Cellulose is the most abundant component of plant cell walls and its importance is well documented. In the primary cell wall it forms part of the load-bearing network that both controls and maintains cell shape, while allowing regulated cell expansion that is essential during growth. Cellulose is also the major component of secondary cell walls where it is required for mechanical strength for the plant (for review, see Cosgrove, 2005; Taylor, 2008).
Most plant cell wall matrix polysaccharides are first synthesized within the cell in the Golgi and subsequently deposited into the wall by exocytosis (Moore and Staehelin, 1988; Reyes and Orellana, 2008). In contrast, cellulose is synthesized at the plasma membrane by a very large membrane-bound complex known as the cellulose synthase complex (CSC). The complex extrudes up to 36 individual cellulose chains that are bound together to form the cellulose microfibril. It is believed that the microfibril forms a rigid structure such that the energy from cellulose chain elongation pushes the CSC through the plane of the plasma membrane (Herth, 1980). Freeze fracture studies have shown the CSC to be a six-lobed rosette structure of 25 to 30 nm in diameter (Mueller and Brown, 1980; Herth, 1985), although more recent analysis suggests that it extends into the cytoplasm, reaching a maximum diameter of about 45 nm (Bowling and Brown, 2008). The large size of the CSC and the need to target it to specific sites in the plasma membrane represent a particular logistical problem for plant cells. Recent evidence from live cell imaging suggests CSC trafficking is both highly dynamic and that it does not easily fit into our understanding of the conventional secretory pathway. In this article we will focus on recent studies that have contributed to improving our understanding of the intracellular trafficking of the CSC. For other aspects of cellulose synthesis, including biochemistry, structure, and microfibril orientation readers are referred to several recent reviews (Somerville, 2006; Lloyd and Chan, 2008; Taylor, 2008).
THE CSC AND ITS VISUALIZATION IN LIVING CELLS
The Arabidopsis (Arabidopsis thaliana) radial swelling1 (rsw1) mutant is a temperature-sensitive mutant in the CESA1 gene. When grown at the restrictive temperature, rsw1 mutants have less cellulose and the CSCs are lost from the plasma membrane (Arioli et al., 1998). Additionally, immunolabeling has shown that the rosette structures that are visualized by freeze fracture can be labeled with anti-CESA antibodies (Kimura et al., 1999). These experiments demonstrate that not only are CESA proteins essential for cellulose deposition, but they are also an integral part of the CSC. Although genetic analysis has identified several proteins required for cellulose synthesis (for review, see Somerville, 2006, Liepman et al., 2010), none of them have definitively been shown to be an essential and integral component of the CSC. Consequently, live cell imaging studies to look at CSC trafficking have necessarily focused upon labeling of CESA proteins.
Three different CESA proteins, encoded by members of the CESA gene family, are required for formation of a functional CSC (for review, see Taylor, 2008). The CESA proteins that make up the CSC responsible for primary cell wall formation consist of CESA1 and CESA3, together with some combination of CESA2, CESA5, CESA6, or CESA9 (Desprez et al., 2007; Persson et al., 2007). Cellulose biosynthesis at the secondary wall requires CESA4, CESA7, and CESA8. In the absence of any one of these three subunits, the CSC is not transported to sites of cell wall formation (Taylor et al., 1999, 2000, 2003; Gardiner et al., 2003). Fusions between GFP variants and the N terminus of several of the CESA subunits, namely CESA3, CESA6, and CESA7, have been demonstrated to not interfere with the protein function and this has allowed their dynamics to be viewed in vivo (Gardiner et al., 2003; Paredez et al., 2006; Desprez et al., 2007; Wightman and Turner, 2008). While only a proportion of CESA proteins are labeled within a single CSC, this has still permitted the detection of single CSCs within the plasma membrane (Paredez et al., 2006; Desprez et al., 2007) and has also allowed detailed examination of intracellular CSC trafficking (Fig. 1).
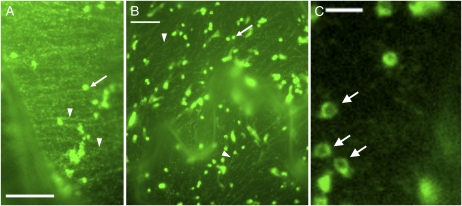
Figure 1.
Live cell imaging of the CSC. The images are taken of YFP-CESA6 fusion within the epidermis of a cotyledon petiole cell (A) and pavement cells (B). Bars = 10 μm. Examples of linear arrays, consisting of CESA6-containing complexes and cortical compartments, are shown by arrowheads. Arrows show Golgi that, in these pictures, yield a saturated signal. A close-up of Golgi taken with a lower exposure is shown in section C, where the characteristic ring-like appearance is observed (arrows). Bar = 3 μm.
CESA COMPLEXES IN THE GOLGI
Live imaging of YFP-CESA protein fusions during both primary wall and secondary wall cellulose synthesis are seen to clearly label internal ring-like structures that colocalize with a Golgi marker (Paredez et al., 2006; Wightman and Turner, 2008; Fig. 1). The ring-like labeling is different to the uniform labeling observed for common Golgi markers such as mannosidase 1 (Nebenfuhr et al., 1999) and the rat sialyltransferase (Boevink et al., 1998). It appears that the ring-like labeling represents labeling of the Golgi periphery. This has been confirmed using immunogold labeling of a GFP-CESA3 fusion in which labeling was observed at the ends of medial- and trans-Golgi cisternae in addition to the cell cortex (Crowell et al., 2009). This work is also consistent with earlier freeze fractures studies. Using a suspension of Zinnia elegans cells undergoing treachery element differentiation, Haigler and Brown (1986) were able to directly visualize rosettes within the periphery of the trans face of the Golgi.
The rings of labeled CSCs within the Golgi are observed to include bright punctae (Fig. 1C). The concentration of CSCs that form these bright spots may represent either an event just prior to vesicle formation or an accumulation following fusion from an endocytic compartment.
NOVEL CESA-CONTAINING COMPARTMENTS
Haigler and Brown (1986) were able to identify CSC-containing vesicles at the cell cortex at sites of secondary cell wall deposition. The similar size of these vesicles to the invaginations around the Golgi periphery suggest that these vesicles arise from the Golgi and that their position at the cell cortex suggests that they are responsible for delivery of assembled CSCs to sites of cellulose deposition (Haigler and Brown, 1986). The presence of CSC-containing vesicles beneath sites of secondary wall deposition has additionally been inferred from indirect visualization of CSC movement during fluorescence loss in photobleaching (Wightman et al., 2009). In these experiments, calculated velocities of CSCs appear to be too rapid to be explained simply by movement of CSCs synthesizing cellulose at the plasma membrane. The high velocities likely represent the movement of the CSC within a cortical intracellular compartment. More direct evidence of a role for small subcellular compartments in CSC transport studies has come from live cell imaging during formation of the primary cell wall. In addition to identifying individual CSCs at the plasma membrane, Paredez et al. (2006) were also able to identify a small subcellular compartment that exhibited more erratic movement. This nature of this compartment has been described in some detail in two recent studies where they have been named either as small CESA compartments (SmaCCs; Gutierrez et al., 2009) or microtubule-associated cellulose synthase compartments (MASCs; Crowell et al., 2009).
Examination of the epidermis from rapidly growing regions of the seedling hypocotyls are characterized by a high density of CSCs at the plasma membrane consistent with a need to synthesize cellulose during cell expansion. In contrast, at the base of the hypocotyl that is not actively growing there were many fewer CSCs at the plasma membrane and most CSCs localized within the SmaCCs/MASCs (Crowell et al., 2009). Furthermore, osmotic stress and some drug treatments result in rapid loss of the CSC from the plasma membrane and accumulation within the SmaCCs/MASCs (Crowell et al., 2009; Gutierrez et al., 2009). These observations have been interpreted in two different ways, either SmaCC/MASCs represent solely delivery compartments that accumulate when insertion of complexes to the plasma membrane is prevented (Gutierrez et al., 2009), or that these compartments also function as an intracellular store of internalized CESA proteins (Crowell et al., 2009; Fig. 2). While the localization of SmaCCs/MASCs close to the cell cortex suggests a role in CSC delivery, the fact that plasma membrane-localized CESA proteins can be internalized into SmaCCs/MASCs within as little as 6 min following osmotic stress, suggests that SmaCCs/MASCs are also involved in removal of the complexes (Crowell et al., 2009). SmaCC/MASCs are frequently associated with microtubules and this would make the SmaCC/MASC a type of recycling compartment, possibly serving as a microtubule-associated intracellular store of complexes (Fig. 2). Live imaging indicates that each SmaCC/MASC contains one or two fully formed complexes (Gutierrez et al., 2009). This would be consistent with SmaCC/MASC being synonymous with the rosette-containing transport vesicles seen by Haigler and Brown (1986).
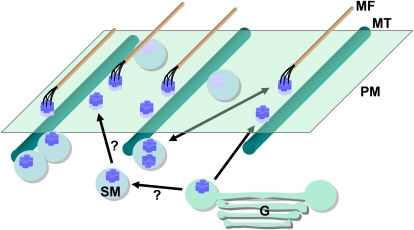
Figure 2.
Overview of CSC intracellular trafficking. Shown in purple are hexameric CSC rosettes that are targeted to the plasma membrane (PM) directly from the Golgi (G) or from SmaCC/MASCs (SM). Recycling occurs from the plasma membrane to SmaCC/MASCs (double arrow), and CSC insertion appears to occur only in the vicinity of microtubules (MT). There is a delay following CSC insertion in the plasma membrane and the start of microfibril (MF) biosynthesis. SmaCC/MASCs track along both ends of depolymerizing microtubules.
Interestingly, SmaCC/MASC movement coincides with microtubule-depolymerizing ends, achieving speeds of up to 9 μm−1 min−1 (Crowell et al., 2009; Gutierrez et al., 2009). Where several SmaCCs are seen to occur along the length of a single microtubule, the passing of a depolymerizing end results in the gathering and aggregation of the compartments and this end-tracking ability occurs regardless of whether it is the plus end or minus end that is undergoing the catastrophe (Gutierrez et al., 2009).
Crowell et al. (2009) also reported an additional CSC-containing compartment that colocalizes with a VHA-a1-based endosomal marker that marks the trans-Golgi network. It has also been shown that a subpopulation of SmaCC/MASCs localize with the trans-Golgi network (Gutierrez et al., 2009) and this may be the same compartment as that described by Crowell et al. (2009). The function of this compartment is unclear, but may represent complexes that are being removed from the plasma membrane to the trans-Golgi for recycling (Crowell et al., 2009).
DELIVERY OF CESA COMPLEXES TO THE PLASMA MEMBRANE
In developing xylem, CSC-containing Golgi move very rapidly along thick actin cables. When lantrunculin B is added to depolymerize the actin, Golgi movement ceases. This also results in loss of the characteristic bands of fluorescently labeled cellulose synthase from beneath the sites of cellulose deposition into the secondary walls (Wightman and Turner, 2008), demonstrating that CSCs are constantly being lost from the plasma membrane and that this loss must be matched by the insertion of new complexes. A similar situation appears to exist in the primary cell wall where clearer imaging has allowed a more detailed description of this phenomenon. The depolymerization of actin results in cessation of Golgi movement (Crowell et al., 2009; Gutierrez et al., 2009). Furthermore, this disruption of the actin network results in the aggregation of Golgi and much higher densities of CSCs are observed in the plasma membrane directly above the Golgi aggregates than in regions where Golgi bodies are largely absent (Crowell et al., 2009; Gutierrez et al., 2009). This suggests that local delivery from the Golgi to the plasma membrane is largely unaffected but Golgi movement along actin is required for equivalent distribution of complexes all around the cell cortex.
During secondary wall synthesis in developing xylem vessels, movement of YFP-CESA7-labeled Golgi occurs unidirectionally and in an actin-dependent manner (Wightman and Turner, 2008). Concurrent imaging with a xylem-specific marker of actin reveals the Golgi to move along the same path on longitudinal thick actin cables and to undergo short pause events, often lasting only fractions of a second. The vast majority (90%) of pause events occur below sites of secondary wall deposition and appear to coincide with the Golgi delivering the CESA complex. What marks these pause sites is not yet fully understood. It is unlikely, however, that these pauses are caused merely by colliding with the membrane invaginations caused by the secondary wall thickenings since different Golgi traveling along identical paths can be seen to skip sites of wall synthesis at which other Golgi pause (Wightman and Turner, 2008). Microtubules are ideally positioned to mark these sites but their removal does not affect the incidences of Golgi pause events. The best candidates for marking these sites are the narrow transverse actin fibers that emerge from the longitudinal cables close to the secondary walls (Wightman and Turner, 2008).
Insertions of the CSC into the plasma membrane during primary wall deposition have been seen to occur directly from the Golgi (Crowell et al., 2009). Golgi in cells of the hypocotyl epidermis paused for up to 73 s. These pause events coincided with the appearance of up to four CSCs at the plasma membrane (Crowell et al., 2009).
Following removal of the isoxaben treatment or osmotic shock, CSCs repopulate the plasma membrane. Insertion of CSCs into the plasma membrane was observed to occur directly from SmaCC/MASCs (Gutierrez et al., 2009). It is assumed, therefore, that the SmaCCs are also involved in CSC delivery during normal plant growth (Gutierrez et al., 2009). CSC insertion sites whether from Golgi or from SmaCC/MASCs always coincided with cortical microtubules. When microtubules were removed with depolymerizing drugs, CSC insertion was maintained at the same rate. The data suggest that microtubules are required for targeting but not insertion of the CSC.
Delivery of cellulose synthase to the plasma membrane, at a rate of 4.8 delivery events μm−2 h−1, is observed to proceed in three stages: (1) appearance of the intracellular delivery compartment; (2) pause of the particle, representing either the stabilized intracellular compartment or the appearance of nonactive cellulose synthase; and (3) steady linear movement characteristic of the complexes making cellulose (Gutierrez et al., 2009).
Many questions remain with regard to the role of the Golgi in inserting CSCs in the plasma membrane. It is possible that the Golgi is responsible for delivery of de novo CSCs into the plasma membrane, while SmaCCs/MASCs represent an intracellular store that recycles CSCs with the plasma membrane. It could also be that SmaCC/MASCs arise directly from the Golgi and, in some cases, the gap between SmaCC/MASCs biogenesis and insertion of the CSC into the plasma membrane is very short such that it appears as a single event (Fig. 2).
The mechanisms of CSC removal from the plasma membrane, either during primary or secondary wall synthesis, are yet to be determined; however, due to the large size of the CSC, removal is likely not to involve clathrin-mediated endocytosis (Crowell et al., 2009).
THE INVOLVEMENT OF EFFECTORS OF COMPARTMENT-CYTOSKELETON ASSOCIATION AND DOCKING FACTORS
Given the importance of the actin cytoskeleton to global trafficking of the cellulose synthase-containing Golgi and the involvement of the cortical microtubules in tethering the SmaCC/MASC compartment, it is likely that motor and other cytoskeletal-associated proteins will be critical for CSC functioning. The reported observations that the SmaCC/MASC compartment moves coincidently with depolymerizing microtubule ends already implicates a type of tip-tracking protein that is not restricted by microtubule polarity (Crowell et al., 2009; Gutierrez et al., 2009). Movement of the Golgi via the actin network in epidermal cells or in a unidirectional fashion along the thick actin bundles in developing xylem likely involves one or more myosins (Avisar et al., 2009). One factor involved in organizing actin is encoded by the Arabidopsis FRAGILE FIBER4/ROOT HAIR DEFECTIVE3 (FRA4/RHD3) gene. The fra4 mutant exhibits an 18% reduction of crystalline cellulose compared to wild type and defective secondary cell walls in fibers and xylem vessels (Hu et al., 2003). Elongating cells in the fra4 mutant also show abnormal actin bundling that might prevent proper delivery of CSCs for secondary wall synthesis and result in reduced cellulose crystallinity. An allele of fra4, rhd3 had originally been identified on the basis of its short, wavy root hair phenotype; the actin defect is also likely to be the cause of altered vesicle trafficking during tip growth (Galway et al., 1997). Another fra mutant, fra7, encodes the PtdIns(3,5)P2-specific phosphoinositide phosphatase called AtSac1 (Zhong et al., 2005). Phosphoinositides in general are known to play important roles in regulating trafficking in the plant cell and phosphinositide phosphatases help to regulate the distribution of phosphoinositides to particular subcellular compartments (Thole and Nielsen, 2008). fra7, like other fra mutants, exhibits both secondary cell wall defects and defects in actin organization. In addition, AtSac1 localizes to the Golgi, making it likely that it is involved in the intracellular trafficking required during cell wall deposition.
The movement of the cell wall cargo, including the CSC, involves the docking and exchange of material between several compartments en route to the plasma membrane and also during internalization of the complexes. Docking between Golgi and post-Golgi compartments needs to be tightly regulated so that the complexes are transported to a final endomembrane compartment that fuses with the plasma membrane. Several docking factors have roles that include the delivery of cell wall cargo, although their docking partners and precise roles are not well understood. Syntaxins are part of the diverse SNARE complexes that mediate docking between intracellular compartments and their target membranes (Teng et al., 2001). One of the best-characterized syntaxins is Knolle, a cytokinesis-specific syntaxin that mediates vesicle fusion with the cell plate. Some of the cell plate cargo that depends on functional Knolle includes cell wall material (Lukowitz et al., 1996; Lauber et al., 1997). Mutants in another syntaxin, SYP122, exhibit alterations in cell wall Fourier transform-infrared spectra, suggesting it has a role in primary wall synthesis (Assaad et al., 2004). Whereas syntaxins are known to be directly involved in vesicle docking, an additional level of regulation is carried out by members of the various Rab GTPase families. Different Rab GTPases regulate trafficking between different membrane compartments (for review, see Lycett, 2008). The RabA4b protein localizes to Golgi and another novel compartment during tip growth in root hairs, suggesting a role in the regulated secretion of wall material to the root tip (Preuss et al., 2004). It appears likely that some Rab GTPases have specific functions during secondary cell wall deposition. RabA6a has been identified as part of a gene expression network that shows very good coexpression with the secondary cell wall CESAs (Srinivasasainagendra et al., 2008).
Mutants in a V-ATPase isoform that is normally localized to several compartments, including the trans-Golgi, has been shown to exhibit cell expansion abnormalities that are a result of cellulose defects (Brux et al., 2008). It is proposed that this V-ATPase is involved in endomembrane organization and is part of a mechanism linking the monitoring of cell wall integrity with the process of cell wall deposition (Brux et al., 2008). Curiously, it is this same V-ATPase isoform, called VHA-a1, that is found to label the novel CESA3 endosome-type compartment described recently by Crowell et al. (2009).
ASSEMBLY OF THE CSC
Little is known about how the CSC assembles. It is known that in plants containing a mutation in any one of the secondary cell wall CESA genes, the two remaining subunits appear not to assemble and are not trafficked to secondary cell wall deposition sites (Gardiner et al., 2003; Taylor et al., 2003). Attempts to affinity purify an intact epitope-tagged CSC led to isolation of CESA oligomers but apparently no intact complexes (Atanassov et al., 2009). These oligomers are likely to be intermediates in the assembly of the complex, but the role of other proteins and the location of CSC assembly are still unclear.
Despite several groups using fusions between fluorescent proteins and different CESA subunits, no pre-Golgi compartments, including the endoplasmic reticulum (ER), are found to be labeled (Paredez et al., 2006; DeBolt et al., 2007a, 2007b; Desprez et al., 2007; Wightman and Turner, 2008; Crowell et al., 2009; Gutierrez et al., 2009). Furthermore, electron microscopy has revealed intact complexes within the Golgi but no recognizable rosette structures have been observed in the ER (Haigler and Brown, 1986). One explanation is that assembly actually takes place within the Golgi, although this would be unprecedented for membrane proteins and does not explain why a newly translated CESA monomer in the ER membrane should not fluoresce. An alternative explanation for this apparent absence is that the complexes reside in one or several compartments where fluorescence is quenched or otherwise inhibited. The inability to see labeled CESA subunits within the ER is particularly surprising in developing xylem vessels. In these cells, large numbers of CSCs localize to sites of secondary walls synthesis, prior to the vessel undergoing programmed cell death. Conservative estimates of the number of 30 nm wide complexes based on freeze fracture and electron microscopy data suggest there are over 3,000 complexes in the region of the plasma membrane beneath a complete hoop of secondary wall in the narrow protoxylem vessels of the root (Wightman et al., 2009). This number can be doubled for the much wider vessels observed in stems. Based on a xylem cell having 40 hoops or spirals of secondary wall, this suggests that several hundred thousand complexes are located to the plasma membrane at any one time during cellulose deposition in a single vessel. We have recently identified a very large compartment that appears to provide storage for assembled CSCs prior to transport to the plasma membrane (R. Wightman and S. Turner, unpublished data). Characterization of this compartment may provide clues as to where in the cell the different CESA subunits associate.
CONCLUDING REMARKS
Recent progress in the field of live cell imaging has allowed the identification of several novel compartments required for delivery of the CSC. In particular SmaCCs/MASCs are highly dynamic compartments that appear to play key roles both as intracellular stores of the CSC and in its delivery to the plasma membrane. It now remains to be understood what factors control the interaction of SmaCCs/MASCs with other compartments such as the Golgi and the plasma membrane and how these interactions are regulated during normal growth.
References
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Hofte H, Plazinski J, Birch R, et al. (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279: 717–720 [DOI] [PubMed] [Google Scholar]
- Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, et al. (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15: 5118–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassov II, Pittman JK, Turner SR. (2009) Elucidating the mechanisms of assembly and subunit interaction of the cellulose synthase complex of Arabidopsis secondary cell walls. J Biol Chem 284: 3833–3841 [DOI] [PubMed] [Google Scholar]
- Avisar D, Abu-Abied M, Belausov E, Sadot E, Hawes C, Sparkes IA. (2009) A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol 150: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15: 441–447 [DOI] [PubMed] [Google Scholar]
- Bowling AJ, Brown RM., Jr (2008) The cytoplasmic domain of the cellulose-synthesizing complex in vascular plants. Protoplasma 233: 115–127 [DOI] [PubMed] [Google Scholar]
- Brux A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K. (2008) Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell 20: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof YD, Schumacher K, Gonneau M, Hofte H, Vernhettes S. (2009) Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S, Gutierrez R, Ehrhardt DW, Melo CV, Ross L, Cutler SR, Somerville C, Bonetta D. (2007a) Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc Natl Acad Sci USA 104: 5854–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S, Gutierrez R, Ehrhardt DW, Somerville C. (2007b) Nonmotile cellulose synthase subunits repeatedly accumulate within localized regions at the plasma membrane in Arabidopsis hypocotyl cells following 2,6-dichlorobenzonitrile treatment. Plant Physiol 145: 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Hofte H, Gonneau M, Vernhettes S. (2007) Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 15572–15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway ME, Heckman JW, Jr, Schiefelbein JW. (1997) Growth and ultrastructure of Arabidopsis root hairs: the rhd3 mutation alters vacuole enlargement and tip growth. Planta 201: 209–218 [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Taylor NG, Turner SR. (2003) Control of cellulose synthase complex localization in developing xylem. Plant Cell 15: 1740–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. (2009) Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11: 797–806 [DOI] [PubMed] [Google Scholar]
- Haigler CH, Brown RM. (1986) Transport of rosettes from the golgi apparatus to the plasma membrane in isolated mesophyll cells of Zinnia elegans during differentiation to tracheary elements in suspension culture. Protoplasma 134: 111–120 [Google Scholar]
- Herth W. (1980) Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J Cell Biol 87: 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth W. (1985) Plasma-membrane rosettes involved in localized wall thickening during xylem vessel formation of Lepidium sativum L. Planta 164: 12–21 [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhong R, Morrison WH, III, Ye ZH. (2003) The Arabidopsis RHD3 gene is required for cell wall biosynthesis and actin organization. Planta 217: 912–921 [DOI] [PubMed] [Google Scholar]
- Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown RM., Jr (1999) Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell 11: 2075–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jurgens G. (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 139: 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. (2010) Arabidopsis—a powerful model system for plant cell wall research. Plant J 61: 1107–1121 [DOI] [PubMed] [Google Scholar]
- Lloyd C, Chan J. (2008) The parallel lives of microtubules and cellulose microfibrils. Curr Opin Plant Biol 11: 641–646 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jurgens G. (1996) Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84: 61–71 [DOI] [PubMed] [Google Scholar]
- Lycett G. (2008) The role of Rab GTPases in cell wall metabolism. J Exp Bot 59: 4061–4074 [DOI] [PubMed] [Google Scholar]
- Moore PJ, Staehelin LA. (1988) Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pratense L.; implication for secretory pathways. Planta 174: 433–445 [DOI] [PubMed] [Google Scholar]
- Mueller SC, Brown RM., Jr (1980) Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J Cell Biol 84: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuhr A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA. (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW. (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR. (2007) Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci USA 104: 15566–15571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E. (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16: 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes F, Orellana A. (2008) Golgi transporters: opening the gate to cell wall polysaccharide biosynthesis. Curr Opin Plant Biol 11: 244–251 [DOI] [PubMed] [Google Scholar]
- Somerville C. (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22: 53–78 [DOI] [PubMed] [Google Scholar]
- Srinivasasainagendra V, Page GP, Mehta T, Coulibaly I, Loraine AE. (2008) CressExpress: a tool for large-scale mining of expression data from Arabidopsis. Plant Physiol 147: 1004–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG. (2008) Cellulose biosynthesis and deposition in higher plants. New Phytol 178: 239–252 [DOI] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. (2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci USA 100: 1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Laurie S, Turner SR. (2000) Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. Plant Cell 12: 2529–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Scheible WR, Cutler S, Somerville CR, Turner SR. (1999) The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. Plant Cell 11: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng FY, Wang Y, Tang BL. (2001) The syntaxins. Genome Biol 2: REVIEWS3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole JM, Nielsen E. (2008) Phosphoinositides in plants: novel functions in membrane trafficking. Curr Opin Plant Biol 11: 620–631 [DOI] [PubMed] [Google Scholar]
- Wightman R, Marshall R, Turner SR. (2009) A cellulose synthase-containing compartment moves rapidly beneath sites of secondary wall synthesis. Plant Cell Physiol 50: 584–594 [DOI] [PubMed] [Google Scholar]
- Wightman R, Turner SR. (2008) The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J 54: 794–805 [DOI] [PubMed] [Google Scholar]
- Zhong R, Burk DH, Nairn CJ, Wood-Jones A, Morrison WH, III, Ye ZH. (2005) Mutation of SAC1, an Arabidopsis SAC domain phosphoinositide phosphatase, causes alterations in cell morphogenesis, cell wall synthesis, and actin organization. Plant Cell 17: 1449–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]