Abstract
Plant derived products are consumed by a large percentage of the population to prevent, delay and ameliorate disease burden; however, relatively little is known about the efficacy, safety and underlying mechanisms of these traditional health products, especially when taken in concert with pharmaceutical agents. The flavonoids are a group of plant metabolites that are common in the diet and appear to provide some health benefits. While flavonoids are primarily derived from soy, many are found in fruits, nuts and more exotic sources, e.g., kudzu. Perhaps the strongest evidence for the benefits of flavonoids in diseases of aging relates to their effect on components of the metabolic syndrome. Flavonoids from soy, grape seed, kudzu and other sources all lower arterial pressure in hypertensive animal models and in a limited number of tests in humans. They also decrease the plasma concentration of lipids and buffer plasma glucose. The underlying mechanisms appear to include antioxidant actions, central nervous system effects, gut transport alterations, fatty acid sequestration and processing, PPAR activation and increases in insulin sensitivity. In animal models of disease, dietary flavonoids also demonstrate a protective effect against cognitive decline, cancer and metabolic disease. However, research also indicates that the flavonoids can be detrimental in some settings and, therefore, are not universally safe. Thus, as the population ages, it is important to determine the impact of these agents on prevention/attenuation of disease, including optimal exposure (intake, timing/duration) and potential contraindications.
Keywords: soy, kudzu, metabolic syndrome, complimentary medicine, dietary supplements
Introduction
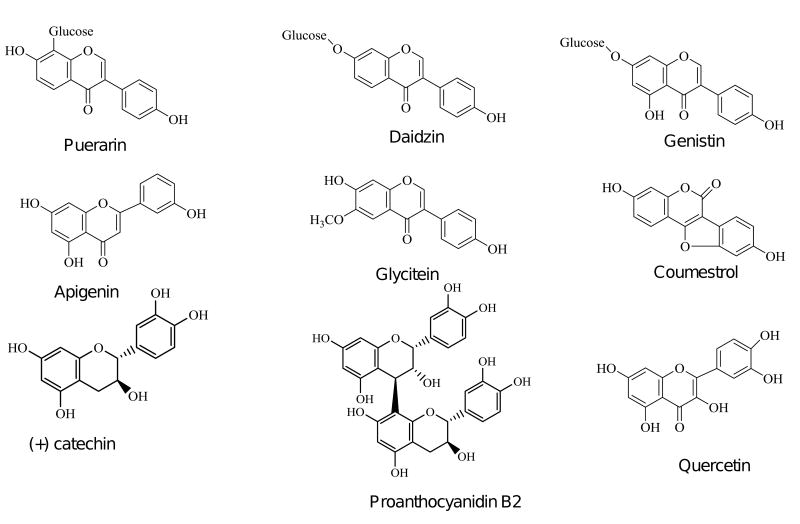
Flavonoids are important secondary metabolites of plants that are the most common group of polyphenolics in the human diet (Figure 1). They are subdivided into several other groups including flavone, flavonol, flavanone, and isoflavones. Isoflavonoids differ from other flavonoids by having ring B attached to C-3 position of ring C. In plants, they are especially important in guarding against oxidant damage, and they provide to the plant the color that attracts pollinators and repels attacks by insects and microbes. Recent research suggests that in humans, these plant polyphenols provide important health benefits related to metabolic syndrome, cancer, brain health and the immune system. While many of these effects are of interest in youth, they are even of greater interest for treating the fastest growing sector of the population, i.e., aging adults. The relatively low toxicity and potential efficacy of most of these agents make them attractive to a large sector of the population. Further, there has been a growing public openness to non-traditional therapies such as botanical supplements. An estimated 42% of adults in the US take some form of dietary supplement for their health [1] and most studies suggest that about 33% of women regularly take a botanical supplement [2].
Figure 1.
Chemical structures of some dietary flavonoids.
Research investigating botanical compounds focuses on elucidating beneficial and adverse effects, as witnessed by the publication in 2009 of over 60 scientific review articles on the health effects of dietary botanicals. The emerging scientific evidence indicates that many of these compounds are effective and have few adverse effects, but some adverse effects and adverse drug interactions have been identified [3, 4]. No polyphenol has been identified as a primary treatment for any disease, but many flavonoids appear to provide potentially important adjuvant and preventative treatments. In response, there has been phenomenal rise in use of isoflavones over the last decade. While soybeans and soy products have been the major sources of commercially available isoflavones, increasing consumer interest led to supplements based on soy, red clover, and kudzu. Similarly, these sources have been the target of many ongoing studies investigating their roles in the prevention/delay of age-related diseases, such as atherosclerosis [5], cancer [6, 7] and osteoporosis [8, 9].
This paper reviews the broad health effects of flavonoids in disease, but it also focuses more specifically on their role in the metabolic syndrome and its tripartite contributors, i.e., diabetes, hypercholesterolemia (and obesity) and hypertension. Whereas there is an extraordinary rise in the incidence of type 2 diabetes and insulin resistance among teens, the incidence of type 2 diabetes is increasing nearly as rapidly among the aging and is highest among aged Hispanics and African-Americans (>70% greater than in age-matched Caucasians [10]). Further, lifestyle modifications that decrease diabetes in the young (e.g., weight loss, smoking cessation and increased exercise) are often unavailable or not well tolerated in older adults. In aged adults, compared to youth, hypertension and dyslipidemia are much more likely to synergize with diabetes to produce the metabolic syndrome, thus requiring many daily medications and. It is well established that an increase in anti-diabetic medications leads to a concomitant decrease in compliance in adults [11]. In contrast, adults appear to much more consistently take botanical supplements than pharmaceuticals [1]. Thus, botanical supplements that exhibit anti-diabetic actions may be an important component of successful diabetes therapy.
Flavonoid biochemistry
To verify the in vivo health effects of isoflavones, it is important to understand how isoflavones are absorbed from the gastrointestinal tract and why some isoflavones are absorbed more efficiently than others. Equally important is the determination of how much and how long isoflavone exposure is required to provide health effects. Although there has been substantial progress in the elucidation of the pharmacokinetics and bioavailability of isoflavones, there is yet no clear understanding of why isoflavones have relatively poor bioavailability.
Uptake and bioavailability of isoflavones
Soy products, the major source of dietary isoflavones for most people, contain 12 known isoflavone compounds (three aglycones, three glucosides, three acetyl-ester glucosides, and three malonyl-ester glucosides). Genistein (5,7,4′-trihydroxyisoflavone) and daidzein (7,4′-dihydroxyisoflavone) are the primary isoflavones and are commonly regarded as phytoestrogens because of their estrogenic-like properties. A series of reports from in vivo studies document the bioavailability of these two isoflavones at target tissues, but the bioavailablity is much lower than the concentration typically used for in vitro studies that are designed to evaluate the botanicals' biological effects, e.g., antioxidant properties [12], estrogen receptor binding [13, 14] and antiproliferative and growth inhibiting effects [15]. Since most isoflavones undergo extensive metabolism in vivo, much of the in vitro data are complicated to interpret relative to in vivo systems. Therefore, a clear understanding of uptake, metabolism and bioavailability of these compounds is crucial to elucidating their heath benefits/adverse effects.
Most common isoflavones exist as O-glucosides (e.g., daidzein and genistein), and, compared to their aglycones form, the glycoside forms are poorly absorbed from the small intestine into the blood due in large part to their high hydrophilicity [16, 17]. Intestinal uptake of majority of isoflavones is by non-ionic passive diffusion, and the glycosidic moieties of isoflavone glucosides are substantial hydrophilic, thus reducing passive transport across the membrane. Some flavonoid glucosides may utilize the Na+-dependent glucose transporters [18, 19]. Interestingly, soy may inhibit glucose transport in isolated rat intestinal vesicles via inhibition of GLUT2 [20].
Poor absorption of O-glycosylated isoflavones poses a potential barrier for their clinical application. Like other flavonoids, isoflavones undergo intestinal absorption and first-pass metabolism before entering the peripheral blood compartment and reaching most target organs. Isoflavones are substrates for β-glucosidase, UDP-glucuronosyltransferase, and sulfotransferase in the small intestine as well as for a number of phase I and II enzymes. Lactase phlorizin hydrolase (LPH) is a membrane-bound β-glucosidase enzyme located in the brush-border of small intestine and is primarily responsible for hydrolysis of lactose and glycosides. Isoflavones in blood circulation mostly exist in the form of glucuronide conjugates. For example, oral bioavailability of genistein in cats is 1.379% for free form and 29.85% for the conjugated forms [21]. It is also important to note that genistein and its principal metabolite, genistein 7-O-β-glucuronide are well absorbed from the intestine and transported from portal blood into the liver and bile and thus undergo enterohepatic circulation [22, 23]. In view of enterohepatic circulation of genistein and its metabolites, genistein is highly bioavailable in rats as it may accumulate within gastrointestinal tract [22].
Ingested isoflavones are subjected to hydrolysis and degradation in the colon due to microbial enzyme catalysis. The pharmacokinetic behavior of isoflavones genistein and daidzein and their respective β-glucosides in healthy humans was first studied by Setchell [24]. The studies indicated that the peak plasma concentrations (Tmax) for aglycones genistein and daidzein were 5.2 and 6.6 h, respectively, whereas in the case of their β-glucosides, the Tmax values were delayed to 9.3 and 9.0 h, respectively [24]. According to this study, daidzein showed extensive tissue distribution compared to genistein as the apparent volume of distribution of daidzein is 236 L compared with genistein (161 L). These data indicate that the systemic bioavailability of genistein (plasma concentration) is higher than daidzein, and the half life (T½) for daidzein and genistein are about 9.3 h and 7.1 h, respectively.
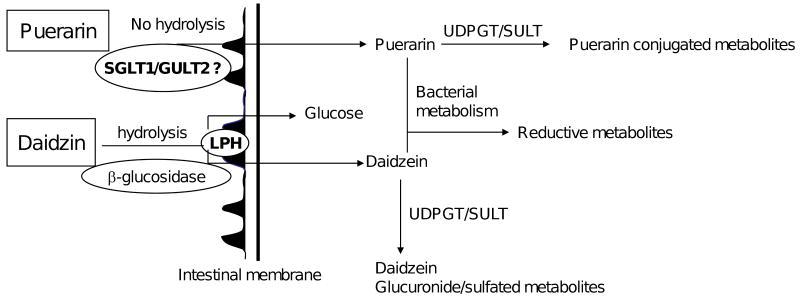
In contrast to isoflavone O-glucosides, puerarin (daidzein 8-C-glucoside) an isoflavone C-glucoside found in kudzu root, is resistant to intestinal hydrolysis and absorbed intact. Puerarin is rapidly absorbed into the blood, reaching a maximum and then declining within 1 h [25]. It undergo limited phase I and phase II reaction and widely distributes to various organs such as liver, kidney, lungs, pancreas, heart, brain and eyes [26, 27]. The mechanism for this action may involve intestinal glucose co-transporters (SGLT1 and/or GLUT2). These observations clearly demonstrate that deconjugation of isoflavone-C-glycosides is not a prerequisite for its absorption in rats. Schematic of possible mechanism involved in intestinal transport of puerarin and daidzin is shown in Fig. 2.
Figure 2.
Schematic outline of uptake and metabolism of daidzin (isoflavone O-glucoside) and puerarin (isoflavone C-glucoside). SGLT1 = sodium dependent glucose transporter 1, GULT2 = glucose transporter 2, LPH = lactase phlorizin hydrolase, UDPGT = uridine diphosphate glucuronosyl transferase,, SULT = sulfotransferase.
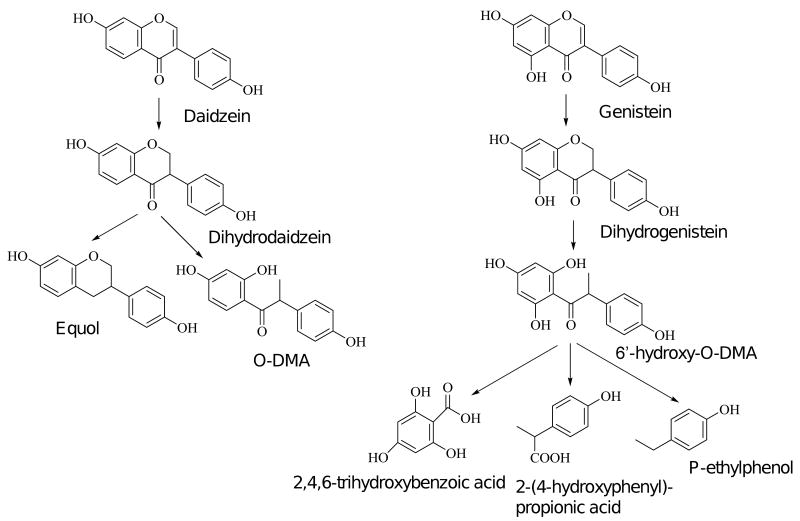
Colonic metabolism of isoflavones by the large number of microorganisms in the lumen of the colon is a major factor affecting their bioavailability. After intestinal hydrolysis, the aglycone daidzein undergoes extensive reductive metabolism to make dihydrodaidzein, dihydrogenistein, O-desmethylangolensin (O-DMA) and equol. Equol-a potential ligand for estrogen receptor (ER)beta, was first identified in urine and blood as metabolites of daidzein [28]. It has attracted interest for its potential application in hormone-dependent therapeutic and is under development as a nutraceutical [29]. Metabolism of genistein includes dihydrogenistein, 6′-hydroxy-O-DMA, 2-(4-hydroxyphenyl)-propionic acid, and 4-ethyl phenol [30] (Fig. 3). The extraction output of these metabolites varies tremendously among individual's gut microbial ecology, i.e. equol producer or non producer. Only 30-40% of the Western populations are equol producers [31].
Figure 3.
Bacterial metabolism of isoflavones daidzein and genistein. Daidzein and genistein undergo reduction to form their dihydro metabolites and further metabolize to O-DMA and 6′-hydroxy-O-DMA, respectively. Daidzein is converted to equol, whereas genistein is metabolized to 2,4,6-trihydroxybenzoic acid, p-ethylphenol and 2-(4-hydroxyphenyl)-propionic acid.
Bacterial metabolism of isoflavones is important relative to generating chiral metabolites from achiral molecules. Setchell has shown that in human the naturally occurring isomer of equol is the S(-)- enantiomer [32]. It is interesting to note that only daidzein metabolites are detected in the prostate of male rats [7]. Because of the differences in absorption and metabolism of each of the isoflavones, the isoflavone composition of soy preparations or dietary supplements plays crucial roles in determination of their health beneficial effects.
Metabolic Syndrome and Botanicals
The metabolic syndrome has three major contributors: hypertension, dyslipidemia/obesity and hyperglycemia/hyperinsulinemia, all of which act synergistically to greatly increase morbidity and mortality. While the incidence of all three contributors is increasing exponentially in adults, a parallel rise is also occurring in children [33]. It is estimated that clustering of these metabolic risk factors occurs in up to 50% of overweight adolescents, leading to an increased appearance in early onset type-2 diabetes and cardiovascular disease [34].
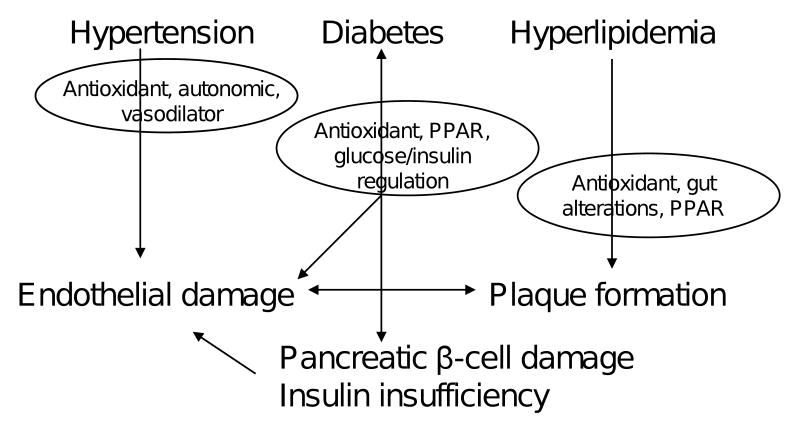
The treatment of metabolic syndrome in both young and aging populations has greatly increased pharmaceutical expenditures, and antihyperglycemic drugs are projected to become the largest single component of all prescription drug spending in the near future [35], making the metabolic syndrome a very significant burden on individual health and the economy. Research is increasingly exploring the ability of botanical supplements to reduce metabolic syndrome risk factors, since these compounds could provide greater efficacy and tolerability at lower cost, compared to current pharmaceutical options (Fig. 4).
Figure 4.
Mechanisms by which flavonoids are most likely to reduce the three main contributors to metabolic syndrome in aging adults.
Metabolic syndrome displays a strong gender disparity. Relative to age-matched males, the rate of metabolic syndrome is significantly lower in premenopausal women, but after menopause women display a large increase in dyslipidaemia, arterial pressure and other risk factors, so that their risk profile for metabolic syndrome approximates that observed in age-matched males [36]. This lends support to the hypothesis that estrogen protects premenopausal women from metabolic disease, adds interest to the search for alternatives to estrogen therapy to reduce metabolic disease and increases interest in favonoids as potential estrogen mimetics.
Hypertension and Botanicals
A major contributor to metabolic syndrome is hypertension, which also significantly increases the incidence of heart disease and stroke, amplifies the adverse effects of other cardiovascular risk factors and is highly age-related [37, 38]. Hypertension is present in 30% of all adults and in more than 60% of adults over 65 years of age [39]. The mechanisms underlying the development of most hypertension are polygenic, and most are only partially understood.
The prevalence of hypertension is also sexually dimorphic. Premenopausal females consistently have lower blood pressure than age-matched males; however, during the perimenopausal period, arterial pressure increases rapidly in females and by age 60 the average arterial pressure is equal to that in males [36, 40]. These observations have long suggested that circulating estrogen and/or progesterone exert cardioprotective effects on premenopausal women and that loss of this protective estrogenic effect at menopause contributes to the rapid increase in arterial pressure, hypertension and cardiovascular disease. In response to these findings, during the last 2 decades of the 20th century there was a tremendous expansion in the use of hormone replacement therapy (HRT) to decrease cardiovascular disease in postmenopausal women; however, the findings of the Women's Health Initiative indicated significant potential adverse effects of HRT [41]. These findings increased interest in the use of alternative methods, especially dietary supplements that were estrogen-like, but appeared to lack the associated adverse effects of estrogen.
Probably the most studied and most widely used botanical for cardiovascular protection is soy, largely due to the ability of soy isoflavones to activate estrogen receptors. A number of studies indicate that dietary soy lowers arterial pressure in both postmenopausal women and age-matched men, e.g., [42, 43], regardless of whether individuals have established hypertension or are normotensive [44]. Several studies have tried to elucidate the roles of genistein and daidzein in these effects. Both genistein and daidzein bind estrogen receptors (ER), especially ERβ receptors [45, 46], and the antihypertensive effects of both of these isoflavones mirrors the effects of whole soy in clinical [47, 48] and animal studies [49, 50]. Our laboratory has focused on the effects of soy isoflavones on blood pressure control in spontaneously hypertensive rats (SHR) and stroke prone SHR (SHRSP), both commonly used genetic model of hypertension that exhibit gender disparity similar to that observed in human subjects [51, 52]. These findings suggested that both estrogen and flavonoid “phytoestrogens” exert a protective effect on arterial pressure in these models. Soy flavonoids are present in high concentrations in normal commercial rodent diet (typically soy based), and they decrease blood pressure in ovariectomized SHR that are on an otherwise phytoestrogen-free diet [52]. Supplementation of the diet with genistein alone at dietary concentrations (0.06% w/w) causes a 50% (>30 mm Hg) decrease in the hypertensive response to a high NaCl diet in these animals [53], and the protective effect of soy isoflavones on salt-sensitivity also extends to male rats and thus the effect is not gender specific [53].
Grape seed extract is another well studied flavonoids, largely due to epidemiological observations that the red wine drinking French display better vascular health and lower cardiac mortality rates relative to their American counterparts, despite consuming a diet similar in fat [54], i.e., the “French Paradox” [55], These observations suggest that the flavonoids in red wine might confer a protective effect against coronary heart disease. Results from ensuing studies indicate that red grapes (both skins and seeds) contain a significant number of polyphenolic compounds, including phytoalexins (e.g., resveratrol), proanthocyanidins and flavonoids, that significantly reduce cardiovascular disease and stroke [54, 56].
Grape seed extract is composed of multiple polyphenolic compounds, including monomeric catechins and proanthocyanidins (oligomeric or polymeric catechins). Similar to soy flavonoids, in combination with a otherwise flavonoids-free diet, grape seed extract reduces NaCl-sensitive hypertension in male and female SHR and decreases blood pressure in ovariectomized SHR on a basal NaCl diet [57]. It appears that these actions are not mediated by estrogenic pathways. In contrast, grape seed extracts provide significant antioxidants and may reduce the amount of reactive oxygen species, which are elevated in cardiovascular disease and cancer along. A similar study using grape skin extract demonstrated that dietary supplementation lowered arterial pressure in both DOCA-NaCl and L-NAME-induced hypertensive rats, likely as a result of vasodilatory effects of grape skin polyphenols [58]. Taken together, these rodent studies indicate that the polyphenolic compounds found in both the skin and seeds of red grapes favorably impact hypertension and control of arterial pressure.
Kudzu root is a botanical that has been extensively used in traditional Asian cultures for centuries. In the SHR, kudzu root extract (compared to control diet) induces a relatively small (< 15 mm Hg) decrease in hypertension compared to that provided by other flavonoids [59]. Further, kudzu root show no greater effect in SHR on a high salt diet, old SHR or male versus intact female versus ovariectomized female rats. Other flavonoids tested have a greater effect in many of these settings. Together, these findings suggest that the mechanisms of action of these botanicals differ, with grape seeds favorably affecting hypertension and control of arterial pressure.
Botanicals and Dyslipidemia
Dyslipidemia, including elevated plasma concentrations of total cholesterol (TC) and low-density lipoprotein (LDL) cholesterol (and/or reduced high-density lipoprotein [HDL]), is strongly associated with cardiovascular disease, peripheral vascular disease and stroke[60]. Further, many observational studies have demonstrated that populations consuming plant-based diets typically have significantly lower TC and LDL levels, corresponding to reduced rates of heart disease compared to the general population. These observations have resulted in dietary guidelines that limit total and saturated fats and dietary cholesterol and are higher in plant products.
Early results indicate that multiple botanical compounds may improve plasma lipid profiles, e.g., plant stanols and sterols, tea-based catechins and theaflavins [61], although clinical data related to the efficacy of many of these substances is lacking. One dietary substance that demonstrates potent beneficial effects on lipid profile is niacin, which increases HDLs and decreases LDL levels. Given the number of clinical studies supporting an effect of niacin on lipid profile (e.g., [62]), it has been increasingly used in hyperlipidemia therapy. Other supplements that demonstrate early clinical success in improving lipid profiles include red yeast rice extract [63, 63] and omega-3 fatty acids[61].
In Asia, where diets generally contain relatively large amounts of soy, lipid profiles and coronary heart disease are low compared to Western societies. Studies have demonstrated that soy isoflavones reduce LDL levels [64-71], triglycerides[72-75] and apolipoprotein B plasma concentrations[76] while increasing HDL levels[77]. In addition, soy intake also lowers body weight and BMI in obese individuals[78].
The mechanism(s) by which soy isoflavones act may include both estrogen-like effects and alternative pathways. Similar to estrogen, the phytoestrogen genistein activates ERβ receptors, decreasing the activity of lipoprotein lipase and thereby decreasing lipogenesis[79]. Soy isoflavones may also positively influence lipid levels through PPAR-mediated pathways, which control the transcription of several genes involved in lipid catabolism and adipocyte differentiation, and as a tyrosine kinase inhibitor by which they can block phosphorylation of elements necessary for adipocyte differentiation[79]Consumption of red grapes and red wine also has been shown to improve plasma lipid profiles and reduce vascular damage and atherosclerosis[80]. Whether these effects are a broad result of general alcohol intake, including white wine or other forms of alcohol, or are more specific to the red grape polyphenols has been widely studied, and most studies indicate that red wine polyphenolic extracts exert the strongest effects[80]. Research to date has indicated that red wine polyphenols improved plasma lipid profiles by increasing HDL cholesterol levels and improve LDL oxidation[81-83]. Further, polyphenols in red wine decrease lipoprotein oxidation in human subjects[84, 85] and increase cellular and plasma antioxidant levels, increasing resistance to oxidative stress[86, 87] and reducing oxidative LDL-induced dysfunction of endothelial cells[88]. The net result of these effects is a decrease in the risk of major cardiovascular events[81].
But, while light to moderate alcohol intake improves risk factors for cardiovascular disease, intake of more alcohol does not potentiate the positive effects on lipid profiles and antioxidant status. It is increasingly clear that a J- or U-shaped curve exists regarding alcohol intake and occurrence of risk factors such as arterial pressure, dyslipidemia, blood glucose levels and markers of oxidative stress[89, 90].
Flavonoid effects on glucose control
Type-2 diabetes has increased greatly in frequency over the last decade resulting in an increased interest of patients in using dietary supplements to improve glycemic control; approximately one third of diabetic individuals use some type of botanical supplement or complementary therapy[91]. Despite a vast array of plant products used in controlling blood glucose levels, such as the botanical alkaloid berberine[92], Russian tarragon[93], plant anthocyanins[93], there is very little research in either diabetic individuals or rodent models of diabetes as to the efficacy or safety of most of these compounds.
The spice cinnamon has demonstrated a strong potential for improving diabetic control in limited number of clinical studies, e.g., [94, 95] The mechanisms by which cinnamon exerts this effect is currently under investigation, with studies suggesting activation of PPAR signaling [96], and regulation of genes related to insulin signaling and lipogenesis in adipose tissue[97]. Similarly, the trace element chromium also improves glycemic control in diabetic individuals, and chromium deficiency induces reversible diabetes[98]. Research from our laboratory has focused on the anti-glycemic effects of extracts derived from kudzu (Pueraria lobata). Kuzdu, originally imported from Japan in 1876, is an invasive species. Kudzu root extract contains several isoflavons, including puerarin (the most dominant compound) and daidzin and genistein (similar to that found in soy). In control and diabetic ob/ob mice, whole kudzu extract and puerarin supplementation improve glucose tolerance (unpublished data). We similarly demonstrated that glycemic control is improved by both acute and long-term puerarin supplementation in stroke-prone SHR[59]. Whether kudzu elicits similar anti-glycemic effects in diabetic individuals remains to be determined.
Flavonoid effects on other age-related diseases
Osteoporosis
Aging decreases bone mass in all adults, but this loss is much more significant in postmenopausal women who do not take replacement therapy or an alternative. While many women take botanical products to reduce bone loss (either by increasing bone formation or decreasing bone resorption), the effectiveness of these agents remains debatable. Yamaguchi suggests that various carotenoids, β-cryptoxanthin from Citrus unchiu MARC increases bone formation and inhibits bone resorption, thus potentially stabilizing bone mass in osteoporosis rat models and perhaps in humans[99]. He indicates that extracts from wasabi, marine alga and bee pollen and p-hydroxycinnamic acid also preserve bone integrity in aged rat and in humans[99]. Soy is by far the most used isoflavone and has been touted for its positive effects on bone health in aging woman, largely due to its putative estrogenic effects. Several groups have shown that soy isoflavones (especially genistein) have a positive effect on bone health in postmenopausal woman[4, 31, 100-103]. However, these effects appear to be modest compared to the effects of estrogen replacement[104], and the animal studies are almost exclusively in young ovariectomized rats. The findings of the NIH Women's Health Initiative suggest that timing is everything in hormone replacement therapy, and by analogy for flavonoid therapy, e.g.[41, 105]. Data from animals suggest that very high intake of soy isoflavones, especially if initiated long after menopause (a time when estrogen receptors are altered in their responsiveness) can worsen bone loss, thus suggesting that flavonoids may be a double-edged sword[104].
Cancer
The use of flavonoids for both cancer prevention and treatment has also increased dramatically over the past decade[106], in general due to the belief that such treatments are effective and much safer than alternative pharmaceutical treatments. Both of these assumptions must be considered with care. Clearly, preclinical and clinical data demonstrate the potential for some botanicals and flavonoids to be beneficial against cancers. In humans, genistein appears to negatively regulate the proliferation of hormone-sensitive breast cancers[107-109], likely by acting directly on estrogen pathways. However, other reports indicate that in rodent models genistein increases the growth of estrogen-positive breast tumors[110, 111]. A similar debate also surrounds the use of soy isoflavones and other botanicals in the prevention and treatment of prostate cancer.
In colorectal cancer, green tea consumption is reported to have a protective effect that is directly correlated with duration of exposure to the green tea catechins[112]. In contrast, soy supplementation appears to increase colon cell proliferation. Curcumin induces apoptosis in colon cell cancer lines, but not in normal colon cells[113], and green tea causes apoptosis in oral cancer cells but not normal oral cells[113].
All of these studies point out clearly that the effects of botanicals on cancers are greatly influenced by dosage, duration and timing of the botanical, supplement type, experiment design (e.g., in vivo or in vitro) and the type of cancer line tested. The mechanisms of these anti-carcinogenic and anti-mutagenic effects of polyphenols appears to be in large part due to the antioxidant and anti-inflammatory properties of these agents and their bioavailability and molecular targets, e.g., NK-κB, caspases, cytokines, angiogenic regulators, etc.[25, 114].
Clearly, there is no single botanical that can be considered efficacious or even safe for all cancer patients. Further, as indicated by Cassileth, et al., there are significant interactions between botanicals and anti-cancer pharmaceuticals that can appreciably decrease the potency of the drugs or even worsen the cancers[115]. Also, each botanical seems to act uniquely in the setting of each cancer[115]. Thus, an agent that protects against one cancer may potentiate another. Further, many of the botanicals act differently depending on circulating hormones or sex steroid sensitivity of the cancer. Better differentiation of the genotype and phenotype of each cancer should greatly improve the effective use of botanicals as complimentary agents in the prevention and amelioration of cancer.
The brain
As longevity dramatically increases in the population, the number of individuals displaying neurodegenerative diseases is greatly increasing. Most of these diseases like Alzheimer's disease (AD) and Parkinson disease (PD) are relatively slow in onset and progression in the over 65-year-old population, but once these individuals become incapacitated from normal competencies of everyday life, they become both a massive burden to the caregiver(s) and a tremendous economic burden, in that the caregiver often must exit the workforce and long-term care typically moves to institutional care as the individual progresses into late stages of the disease. Many of these patients are otherwise healthy, and thus live with AD and to a lesser extent PD for a decade or more. Thus, any mechanism that could delay the onset of neurodegenerative disease or decrease its severity would greatly benefit the health and economic welfare of everyone.
Perhaps the best-publicized example of polyphenols protecting the brain comes from the work of James Joseph and colleagues on blueberries. That work demonstrates that feeding aged rodents blueberries or strawberries significantly reverses age-related motor and cognitive dysfunction[116-118]. While these effects were initially considered to be the result of antioxidative actions of the botanicals, the effects of the botanicals were greater than would that produced by antioxidant treatment alone[116]. These treatments appear to both delay and reverse the age-related decline of rodent brain function.
Compared to their premenopausal counterparts, postmenopausal women are at relatively higher risk for cognitive impairment. The standard therapy of a decade ago promoted the pharmaceutical replacement of the lost hormones in these women; however, extensive studies have demonstrated that long-term use of HRT is associated with significant adverse effects in many women[41, 119], (see[120] for counterpoint). In contrast, dietary polyphenols appear to provide some of the beneficial effects of hormone replacement therapy without appreciable adverse effects.
Grapes (Vitis vinifera) are one of the most widely consumed fruits worldwide and are rich in polyphenols. Grape seed polyphenols have antihypertensive and cognitive enhancement effects in ovariectomized, aged female rodents similar to the effects of several other polyphenols[57]. While the mechanism(s) remains unclear, several lines of evidence suggest that they do not primarily act via estrogen receptor binding[121-123]. Grape seed polyphenols are powerful antioxidants with greater potency than vitamin E and C[124], and upregulation of reactive oxygen species (ROS) appears to play an important role in some forms of cardiovascular diseases, including hypertension, e.g., [125-127]. Grape seed proanthrocyanidins can significantly reduce vascular endothelial superoxide production in the body and importantly in the brain.
Treatment of aging normotensive rats with angiotensin converting enzyme inhibitors improves memory despite negligible effects on arterial pressure[128]. and chronic antihypertensive treatment of hypertensive rats with hydralazine does not improve their age-related memory impairment [129]. Thus, it seems unlikely that arterial pressure is the only factor underlying the improved spatial learning performance of the rats supplemented with grape seed or other isoflavones.
Potential toxicities and quality control of dietary supplements
While there is an increased interest in the use of flavonoids alone or in combination with other medicines, there is a possibility of botanical-drug interactions. For example, silybin- a flavonoid from milk thistel inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic enzymes glucuronosyltransferases [130]. When this compound is taken alone or in dietary supplements in high doses, there is a concern of potential drug-drug interaction. Similarly, G. biloba extract or supplements containing quercetin or kaempferol may inhibits intestinal or hepatic glucuronidation of mycophenolic acid [131]. Kava-kava (Piper methysticum) is well know for its hepato-toxicity, and flavonoid C-glycosides isolated from Kava extract show mutagenic effects [132]. These results indicate that some dietary flavonoids may have the potential to negatively interact with clinical drugs.
Toxicity associated with botanical dietary supplements in part, may result from fungal or bacterial contamination or contamination with pesticides, herbicides, and heavy metals. There are plethora of botanical products are being marketed in the United States with little or no regulation regarding their validation of chemical composition and efficacy [3]. Proper identification and quantification of major bioactive principles is crucial for preclinical and clinical application of dietary supplements. Studies by Prasain, et al., indicated that some manufactures of kudzu dietary supplements did not state puerarin as the most abundant compound; the compound assumed to be daidzin was in fact puerarin [133].
Summary
Flavonoids show promise as useful adjuvants to prevent, delay and/or ameliorate several chronic diseases in aging humans. Recent studies have elucidated their bioavailability, a first step in understanding their mechanisms of action. In vivo experiments have demonstrated that they are effective in reducing disease burden in humans and in animal models of disease, including cancer, cardiovascular disease, cognitive impairments. Perhaps the greatest future impact of botanicals in age-related disease will be related to reducing the impact of the three major contributors to the metabolic syndrome, since each of them is very closely linked to diet (e.g., excess fat and carbohydrate ingestion). Studies are also elucidating the mechanisms by which botanicals act. However, not all botanicals are effective nor are they all beneficial for all aged individuals. Given the high percentage of aging individuals regularly taking these flavonoids, it is incumbent on the medical community to more clearly understand the benefits, adverse effects and related dosing and timing issues, so that flavonoids can compliment other mechanisms to increase patient health.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant AT 00477 from the National Center for Complementary and Alternative Medicine (to J.M.W.) and the Office of Dietary Supplements and Grants NS 041071 (to J.M.W.) and NS 047466 and NS 057098 (to J.M.W.) from the National Institute of Neurological Disorders and Stroke. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements or the NIH.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002 2. Adv Data. 2004 May 27;(343):1–19. [PubMed] [Google Scholar]
- 2.Radimer KL. National nutrition data: contributions and challenges to monitoring dietary supplement use in women 285. J Nutr. 2003 Jun;133(6):2003S–7S. doi: 10.1093/jn/133.6.2003S. [DOI] [PubMed] [Google Scholar]
- 3.van Breemen RB, Fong HH, Farnsworth NR. Ensuring the safety of botanical dietary supplements 7. Am J Clin Nutr. 2008 Feb;87(2):509S–13S. doi: 10.1093/ajcn/87.2.509S. [DOI] [PubMed] [Google Scholar]
- 4.Weaver CM, Barnes S, Wyss JM, et al. Botanicals for age-related diseases: from field to practice 2. Am J Clin Nutr. 2008 Feb;87(2):493S–7S. doi: 10.1093/ajcn/87.2.493S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony MS, Clarkson TB, Hughes CL, Jr, Morgan TM, Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys 4. J Nutr. 1996 Jan;126(1):43–50. doi: 10.1093/jn/126.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Barnes S, Grubbs C, Setchell KD, Carlson J. Soybeans inhibit mammary tumors in models of breast cancer 6. Prog Clin Biol Res. 1990;347:239–53. [PubMed] [Google Scholar]
- 7.Hedlund TE, Maroni PD, Ferucci PG, et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men 2. J Nutr. 2005 Jun;135(6):1400–6. doi: 10.1093/jn/135.6.1400. [DOI] [PubMed] [Google Scholar]
- 8.Harkness LS, Fiedler K, Sehgal AR, Oravec D, Lerner E. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women 1. J Womens Health (Larchmt) 2004 Nov;13(9):1000–7. doi: 10.1089/jwh.2004.13.1000. [DOI] [PubMed] [Google Scholar]
- 9.Arjmandi BH, Alekel L, Hollis BW, et al. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis 1. J Nutr. 1996 Jan;126(1):161–7. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Miech RA, Kim J, McConnell C, Hamman RF. A growing disparity in diabetes-related mortality U.S. trends, 1989-2005. Am J Prev Med. 2009 Feb;36(2):126–32. doi: 10.1016/j.amepre.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence DB, Allison W, Chen JC, Demand M. Improving medication adherence with a targeted, technology-driven disease management intervention. Dis Manag. 2008 Jun;11(3):141–4. doi: 10.1089/dis.2007.0013. [DOI] [PubMed] [Google Scholar]
- 12.Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein11. Nutr Cancer. 1993;20(1):1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- 13.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro 3. Nutr Cancer. 1997;27(1):31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 14.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations 6. Scand J Clin Lab Invest Suppl. 1990;201:3–23. [PubMed] [Google Scholar]
- 15.Peterson G, Barnes S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells 16. Cell Growth Differ. 1996 Oct;7(10):1345–51. [PubMed] [Google Scholar]
- 16.Piskula MK, Yamakoshi J, Iwai Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach 2. FEBS Lett. 1999 Mar 26;447(2-3):287–91. doi: 10.1016/s0014-5793(99)00307-5. [DOI] [PubMed] [Google Scholar]
- 17.Setchell KD. Soy isoflavones--benefits and risks from nature's selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001 Oct;20(5 Suppl):354S–62S. doi: 10.1080/07315724.2001.10719168. [DOI] [PubMed] [Google Scholar]
- 18.Ader P, Block M, Pietzsch S, Wolffram S. Interaction of quercetin glucosides with the intestinal sodium/glucose co-transporter (SGLT-1) Cancer Lett. 2001 Jan 26;162(2):175–80. doi: 10.1016/s0304-3835(00)00645-5. [DOI] [PubMed] [Google Scholar]
- 19.Cermak R, Landgraf S, Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br J Nutr. 2004 Jun;91(6):849–55. doi: 10.1079/BJN20041128. [DOI] [PubMed] [Google Scholar]
- 20.Vedavanam K, Srijayanta S, O'Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE) 1. Phytother Res. 1999 Nov;13(7):601–8. doi: 10.1002/(sici)1099-1573(199911)13:7<601::aid-ptr550>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Cave NJ, Backus RC, Marks SL, Klasing KC. The bioavailability and disposition kinetics of genistein in cats 2. J Vet Pharmacol Ther. 2007 Aug;30(4):327–35. doi: 10.1111/j.1365-2885.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 22.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats 1. J Nutr. 1997 Jul;127(7):1260–8. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 23.Prasain JK, Xu J, Kirk M, Smith JM, Sfakianos J, Barnes S. Differential biliary excretion of genistein metabolites following intraduodenal and intravenous infusion of genistin in female rats 1. J Nutr. 2006 Dec;136(12):2975–9. doi: 10.1093/jn/136.12.2975. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements11. J Nutr. 2001 Apr;131(4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 25.Prasain JK, Barnes S. Metabolism and bioavailability of flavonoids in chemoprevention: current analytical strategies and future prospectus 5. Mol Pharm. 2007 Nov;4(6):846–64. doi: 10.1021/mp700116u. [DOI] [PubMed] [Google Scholar]
- 26.Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004 Jun 16;52(12):3708–12. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- 27.Prasain JK, Peng N, Moore R, Arabshahi A, Barnes S, Wyss JM. Tissue distribution of puerarin and its conjugated metabolites in rats assessed by liquid chromatography-tandem mass spectrometry. Phytomedicine. 2009 Jan;16(1):65–71. doi: 10.1016/j.phymed.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Soya--a dietary source of the non-steroidal oestrogen equol in man and animals 1. J Endocrinol. 1984 Jul;102(1):49–56. doi: 10.1677/joe.0.1020049. [DOI] [PubMed] [Google Scholar]
- 29.Setchell KD, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women 1. J Nutr. 2009 Nov;139(11):2037–43. doi: 10.3945/jn.109.110874. [DOI] [PubMed] [Google Scholar]
- 30.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life 1. Am J Clin Nutr. 1998 Dec;68(6 Suppl):1453S–61S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 31.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones 2. J Nutr. 2002 Dec;132(12):3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 32.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians 1. J Nutr. 2006 Aug;136(8):2188–93. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 33.Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes 278. Curr Opin Endocrinol Diabetes Obes. 2008 Feb;15(1):21–9. doi: 10.1097/MED.0b013e3282f43d19. [DOI] [PubMed] [Google Scholar]
- 34.Nelson RA, Bremer AA. Insulin Resistance and Metabolic Syndrome in the Pediatric Population 279. Metab Syndr Relat Disord. 2009 Nov 29; doi: 10.1089/met.2009.0068. [DOI] [PubMed] [Google Scholar]
- 35.Hoerger TJ, Ahmann AJ. The impact of diabetes and associated cardiometabolic risk factors on members: strategies for optimizing outcomes 280. J Manag Care Pharm. 2008 Feb;14(1 Suppl C):S2–14. doi: 10.18553/jmcp.2008.14.S1-B.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henneman P, Janssens AC, Zillikens MC, et al. Menopause impacts the relation of plasma adiponectin levels with the metabolic syndrome 281. J Intern Med. 2009 Aug 26; doi: 10.1111/j.1365-2796.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 37.Ledingham JM. The vascular fault in hypertension: Byrom's work revisited. Hypertension: Pathophysiology, Diagnosis, and Management. Second. Raven Press Ltd.; New York: 1995. pp. 1pp. 37–53. [Google Scholar]
- 38.Wilson PWF, Kannel WB. Hypertension: Pathophysiology, Diagnosis, and Management. Second. Raven Press Ltd.; New York; 1995. Hypertension, other risk factors, and the risk of cardiovascular disease; pp. 1pp. 99–114. [Google Scholar]
- 39.Cohen E, Wheat ME, Swiderski DM, Charney P. Hypertension in women. Hypertension: Pathophysiology, Diagnosis, and Management. Second. Raven Press Ltd.; New York: 1995. pp. 1pp. 159–69. [Google Scholar]
- 40.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease 282. Clin Res Cardiol. 2006 Mar;95(3):136–47. doi: 10.1007/s00392-006-0351-5. [DOI] [PubMed] [Google Scholar]
- 41.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial 1. JAMA. 2002 Jul 17;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 42.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Association of blood pressure with intake of soy products and other food groups in Japanese men and women 235. Prev Med. 2003 Jun;36(6):692–7. doi: 10.1016/s0091-7435(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura M, Aoki N, Yamada T, Kubo N. Feasibility and effect on blood pressure of 6-week trial of low sodium soy sauce and miso (fermented soybean paste) 236. Circ J. 2003 Jun;67(6):530–4. doi: 10.1253/circj.67.530. [DOI] [PubMed] [Google Scholar]
- 44.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007 May 28;167(10):1060–7. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta 243. Endocrinology. 1997 Mar;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 46.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta 244. Endocrinology. 1998 Oct;139(10):4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 47.Liang YL, Teede H, Dalais F, McGrath BP. [The effects of phytoestrogen on blood pressure and lipids in healthy volunteers] 97. Zhonghua Xin Xue Guan Bing Za Zhi. 2006 Aug;34(8):726–9. [PubMed] [Google Scholar]
- 48.Rivas M, Garay RP, Escanero JF, Cia P, Jr, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension 155. J Nutr. 2002 Jul;132(7):1900–2. doi: 10.1093/jn/132.7.1900. [DOI] [PubMed] [Google Scholar]
- 49.Mahn K, Borras C, Knock GA, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo 118. FASEB J. 2005 Oct;19(12):1755–7. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 50.Li P, Ferrario CM, Ganten D, Brosnihan KB. Chronic estrogen treatment in female transgenic (mRen2)27 hypertensive rats augments endothelium-derived nitric oxide release. American Journal of Hypertension. 1997 Jun;10(6):662–70. doi: 10.1016/s0895-7061(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 51.Calhoun DA, Oparil S. The Sexual Dimorphism of High Blood Pressure 318. Cardiol Rev. 1998 Nov;6(6):356–63. doi: 10.1097/00045415-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Fang Z, Carlson SH, Chen YF, Oparil S, Wyss JM. Estrogen depletion induces NaCl-sensitive hypertension in female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001 Dec;281(6):R1934–R1939. doi: 10.1152/ajpregu.2001.281.6.R1934. [DOI] [PubMed] [Google Scholar]
- 53.Cho TM, Peng N, Clark JT, et al. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology. 2007 Nov;148(11):5396–402. doi: 10.1210/en.2007-0245. [DOI] [PubMed] [Google Scholar]
- 54.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols 2375. Nutr Res. 2008 Nov;28(11):729–37. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease 1842. Lancet. 1992 Jun 20;339(8808):1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 56.Freedman JE, Parker C, III, Li L, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release 2376. Circulation. 2001 Jun 12;103(23):2792–8. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 57.Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005 Sep;289(3):R771–R775. doi: 10.1152/ajpregu.00147.2005. [DOI] [PubMed] [Google Scholar]
- 58.Soares De MR, Costa Viana FS, Souza MA, et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract 2379. J Pharm Pharmacol. 2002 Nov;54(11):1515–20. doi: 10.1211/002235702153. [DOI] [PubMed] [Google Scholar]
- 59.Peng N, Prasain J, Dai Y, et al. Chronic Dietary Kudzu Isoflavones Improve Components of Metabolic Syndrome in Stroke-Prone Spontaneously Hypertensive Rats. J Agric Food Chem. 2009;57:7268–73. doi: 10.1021/jf901169y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids 2380. Am J Cardiol. 2009 Oct 1;104(7):947–56. doi: 10.1016/j.amjcard.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 61.McGowan MP, Proulx S. Nutritional supplements and serum lipids: does anything work? 275. Curr Atheroscler Rep. 2009 Nov;11(6):470–6. doi: 10.1007/s11883-009-0070-2. [DOI] [PubMed] [Google Scholar]
- 62.Charland SL, Malone DC. Prediction of cardiovascular event risk reduction from lipid changes associated with high potency dyslipidemia therapy 276. Curr Med Res Opin. 2009 Dec 9; doi: 10.1185/03007990903484802. [DOI] [PubMed] [Google Scholar]
- 63.Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial 2381. Ann Intern Med. 2009 Jun 16;150(12):830–9. doi: 10.7326/0003-4819-150-12-200906160-00006. [DOI] [PubMed] [Google Scholar]
- 64.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995 Aug 3;333(5):276–82. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 65.Demonty I, Lamarche B, Jones PJ. Role of isoflavones in the hypocholesterolemic effect of soy. Nutr Rev. 2003 Jun;61(6 Pt 1):189–203. doi: 10.1301/nr.2003.jun.189-203. [DOI] [PubMed] [Google Scholar]
- 66.Demonty I, Lamarche B, Deshaies Y, Jacques H. Role of soy isoflavones in the hypotriglyceridemic effect of soy protein in the rat. J Nutr Biochem. 2002 Nov;13(11):671–7. doi: 10.1016/s0955-2863(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 67.Merz-Demlow BE, Duncan AM, Wangen KE, et al. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000 Jun;71(6):1462–9. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- 68.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001 Feb;73(2):225–31. doi: 10.1093/ajcn/73.2.225. [DOI] [PubMed] [Google Scholar]
- 69.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007 Apr;85(4):1148–56. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 70.Allen JK, Becker DM, Kwiterovich PO, Lindenstruth KA, Curtis C. Effect of soy protein-containing isoflavones on lipoproteins in postmenopausal women. Menopause. 2007 Jan;14(1):106–14. doi: 10.1097/01.gme.0000229572.21635.49. [DOI] [PubMed] [Google Scholar]
- 71.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007 May 28;167(10):1060–7. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 72.Merz-Demlow BE, Duncan AM, Wangen KE, et al. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000 Jun;71(6):1462–9. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- 73.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001 Feb;73(2):225–31. doi: 10.1093/ajcn/73.2.225. [DOI] [PubMed] [Google Scholar]
- 74.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007 Apr;85(4):1148–56. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 75.Allen JK, Becker DM, Kwiterovich PO, Lindenstruth KA, Curtis C. Effect of soy protein-containing isoflavones on lipoproteins in postmenopausal women. Menopause. 2007 Jan;14(1):106–14. doi: 10.1097/01.gme.0000229572.21635.49. [DOI] [PubMed] [Google Scholar]
- 76.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007 May 28;167(10):1060–7. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 77.Crawford P, Paden SL, Park MK. Clinical inquiries: What is the dietary treatment for low HDL cholesterol? J Fam Pract. 2006 Dec;55(12):1076–8. [PubMed] [Google Scholar]
- 78.Allison DB, Gadbury G, Schwartz LG, et al. A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial 2384. Eur J Clin Nutr. 2003 Apr;57(4):514–22. doi: 10.1038/sj.ejcn.1601587. [DOI] [PubMed] [Google Scholar]
- 79.Orgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood) 2008 Sep;233(9):1066–80. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- 80.Saremi A, Arora R. The cardiovascular implications of alcohol and red wine 2386. Am J Ther. 2008 May;15(3):265–77. doi: 10.1097/MJT.0b013e3180a5e61a. [DOI] [PubMed] [Google Scholar]
- 81.de GG, Cerletti C. Wine and cardiovascular disease 2387. Nutr Metab Cardiovasc Dis. 2001 Aug;11(4 Suppl):47–50. [PubMed] [Google Scholar]
- 82.Hansen AS, Marckmann P, Dragsted LO, Finne NI, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease 2388. Eur J Clin Nutr. 2005 Mar;59(3):449–55. doi: 10.1038/sj.ejcn.1602107. [DOI] [PubMed] [Google Scholar]
- 83.Tsang C, Higgins S, Duthie GG, et al. The influence of moderate red wine consumption on antioxidant status and indices of oxidative stress associated with CHD in healthy volunteers 2389. Br J Nutr. 2005 Feb;93(2):233–40. doi: 10.1079/bjn20041311. [DOI] [PubMed] [Google Scholar]
- 84.Nigdikar SV, Williams NR, Griffin BA, Howard AN. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo 2840. Am J Clin Nutr. 1998 Aug;68(2):258–65. doi: 10.1093/ajcn/68.2.258. [DOI] [PubMed] [Google Scholar]
- 85.Guarda E, Godoy I, Foncea R, et al. Red wine reduces oxidative stress in patients with acute coronary syndrome 2841. Int J Cardiol. 2005 Sep 15;104(1):35–8. doi: 10.1016/j.ijcard.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Pignatelli P, Ghiselli A, Buchetti B, et al. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine 2842. Atherosclerosis. 2006 Sep;188(1):77–83. doi: 10.1016/j.atherosclerosis.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 87.Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans 2843. Nutr J. 2007;6:27. doi: 10.1186/1475-2891-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ou HC, Chou FP, Sheen HM, Lin TM, Yang CH, Huey-Herng SW. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells 2848. Clin Chim Acta. 2006 Feb;364(1-2):196–204. doi: 10.1016/j.cccn.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. Effects of chronic alcohol consumption on lipid levels, inflammatory and haemostatic factors in the general population: the ‘ATTICA’ Study 1772. Eur J Cardiovasc Prev Rehabil. 2003 Oct;10(5):355–61. doi: 10.1097/01.hjr.0000065928.57001.4d. [DOI] [PubMed] [Google Scholar]
- 90.Spaak J, Merlocco AC, Soleas GJ, et al. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter 2846. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H605–H612. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- 91.Shane-McWhorter L. Botanical dietary supplements and the treatment of diabetes: what is the evidence? 2411. Curr Diab Rep. 2005 Oct;5(5):391–8. doi: 10.1007/s11892-005-0099-8. [DOI] [PubMed] [Google Scholar]
- 92.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis 2403. Am J Physiol Endocrinol Metab. 2008 Jan;294(1):E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cefalu WT, Ye J, Zuberi A, et al. Botanicals and the metabolic syndrome. Am J Clin Nutr. 2008 Feb;87(2):481S–7S. doi: 10.1093/ajcn/87.2.481S. [DOI] [PubMed] [Google Scholar]
- 94.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance 2775. Diabetes Obes Metab. 2009 Dec;11(12):1100–13. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 95.Crawford P, Paden SL, Park MK. Clinical inquiries: What is the dietary treatment for low HDL cholesterol? J Fam Pract. 2006 Dec;55(12):1076–8. [PubMed] [Google Scholar]
- 96.Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008;2008:581348. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin B, Polansky MM, Anderson RA. Cinnamon Extract Regulates Plasma Levels of Adipose-derived Factors and Expression of Multiple Genes Related to Carbohydrate Metabolism and Lipogenesis in Adipose Tissue of Fructose-fed Rats. Horm Metab Res. 2009 Nov 23; doi: 10.1055/s-0029-1242746. [DOI] [PubMed] [Google Scholar]
- 98.Nahas R, Moher M. Complementary and alternative medicine for the treatment of type 2 diabetes 2782. Can Fam Physician. 2009 Jun;55(6):591–6. [PMC free article] [PubMed] [Google Scholar]
- 99.Yamaguchi M. Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis 1. Yakugaku Zasshi. 2006 Nov;126(11):1117–37. doi: 10.1248/yakushi.126.1117. [DOI] [PubMed] [Google Scholar]
- 100.Messina M, Ho S, Alekel DL. Skeletal benefits of soy isoflavones: a review of the clinical trial and epidemiologic data 1. Curr Opin Clin Nutr Metab Care. 2004 Nov;7(6):649–58. doi: 10.1097/00075197-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 101.Atteritano M, Marini H, Minutoli L, et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study 1. J Clin Endocrinol Metab. 2007 Aug;92(8):3068–75. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 102.Marini H, Minutoli L, Polito F, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial 2. Ann Intern Med. 2007 Jun 19;146(12):839–47. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 103.Weaver CM, Martin BR, Jackson GS, et al. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using (41)Ca methodology 1. J Clin Endocrinol Metab. 2009 Oct;94(10):3798–805. doi: 10.1210/jc.2009-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reinwald S, Weaver CM. Soy isoflavones and bone health: a double-edged sword? 3. J Nat Prod. 2006 Mar;69(3):450–9. doi: 10.1021/np058104g. [DOI] [PubMed] [Google Scholar]
- 105.Bittner V. Menopause and cardiovascular risk cause or consequence? 10. J Am Coll Cardiol. 2006 May 16;47(10):1984–6. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 106.Deng G, Cassileth BR. To what extent do cancer patients use complementary and alternative medicine? Nat Clin Pract Oncol. 2005 Oct;2(10):496–7. doi: 10.1038/ncponc0317. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki T, Matsuo K, Tsunoda N, et al. Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. Int J Cancer. 2008 Oct 1;123(7):1674–80. doi: 10.1002/ijc.23644. [DOI] [PubMed] [Google Scholar]
- 108.Dai Q, Shu XO, Jin F, et al. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer. 2001 Aug 3;85(3):372–8. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Touillaud MS, Pillow PC, Jakovljevic J, et al. Effect of dietary intake of phytoestrogens on estrogen receptor status in premenopausal women with breast cancer. Nutr Cancer. 2005;51(2):162–9. doi: 10.1207/s15327914nc5102_6. [DOI] [PubMed] [Google Scholar]
- 110.Allred CD, Ju YH, Allred KF, Chang J, Helferich WG. Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis. 2001 Oct;22(10):1667–73. doi: 10.1093/carcin/22.10.1667. [DOI] [PubMed] [Google Scholar]
- 111.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001 Jul 1;61(13):5045–50. [PubMed] [Google Scholar]
- 112.Yang G, Shu XO, Li H, et al. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2007 Jun;16(6):1219–23. doi: 10.1158/1055-9965.EPI-07-0097. [DOI] [PubMed] [Google Scholar]
- 113.Howells LM, Mitra A, Manson MM. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int J Cancer. 2007 Jul 1;121(1):175–83. doi: 10.1002/ijc.22645. [DOI] [PubMed] [Google Scholar]
- 114.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins 4. Cancer Lett. 2008 Oct 8;269(2):378–87. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cassileth BR, Heitzer M, Wesa K. The Public Health Impact of Herbs and Nutritional Supplements. Pharm Biol. 2009 Aug 1;47(8):761–7. doi: 10.1080/13880200902991581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation 12. J Neurosci. 1999 Sep 15;19(18):8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coleman P, Finch C, Joseph J. The need for multiple time points in aging studies [editorial] Neurobiol Aging. 1990 Jan;11(1):1–2. doi: 10.1016/0197-4580(90)90055-5. [DOI] [PubMed] [Google Scholar]
- 118.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits 14. J Neurosci. 1998 Oct 1;18(19):8047–55. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wharton W, Dowling M, Khosropour CM, Carlsson C, Asthana S, Gleason CE. Cognitive benefits of hormone therapy: cardiovascular factors and healthy-user bias 1. Maturitas. 2009 Nov 20;64(3):182–7. doi: 10.1016/j.maturitas.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy 2. J Clin Endocrinol Metab. 2009 Nov;94(11):4152–61. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eng ET, Ye J, Williams D, et al. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res. 2003 Dec 1;63(23):8516–22. [PubMed] [Google Scholar]
- 122.Agarwal C, Sharma Y, Zhao J, Agarwal R. A polyphenolic fraction from grape seeds causes irreversible growth inhibition of breast carcinoma MDA-MB468 cells by inhibiting mitogen-activated protein kinases activation and inducing G1 arrest and differentiation. Clin Cancer Res. 2000 Jul;6(7):2921–30. [PubMed] [Google Scholar]
- 123.Sharma G, Tyagi AK, Singh RP, Chan DC, Agarwal R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat. 2004 May;85(1):1–12. doi: 10.1023/B:BREA.0000020991.55659.59. [DOI] [PubMed] [Google Scholar]
- 124.Aldini G, Carini M, Piccoli A, Rossoni G, Facino RM. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sci. 2003 Oct 17;73(22):2883–98. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- 125.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003 Jun;41(6):1346–52. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 126.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004 May 18;109(19):2357–62. doi: 10.1161/01.CIR.0000128695.49900.12. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Croft KD, Mori TA, Schyvens CG, McKenzie KU, Whitworth JA. The antioxidant tempol prevents and partially reverses dexamethasone-induced hypertension in the rat. Am J Hypertens. 2004 Mar;17(3):260–5. doi: 10.1016/j.amjhyper.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 128.Wyss JM, Kadish I, Van Groen T. Age-related decline in spatial learning and memory: attenuation by captopril. Clin Exp Hypertens. 2003 Oct;25(7):455–74. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- 129.Wyss JM, Kadish I, Van Groen T. Age-related decline in spatial learning and memory: attenuation by captopril. Clin Exp Hypertens. 2003 Oct;25(7):455–74. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- 130.Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases 2. Drug Metab Dispos. 2004 Jun;32(6):587–94. doi: 10.1124/dmd.32.6.587. [DOI] [PubMed] [Google Scholar]
- 131.Mohamed ME, Frye RF. Inhibition of intestinal and hepatic glucuronidation of mycophenolic acid by Ginkgo biloba extract and flavonoids 1. Drug Metab Dispos. 2009 Nov 4; doi: 10.1124/dmd.109.030080. [DOI] [PubMed] [Google Scholar]
- 132.Jhoo JW, Ang CY, Heinze TM, et al. Identification of C-glycoside flavonoids as potential mutagenic compounds in kava 1. J Food Sci. 2007 Mar;72(2):C120–C125. doi: 10.1111/j.1750-3841.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 133.Prasain JK, Jones K, Kirk M, et al. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2003 Jul 16;51(15):4213–8. doi: 10.1021/jf030174a. [DOI] [PubMed] [Google Scholar]