMSO respectively in buffer B. Eluted fractions containing the ∼190kDa gelatinase were concentrated (Vivaspin 500, Sartorius, Hanover Germany) and resolved using SDS-PAGE. Gels were stained with Sypro Ruby (Bio-Rad, Hercules CA) and protein bands were excised from the gel, subjected to tryptic digestion and analyzed by Tandem MS/MS mass spectrometry (Perceptive STR, Applied Biosystems, Framingham MA). Using the SEQUEST (32) search program, the peptide maps generated were searched against a FASTA database of public domain proteins. Peptide matches were filtered according to their xCorrelation values, percentage of masses matched, molecular weight and number of observations of peptides and proteins.
Statistical Analyses
Percentages of individuals with positive expression for different uMMP species (MMP-9, MMP-2, MMP-9/NGAL complex and MMP-9 dimer) were compared between cancer patients and controls using Fisher's exact test. Power analysis indicated that a sample size of 40 patients in each cancer group (prostate and bladder) and 40 controls would provide 80% power (2-tailed α=0.01, β=0.20) to detect significant differences in expression for each uMMP species (version 6.0, nQuery Advisor, Statistical Solutions, Boston MA). Sensitivity, specificity, and accuracy were determined for each uMMP species using standard formulae. Sensitivity (true-positive rate) is defined as the percentage of patients with the target disease who have a positive MMP test result; specificity (true-negative rate) is defined as the percentage of individuals without the target disease who have a negative MMP test result; accuracy is determined as the percentage of correct test results (true-positives and true-negatives divided by the number of tests). Likelihood ratio for a positive test result (LR+) was defined as the ratio of its probability of occurrence if cancer is present to its probability of occurrence if cancer is absent (sensitivity/1–specificity) (33). Multiple logistic regression was applied to estimate the probability of prostate and bladder cancer based on the MMPs that were identified as multivariate predictors (34). Statistical analysis was performed using SPSS (version 16.0, SPSS Inc., Chicago, IL). Conservative two-tailed P<0.01 were considered statistically significant to protect against Type I errors arising from multiple comparisons (34).
Results
Identification of HMW gelatinase species in urine of cancer patients
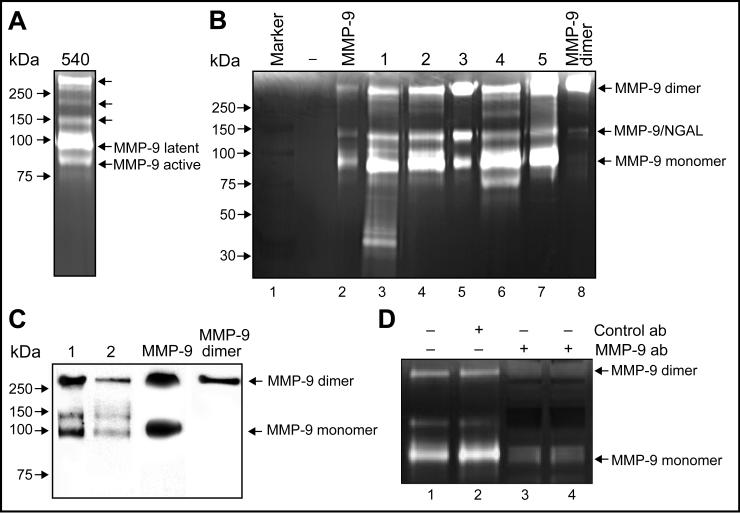
In addition to MMP-2 (∼72kDa,18), MMP-9 (∼92kDa,18) and MMP-9/NGAL complex (∼125kDa,19−22) urine samples from cancer patients also contain several previously unidentified HMW gelatinases (Fig.1A). These include species migrating at >220, ∼190, and ∼140kDa, respectively. The first HMW gelatinase identified in this study was the >220kDa species. When urine containing this activity was subjected to zymography, we found that it co-migrated with pure MMP-9 dimer (Fig. 1B). Urine contained three immunoreactive forms of MMP-9. As previously reported, the ∼92kDa and ∼125kDa gelatinase species were the monomeric and MMP-9/NGAL complex form of MMP-9 respectively (18,19). The >220kDa species was identified as MMP-9 dimer (Fig. 1C). These results were further verified by immunoprecipitation using anti-MMP-9 antibodies. The non-immune control antibody failed to immunoprecipitate any of the MMP activities, however, MMP-9 dimer, MMP-9/NGAL and MMP-9 were all specifically precipitated by the anti-MMP-9 antibody (Fig. 1D). Taken together, these data confirm the identity of the >220kDa gelatinase in urine of cancer patients as being MMP-9 dimer.
Figure 1. HMW gelatinase species in urine from cancer patients.
(A) Representative urine samples from cancer patient analyzed by zymography. Arrows indicate previously unidentified uMMPs. Latent and active forms of MMP-9 are indicated. (B) Substrate gel electrophoresis and co-migration studies of urine from cancer patients (#1−5) with pure MMP-9 monomer and dimer (lane2 & 8). (C) MMP-9 dimer detected in urine from cancer patients (#1−2) by immunoblot. (D) Immunoprecipitation of MMP-9 dimer. Urine containing MMP-9 dimer activity (lane1) was treated with non-immune control antibodies (lane2) or anti-MMP-9 antibodies for 30 min (lane3) or 1 h (lane4). The MMP-9 dimer, MMP-9/NGAL and MMP-9 species were specifically precipitated by the anti-MMP-9 antibody but not by the control antibody.
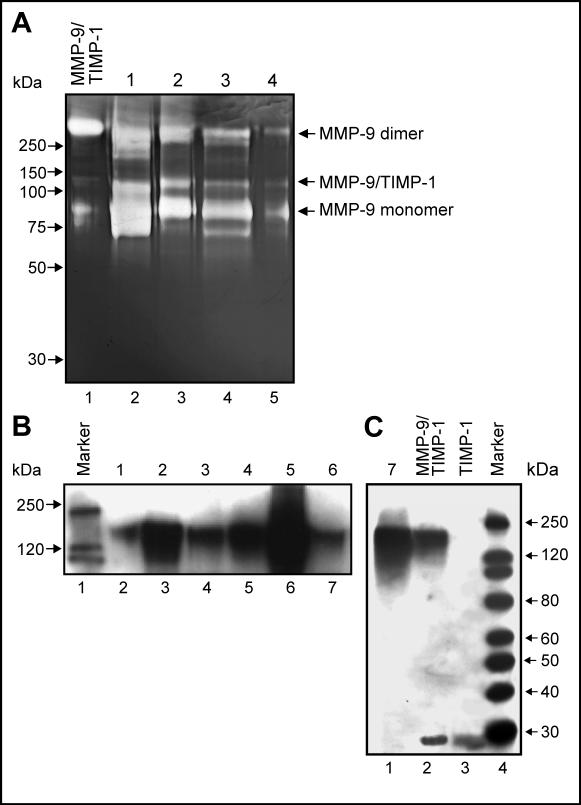
Interestingly, the ∼140kDa gelatinase appeared to co-migrate with purified preparation of MMP-9/TIMP-1 complex (Fig. 2A). Under physiological conditions, active MMP-9 is known to form a complex with TIMP-1, an inhibitor of MMPs (35). Anti-TIMP-1 antibodies detected the ∼140kDa band in urine from cancer patients (Fig. 2B). This antibody also detected pure MMP-9/TIMP-1 complex and TIMP-1 (∼28kDa) (Fig. 2C). To validate this finding, 15 urine samples containing the ∼140kDa gelatinase were subjected to immunoblotting. Six of these samples (40%) indicated the presence of MMP-9/TIMP-1 complex (Fig. 2B,C).
Figure 2. Detection of MMP-9/TIMP-1 complex in urine.
(A) ∼140kDa gelatinase in representative urine samples (#1−4, lane2−5) co-migrates with MMP-9/TIMP-1 complex (lane1). (B,C) Immunoblot of TIMP-1. Presence of MMP-9/TIMP-1 complex in urine from cancer patients (#1−7, lane2−7 (B) and lane1 (C)). Pure TIMP-1 (∼28 kDa) and MMP-9/TIMP-1 complex (∼140 kDa) are indicated (C).
Identification of the ∼190kDa gelatinase in urine
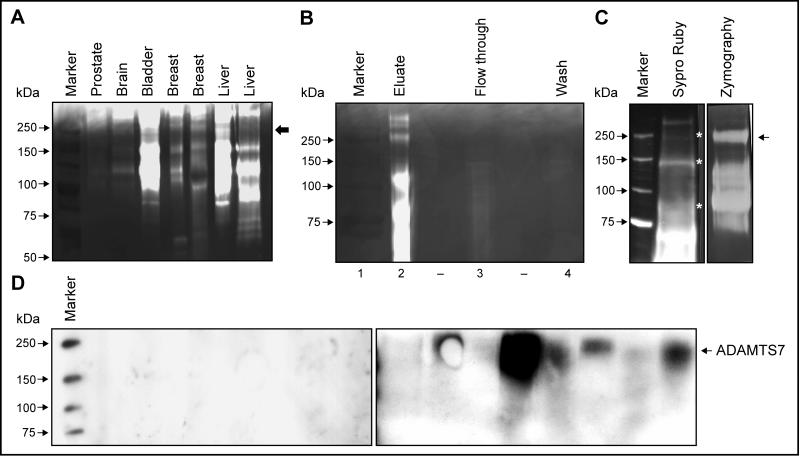
An ∼190kDa gelatinase species was detected in urine from patients with a variety of cancers including prostate, brain, bladder, breast and liver carcinomas (Fig. 3A). To identify this species, we pooled and fractionated urine. The enriched fraction collected from a Gelatin Sepharose column contained several distinct ∼68, 92, 125, 190 and >250kDa gelatinases (Fig. 3B, lane2) whereas the flow through and wash fractions did not contain these activities (Fig. 3B, lane3 & 4). Enrichment of the ∼190kDa band in the fractionated sample was evident from the appearance of strong gelatinase activity (Fig. 3C, right panel). Distinct protein bands of ∼92, 125, 190 and >220kDa (Fig. 3C, left panel) were excised and subjected to Tandem MS/MS. Consistent with previous reports, the ∼92kDa band was identified as human MMP-9 (NP_004985, 11 peptides), the ∼125kDa species contained both MMP-9 (9 peptides) and NGAL (NP_005555, 4 peptides) (18-22). The ∼190kDa gelatinase (Fig. 3C, lane2) was identified as disintegrin and metalloprotease with thrombospondin motifs-7 (ADAMTS-7) (NP_055087). Three distinct peptides spanning the amino acid sequence of human ADAMTS-7 were identified (data not shown). To confirm these results, urine from patients with breast, bladder or prostate carcinoma and age-matched controls was analyzed. An ADAMTS-7-specific antibody (ab28596) recognized the ∼190kDa species (Fig. 3D, right panel) in these urines, whereas no such species was detected in control urines (Fig. 3D, left panel). A second ADAMTS-7 antibody (CL1ADAMTS-7) gave similar results (data not shown). Based on this cross reactivity and molecular weight, the ∼190kDa gelatinase in urine was identified as the mature form of ADAMTS-7.
Figure 3. Identification of ∼190kDa gelatinase species in urine.
(A) The ∼190kDa gelatinase species (arrow) was detected in representative urine samples by zymography. (B) Enrichment of the ∼190kDa species via fractionation. Fractions eluted from gelatin sepharose column were analyzed by zymography and the ∼190kDa gelatinolytic activity was detected in the fraction eluted after the application of 20% DMSO (lane2) but not in the flow through or wash fractions (lane3 and 4). (C) Sypro Ruby staining (left) and zymography (right) of ∼190kDa species. (D) Immunoblot analysis of ADAMTS-7. ADAMTS-7 was detected in urines from cancer patients (right) but not controls (left).
Tumor-specific fingerprinting of uMMPs
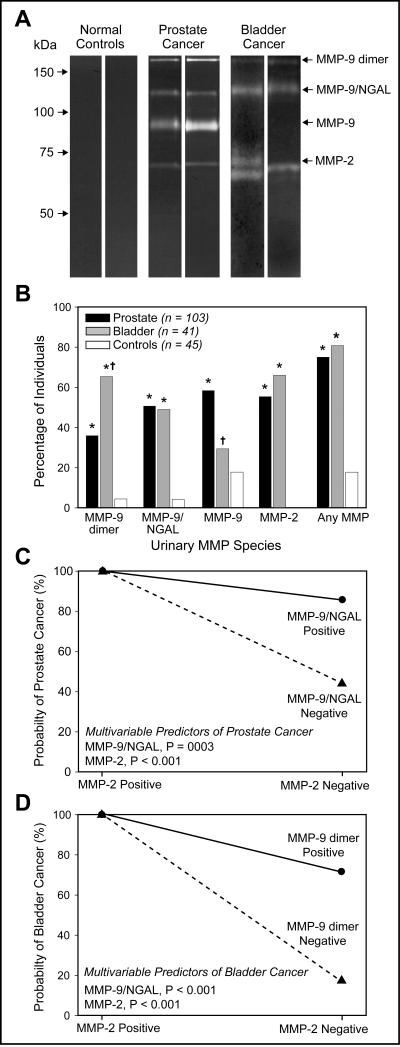
Analysis of 189 urine samples was conducted via zymography (n=144 patients with organ-confined prostate or bladder cancers; n=45 healthy volunteers). Representative zymographic results indicated low or no gelatinase activity in control urines (Fig. 4A). Predominant MMPs detected reproducibly in a binary fashion, in both types of cancer specimens were MMP-9 dimer (>220kDa), MMP-9/NGAL complex (∼125kDa) and MMP-2 (∼68kDa).
Figure 4. Tumor-specific fingerprinting of uMMPs.
(A) Substrate gel electrophoresis of MMPs in representative urine samples from healthy controls, prostate and bladder cancer patients. Gelatinase activity was detected in the urine of both types of cancer patients at approximately ∼ 68 kDa, 125 kDa, and >220 kDa, which corresponds to MMP-2, MMP-9/NGAL complex and MMP-9 dimer, respectively. MMP-9 (∼92 kDa) was detected with significantly higher frequency in the urine of prostate cancer patients. (B) Positive expression for different MMP species for cancer patients and controls. Asterisks (*) denote a significantly higher rate of expression for cancer group compared to controls (P < 0.001 for each, Fisher's exact test). No significant difference was observed in the MMP-9 positive expression rate between patients with bladder cancer and controls (29% vs. 18%, P = 0.31). † denote a significantly different rate of expression between and prostate and bladder cancer groups. Model indicating probability of prostate (C) and bladder (D) cancer based on combinations of two multivariate biomarkers. For both cancer groups the estimated probability is 100% for positive MMP-2 expression, however, when MMP-2 is negative a positive MMP-9/NGAL expression indicates a predicted probability of 86% for prostate cancer (C) whereas positive MMP-9 dimer expression indicates a predicted probability of 71% for bladder cancer (D).
Positive expression of MMPs was compared between prostate and bladder cancer by Fisher's exact test (Fig. 4B). Higher percentages of urines from prostate and bladder cancer patients were positive for MMP-9 dimer and MMP-9/NGAL compared to controls (Table 1). MMP-9 dimer was present with significantly higher frequency in urine of bladder compared to prostate cancer (66% vs. 36%, P<0.001) patients, whereas MMP-9/NGAL expression was similar between cancer subgroups (50% vs. 49%). As compared to controls, positive rates for MMP-9 were significantly higher in patients with prostate cancer (18% vs. 58%, P<0.001), but not with bladder cancer (18% vs. 29%, P=0.31). No controls were positive for MMP-2 compared to 55% of prostate and 66% of bladder cancer patients respectively (P<0.001).
Table 1.
MMP Diagnostic Performance Characteristics in Differentiating Prostate and Bladder Cancer Patients from Each Other and from Controls
| MMP Species | Sensitivity | Specificity | Accuracy | LR (+) | LR (−) |
|---|---|---|---|---|---|
| Prostate Cancer | |||||
| MMP-9 dimer | 36% (37/103) | 96% (43/45) | 54% (80/148) | 9.0 | 0.67 |
| MMP-9/NGAL | 50% (52/103) | 96% (43/45) | 64% (95/148) | 12.5 | 0.52 |
| MMP-9 | 58% (60/103) | 82% (37/45) | 65% (97/148) | 3.2 | 0.51 |
| MMP-2 | 55% (57/103) | 100% (45/45) | 69% (102/148) | ND | ND |
| Any MMP | 74% (77/103) | 82% (37/45) | 77% (114/118) | 4.1 | 0.32 |
| Bladder Cancer | |||||
| MMP-9 dimer | 66% (27/41) | 96% (43/45) | 81% (70/86) | 16.5 | 0.35 |
| MMP-9/NGAL | 49% (20/41) | 96% (43/45) | 73% (63/86) | 12.3 | 0.53 |
| MMP-9 | 29% (12/41) | 82% (37/45) | 57% (49/86) | 1.6 | 0.87 |
| MMP-2 | 66% (27/41) | 100% (45/45) | 84% (72/86) | ND | ND |
| Any MMP | 81% (33/41) | 82% (37/45) | 81% (70/86) | 4.5 | 0.23 |
LR (+) = likelihood ratio for a positive test; LR (−) = likelihood ratio for a negative test; ND = not defined.
MMP diagnostic characteristics are provided in Table 1. Likelihood ratios summarize probability of a test result for individuals with and without cancer. For example, 52 of 103 prostate cancer patients were positive for MMP-9/NGAL (sensitivity = 50%) whereas 2 of 45 controls were positive (FPR = 4%). This represents a likelihood ratio for a positive test (LR+) of 12.5 (50/4), indicating that a positive MMP-9/NGAL test result is over 12 times as likely to come from patients with prostate cancer than controls. Likelihood ratio for a negative test (LR-) calculated as the false negative rate (50%, 52/103) divided by specificity (96%, 43/45) was determined to be 0.52. Thus a negative MMP-9/NGAL result is about half as likely to be from prostate cancer patients than controls. For bladder cancer, the sensitivity and FPR were determined to be 66% and 4% respectively with a LR+ of 16.5 (66/4). Therefore, urine from a bladder cancer patient would be over 16 times as likely to be positive for MMP-9 dimer than controls. Similarly, a LR- of 0.35 indicates that a negative MMP-9 dimer result is about a third as likely to come from bladder cancer patients as controls.
Multivariable regression confirmed two MMPs (MMP-2, P<0.001; MMP-9/NGAL, P=0.003) as independently predictive in differentiating patients with prostate cancer from controls. When MMP-2 is positive the probability of prostate cancer is 100% regardless of MMP-9/NGAL status (Fig. 4C). When MMP-2 is negative, the probability of prostate cancer is 86% when MMP-9/NGAL is positive and 44% when MMP-9/NGAL is negative. On the other hand, for bladder cancer MMP-2 and MMP-9 dimer proved to be independently predictive (Fig. 4D). Specifically, when MMP-2 is positive, the probability of bladder cancer is 100% regardless of whether MMP-9 dimer is positive or negative; however, when MMP-2 is negative the probability is high (71%) if MMP-9 dimer is positive and low (17%) when it is negative. Clearly, MMP-2 is the most powerful diagnostic predictor for both cancer groups.
We also identified MMP-9 dimer and MMP-9 as multivariable predictors for differentiating prostate from bladder cancer (P<0.001 each). Positive expression of MMP-9 dimer was 1.5-fold higher in frequency in urine from bladder compared to prostate cancer patients, while MMP-9 expression was 2-fold higher in prostate as compared to bladder cancer patients, respectively. MMP-2 (P=0.73) and MMP-9/NGAL (P=0.69) were not discriminatory. In short, while prostate and bladder cancer patients often test positive for MMP-2 and MMP-9/NGAL, differences relate to MMP-9 monomer and dimer.
Discussion
In this study, we report for the first time the identity of several HMW species in urine from cancer patients. These are MMP-9 dimer, MMP-9/TIMP-1 complex and ADAMTS-7. Among the members of the MMP family, MMP-9 is unique in that when present in excess relative to its endogenous inhibitor TIMP-1, it can form dimers. MMP-9 dimer has been identified in a variety of MMP-9-producing cells including neutrophils and normal breast epithelial cells (36,37) and is a component of normal plasma (38). Enzymatic activity of the monomeric and dimeric forms of MMP-9 does not differ, however the dimer can be activated by stromelysin with much lower efficiency (10-fold) than the monomer (39). The existence of the more stable, slow-activating MMP-9 dimer might serve as a regulatory mechanism during ECM degradation (39). Therefore, it is conceivable, that in our study, expression of an excess level of MMP-9 relative to TIMP-1 by the primary tumor and surrounding stroma resulted in elevated levels of both monomeric and dimeric forms of this protease in urine.
MMP-9 is inhibited by TIMP-1 (37,39). Several studies that measured free MMP-9 and TIMP-1 levels in bladder, breast and gastric tumor tissue (26,40,41) and biological fluids including urine, plasma and serum from cancer patients (15,26,42) reported that ratio of MMP-9 vs. TIMP-1 expression may be an important indicator of tumor progression and a predictor of tumor recurrence. MMP-9/TIMP-1 complex was recently reported to be a serum marker for fibrosis in children with chronic hepatitis B (43). However, very little is known about MMP-9/TIMP-1 complex and its correlation with cancer. We found that urine from cancer patients indicated the presence of ∼140kDa species consistent with MMP-9/TIMP-1 complex when probed with TIMP-1-specific antibody. However, in the current study, MMP-9/TIMP-1 complex was not detected with significant frequency in urine from prostate and bladder cancer patients.
We also report here the identification of ADAMTS-7 as the ∼190kDa gelatinase present in urine. To date, ADAMTS-7 has yet to be associated with human cancers. Therefore, the identification of ADAMTS-7 in urine from cancer patients in this study represents the first report correlating this protease with this disease. ADAMTS are a family of disintegrin zinc-dependent proteases that have at least one Thrombospondin type I motif (1,44). ADAMTS-7 is known to bind and degrade cartilage oligomeric matrix protein (COMP) a prominent matrix component of articular cartilage. Fragments of COMP as well as elevated levels of ADAMTS-7 can be detected in the cartilage, synovial fluid and serum of arthritis patients (45). Given that ADAMTS-7 has a functional catalytic site and can degrade COMP, it is not surprising that the enzyme has gelatinolytic activity as observed in the present study. Interestingly, analysis of urine from patients with prostate, bladder and breast tumors indicated the presence of ADAMTS-7, suggesting a functional role for this protease in tumor growth and invasion.
In this study, we analyzed the expression of four distinct gelatinase species including MMP-2, MMP-9, MMP-9/NGAL complex and MMP-9 dimer in urine of patients with primary tumors in the prostate or bladder. Taken individually, each MMP species was detected at significantly higher rates in urine from cancer patients as compared to controls. Multivariate logistic regression analyses of all four uMMP species indicated that MMP-9 dimer and MMP-9 were independent predictors for distinguishing between prostate and bladder cancer. The difference in detection of MMP-9 dimer, MMP-9 and MMP-2 in the urine of these two cancer subgroups may be an important pattern that can indicate both the presence of a tumor as well as location. This first report of a tumor-specific fingerprinting pattern based on the detection of uMMPs suggests that the detection of these uMMPs may provide useful clinical information regarding the cancer type and status.
Acknowledgements
We dedicate this study to the memory of Dr. Judah Folkman to express our appreciation for his continued support and encouragement of our urinary biomarker research.
Supported by National Institute of Health grants PO1CA4554818 and P50DK065298
Footnotes
Statement of Clinical Relevance
The present study identifies a tumor-specific fingerprinting pattern, based on the detection of MMPs in urine of cancer patients, that may non-invasively facilitate identification of cancer presence and type. This information may be of diagnostic and prognostic value in the detection and/or clinical monitoring of disease progression and therapeutic efficacy in patients with localized bladder or prostate tumors.
References
- 1.Roy R, Zhang B, Moses MA. Making the cut: protease-mediated regulation of angiogenesis. Exp Cell Res. 2006;312:608–22. doi: 10.1016/j.yexcr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. Journal of the National Cancer Institute. 1997;89:1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 3.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 4.Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. The Journal of pathology. 1999;189:300–8. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 6.Fang J, Shing Y, Wiederschain D, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97:3884–9. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature reviews. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 9.Bode W. Structural basis of matrix metalloproteinase function. Biochem Soc Symp. 2003:1–14. doi: 10.1042/bss0700001. [DOI] [PubMed] [Google Scholar]
- 10.Garbisa S, Scagliotti G, Masiero L, et al. Correlation of serum metalloproteinase levels with lung cancer metastasis and response to therapy. Cancer Res. 1992;52:4548–9. [PubMed] [Google Scholar]
- 11.Nakajima M, Welch DR, Wynn DM, Tsuruo T, Nicolson GL. Serum and plasma M(r) 92,000 progelatinase levels correlate with spontaneous metastasis of rat 13762NF mammary adenocarcinoma. Cancer Res. 1993;53:5802–7. [PubMed] [Google Scholar]
- 12.Gohji K, Fujimoto N, Komiyama T, et al. Elevation of serum levels of matrix metalloproteinase-2 and -3 as new predictors of recurrence in patients with urothelial carcinoma. Cancer. 1996;78:2379–87. [PubMed] [Google Scholar]
- 13.Guan KP, Ye HY, Yan Z, Wang Y, Hou SK. Serum levels of endostatin and matrix metalloproteinase-9 associated with high stage and grade primary transitional cell carcinoma of the bladder. Urology. 2003;61:719–23. doi: 10.1016/s0090-4295(02)02429-9. [DOI] [PubMed] [Google Scholar]
- 14.Vasala K, Turpeenniemi-Hujanen T. Serum tissue inhibitor of metalloproteinase-2 (TIMP-2) and matrix metalloproteinase-2 in complex with the inhibitor (MMP-2:TIMP-2) as prognostic markers in bladder cancer. Clinical Biochemistry. 2007;40:640–4. doi: 10.1016/j.clinbiochem.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Wu ZS, Wu Q, Yang JH, et al. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. International journal of cancer. 2008;122:2050–6. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 16.Davies B, Waxman J, Wasan H, et al. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53:5365–9. [PubMed] [Google Scholar]
- 17.Jung K, Krell HW, Ortel B, et al. Plasma matrix metalloproteinase 9 as biomarker of prostate cancer progression in Dunning (Copenhagen) rats. The Prostate. 2003;54:206–11. doi: 10.1002/pros.10183. [DOI] [PubMed] [Google Scholar]
- 18.Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998;58:1395–9. [PubMed] [Google Scholar]
- 19.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11:5390–5. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- 21.Smith E, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14:2378–86. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 22.Smith ER, Manfredi M, Scott RM, Black PM, Moses MA. A recurrent craniopharyngioma illustrates the potential usefulness of urinary matrix metalloproteinases as noninvasive biomarkers: case report. Neurosurgery. 2007;60:E1148–9. doi: 10.1227/01.NEU.0000255464.37634.3C. discussion E9. [DOI] [PubMed] [Google Scholar]
- 23.Sier CF, Casetta G, Verheijen JH, et al. Enhanced urinary gelatinase activities (matrix metalloproteinases 2 and 9) are associated with early-stage bladder carcinoma: a comparison with clinically used tumor markers. Clin Cancer Res. 2000;6:2333–40. [PubMed] [Google Scholar]
- 24.Monier F, Surla A, Guillot M, Morel F. Gelatinase isoforms in urine from bladder cancer patients. Clinica chimica acta; international journal of clinical chemistry. 2000;299:11–23. doi: 10.1016/s0009-8981(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 25.Gerhards S, Jung K, Koenig F, et al. Excretion of matrix metalloproteinases 2 and 9 in urine is associated with a high stage and grade of bladder carcinoma. Urology. 2001;57:675–9. doi: 10.1016/s0090-4295(00)01087-6. [DOI] [PubMed] [Google Scholar]
- 26.Durkan GC, Nutt JE, Rajjayabun PH, Neal DE, Lunec J, Mellon JK. Prognostic significance of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in voided urine samples from patients with transitional cell carcinoma of the bladder. Clin Cancer Res. 2001;7:3450–6. [PubMed] [Google Scholar]
- 27.Monier F, Mollier S, Guillot M, Rambeaud JJ, Morel F, Zaoui P. Urinary release of 72 and 92 kDa gelatinases, TIMPs, N-GAL and conventional prognostic factors in urothelial carcinomas. European urology. 2002;42:356–63. doi: 10.1016/s0302-2838(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 28.Eissa S, Swellam M, el-Mosallamy H, et al. Diagnostic value of urinary molecular markers in bladder cancer. Anticancer research. 2003;23:4347–55. [PubMed] [Google Scholar]
- 29.Nutt JE, Durkan GC, Mellon JK, Lunec J. Matrix metalloproteinases (MMPs) in bladder cancer: the induction of MMP9 by epidermal growth factor and its detection in urine. BJU international. 2003;91:99–104. doi: 10.1046/j.1464-410x.2003.04020.x. [DOI] [PubMed] [Google Scholar]
- 30.Saito M, Kimoto M, Araki T, et al. Proteome analysis of gelatin-bound urinary proteins from patients with bladder cancers. European urology. 2005;48:865–71. doi: 10.1016/j.eururo.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004;279:51323–30. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- 32.MacCoss MJ, Wu CC, Yates JR., 3rd Probability-based validation of protein identifications using a modified SEQUEST algorithm. Analytical chemistry. 2002;74:5593–9. doi: 10.1021/ac025826t. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. Jama. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee SaH AS. Regression analysis by example. 4 ed John Wiley; New York: 2006. pp. 317–40. [Google Scholar]
- 35.Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992;267:4583–91. [PubMed] [Google Scholar]
- 36.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 37.Toth M, Gervasi DC, Fridman R. Phorbol ester-induced cell surface association of matrix metalloproteinase-9 in human MCF10A breast epithelial cells. Cancer Res. 1997;57:3159–67. [PubMed] [Google Scholar]
- 38.Vartio T, Baumann M. Human gelatinase/type IV procollagenase is a regular plasma component. FEBS letters. 1989;255:285–9. doi: 10.1016/0014-5793(89)81107-x. [DOI] [PubMed] [Google Scholar]
- 39.Olson MW, Bernardo MM, Pietila M, et al. Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase-9. Differential rates for activation by stromelysin 1. J Biol Chem. 2000;275:2661–8. doi: 10.1074/jbc.275.4.2661. [DOI] [PubMed] [Google Scholar]
- 40.Jinga DC, Blidaru A, Condrea I, et al. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: correlations with prognostic factors. Journal of cellular and molecular medicine. 2006;10:499–510. doi: 10.1111/j.1582-4934.2006.tb00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo YS, Park JJ, Kim JH, et al. Usefulness of MMP-9/TIMP-1 in predicting tumor recurrence in patients undergoing curative surgical resection for gastric carcinoma. Digestive diseases and sciences. 2007;52:753–9. doi: 10.1007/s10620-006-9535-0. [DOI] [PubMed] [Google Scholar]
- 42.Morgia G, Falsaperla M, Malaponte G, et al. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urological research. 2005;33:44–50. doi: 10.1007/s00240-004-0440-8. [DOI] [PubMed] [Google Scholar]
- 43.Lebensztejn DM, Sobaniec-Lotowska ME, Bauer M, Kaczmarski M, Voelker M, Schuppan D. Serum fibrosis markers as predictors of an antifibrotic effect of interferon alfa in children with chronic hepatitis B. European journal of gastroenterology & hepatology. 2005;17:843–8. doi: 10.1097/00042737-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–5. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Liu CJ, Kong W, Ilalov K, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. Faseb J. 2006;20:988–90. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]