Abstract
A dysregulation of the mesolimbic dopamine system in schizophrenia patients may lead to aberrant attribution of incentive salience and contribute to the emergence of psychopathological symptoms like delusions. The dopaminergic signal has been conceptualized to represent a prediction error that indicates the difference between received and predicted reward. The incentive salience hypothesis states that dopamine mediates the attribution of “incentive salience” to conditioned cues that predict reward. This hypothesis was initially applied in the context of drug addiction and then transferred to schizophrenic psychosis. It was hypothesized that increased firing (chaotic or stress associated) of dopaminergic neurons in the striatum of schizophrenia patients attributes incentive salience to otherwise irrelevant stimuli. Here, we review recent neuroimaging studies directly addressing this hypothesis. They suggest that neuronal functions associated with dopaminergic signaling, such as the attribution of salience to reward-predicting stimuli and the computation of prediction errors, are indeed altered in schizophrenia patients and that this impairment appears to contribute to delusion formation.
Keywords: psychosis, delusion, reward, ventral striatum, fMRI, FDOPA
It has long been suggested that dopamine dysfunction plays a major role in the pathogenesis of schizophrenic psychosis.1 First studies with positron emission tomography (PET) suggested that dopamine D2 receptors are indeed upregulated in schizophrenia patients2; however, this finding was not confirmed in further studies.3 Also, studies on dopamine D2 receptor genotype failed to find an association between functional variance and schizophrenia.4 It was not until 1996 that in vivo imaging studies first found convincing evidence of dopamine dysregulation in acute psychosis: Laruelle et al5 and Breier et al6 used the fact that radioligands, such as raclopride, are displaced by endogenous dopamine due to competition for binding at dopamine D2 receptors (figure 1 and figure 2). They applied psychostimulants to release dopamine and observed increased displacement of dopamine D2 receptor ligands in unmedicated schizophrenia patients compared with healthy controls, suggesting that the pool of releasable dopamine is increased in patients suffering from schizophrenic psychosis. This hypothesis was further supported by studies using the radioligand [18F]fluoro-3,4-dihydroxyphenyl-L-alanine (FDOPA), which is absorbed by dopaminergic neurons and metabolized into dopamine and subsequently stored in the presynaptic terminal. Studies with this radioligand suggested increased striatal dopamine synthesis capacity in unmedicated schizophrenia patients8–10; a recent study by Kumakura et al11 used a refined technology that takes into account that presynaptical dopamine is not simply trapped but instead released into the extracellular space in association with neuronal activation. This study found considerable differences in dopamine storage capacity between schizophrenia patients and healthy controls in the striatum and other limbic areas, such as the amygdala. However, such studies point to increased presynaptic dopamine storage and do not necessarily address whether dopamine is definitely released in higher amounts in vivo in unmedicated schizophrenia patients. A landmark study directly addressing this question was published by Abi-Dargham and colleagues,12 who again used the fact that dopamine D2 receptor radioligands are displaced by endogenous dopamine. The groups of Abi-Dargham and Laruelle depleted dopamine by applying the drug alphamethyl-paratyrosine, thus reducing extracellular dopamine levels, and observed a larger increase (higher level) in dopamine D2 receptor radioligand binding (of unoccupied D2 receptors) in schizophrenia patients compared with healthy controls (figure 3). These findings suggest that extracellular dopamine levels are indeed increased by about 10%–20% in the striatum of unmedicated schizophrenia patients and that this increase is associated with the severity of positive symptoms.12 However, how could striatal dopamine induce complex positive symptoms such as delusions, acoustic hallucinations, or phenomena such as thought insertion?
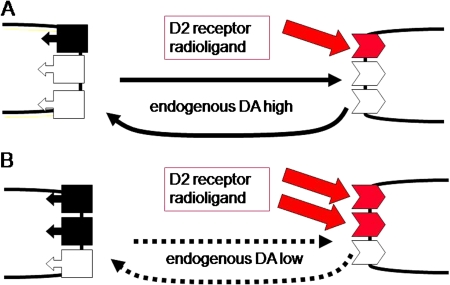
Fig. 1.
Blockade of Radioligand Binding by Endogenous Neurotransmitter. Competition between radioligand binding to dopamine D2 receptors and endogenous dopamine can be used to measure dopamine concentrations in vivo. (A) Presynaptic dopamine release is depicted on the left side (with dopamine transporters for reuptake), and postsynaptic dopamine D2 receptors are indicated by boxes on the right side. High endogenous dopamine release blocks a considerable amount of dopamine D2 receptors, which then cannot bind a radioligand tracer (eg, the neuroleptic drug [123I]Iodobenzamid [IBZM]), resulting in low radioligand binding. (B) Reduced dopamine release (eg, due to blockade of dopamine synthesis) decreases dopamine concentrations and results in less binding of endogenous dopamine to D2 receptors, which can now be marked by radioligand tracers. Alterations in radioligand binding can be quantified and reflect changes in endogenous dopamine concentrations.
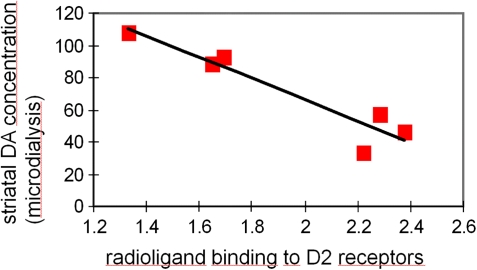
Fig. 2.
Proof of Concept That Alterations in Radioligand Binding Can Reflect Endogenous Neurotransmitter Concentrations. A direct negative correlation can be observed between radioligand binding to dopamine D2 receptors (measured with the neuroleptic radioligand tracer IBZM) and endogenous dopamine concentrations (measured with microdialysis) following prefrontal stimulation with amphetamine to mimic stress effects in nonhuman primates (7).
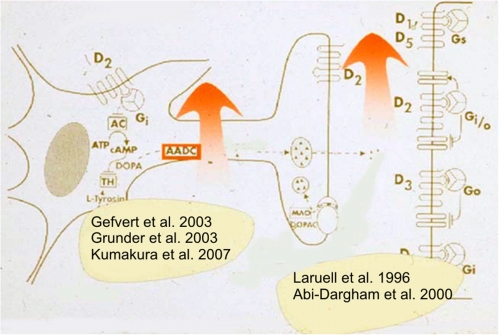
Fig. 3.
Dopamine Dysfunction in Schizophrenia. A series of studies using positron emission tomography (PET) indicated that presynaptic dopamine synthesis and extracellular dopamine concentrations are increased in unmedicated patients suffering from acute schizophrenia.5,9,11–13
The Incentive Salience Hypothesis: Created Within an Addiction Model and Transferred into Schizophrenia Research
Dopamine dysfunction has also been suggested to play a prominent role in addictive disorders, particularly because it is known that all drugs of abuse induce dopamine release in the ventral striatum, which is thought to reinforce behaviors that elicited dopamine release.14,15 Originally, it was suggested that dopamine release is directly rewarding and associated with hedonic feelings of pleasure, whereas blockade of dopamine D2 receptors by neuroleptics could induce anhedonia.16 However, further animal research suggested that dopamine release is not directly rewarding but instead reflects an error of reward prediction: According to this hypothesis, dopamine is released whenever an incoming reward exceeds the predicted reward and the positive difference between received and predicted reward is reflected in dopamine firing.17 Likewise, dopamine firing is reduced whenever the outcome is worse than expected.17 Conditioned cues that indicate upcoming reward at a certain time point following their appearance acquires the same ability to elicit a short phasic increase in dopamine firing because again their appearance is unpredicted and exceeds the individuals expectation, whereas a reward that arrives exactly as predicted by the previous conditioned cue will no longer elicit dopamine release because the difference between the incoming and the expected reward is zero (figure 4).17 This latter finding was a cornerstone of the hypothesis that the hedonic pleasure is associated with the consumption of a predicted reward independent of dopamine. Instead, Robinson and Berridge18 suggested that a phasic increase in dopamine reflects a prediction error and is associated with the attribution of “incentive salience” to conditioned cues that predict reward; the individual will thus be motivated to search for this reward. This hypothesis was applied by our group and others19–22 for schizophrenic psychosis, and it was hypothesized that increased chaotic or stress-associated firing of dopaminergic afferents to the striatum of schizophrenia patients attributes increased incentive salience to otherwise irrelevant stimuli. This overattribution of meaning to otherwise irrelevant cues can play a prominent role in early stages of psychosis, particularly when patients develop a delusional mood23 and feel that the world is full of signs that point to a yet unrevealed secret. Related ideas were suggested by Miller,24 who stated that aberrant learning mechanisms play a prominent role in the development of positive symptoms, and by Maher,25 who conceptualized delusional thinking as a consequence of aberrant perceptions due to altered gating of sensory inputs. Particularly, the latter theory fits well with the model proposed here because it was suggested that in the basal ganglia, dopamine plays a decisive role in gating information to the prefrontal cortex (PFC).26 Altogether, these hypotheses suggest that dopamine dysfunction may be particularly prominent during the early stages of schizophrenia before delusional mood is transformed into fixed and rigid patterns of delusional explanatory models; the model implicitly rests on the assumption that dopamine firing can be increased by environmental stress.19,20 How plausible is this idea?
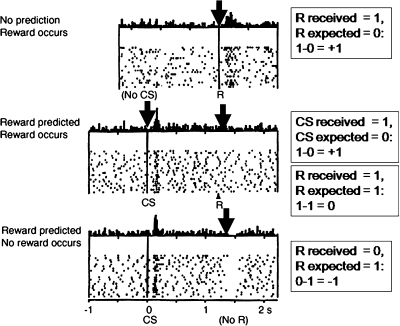
Fig. 4.
Phasic Dopamine Release Reflects an Error of Reward Prediction. Upper part: When no reward is expected, it comes as a surprise and hence the difference between the received and the expected reward (arbitrarily set at 1) is positive, which according to Schultz and coworkers17 is reflected in an increase in dopamine firing. Middle part: A conditioned stimulus that reliably predicts that reward is attributed with incentive salience; whenever it appears unexpectedly, it elicits a phasic dopamine response due to a positive difference between the received and the expected value of the cue. Arrival of the reward itself, on the other hand, does not elicit dopamine firing as long as this reward is fully predicted by the preceding salient stimulus (because the reward received is exactly the same as the expected reward).17 Lower part: When the expected reward fails to be received, the difference between the received and the expected reward is negative, reflected in a phasic decrease of dopamine firing.17
Stress-Induced Dopamine Firing: Animal Findings and Human Observations
In a series of studies in nonhuman primates, Nader and colleagues observed that dopaminergic neurotransmission is indeed affected by social stress factors, such as the presence of dominant competitors or social isolation.27,28 Nonhuman primates in dominant positions showed more dopamine D2 receptor availability than primates in subordinate positions, particularly in males, and the authors suggested that these differences in dopamine D2 receptor availability reflect a low dopamine turnover in the dominant monkeys and an increased dopamine turnover (with increased competition for D2 receptor binding and hence lower dopamine D2 radioligand binding in the striatum; see figures 1 and 2) among subordinate and high-stressed monkeys.27,28
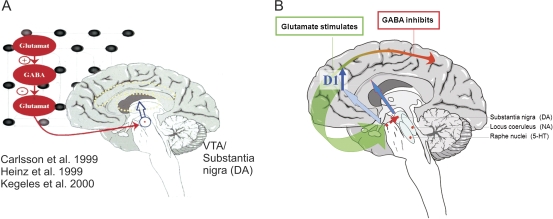
These observations fit well with older accounts of stress effects on in vivo dopamine release in the ventral and dorsal striatum and in the PFC.29 Thus far, direct evidence for altered dopamine release in the PFC of patients suffering from schizophrenia is lacking; however, one study observed that dopamine D1 receptors were upregulated in the dorsolateral PFC of unmedicated schizophrenia patients,30 which may be due to a deficit in (tonic) prefrontal dopamine release.31 Because dopamine D1 receptors in the PFC are thought to stabilize neuronal network representations,32 a lack of overall dopamine input in this brain area may contribute to a dysfunction of the prefrontal neuronal correlates of working memory and other executive functions, and such impairments have regularly been observed in schizophrenia patients.20,33–35 It is possible that altered PFC activation during working memory tasks as observed in functional imaging studies30,34 reflects a dysfunction of dopamine-glutamate interactions, eg, via effects of dopamine D1/5 receptors on the N-methyl-D-aspartic acid (NMDA)-mediated component of excitatory postsynaptic currents of glutamate receptors36 or on local GABAergic (γ-aminobutyric acid) interneurons.37 Such dopamine-glutamate dysfunction in the PFC can interfere with prefrontal-striatal circuits, particularly with glutamatergic projections from the PFC to the ventral tegmental area (VTA) (figure 5).39,40 If this results in reduced glutamatergic input to the VTA, it can further impair overall prefrontal dopamine release.39 Due to differential effects of glutamatergic projection inputs on GABAergic interneurons in the brainstem, the same reduction of glutamatergic input from the PFC can additionally result in increased dopamine release in the ventral and potentially associative (central) striatum (figure 5).41 Indeed, one imaging study observed that reduced prefrontal brain activation during a working memory task was associated with increased striatal dopamine synthesis capacity.42 However, dysfunctional prefrontal activation during a working memory task can of course be associated with a variety of neurotransmitter aberrations and does not necessarily indicate a specific dopamine or glutamate deficit.43–45
Fig. 5.
Increased Dopamine Release in Acute Schizophrenia Is Hypothetically Due To Altered Cortico-Striatal-Thalamic Neurocircuits. Left panel (A): Dysfunction of the interaction between excitatory glutamatergic and inhibitory GABAergic (γ-aminobutyric acid) neurotransmission in cortical brain areas can disinhibit subcortical dopamine release, as suggested by studies using ketamine to block glutamatergic neurotransmission via NMDA receptors in humans and nonhuman primates.1,7,38 Right panel (B): Brain imaging studies suggest that in schizophrenia, low dopamine release in the prefrontal cortex (PFC) results in an upregulation of dopamine D1 receptors,12 which can destabilize information processing and interfere with a glutamatergic projection to midbrain dopamine neurons. According to Sesack and Carr,39 these glutamatergic projections can directly stimulate prefrontal dopamine release but inhibit midbrain dopamine neurons projecting to the striatum (via GABAergic interneurons). Dysfunctional glutamatergic input from the PFC can thus (further) impair prefrontal dopamine release while at the same time disinhibiting striatal dopaminergic neurotransmission. Glutamatergic projections are depicted in green and GABAergic in red; reduced neurotransmission is indicated by shaded and dotted lines.
Nonhuman primate studies suggested that a reduction of prefrontal control of subcortical dopamine release does not necessarily have its origin solely in the PFC. Instead, an early neonatal developmental lesion of the temporal-limbic cortex was associated with reduced prefrontal control of subcortical dopamine release, particularly when primates were medicated with ketamine, an agent that blocks glutamatergic neurotransmission via NMDA receptors. In a study comparing the effect of neonatal vs adult lesions of the temporal-limbic cortex in rhesus monkeys with a group of healthy, age-matched nonhuman primates, only those with neonatal lesions of the temporolimbic cortex showed an increased striatal dopamine release after ketamine application.7 An increase in striatal dopamine release was also observed in healthy human volunteers after ketamine application: The subsequent administration of amphetamine induced a similar increase in striatal dopamine, as found in schizophrenia patients without ketamine application, supporting the hypothesis that schizophrenia patients suffer from a glutamate deficit that affects the function of NMDA receptors.38 Altogether, these studies support the hypothesis that social stress factors increase dopamine release and suggest that developmentally specific disruptions of frontocortical-striatal-thalamic networks may play a role in increasing the vulnerability of individual subjects toward such stress effects.20
Increased Striatal Dopamine Release: Interference with Salience Attribution?
If dopamine release is increased in schizophrenia patients, particularly during early psychotic stages, can this indeed interfere with salience attribution to stimuli that predict reward? A series of studies tried to directly assess this hypothesis (see table 1). Knutson et al54 showed that in healthy volunteers, presentation of a salient stimulus that has reliably been shown to predict reward 1) increases the speed of the motor response to obtain the reward (compared with a neutral stimulus) and 2) evokes a phasic activation of the ventral striatum (figure 6). Of course, a phasic activation of the ventral striatum measured with the BOLD response using functional magnetic resonance imaging (fMRI) appears on a much larger time scale (around 10 s) compared with the phasic increase in dopamine firing suggested by Schultz et al, which ranges in the milliseconds scale. Therefore, fMRI cannot directly assess alterations in phasic dopamine firing but rather measures changes in neuronal network activation, which may reflect a briefer dopaminergic input. Indeed, the application of dopamine D1/D2 receptor agonists has been shown to affect the BOLD response in fMRI.55,56 If the hypothesis is correct, which suggests that schizophrenia patients show a chaotic or stress-induced increase in dopaminergic activation, how could this interfere with functional activation elicited by reward-predicting cues? Knutson et al57 directly addressed this question when they applied psychostimulants to healthy volunteers; the resulting massive increase in striatal dopamine was associated with a “reduced” BOLD response following the presentation of reward-predicting cues. This finding suggests that a strong increase in dopamine release following psychostimulants, which last over a considerable amount of time, may “drown out” the phasic increase in dopamine elicited by reward-predicting cues. If this interpretation is correct, then unmedicated schizophrenia patients with increased striatal dopamine release should also show a blunted, ie, reduced brain activation following the presentation of salient reward-predicting stimuli (figure 7). This hypothesis was tested in a study by Juckel et al,46 who showed that brain activation following reward-predicting stimuli is reduced in unmedicated schizophrenia patients and that the reduction in brain activation is directly associated with motivational dysfunction and other negative symptoms. On the other hand, a high degree of dopamine D2 receptor blockage, which follows relatively high doses of first-generation (typically) neuroleptics, was also associated with a lack of activation of the ventral striatum during the presentation of reward-predicting stimuli,47 whereas switching to a lower dose of second-generation (atypical) neuroleptics to some degree restored activation of the ventral striatum following reward-predicting cues.51 For the first time, these studies showed that a potential correlate of salience attribution to reward-predicting cues, cue-induced functional activation of the ventral striatum, is indeed reduced in unmedicated schizophrenia patients and that a similar dysfunction can be induced by higher doses of antipsychotic drugs that block dopamine D2 receptors. This observation is in accordance with previous studies, which showed that the degree of dopamine D2 receptor blocked in the striatum is directly correlated both with psychomotor slowing and with the negative symptom “apathy,” reflecting a motivational deficit associated with dysfunction of striatal dopaminergic neurotransmission.59
Table 1.
Imaging Studies of Reward Anticipation and Prediction in Schizophrenia Patients Using Functional MRI
| Author | Pub Date | Group | PANSS Total | Paradigm | Analysis | Main Finding | Interpretation |
| Juckel et al46 | 2006 | 10 unmedicated SV (7 drug naive), 10 HC | 92.8 ± 23.7 | Monetary incentive delay task | SPM2, contrast of anticipation of reward, respectably, loss compared with neutral trials, SVC for VS | Reduced VS activation during the presentation of reward-indicating cues in SV compared with HC. Reduced VS activation inversely correlated with psychopathology (significant for negative symptoms and trendwise for positive symptoms) | High striatal DA turnover in unmedicated SV may increase “noise” in the reward system and contribute to psychopathology |
| Juckel et al47 | 2006 | 10 SV with FGAs, 10 SV with SGAs, 10 HC | FGA: 70.1 ± 20.3, SGA: 64.4 ± 22.6 | Monetary incentive delay task | SPM2, contrast of anticipation of reward, resp., loss compared with neutral trials, SVC for VS | HC showed stronger left VS activation compared with SV with FGAs but with SV with SGAs. Reduced VS activation of SV with FGAs was correlated with negative symptoms | Failure to normalize VS reward anticipation may limit effectiveness of FGAs in treating negative symptoms. Efficacy of some SGAs in treating negative symptoms may partly result from their effects on the brain reward system |
| Jensen et al48 | 2007 | 13 medicated SV (11 with SGAs), 13 HC | 61.4 ± 20.6 | Classical passive Pavlovian conditioning task: with aversive (load noise as unconditioned stimulus US) and neutral events (visual display of a star as conditioned stimulus CS), which are associated to cues (CS+, respectively, CS−). | SPM2, modeling of CS+ and CS−. SVC for VS | Patients did not distinguish between aversive and neutral events in subjective ratings and showed lesser galvanic skin response to the CS+; HC > SV for CS− baseline contrast in the R VS, middle cingulate, R thalamus, R PFC, R hippocampus. HC > SV for CS + > CS− in L VS. No correlation with PANSS | Stronger responses to the neutral stimulus may reflect aberrant attribution of motivational salience to neutral stimuli; context-inappropriate associations are reinforced; supports the idea of aberrant conditioning in SV |
| Murray et al49 | 2007 | 13 psychotic patients (1 bipolar) with current psychotic symptoms (5 unmedicated, 8 SGAs), 12 HC | — | Instrumental reward conditioning task | SPM2, prediction error derived from a Q learning algorithm used as regressor for rewarding and neutral feedback. Mask for VS and midbrain | Faster RT in neutral trials in SV > HC; functional activation: HC > SV in VS and midbrain for reward PE compared with neutral PE (attenuated response to reward PE and augmented response to neutral PE in psychosis). No correlation with psychotic symptoms | Psychotic patients fail behaviorally to distinguish between salient events. Abnormal DA-dependent motivational salience in SV |
| Corrlett et al50 | 2007 | 12 psychotic patients (1 bipolar, 8 SGAs, 4 unmedicated), 12 HC | — | Associative learning task: learning of associations between different food cues and aversive outcomes, violation of expectancies to produce a PE | SPM2, contrast for violation of expected compared with well-learned control items. VOI analysis in 5 predefined regions (R lateral PFC, bilateral VS, and substantia nigra) with 2 sample t-tests of parameter estimates within SPSS | Both groups acquired the associative relationships; SV showed disturbed PE signal in R dorsal PFC due to attenuation to unexpected events and augmentation to predictable events. Inverse correlation of functional activation with delusion (unusual thought content BPRS) in R dorsal PFC | Support for learning-based accounts of delusion formation and fronto-basal-ganglia disruption in psychosis. Inappropriate PE signal and maladaptive update of prefrontal representation of the world with irrelevant information; PFC responds to physiological noise as if it were salient biological signal |
| Schlagenhauf et al51 | 2008 | 10 SV: first scan (T1) with FGA and second scan (T2) with SGA, 10 HC at corresponding time points | T1: 74.0 ± 18.0, T2: 63.6 ± 14.5 | Monetary incentive delay task | SPM2, contrast of anticipation of reward, respectably, loss compared with neutral trials, SVC for VS and VOI analysis of VS | Group by session interaction in the right VS due to an increase among the SV and a decrease among the HC in a VOI analysis. Left VS activation during reward anticipation was correlated with negative symptoms in SV at T1 (with FGAs) | D2 receptor blockade in VS by FGAs may interfere with salience attribution. SGA olanzapine may preserve some degree of dopaminergic neurotransmission in the VS, leading to less secondary, neuroleptic-induced negative symptoms |
| Walter et al52 | 2009 | 16 atypical medicated SV; 16 HC | 71.9 ± 6.2 | Monetary incentive delay task with parametric variation of reward probability | SPM2, anticipation: contrast of different reward magnitudes (high, low, or no reward; no loss condition), outcome: U-shaped salience contrast of gain or omission of high reward vs low/no reward | Anticipation: HC but not SV showed increased ACC activation with increasing reward. Blunted ACC activation correlates with high positive symptoms in SV. Outcome: HC but not SV display higher activation in R ventrolateral PFC with increased salience (omission or receipt of reward vs no reward) | Normal mesolimbic DA system in remitted SV; hypoactive cortical regions mediating attentional processes and action selection during reward processing |
| Schlagenhauf et al53 | 2009 | 15 unmedicated SV, 15 HC | 99.4 ± 20.3 | Monetary incentive delay task | SPM5, contrasts for feedback (outcome) of successful vs unsuccessful reward and loss avoidance | SV displayed exaggerated responses when expected reward was not delivered in MFPC; reduction of neural responses during unsuccessful loss-avoidance feedback in VS was abolished in SV. Reduced functional connectivity between the MPFC and the VS in unmedicated schizophrenia patients compared with healthy controls. Correlation between delusions and MPFC responses to feedback of successful vs unsuccessful loss avoidance | In drug-free SV, processing of reward and loss-avoidance feedback is differentially affected in MPFC and VS. Blunted neuronal differences between successful and unsuccessful loss feedback could exacerbate delusion |
Note: SV, schizophrenia volunteers; HC, healthy controls; SVC, small volume correction; VS, ventral striatum; FGAs, first-generation antipsychotics; SGAs, second-generation antipsychotics; R, right; PFC, prefrontal cortex; L, left; PE, prediction error; ACC, anterior cingulate cortex; MPFC, medial prefrontal cortex; PANSS, Positive and Negative Symptom Scale; BPRS, brief psychiatric rating scale; SPM, Statistical Parametric Mapping; DA, dopamine; RT, reaction times; VOI, volume of interest.
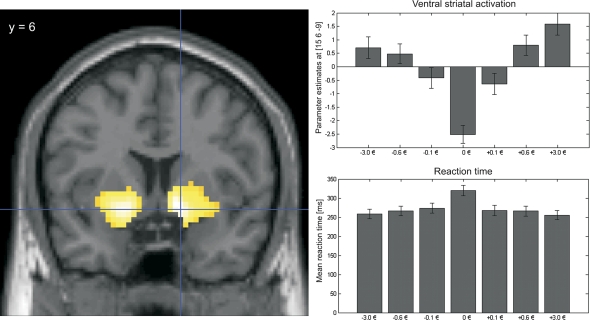
Fig. 6.
Ventral Striatal Activation During Incentive Anticipation Represents Incentive Salience. Left panel: BOLD response in the bilateral ventral striatum during anticipation of potential monetary reward and loss avoidance compared with the neutral condition in 44 healthy controls (P < .05 family wise error corrected for the whole brain; displayed at Montreal Neurological Institute coordinate y = 6). Right panel upper part: Higher activation during anticipation of larger amounts of monetary gain resp. loss avoidance: Parameter estimates for the different cues indicating different amounts of loss avoidance and reward or no monetary consequences (−3.0€, −0.6€, −0.1€, ±0€, +0.6€, +3.0€, +0.1€): Cues that indicate higher gain or higher loss-avoidance trials elicited stronger activation compared with lower amounts of money (parameter estimates from the peak voxel of the right ventral striatum at x = 15, y = 6, z = −9, t = 7.57). Right panel lower part: Cues that predict higher gains or the possibility to avoid higher losses elicited faster reaction times than cues predicting lower gains (respectably loss avoidance) with the lowest reaction times following neutral cues. This indicated that these stimuli actually represent an incentive to adjust goal-directed behavior according to the predicted gain or loss avoidance.
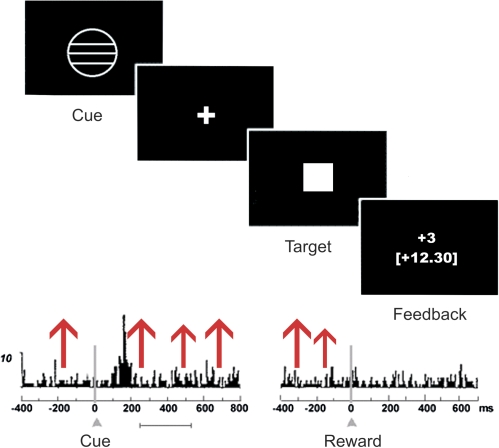
Fig. 7.
Hypothesized Association Between Dysfunction of Reward Anticipation and Altered Dopaminergic Neurotransmission in the Hyperdopaminergic State of Acute Schizophrenic Psychosis. Upper part: Task structure for the monetary incentive delay task58: During each trial, a cue indicated potential reward, loss avoidance, or a neutral trial. After cue presentation, volunteers waited a variable interval (fixation cross) and then responded to a white target square with a button press. To succeed in a given trial, volunteers had to press the button while the target was visible. Chance of winning was 66% due to an individual adjustment to response performance. After target presentation, feedback appeared, notifying volunteers whether they had successfully won or avoided losing money. Lower part: Firing rate of dopaminergic midbrain neurons according to Schultz et al.17 A phasic dopamine firing increase occurs during presentation of reward-indicating stimuli once learning has taken place (left panel), which corresponds to the anticipation phase of the Monetary Incentive Delay (MID) task. No increase is observed after the delivery of fully predicted (expected) reward, which corresponds to the feedback phase of the MID task (right panel). Chaotic firing due to the hyperdopaminergic state in unmedicated schizophrenia patients indicated by the red arrows may lead to increased “noise” and “drown out” the neuronal response measured with functional magnetic resonance imaging following the reward-indicating cue.
However, the process of learning, which occurred before previously neutral cues are attributed with incentive salience in the above-mentioned studies, occurred “before” subjects entered the scanner. They were trained for about 15 min and learned that an abstract cue, such as a circle, for the rest of the task will represent a conditioned stimulus that predicts reward.46,47,51 These studies suggest that dopamine dysfunction interferes with learning of reward-predicting stimuli; however, to directly prove learning dysfunctions in schizophrenic psychosis, the learning process itself should be assessed during the imaging session.
A series of studies went further in elucidating the neuronal correlates of learning dysfunction in schizophrenia. These studies directly addressed the prediction error that occurs when a reward or punishment does not arrive as anticipated. In a classical Pavlovian conditioning task with aversive and neutral events, Jensen et al48 showed that cues, which were not followed by the expected aversive outcome, elicited a significant activation of the right ventral striatum, the middle cingulate and right PFC, the right hippocampus, and the thalamus in healthy controls; this pattern of activation was not found in schizophrenia patients. Instead, schizophrenia patients showed a stronger response to neutral stimuli, which may reflect aberrant attribution of motivational salience to these neutral stimuli. A similar finding was reported by Corlett et al,50 who also observed a diminished difference in brain activation in the right lateral PFC between unexpected vs predictable events. A study of Walter et al52 pointed in a similar direction and observed that healthy controls, but not medicated schizophrenia patients, showed increased anterior cingulate cortex (ACC) activation with increasing reward anticipation. During reward feedback, schizophrenia patients failed to display a higher activation in the right ventrolateral PFC with increased salience.
Again, the blunted difference between relevant and irrelevant stimuli and outcomes may reflect chaotic attribution of salience to otherwise irrelevant cues, an interpretation that is in accordance with the idea that chaotic or stress-induced dopamine firing can interfere with salience attribution in schizophrenia.19–22 Finally, Murray et al49 used an instrumental reward conditioning task and observed that schizophrenia patients showed faster reaction times in neutral trials compared with healthy controls, which again potentially reflects increased salience attribution to irrelevant stimuli. Schizophrenia patients also showed reduced brain activation in the ventral striatum and midbrain for reward-associated prediction errors compared with neutral prediction errors. However, these latter studies48–50 only included medicated patients or a combination of medicated and unmedicated patients. Given the fact that neuroleptic medication directly interferes with dopamine D2 receptor functioning, altered brain activation, following an error of reward prediction, may in part be due to direct medication effects on dopamine-mediated neuronal activation. One recent study observed reward feedback alteration in “unmedicated” schizophrenia patients and supported the hypothesis that a blunted difference in neuronal responses to relevant vs irrelevant events contributes to delusion formation.53 In this study, responses to rewards and punishments were compared and it was observed that neuronal responses to successful vs unsuccessful avoidance of loss were blunted in the ventral striatum of unmedicated schizophrenia patients compared with healthy controls.53 Interestingly, functional responses to negative outcomes in reward trials, ie, when the omission of an expected reward occurred, were increased in the medial PFC of patients with schizophrenia. If independently confirmed, this observation suggests that neuronal responses to negative outcomes are exaggerated in unmedicated schizophrenia patients and that these responses may bias the individuals to focus on aversive outcomes rather than on the successful avoidance of such events. Patients may thus be inclined to look at the negative side of events, and this may increase their propensity to distrust their environment and to attribute negative intentions to others. Indeed, Schlagenhauf et al53 observed that an increased severity of delusion among schizophrenia patients was associated with a decrease in medial PFC activation elicited by successful vs unsuccessful avoidance of loss. This observation suggests that positive symptoms, such as delusion formation, can result from altered representations of an individual’s ability to successfully avoid aversive outcomes: If the neuronal activation associated with such a successful avoidance of negative outcomes is impaired, subjects may feel more helpless and under the control of a threatening environment.
Thus far, these studies did not directly use reinforcement learning algorithms during learning task with changing reward contingencies to model learning rates and trial-by-trial prediction errors in unmedicated schizophrenia patients. In addressing this issue, we recently found evidence that both learning rates and success are reduced in schizophrenia patients, which was reflected in brain activations elicited by these psychometric indices.60 One recent fMRI study showed that during a reward-based decision-making task, the dorsal and ventral striatum were differentially connected to different midbrain regions (possibly corresponding to the substantia nigra [SN] and the VTA, respectively). However, only individual differences in the strength of the functional connectivity between the dorsal striatum and the (putative) SN predicted the impact of different reinforcement types on individual learning rates.61 Because dopaminergic neurons arising from the VTA and the SN directly innervate the ventral and dorsal striatum, it will be interesting to examine whether functional connectivity between these brain areas is impaired in schizophrenia patients and whether this impairment contributes to learning dysfunctions. Comparison between brain activation of the dorsal and ventral striatum appears even more warranted because the group of Abi-Dargham and Laruelle recently reported that increased dopamine turnover in schizophrenia may not be most prominent in the ventral but rather in the central or associate striatum.62 The ventral, central, and dorsal striatum receive projections from cortical areas, which are organized in a topographical distinct as well as an overlapping manner63 from dopaminergic neurons in the midbrain and further subcortical regions.64–66 Specifically, the amygdala, hippocampus, orbitofrontal, and medial prefrontal cortices including the ACC send inputs to the ventral striatum,67 whereas the central (associative) striatum receives input from the dorsal ACC and dorsolateral PFC and the dorsal striatum is innervated by (pre-)motor cortical areas.40,41,67 Multimodal imaging studies also showed that dopamine synthesis in the ventral vs dorsal striatum correlated with functional processing of affective stimuli in the ACC vs dorsolateral PFC.68,69
It has been suggested that the ventral striatum is activated by novel cues, as well as during the acquisition of reward contingencies, whereas the associative and dorsal striatum is implicated in habit formation, eg, an automatic elicitation of responses that become increasingly independent from reward feedback.15,70 If indeed dopamine dysfunction in schizophrenia is stronger in (central) striatal regions associated with habit formation, delusions may not result only from impaired neuronal representation of reward and punishment feedback but also from a breakdown of automatic responses. In his famous theoretic account of schizophrenia, Blankenburg71 suggested that schizophrenia is characterized by a breakdown of implicit responses in well-known everyday situations, which in healthy subjects do not require thoughtful considerations (“Der Verlust der natürlichen Selbstverständlichkeit”). Therefore, it would be very interesting to simultaneously assess dopamine dysfunction with PET and functional brain activation (with fMRI) to test whether dopamine dysfunctions in the ventral, central, or more dorsal striatum are indeed associated with differential impairments in the acquisition of conditioned responses and the execution of habits.
Summary and Outlook
Altogether, a series of studies demonstrated a dysfunction of dopaminergic neurotransmission in the striatum of schizophrenia patients. The studies also suggest that functional activation potentially associated with striatal dopaminergic signaling, such as the attribution of salience to reward-predicting stimuli and the computation of prediction errors,17,18,72 is indeed impaired in schizophrenia patients and that this impairment may contribute to delusion formation.20,21 However, direct proof is lacking for the role of dopamine in these functional impairments. Also, the exact location of dopamine dysfunction within the striatum remains to be addressed. Electrophysiological studies have shown that dopamine plays a key role in regulating cortico-striatal synaptic plasticity via both long-term potentiation and depression within the striatal microcircuits.73,74 Dopamine acts on D1 and D2 receptors, which are located on distinct populations of medium spiny interneurons in the striatum; together with other neurotransmitters like glutamate, adenosine, and endocannabinoid, dopamine engenders bidirectional effects (ie, both long-term depression and potentiation) and thus influences Hebbian synaptic plasticity. It was suggested that in hyperdopaminergic states like schizophrenia, alterations of this bidirectional mechanism may lead to the formation of inappropriate associations in these microcircuits.73 Indeed, Robinson and Kolb75 observed that dopamine stimulation of medium spiny neurons in the striatum results in structural changes in striatal GABAergic interneurons, which can induce long-lasting alterations in habitual behavior. It remains to be tested whether dopamine dysfunction in the associative (central) striatum is indeed associated with a breakdown of automatic responses and thus contributes to behavioral disorganization in schizophrenia.
Affectively, schizophrenic delusions are often characterized by anxiety and other negative mood states, resulting in delusions of prosecution rather than grandiosity. Therefore, further brain areas innervated by dopamine such as the amygdala and the PFC appear highly relevant for the formation of delusions.50,53,76 Indeed, altered dopamine storage capacity in the amygdala of schizophrenia patients has recently been reported.11 Further studies need to elucidate whether dopamine dysfunction in different brain areas (eg, the striatum, PFC, and amygdala) is associated with distinguishable aspects of delusion formation, eg, whether the ventral striatum is generally implicated in errors of salience attribution and the PFC in delusion formation (eg, due to misrepresentation of successful avoidance of aversive outcomes53 and the amygdala in anxiety and other negative mood states associated with delusions of persecution). Also, it is not clear whether the model suggested here for schizophrenic psychosis can also be applied to delusion formation associated with other psychotic disorders (eg, mania) as may be suggested by the work of Corlett et al50 who included patients with schizoaffective disorders in their studies.
Finally, computational models of human reward-based learning have successfully been applied in healthy volunteers77,78 and can easily be combined with PET studies of dopaminergic neurotransmission, eg, in the ventral and dorsal striatum and the amygdala. Such studies will not only help to elucidate the neurobiological correlates of psychotic behavior but also caution against high blockade of dopamine receptors due to high doses of neuroleptic medication because the resulting striatal dopamine dysfunction can further impair reward expectation and reward-based learning. These studies thus emphasize the need for individually adjusted neuroleptic doses and for new therapeutic approaches that do not severely interfere with dopamine functioning.
Funding
This work was supported by the German Research Foundation (Deutsche Forschungs-gemeinschaft; HE 2597/4-3 und /4-4).
References
- 1.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1(3):179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 2.Wong DF, Wagner HN, Jr, Tune LE, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234:1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 3.Farde L, Wiesel FA, Hall H, Halldin C, Stone-Elander S, Sedvall G. No D2 receptor increase in PET study of schizophrenia. Arch Gen Psychiatry. 1987;44:671–672. doi: 10.1001/archpsyc.1987.01800190091013. [DOI] [PubMed] [Google Scholar]
- 4.Talkowski ME, Bamne M, Mansour H, Nimgaonkar VL. Dopamine genes and schizophrenia: case closed or evidence pending? Schizophr Bull. 2007;33:1071–1081. doi: 10.1093/schbul/sbm076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laruelle M, bi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breier A, Su TP, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinz A, Saunders RC, Kolachana BS, et al. Striatal dopamine receptors and transporters in monkeys with neonatal temporal limbic damage. Synapse. 1999;32(2):71–79. doi: 10.1002/(SICI)1098-2396(199905)32:2<71::AID-SYN1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 9.Grunder G, Vernaleken I, Muller MJ, et al. Subchronic haloperidol downregulates dopamine synthesis capacity in the brain of schizophrenic patients in vivo. Neuropsychopharmacology. 2003;28:787–794. doi: 10.1038/sj.npp.1300103. [DOI] [PubMed] [Google Scholar]
- 10.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 11.Kumakura Y, Cumming P, Vernaleken I, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–8087. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gefvert O, Lindstrom LH, Waters N, Waters S, Carlsson A, Tedroff J. Different corticostriatal patterns of L-DOPA utilization in patients with untreated schizophrenia and patients treated with classical antipsychotics or clozapine. Scand J Psychol. 2003;44:289–292. doi: 10.1111/1467-9450.00347. [DOI] [PubMed] [Google Scholar]
- 14.Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 15.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 16.Wise RA. Neuroleptics and operant-behavior—the Anhedonia hypothesis. Behav Brain Sci. 1982;5:39–53. [Google Scholar]
- 17.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 19.Heinz A. [Psychopathological correlates of dopaminergic dysfunction in alcoholic and schizophrenic patients] Nervenarzt. 1999;70:399–407. doi: 10.1007/s001150050455. [DOI] [PubMed] [Google Scholar]
- 20.Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia—psychopathological and behavioral correlates. Eur Psychiatry. 2002;17(1):9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 22.Morrison PD, Murray RM. From real-world events to psychosis: the emerging neuropharmacology of delusions. Schizophr Bull. 2009;35:668–674. doi: 10.1093/schbul/sbp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conrad C. Die beginnende Schizophrenie. Stuttgart, Germany: Thieme; 1992. [Google Scholar]
- 24.Miller R. Schizophrenic psychology, associative learning and the role of forebrain dopamine. Med Hypotheses. 1976;2:203–211. doi: 10.1016/0306-9877(76)90040-2. [DOI] [PubMed] [Google Scholar]
- 25.Maher BA. Delusional thinking and perceptual disorder. J Individ Psychol. 1974;30(1):98–113. [PubMed] [Google Scholar]
- 26.Dodds CM, Muller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant KA, Shively CA, Nader MA, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29(1):80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Morgan D, Grant KA, Gage HD, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 29.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 30.Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinz A, Romero B, Gallinat J, Juckel G, Weinberger DR. Molecular brain imaging and the neurobiology and genetics of schizophrenia. Pharmacopsychiatry. 2003;36(suppl 3):S152–S157. doi: 10.1055/s-2003-45123. [DOI] [PubMed] [Google Scholar]
- 32.Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 33.Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 35.Tan HY, Sust S, Buckholtz JW, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- 36.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 39.Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 40.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 43.Schlagenhauf F, Wustenberg T, Schmack K, et al. Switching schizophrenia patients from typical neuroleptics to olanzapine: effects on BOLD response during attention and working memory. Eur Neuropsychopharmacol. 2008;18:589–599. doi: 10.1016/j.euroneuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 45.Allen PP, Cleare AJ, Lee F, et al. Effect of acute tryptophan depletion on pre-frontal engagement. Psychopharmacology (Berl) 2006;187:486–497. doi: 10.1007/s00213-006-0444-x. [DOI] [PubMed] [Google Scholar]
- 46.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 47.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 48.Jensen J, Willeit M, Zipursky RB, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2007;28:294–302. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 49.Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:239. doi: 10.1038/sj.mp.4002058. 267–239, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corlett PR, Murray GK, Honey GD, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130(pt 9):2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlagenhauf F, Juckel G, Koslowski M, et al. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196:673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- 52.Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl) 2009;206:121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- 53.Schlagenhauf F, Sterzer P, Schmack K, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, Andersen A, Grondin R, et al. Pharmacological MRI mapping of age-associated changes in basal ganglia circuitry of awake rhesus monkeys. Neuroimage. 2001;14:1159–1167. doi: 10.1006/nimg.2001.0902. [DOI] [PubMed] [Google Scholar]
- 56.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 57.Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR. Amphetamine modulates human incentive processing. Neuron. 2004;43:261–269. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 58.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 59.Heinz A, Knable MB, Coppola R, et al. Psychomotor slowing, negative symptoms and dopamine receptor availability—an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophr Res. 1998;31:19–26. doi: 10.1016/s0920-9964(98)00003-6. [DOI] [PubMed] [Google Scholar]
- 60.Schlagenhauf F, Beck A, Deserno L, et al. Altered prediction error signal during reversal learning in drug-free schizophrenia patients. 2009 Program No. 838.15/M4. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience. October 17–21. [Google Scholar]
- 61.Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci. 2009;21:1332–1345. doi: 10.1162/jocn.2009.21092. [DOI] [PubMed] [Google Scholar]
- 62.Kegeles L, Frankle W, Gil R, et al. Schizophrenia is associated with increased synaptic dopamine in associative rather than limbic regions of the striatum: implications for mechanisms of action of antipsychotic drugs. J Nucl Med Meeting Abstracts. 2006;47(suppl 1):139P–139a. [Google Scholar]
- 63.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- 65.Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- 66.Gimenez-Amaya JM, McFarland NR, de Las HS, Haber SN. Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol. 1995;354:127–149. doi: 10.1002/cne.903540109. [DOI] [PubMed] [Google Scholar]
- 67.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4th ed. Berlin, Heidelberg: Springer; 2008. [Google Scholar]
- 68.Siessmeier T, Kienast T, Wrase J, et al. Net influx of plasma 6-[18F]fluoro-L-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur J Neurosci. 2006;24:305–313. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- 69.Kienast T, Siessmeier T, Wrase J, et al. Ratio of dopamine synthesis capacity to D2 receptor availability in ventral striatum correlates with central processing of affective stimuli. Eur J Nucl Med Mol Imaging. 2008;35:1147–1158. doi: 10.1007/s00259-007-0683-z. [DOI] [PubMed] [Google Scholar]
- 70.Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- 71.Blankenburg W. Der Verlust der natürlichen Selbstverständlichkeit: Ein Beitrag zur Psychopathologie symptomarmer Schizophrenien. Stuttgart, Germany: Enke; 1971. [Google Scholar]
- 72.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 75.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kienast T, Hariri AR, Schlagenhauf F, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- 77.O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 78.O'Doherty JP, Hampton A, Kim H. Model-based fMRI and its application to reward learning and decision making. Ann N Y Acad Sci. 2007;1104:35–53. doi: 10.1196/annals.1390.022. [DOI] [PubMed] [Google Scholar]