Abstract
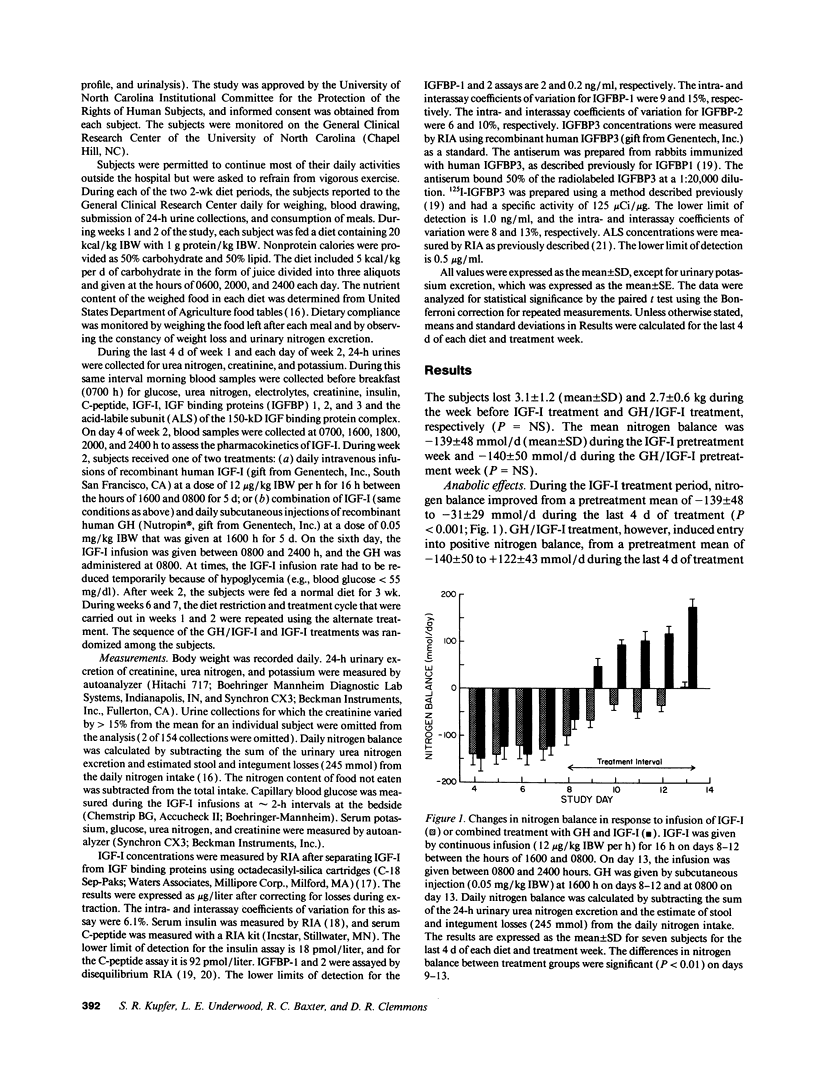
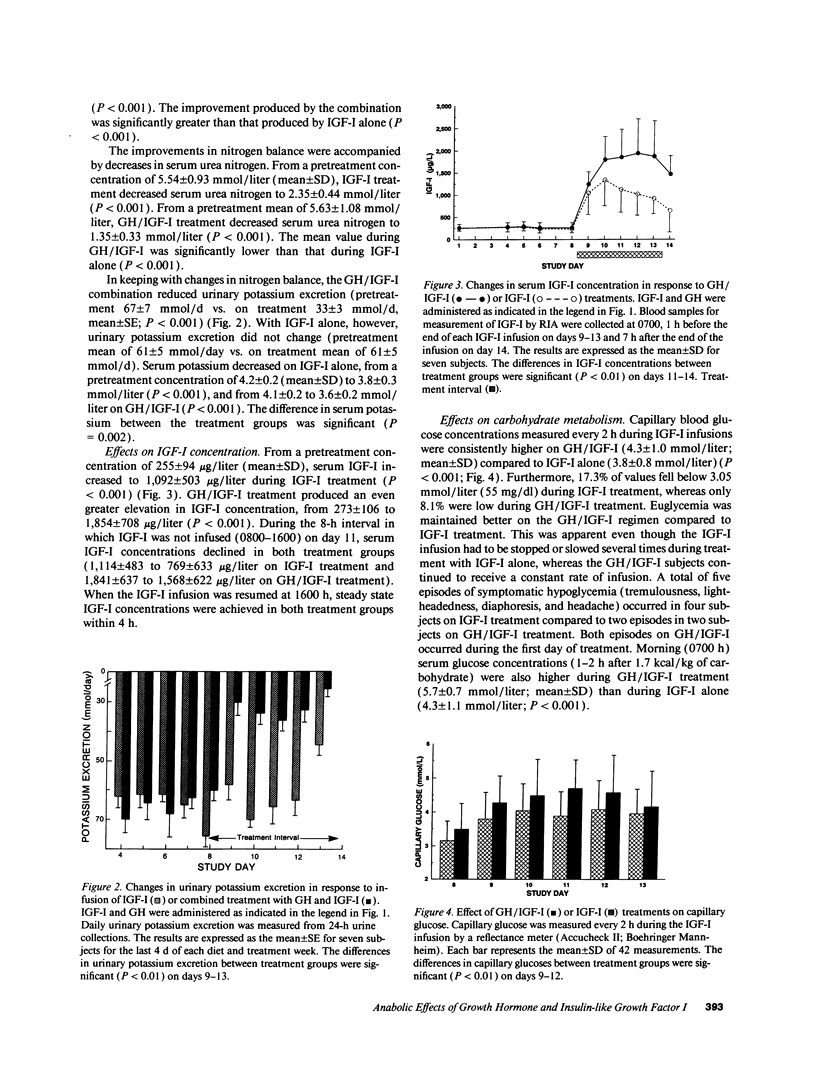
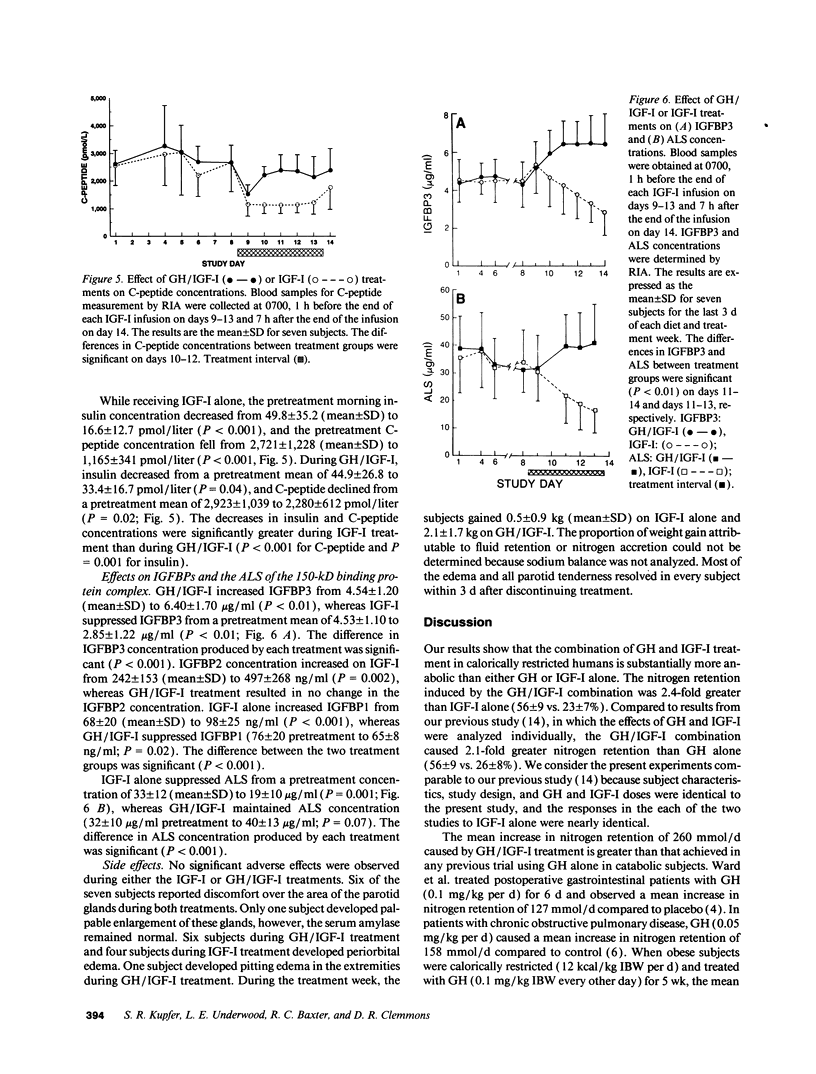
The use of growth hormone (GH) as an anabolic agent is limited by its tendency to cause hyperglycemia and by its inability to reverse nitrogen wasting in some catabolic conditions. In a previous study comparing the anabolic actions of GH and IGF-I (insulin-like growth factor I), we observed that intravenous infusions of IGF-I (12 micrograms/kg ideal body wt [IBW]/h) attenuated nitrogen wasting to a degree comparable to GH given subcutaneously at a standard dose of 0.05 mg/kg IBW per d. IGF-I, however, had a tendency to cause hypoglycemia. In the present study, we treated seven calorically restricted (20 kcal/kg IBW per d) normal volunteers with a combination of GH and IGF-I (using the same doses as in the previous study) and compared its effects on anabolism and carbohydrate metabolism to treatment with IGF-I alone. The GH/IGF-I combination caused significantly greater nitrogen retention (262 +/- 43 mmol/d, mean +/- SD) compared to IGF-I alone (108 +/- 29 mmol/d; P < 0.001). GH/IGF-I treatment resulted in substantial urinary potassium conservation (34 +/- 3 mmol/d, mean +/- SE; P < 0.001), suggesting that most protein accretion occurred in muscle and connective tissue. GH attenuated the hypoglycemia induced by IGF-I as indicated by fewer hypoglycemic episodes and higher capillary blood glucose concentrations on GH/IGF-I (4.3 +/- 1.0 mmol/liter, mean +/- SD) compared to IGF-I alone (3.8 +/- 0.8 mmol/liter; P < 0.001). IGF-I caused a marked decline in C-peptide (1,165 +/- 341 pmol/liter; mean +/- SD) compared to the GH/IGF-I combination (2,280 +/- 612 pmol/liter; P < 0.001), suggesting maintenance of normal carbohydrate metabolism with the latter regimen. GH/IGF-I produced higher serum IGF-I concentrations (1,854 +/- 708 micrograms/liter; mean +/- SD) compared to IGF-I only treatment (1,092 +/- 503 micrograms/liter; P < 0.001). This observation was associated with increased concentrations of IGF binding protein 3 and acid-labile subunit on GH/IGF-I treatment and decreased concentrations on IGF-I alone. These results suggest that the combination of GH and IGF-I treatment is substantially more anabolic than either IGF-I or GH alone. GH/IGF-I treatment also attenuates the hypoglycemia caused by IGF-I alone. GH/IGF-I treatment could have important applications in diseases associated with catabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter R. C. Circulating levels and molecular distribution of the acid-labile (alpha) subunit of the high molecular weight insulin-like growth factor-binding protein complex. J Clin Endocrinol Metab. 1990 May;70(5):1347–1353. doi: 10.1210/jcem-70-5-1347. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Martin J. L. Structure of the Mr 140,000 growth hormone-dependent insulin-like growth factor binding protein complex: determination by reconstitution and affinity-labeling. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6898–6902. doi: 10.1073/pnas.86.18.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher H. J., Mercer D., Judkins K. C., Shalaby S., Wise S., Marks V., Tanner N. S. Biosynthetic human growth hormone in burned patients: a pilot study. Burns. 1989 Apr;15(2):99–107. doi: 10.1016/0305-4179(89)90138-1. [DOI] [PubMed] [Google Scholar]
- Binoux M., Hossenlopp P. Insulin-like growth factor (IGF) and IGF-binding proteins: comparison of human serum and lymph. J Clin Endocrinol Metab. 1988 Sep;67(3):509–514. doi: 10.1210/jcem-67-3-509. [DOI] [PubMed] [Google Scholar]
- Busby W. H., Snyder D. K., Clemmons D. R. Radioimmunoassay of a 26,000-dalton plasma insulin-like growth factor-binding protein: control by nutritional variables. J Clin Endocrinol Metab. 1988 Dec;67(6):1225–1230. doi: 10.1210/jcem-67-6-1225. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Smith-Banks A., Underwood L. E. Reversal of diet-induced catabolism by infusion of recombinant insulin-like growth factor-I in humans. J Clin Endocrinol Metab. 1992 Jul;75(1):234–238. doi: 10.1210/jcem.75.1.1619015. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Snyder D. K., Busby W. H., Jr Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J Clin Endocrinol Metab. 1991 Oct;73(4):727–733. doi: 10.1210/jcem-73-4-727. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Thissen J. P., Maes M., Ketelslegers J. M., Underwood L. E. Insulin-like growth factor-I (IGF-I) infusion into hypophysectomized or protein-deprived rats induces specific IGF-binding proteins in serum. Endocrinology. 1989 Dec;125(6):2967–2972. doi: 10.1210/endo-125-6-2967. [DOI] [PubMed] [Google Scholar]
- Cohn S. H., Vartsky D., Yasumura S., Sawitsky A., Zanzi I., Vaswani A., Ellis K. J. Compartmental body composition based on total-body nitrogen, potassium, and calcium. Am J Physiol. 1980 Dec;239(6):E524–E530. doi: 10.1152/ajpendo.1980.239.6.E524. [DOI] [PubMed] [Google Scholar]
- Dahn M. S., Lange M. P., Jacobs L. A. Insulinlike growth factor 1 production is inhibited in human sepsis. Arch Surg. 1988 Nov;123(11):1409–1414. doi: 10.1001/archsurg.1988.01400350123019. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Ward A. P., Goldberg A. C., Trivedi B., Kapadia M. Characterization of somatomedin binding in human serum by ultracentrifugation and gel filtration. J Clin Endocrinol Metab. 1982 Nov;55(5):916–921. doi: 10.1210/jcem-55-5-916. [DOI] [PubMed] [Google Scholar]
- Davenport M. L., Svoboda M. E., Koerber K. L., Van Wyk J. J., Clemmons D. R., Underwood L. E. Serum concentrations of insulin-like growth factor II are not changed by short-term fasting and refeeding. J Clin Endocrinol Metab. 1988 Dec;67(6):1231–1236. doi: 10.1210/jcem-67-6-1231. [DOI] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Rowe J. W., Goodman M. N., Matthews D. E., Bier D. M., Young V. R. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985 Dec;76(6):2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Eckardt K. U., Zapf J., Bauer C., Froesch E. R. Insulin-like growth factor I increase glomerular filtration rate and renal plasma flow in man. Acta Endocrinol (Copenh) 1989 Jul;121(1):101–106. doi: 10.1530/acta.0.1210101. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Schmid C., Zapf J., Froesch E. R. Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2868–2872. doi: 10.1073/pnas.86.8.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Froesch E. R. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med. 1987 Jul 16;317(3):137–140. doi: 10.1056/NEJM198707163170303. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Scheiwiller E., Froesch E. R. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Schmid C., Froesch E. R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989 Dec;121(6):753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- Ho K. Y., Weissberger A. J. The antinatriuretic action of biosynthetic human growth hormone in man involves activation of the renin-angiotensin system. Metabolism. 1990 Feb;39(2):133–137. doi: 10.1016/0026-0495(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Horber F. F., Haymond M. W. Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J Clin Invest. 1990 Jul;86(1):265–272. doi: 10.1172/JCI114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Barrett E., Plewe G., Fagin K. D., Sherwin R. S. Acute effects of insulin-like growth factor I on glucose and amino acid metabolism in the awake fasted rat. Comparison with insulin. J Clin Invest. 1989 May;83(5):1717–1723. doi: 10.1172/JCI114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson J. M., Wilmore D. W. Positive nitrogen balance with human growth hormone and hypocaloric intravenous feeding. Surgery. 1986 Aug;100(2):188–197. [PubMed] [Google Scholar]
- Marcus R., Butterfield G., Holloway L., Gilliland L., Baylink D. J., Hintz R. L., Sherman B. M. Effects of short term administration of recombinant human growth hormone to elderly people. J Clin Endocrinol Metab. 1990 Feb;70(2):519–527. doi: 10.1210/jcem-70-2-519. [DOI] [PubMed] [Google Scholar]
- Pape G. S., Friedman M., Underwood L. E., Clemmons D. R. The effect of growth hormone on weight gain and pulmonary function in patients with chronic obstructive lung disease. Chest. 1991 Jun;99(6):1495–1500. doi: 10.1378/chest.99.6.1495. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wildbore M., Wolfe R. R. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg. 1987 Mar;205(3):288–294. doi: 10.1097/00000658-198703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Schulman G. A., Hendler R., Walesky M., Belous A., Tamborlane W. Effect of growth hormone on oral glucose tolerance and circulating metabolic fuels in man. Diabetologia. 1983 Mar;24(3):155–161. doi: 10.1007/BF00250154. [DOI] [PubMed] [Google Scholar]
- Snyder D. K., Clemmons D. R., Underwood L. E. Dietary carbohydrate content determines responsiveness to growth hormone in energy-restricted humans. J Clin Endocrinol Metab. 1989 Oct;69(4):745–752. doi: 10.1210/jcem-69-4-745. [DOI] [PubMed] [Google Scholar]
- Snyder D. K., Clemmons D. R., Underwood L. E. Treatment of obese, diet-restricted subjects with growth hormone for 11 weeks: effects on anabolism, lipolysis, and body composition. J Clin Endocrinol Metab. 1988 Jul;67(1):54–61. doi: 10.1210/jcem-67-1-54. [DOI] [PubMed] [Google Scholar]
- Snyder D. K., Underwood L. E., Clemmons D. R. Anabolic effects of growth hormone in obese diet-restricted subjects are dose dependent. Am J Clin Nutr. 1990 Sep;52(3):431–437. doi: 10.1093/ajcn/52.3.431. [DOI] [PubMed] [Google Scholar]
- Stein T. P. Nutrition and protein turnover: a review. JPEN J Parenter Enteral Nutr. 1982 Sep-Oct;6(5):444–454. doi: 10.1177/0148607182006005444. [DOI] [PubMed] [Google Scholar]
- Streat S. J., Beddoe A. H., Hill G. L. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987 Mar;27(3):262–266. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- Voina S. J., Underwood L. E., Van Wyk J. J. Failure of leucine, arginine, and epinephrine to alter plasma insulin levels in vitro. Horm Metab Res. 1971 Mar;3(2):127–128. doi: 10.1055/s-0028-1096764. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Ginalska-Malinowska M., Romer T. E., Pucilowska J. B., Underwood L. E. Effects of the infusion of insulin-like growth factor I in a child with growth hormone insensitivity syndrome (Laron dwarfism). N Engl J Med. 1991 May 23;324(21):1483–1488. doi: 10.1056/NEJM199105233242107. [DOI] [PubMed] [Google Scholar]
- Ward H. C., Halliday D., Sim A. J. Protein and energy metabolism with biosynthetic human growth hormone after gastrointestinal surgery. Ann Surg. 1987 Jul;206(1):56–61. doi: 10.1097/00000658-198707000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow J. C., Golden J., Picou D. The measurements of rates of protein turnover, synthesis, and breakdown in man and the effects of nutritional status and surgical injury. Am J Clin Nutr. 1977 Aug;30(8):1333–1339. doi: 10.1093/ajcn/30.8.1333. [DOI] [PubMed] [Google Scholar]
- Zapf J., Hauri C., Waldvogel M., Futo E., Häsler H., Binz K., Guler H. P., Schmid C., Froesch E. R. Recombinant human insulin-like growth factor I induces its own specific carrier protein in hypophysectomized and diabetic rats. Proc Natl Acad Sci U S A. 1989 May;86(10):3813–3817. doi: 10.1073/pnas.86.10.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Schmid C., Guler H. P., Waldvogel M., Hauri C., Futo E., Hossenlopp P., Binoux M., Froesch E. R. Regulation of binding proteins for insulin-like growth factors (IGF) in humans. Increased expression of IGF binding protein 2 during IGF I treatment of healthy adults and in patients with extrapancreatic tumor hypoglycemia. J Clin Invest. 1990 Sep;86(3):952–961. doi: 10.1172/JCI114797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler T. R., Rombeau J. L., Young L. S., Fong Y., Marano M., Lowry S. F., Wilmore D. W. Recombinant human growth hormone enhances the metabolic efficacy of parenteral nutrition: a double-blind, randomized controlled study. J Clin Endocrinol Metab. 1992 Apr;74(4):865–873. doi: 10.1210/jcem.74.4.1548352. [DOI] [PubMed] [Google Scholar]


