Abstract
Jasmonic acid (JA) and ethylene (ET) are known to play important roles in mediating plant defense against herbivores, but how they affect development in herbivore-attacked plants is unknown. We used JA-deficient (silenced in LIPOXYGENASE3 [asLOX3]) and ET-insensitive (expressing a mutated dominant negative form of ETHYLENE RESPONSE1 [mETR1]) Nicotiana attenuata plants, and their genetic cross (mETR1asLOX3), to examine growth and development of these plants under simulated herbivory conditions. At the whole plant level, both hormones suppressed leaf expansion after the plants had been wounded and the wounds had been immediately treated with Manduca sexta oral secretions (OS). In addition, ectopic cell expansion was observed around both water- and OS-treated wounds in mETR1asLOX3 leaves but not in mETR1, asLOX3, or wild-type leaves. Pretreating asLOX3 leaves with the ET receptor antagonist 1-methylcyclopropane resulted in local cell expansion that closely mimicked the mETR1asLOX3 phenotype. We found higher auxin (indole-3-acetic acid) levels in the elicited leaves of mETR1asLOX3 plants, a trait that is putatively associated with enhanced cell expansion and leaf growth in this genotype. Transcript profiling of OS-elicited mETR1asLOX3 leaves revealed a preferential accumulation of transcripts known to function in cell wall remodeling, suggesting that both JA and ET act as negative regulators of these genes. We propose that in N. attenuata, JA-ET cross talk restrains local cell expansion and growth after herbivore attack, allowing more resources to be allocated to induced defenses against herbivores.
Herbivores substantially affect the accumulation of plant biomass and consequently can threaten plant survival and reproductive success in the natural environment. Plants have countered herbivore attacks by evolving a suite of defense and tolerance responses, which has resulted in what is referred to as the “coevolutionary arms race” in plant-herbivore interactions (Ehrlich and Raven, 1964; Kareiva, 1999). Even though essential for survival, defenses against herbivores are thought to be costly for plants (Baldwin, 1998; Redman et al., 2001; Zavala and Baldwin, 2004; Zavala et al., 2004; Bostock, 2005). It is likely that defense processes directly compete for plant resources allocated to growth and repair of the mechanically wounded tissues. Plant defense-related signals, and their cross talk with development-associated hormones, should therefore play important roles in optimizing resource allocation decisions in plants under biotic stress.
It is well established that plant responses to biotic and abiotic stress stimuli are controlled by defense-related plant hormones (Pieterse et al., 2009); among them, jasmonic acid (JA) is responsible for elicitation of defenses against attack from herbivores and necrotrophic pathogens (Kessler and Baldwin, 2002; Turner et al., 2002; Weber, 2002; Wasternack, 2007). In addition to induction of plant defenses, jasmonates have been shown to play important roles in growth and development, including flower development, tuber formation, tendril coiling, nyctinastic movements, trichome formation, and senescence (Wasternack, 2007). While JA accumulation generally stimulates the above-mentioned processes, it inhibits root and leaf growth and seed germination (Wasternack, 2007; Zhang and Turner, 2008). In conifers, insect attack, mechanical wounding, or treatment with methyl jasmonate (MeJA) and associated increase in ethylene (ET) levels altered the development of xylem cells into traumatic resin ducts, defense-related structures that accumulate defensive terpenoid compounds, oleoresins (Martin et al., 2002; Hudgins and Franceschi, 2004). Large amounts of MeJA also severely inhibited growth of Norway spruce (Picea excelsa) saplings (Martin et al., 2002).
In Arabidopsis (Arabidopsis thaliana), JA biosynthesis is initiated by a wound-mediated release of α-linolenic acid (18:3) from chloroplastic membranes, followed by the activity of several chloroplast-located enzymes, including 13-lipoxygenase (LOX; Delker et al., 2006). Silencing of LOX3, an enzyme responsible for JA biosynthesis in Nicotiana attenuata plants, has been shown to reduce JA levels and impair both direct and indirect defenses in LOX3-silenced plants (Halitschke and Baldwin, 2003), providing a tool for the examination of jasmonate's function in defense regulation as well as its role in growth and development in plants under herbivore attack.
ET gas, a small molecule with hormonal activity in plants, is known to be involved in mediating plant defense responses against herbivores (O'Donnell et al., 1996; Stotz et al., 2000; Ludwig et al., 2005; von Dahl et al., 2007). Like JA, ET is also required for normal plant growth and development, including germination, fruit ripening, senescence, and regulation of cell expansion and elongation (Binder et al., 2004; Chen et al., 2005; Stepanova et al., 2007). In Arabidopsis, ET signal transduction is initiated by ET perception through multiple membrane-bound receptors: ETHYLENE RESPONSE1 (ETR1), ETR2, ETHYLENE RESPONSE SENSOR1 (ERS1), ERS2, and ETHYLENE INSENSITIVE4 (Ecker, 1995; Alonso and Ecker, 2001; Chen et al., 2005). Ectopic heterologous expression of the mutated Arabidopsis ET receptor, etr1-1, has been shown to dominantly repress ET signaling in several plant species, including tomato (Solanum lycopersicum), petunia (Petunia hybrida), Nemesia, and the native tobacco N. attenuata (Bleecker et al., 1988; Chang et al., 1993; Wilkinson et al., 1997; Cui et al., 2004; von Dahl et al., 2007).
In contrast to the defense-related hormones JA and ET, auxin (indole-3-acetic acid [IAA]) is a pleiotropic regulator of plant growth and development, primarily targeting cell division, cell expansion, and cell elongation (Teale et al., 2006). The partially overlapping functions of auxin and JA/ET in controlling growth and development suggest that some of the plant responses to JA and ET could be mediated by changes in auxin concentration and/or perception (for review, see Woodward and Bartel, 2005; Stepanova and Alonso, 2009). JA and ET are known to rapidly accumulate in herbivore-attacked tissues, while leaf IAA concentrations rapidly decline after wounding (Thornburg and Li, 1991), possibly due to effects of ET and JA on IAA metabolism. It has been shown that down-regulation of auxin signaling, mediated by the plant miRNA393, which targets the auxin receptor proteins TRANSPORT INHIBITOR RESPONSE1, AUXIN SIGNALING F-BOX2 (AFB2), and AFB3, is required for resistance of Arabidopsis plants against virulent strains of Pseudomonas syringae (Navarro et al., 2006). In addition, nicotine levels dramatically increase in plants after removing their apical meristems and/or lateral buds, known to be the main sources of auxin in plants (Shi et al., 2006; Wang et al., 2008). In contrast, application of auxin to leaves substantially decreased the accumulation of nicotine in wounded Nicotiana sylvestris plants (Baldwin, 1989; Baldwin et al., 1997), suggesting a general negative role of auxin in JA-mediated accumulation of nicotine.
To examine more closely the hormone cross talk in mechanically wounded and herbivore-attacked plants, namely interactions between JA, ET, and IAA, we used N. attenuata plants silenced in the production of JA (asLOX3) and ET perception (mETR1), and their genetic cross (mETR1asLOX3), and compared growth, transcriptional, and hormonal responses in wounded leaves of these plants. The combination of JA deficiency and ET insensitivity resulted in a novel growth phenotype observed only in mETR1asLOX3 plants, characterized by massive cell expansion around puncture wounds or wounds inflicted by Manduca sexta larvae feeding on the leaves, suggesting that both JA and ET may repress local growth in wild-type N. attenuata leaves after wounding and/or herbivore attack.
RESULTS
Hemizygous mETR1asLOX3 Cross Shows Combined Phenotype of the Parental Lines
Homozygous lines of the native tobacco N. attenuata silenced for LOX3 (asLOX3) and lines ectopically expressing the mutated ETR1 (mETR1) have been characterized previously (Halitschke and Baldwin, 2003; von Dahl et al., 2007). To examine the effects of JA and ET cross talk on plants, we created a new hemizygous cross between these two lines, which was designated in this study as mETR1asLOX3 (Fig. 1A). Because mETR1asLOX3 plants contain only a single allele of the parental transgenes, as LOX3 and mETR1, we first compared the phenotype of the cross with the original homozygous parental lines.
Figure 1.
Hemizygous mETR1asLOX3 plants show combined phenotypes of the parent lines. A, Homozygous mETR1 flowers were pollinated with asLOX3 pollen to produce a hemizygous mETR1asLOX3 cross. B, JA levels in wild-type (WT), mETR1, asLOX3, and mETR1asLOX3 plants at the indicated time points after wounding with a pattern wheel and applying OS from M. sexta (W+OS treatment). C, ET emissions collected from wild-type (white bars), mETR1 (striped bars), asLOX3 (checked bars), and mETR1asLOX3 (black bars) cut leaves over 5 h after wounding with a pattern wheel and applying water (W+W) or after W+OS treatment; control leaves remained untreated. Values in B and C are means of three and five replicate measurements, respectively, with se indicated. FM, Fresh mass.
First, JA concentrations were determined after wounding the plants with a pattern wheel (W) and directly applying 5× diluted oral secretions (OS) from M. sexta larvae to the wounds. The peak of JA accumulation occurred 1 h after elicitation in wild-type and mETR1 plants, reaching more than 1,000 ng g−1 fresh mass in both lines (Fig. 1B). In contrast, JA was found at much lower concentrations, approximately 200 ng g−1 fresh mass, in asLOX3 and mETR1asLOX3 plants, suggesting that LOX3 gene silencing was still as effective in the cross as it was in the parental line (Fig. 1B).
ET accumulation is known to be controlled via ET perception and a feedback loop with ET biosynthesis (Kende, 1993; Zhang et al., 2009). Ectopic expression of the mutated ET receptors thus not only affects ET perception but also increases ET production due to the lack of feedback control, which is often used as an indirect indicator of ET insensitivity (Wilkinson et al., 1997; von Dahl et al., 2007). Prior to elicitation, isolated leaves from all genotypes emitted less than 5 nL ET g−1 fresh mass h−1 (Fig. 1C); wounding with a pattern wheel and directly applying water to the wounds (W+W) showed only moderate effect on ET production in the wild type and asLOX3; however, ET increased to much higher levels in mETR1 and mETR1asLOX3 plants, suggesting that both genotypes are ET insensitive (Fig. 1C). Replacing water with OS had a similar but more profound effect on the ET emissions from wounded N. attenuata leaves (Fig. 1C), consistent with the previous reports of OS strongly potentiating the ET burst in N. attenuata (Kahl et al., 2000; von Dahl et al., 2007).
In summary, despite containing only a single allele of the transgenes, mETR1asLOX3 hemizygous plants showed combined phenotypes of both parental lines, each of them at levels comparable to those found in parental homozygous lines. Therefore, we used wild-type, asLOX3, mETR1, and mETR1asLOX3 plants to examine the cross talk between JA and ET during wounding and herbivory. In this report, we specifically focus on growth-related responses in the plants challenged with simulated herbivory stress and the role of JA and ET in modulating these responses, while defense responses of these plants will be reported elsewhere (C.C. von Dahl, N. Onkokesung, and I.T. Baldwin, unpublished data).
Local Cell Expansion in mETR1asLOX3 Leaves after Wounding and OS Elicitation
JA and ET have been previously implicated in the regulation of plant growth and development (Pierik et al., 2006; Dugardeyn and Van der Straeten, 2008; Zhang and Turner, 2008); therefore, we investigated whether JA or ET, alone or together, could visibly affect N. attenuata growth responses after wounding and/or simulated herbivory using wild-type, mETR1, asLOX3, and mETR1asLOX3 plants. Interestingly, we found an extensive cell expansion around the wounds, which was highly specific to mETR1asLOX3 plants (Fig. 2A). While simple wounding was sufficient to induce ectopic cell expansion on the leaves (see leaf discs in Fig. 3A below), simulated herbivory treatment, which is reportedly associated with significantly higher levels of the putative cell expansion-suppressing signals, JA and ET (Fig. 1, B and C; Halitschke et al., 2001; von Dahl et al., 2007; Wu et al., 2007), showed a comparable phenotypic response. We therefore decided to use OS elicitation in the following experiments because it allowed for stronger contrasts among the responses of the elicited genotypes. Moreover, OS elicitation faithfully simulates most responses elicited by the M. sexta caterpillar feeding on N. attenuata plants (Halitschke et al., 2003). When we closely inspected the wounds caused by a short feeding bout by M. sexta larvae, a response comparable to OS elicitation was observed (data not shown). In contrast, only a very limited development of transparent cells around the wounds and no expansion of these cells were observed in wild-type, mETR1, and asLOX3 leaves, both with simulated (Fig. 2A) and real herbivory treatments (data not shown).
Figure 2.
mETR1asLOX3 leaves expand cells around puncture wounds after W+OS treatment. A, Transparent cells around wounds develop into a large zone of expanded cells in mETR1asLOX3 leaves. WT, Wild type. B, Perimeter size of clear cell zone in W+OS-treated wild-type and mETR1asLOX3 leaves (left; mean from 20 measurements ± se) and puncture holes (right; mean from 20 measurements ± se) measured as indicated by the red lines in the schematics. C, Microscopic analysis of toluidine blue-stained transversal sections of wild-type and mETR1asLOX3 leaf lamina capturing individual puncture wounds at 3, 5, and 7 d after W+OS treatment.
Figure 3.
Lack of JA and ET mediates ectopic cell expansion in mETR1asLOX3. A, Leaf discs from wild-type (WT), mETR1, asLOX3, and mETR1asLOX3 plants were placed on medium containing 0, 5, and 50 μm MeJA, which suppressed local cell expansion in the mETR1asLOX3 genotype in a concentration-dependent manner. B, Leaves from wild-type, mETR1, asLOX3, and mETR1asLOX3 plants were exposed overnight to the ET receptor inhibitor 1-MCP (see “Materials and Methods”) and treated with W+OS the next morning; a larger transparent zone and expanded cells developed in asLOX3 leaves after 5 d, phenocopying the mETR1asLOX3 phenotype. C, Dissection of 1-MCP-treated leaves from 5-d W+OS-elicited wild-type and asLOX3 leaves stained with toluidine blue and observed with a microscope. The red arrow shows enlarged cells in W+OS-elicited asLOX3 leaves treated with 1-MCP.
Time-course microscopic analysis showed that expanded cells in mETR1asLOX3 leaves develop from a transparent cell layer around the wound edges (referred to as the clear zone), which become clearly visible on the 2nd and/or 3rd d after treatment of the leaves with W+OS (Fig. 2B, left). Interestingly, the size of pattern wheel wounds (referred to as puncture holes) began to decrease in mETR1asLOX3 after 3 d (Fig. 2B, right), suggesting that the transparent cells expanded inward, so as to cover the open wound areas. We further dissected the wound sites from mETR1asLOX3 and wild-type leaves to determine the architecture of cells surrounding the wounds. In transverse leaf cross-sections, the transparent cells of mETR1asLOX3 plants appeared as large, expanded, irregularly shaped cells that were completely absent in similarly treated and dissected wild-type leaves (Fig. 2C), suggesting that W+OS-induced cell expansion in mETR1asLOX3 requires the combination of low JA levels and impaired ET signaling after wounding, as confirmed in the following complementation experiments.
JA and ET Deficiency Are Both Indispensable for Local Cell Expansion in mETR1asLOX3 Leaves
Our data suggest that either JA or ET should be sufficient to suppress the development of expanded cells around puncture wounds in mETR1asLOX3 N. attenuata plants. To test this hypothesis, we excised discs from wild-type, mETR1, asLOX3, and mETR1asLOX3 leaves and placed them on agar-containing medium supplemented with two different concentrations of MeJA (5 and 50 μm). On control plates without MeJA, mETR1asLOX3 leaf discs still developed expanded cells at their cut edges, as observed around the puncture wounds on intact leaves. In contrast, wild-type, mETR1, and asLOX3 leaf discs rarely formed any protrusions at their cut edges (Fig. 3A). Consistent with our hypothesis, the development of expanded cells in mETR1asLOX3 leaf discs was substantially reduced by treatment with 5 μm MeJA and completely suppressed in the presence of 50 μm MeJA (Fig. 3A).
To verify the suppressive effect of ET on the mETR1asLOX3 phenotype, we pretreated wild-type, mETR1, asLOX3, and mETR1asLOX3 leaves for 12 h with 1-methylcyclopropane (1-MCP), an ET receptor antagonist (Sisler and Serek, 1997). The leaves were subsequently treated with W+OS and observed 5 d after treatment. During treatment, the leaves were maintained in clip cages and supplemented with fresh 1-MCP in 2-d intervals. No expanded cells developed around wounds on wild-type and mETR1 plants in both control and 1-MCP-treated leaves. In contrast, the number of expanded cells increased in mETR1asLOX3 leaves, and a comparable cell expansion phenotype developed in W+OS-treated and 1-MCP-fumigated asLOX3 leaves (Fig. 3B). The expanded cells around asLOX3 puncture wounds closely resembled those previously observed in mETR1asLOX3 plants, which was further confirmed by dissecting the leaves and observing the wounds with the microscope (Fig. 3C). We conclude from these complementation experiments that ET insensitivity and low JA content after wounding are both indispensable for the appearance of the mETR1asLOX3 phenotype.
Leaf Expansion in Wild-Type Plants Is Significantly Reduced by Simulated Herbivory
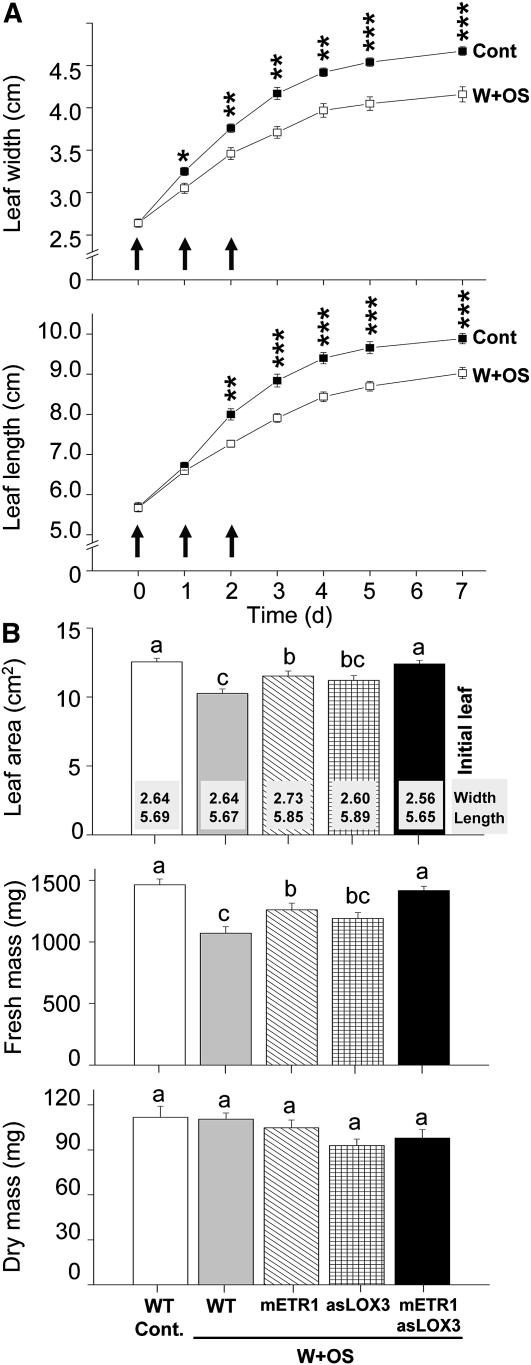
At least two independent studies have reported that leaf growth is suppressed by wounding and that this suppression requires JA signaling (Yan et al., 2007; Zhang and Turner, 2008). However, the contribution of ET and possible cross talk of ET with JA in wound-induced leaf growth inhibition have not yet been examined. To address this question, we first used wild-type leaves at the source-sink transition stage of development with an initial width/length of approximately 2.6/5.7 cm and repeatedly elicited them with W+OS over a 3-d time interval (see “Materials and Methods”). The growth of the treated leaves was compared with the growth of untreated control leaves of the same developmental stage. Previously, leaf width, a nondestructive parameter of leaf growth, and leaf area, which normally requires destructive leaf removal, have been shown to strongly correlate in tobacco plants (Sloan et al., 2009). To obtain leaf growth kinetics, therefore, we first measured leaf width and leaf length after repeated OS elicitations. Both parameters were strongly reduced in the elicited leaves compared with control undamaged leaves, demonstrating a direct inhibitory effect of W+OS-associated signals on leaf growth (Fig. 4A).
Figure 4.
JA and ET negatively affect leaf growth after W+OS treatment. A, A transition leaf of rosette-stage wild-type plants was treated three times with W+OS or left untreated (Cont), and leaf width and length were measured over 7 d. Values are means of 10 replicate leaves from individual OS-elicited wild-type plants with se indicated. Asterisks indicate means that differed significantly between OS-elicited and control leaves at respective time points (Student's t test: *** P < 0.001, ** P < 0.01, * P < 0.05); black arrows indicate days when leaves were treated with W+OS. B, Leaf area, fresh mass, and dry mass were determined 7 d after treatment of leaves with W+OS in wild-type (WT), mETR1, asLOX3, and mETR1asLOX3 plants. Nontreated wild-type leaves (Cont.) are included on the left for comparison. Different letters indicate significant differences between leaves determined by one-way ANOVA with post hoc tests with Bonferroni correction.
After 7 d, the leaves were dissected from the plants and their leaf area and fresh mass were determined. Both parameters were again significantly reduced by W+OS treatment in wild-type leaves (Fig. 4B). We then compared the growth of W+OS-treated leaves from wild-type, mETR1, asLOX3, and mETR1asLOX3 plants, taking care to select leaves with a similar initial size (Fig. 4B; numbers in bars show average leaf widths and lengths in each genotype before treatment) and to subject them to the same amount of damage. The leaves of mETR1asLOX3 plants were the largest and heaviest among all plants treated with W+OS. While W+OS-treated mETR1 and asLOX3 leaves appeared larger than comparably treated wild-type leaves, their growth was intermediate, pointing to the existence of an additive effect between JA and ET (cross talk) in the leaf growth inhibition.
The growth inhibition by W+OS, mediated by JA and ET signaling, can be explained either by decreased rates of cell division or reduced cell expansion, or by a combination of both mechanisms. Because the inhibition of cell division by JA has been shown as a major factor associated with reduced leaf growth in wounded Arabidopsis plants (Zhang and Turner, 2008), we first measured the expression of cyclin B1 in W+OS-elicited N. attenuata leaves. Cyclin B1 is expressed predominantly during G2/M phase of the cell cycle and therefore provides a good marker of cell division in plants (Ito, 2000; Zhang and Turner, 2008). However, the expression of cyclin B1 did not show any significant trends associated with leaves from expanding and nonexpanding tobacco genotypes (Supplemental Fig. S1). Therefore, we measured the dry mass of the leaves and found no statistically significant differences in growth among all treatments and genotypes (Fig. 4B). Even the largest differences in size and fresh mass between W+OS-treated and untreated wild-type leaves were not detectable in the dry mass measured. This disconnection between the leaf fresh and dry mass measurements suggests that JA and ET most likely mediate the suppression of cell expansion, followed by lower water uptake (content) in the smaller cells (Fig. 4B). The difference between Arabidopsis and native tobacco is most probably related to the fact that leaves used in our experiments have already entered the mid growth phase of development, and their growth therefore mainly depended on expansion of the cells (Sloan et al., 2009) that was then affected by W+OS treatments.
Transcriptional Changes in mETR1asLOX3 Leaves
The lack of JA and impaired ET signaling allowed larger leaf growth and the development of an ectopic cell expansion phenotype in W+OS-elicited mETR1asLOX3 leaves, pointing to the modulation of cell wall rigidity by JA and ET as a possible response mechanism. To investigate this possibility, we used a custom cDNA microarray spotted with more than 16,000 cDNA probes from cultivated tobacco cells (Matsuoka et al., 2004; Galis et al., 2006) and investigated global transcriptional changes in the leaves after eliciting them with W+OS. Labeled cDNA probes from mETR1asLOX3 and wild-type leaves at 60 h after W+OS treatment, a time point coinciding with the first visible cell expansion observed around mETR1asLOX3 wounds, were compared and analyzed for differential gene expression. In addition, the expression profiles of mETR1 and asLOX3 leaves relative to those of wild-type plants were analyzed to distinguish among single hormone- and JA/ET cross talk-mediated transcriptional changes after W+OS treatment.
We found 50 genes with 1.5-fold or more induced expression in mETR1asLOX3 compared with wild-type plants, but only five and three genes were induced relative to the wild type in single hormone-deficient plants, mETR1 and asLOX3, respectively (Fig. 5; Supplemental Table S1). As predicted from morphological observations and growth measurements of the leaves, the genes with possible functions in cell wall remodeling were enriched in the up-regulated gene list of W+OS-treated mETR1asLOX3 leaves (13 of 50 genes; Supplemental Table S1). Three up-regulated genes putatively associated with cell wall expansion in mETR1asLOX3 leaves were further analyzed by quantitative real-time (qRT)-PCR using leaf samples collected at 0, 6, 20, and 60 h after W+OS treatment. Pectin methylesterase (+2.7-fold change; BP131988) and expansin (+1.9-fold change; BP531140), previously shown to mediate cell expansion in other plant species (Micheli, 2001; Pien et al., 2001; Gao et al., 2008), showed more abundant transcripts in mETR1asLOX3 compared with wild-type and remaining transgenic lines (Fig. 6). Furthermore, transcripts of the auxin-inducible ROOT SYSTEM INDUCIBLE-1 (RSI-1) gene (+2.8-fold change; BP530317) with a proposed function in the regulation of auxin action (Cheong et al., 1999; Kwon et al., 1999) were more abundant in mETR1asLOX3 leaves after W+OS, suggesting that RSI-1 might be involved in the enhanced cell expansion in mETR1asLOX3 leaves (Fig. 6). In contrast, the up-regulation of two selected genes (BP533791 and BP131199) involved in photosynthesis (Supplemental Table S1) could not be confirmed by qRT-PCR using cDNA samples from an independent experiment.
Figure 5.
cDNA microarray analysis revealed a large number of genes specifically regulated in mETR1asLOX3 leaves. cDNA samples from mETR1, asLOX3, and mETR1asLOX3 were hybridized against wild-type leaf samples prepared at 60 h after W+OS treatment. Venn diagrams of up-regulated (left) and down-regulated (right) transcripts show a large group of genes specifically regulated in mETR1asLOX3 leaves (≥1.5-fold change; P ≤ 0.05; FDR ≤ 5%; n = 3).
Figure 6.
qRT-PCR transcript abundance of selected genes with function related to cell wall remodeling. Relative transcript abundance of pectin methylesterase (PME), expansin, and RSI-1 in wild-type (WT), mETR1, asLOX3, and mETR1asLOX3 leaves was measured by qRT-PCR before and 6, 20, and 60 h after W+OS treatment. PME (BP131988), Expansin (BP531140), and RSI-1 (BP530317) genes show higher transcript abundance at 20 and 60 h after treatment, confirming the initial microarray hybridization data at 60 h. Relative values are means of three biological replicates ± se.
In summary, an elevated expression of two putative cell wall remodeling-associated genes and the RSI-1 gene in mETR1asLOX3 plants, confirmed by qRT-PCR analysis, provided a strong indication that changes in cell wall structure are most likely responsible for the altered leaf growth phenotype in mETR1asLOX3 plants. Interestingly, only the leaves in their mid growth phase were able to respond to ectopically produced expansin from overexpressing the expansin gene in tobacco plants (Sloan et al., 2009), a coincidence consistent with the results of our microarray experiments, and the presence of higher expansin transcript levels in W+OS-elicited mETR1asLOX3 leaves.
IAA Content Is Higher in W+OS-Treated mETR1asLOX3 Leaves
The endogenous auxin (IAA) is known to regulate growth and development in plants (Chen, 2001; Swarup et al., 2002; Woodward and Bartel, 2005), and the exogenous application of auxin is sufficient to induce callus proliferation in plant tissues cultivated in vitro (Skoog and Tsui, 1948). The spontaneous cell expansion on wound edges in mETR1asLOX3 leaf discs and larger leaf size in mETR1asLOX3 plants after W+OS treatment led to the hypothesis that the changes in endogenous IAA content could be responsible for these responses. Therefore, we determined IAA concentrations in wild-type, mETR1, asLOX3, and mETR1asLOX3 transition leaves before and 3, 5, and 7 d after a single treatment with W+OS using six rows of puncture wounds per leaf. Before treatment, leaves showed no statistically significant differences in IAA content among all four genotypes (P ≤ 0.05), even though the levels of IAA tended to be higher in mETR1asLOX3 leaves (Fig. 7A). IAA concentrations uniformly decreased in all genotypes up to 3 d after W+OS treatment; however, while IAA concentrations continued to decline in W+OS-elicited wild-type leaves at 5 and 7 d after treatment, IAA content started to increase again in asLOX3, mETR1, and most markedly in mETR1asLOX3 leaves (Fig. 7A). The kinetic of IAA concentrations correlated with the time-resolved growth of expanded cells on mETR1asLOX3 leaves (Fig. 2A), suggesting that higher IAA concentrations could play an important role in mediating late (approximately 5–7 d) cell expansion of mETR1asLOX3 leaf cells. The whole leaf analysis of IAA levels in the leaves takes into account neither the local distribution of IAA across the leaf lamina nor the local sensitivity of cells to IAA, which may have also differed and contributed to the penetrance of the expanded cell phenotype around mETR1asLOX3 leaf wounds.
Figure 7.
IAA levels are higher in mETR1asLOX3 leaves after W+OS treatment, and mETR1 plants show increased sensitivity to IAA. A, IAA concentrations in wild-type (WT), mETR1, asLOX3, and mETR1asLOX3 leaves measured before and 3, 5, and 7 d after W+OS treatment. Values are means of three biological replicates with se indicated. Different letters indicate means that differed significantly between genotypes at 7 d post treatment; IAA contents between genotypes at 0, 3, and 5 d were not statistically different (Mann-Whitney test: P < 0.05). FM, Fresh mass. B, Wild-type, mETR1, asLOX3, and mETR1asLOX3 leaves were treated with W+OS and supplemented with 50 μL of IAA in water (1 μg mL−1; repeated in 2-d intervals) or water without IAA (mock). The size of the clear zone was measured after 7 d; values are means of 20 measurements with se indicated. Asterisks indicate means that differed significantly between IAA-treated and untreated leaves in respective genotypes (Student's t test: ** P < 0.01). C, Microscopic images of puncture wounds 7 d after treatment with W+OS in wild-type, mETR1, asLOX3, and mETR1asLOX3 leaves maintained in the presence or absence of IAA (1 μg mL−1).
At last, we tested whether the exogenous application of IAA could promote local cell expansion in otherwise “nonexpanding” genotypes: wild-type, asLOX3, and mETR1. While the first two genotypes showed no visible response to IAA, a statistically larger clear zone around puncture wounds (Fig. 7B) and more expanded cells (Fig. 7C) developed in mETR1 plants treated with 1 μg mL−1 IAA, suggesting that ET insensitivity may have altered the sensitivity to IAA in mETR1 leaves, promoting ectopic cell expansion even in the presence of normal wound-induced levels of JA in these plants (Fig. 1B).
DISCUSSION
Parallel evolution of specialist and generalist insect herbivores with plants promoted an accelerated development of defense responses and novel attack strategies in plants and herbivores, respectively (Kareiva, 1999). Our data suggest that, as a result of this process, local leaf growth has been independently concealed in N. attenuata plants, and possibly other terrestrial plants, by two intersecting defense-related signaling pathways mediated by ET and JA, ensuring that local outgrowth would be diminished in herbivore attacked N. attenuata plants. This plant behavior can be interpreted as a means by which strongly defense-oriented plants redirect their resources from further growth into defensive metabolite production (e.g. nicotine or trypsin proteinase inhibitors). We provide experimental evidence that the local growth potential of N. attenuata plants (Fig. 2A), while silently resident in this strongly defended plant species, can be reactivated by simultaneously suppressing JA and ET signaling pathways with transgenic technology. Our findings are consistent with the existence of a trade-off between growth and defense that has been proposed to maximize plant fitness during adverse growth conditions (Baldwin, 1998). Currently, we are also using the mETR1asLOX3 N. attenuata plants to examine the role of ET and its cross talk with JA in plant defense against the specialist herbivore M. sexta (C.C. von Dahl, N. Onkokesung, and I.T. Baldwin, unpublished data).
Documented Growth Inhibitory Effects of JA and ET in Plants
The accumulation of toxic metabolites in local, herbivore-attacked as well as systemic plant tissues has been proposed to result in considerable metabolic cost to the defending plants, lowering their reproductive fitness in the natural environment (McKey, 1974; Baldwin, 1998; Purrington, 2000). In the so called “arms races” between plants and herbivores, plants have evolved inducible defense systems leading to significant resource conservations (Karban and Baldwin, 1997). Therefore, two major wound and herbivory-associated signals, JA and ET, while mediating inducible defenses, may also function as major switches between growth and defense (Fig. 8) and the associated changes in resource allocations.
Figure 8.
Cross talk between JA and ET regulates defense against herbivores and growth in N. attenuata plants. Wounding and herbivore-associated elicitors (fatty acid-amino acid conjugates) trigger biosynthesis of JA and ET, which activate defense against herbivores, while suppressing IAA levels and expansion of the leaves. ET- and JA-mediated suppression of cell wall-modifying enzymes and their transcripts, including expansin and pectin methylesterase (PME) enzymes, is proposed as a mechanism leading to reduced growth and local expansion of leaves after simulated herbivory treatment.
When N. attenuata plants were challenged with simulated herbivory, the lack of JA and ET signals in transgenic plants resulted in greater leaf expansion of W+OS-treated leaves compared with wild-type plants (Fig. 4B). Excessive ET production and dominant mutations in ET signal transduction, for example in constitutive triple response1 plants, have been shown to result in dwarfism and a smaller leaf phenotype in Arabidopsis plants (Kieber et al., 1993; Knoester et al., 1997). In contrast, ET-insensitive plants such as etr1 and ers1 produce larger leaves (Bleecker et al., 1988; Grbic and Bleecker, 1995; Hua et al., 1995), consistent with the proposed role of ET, together with JA, in suppressing leaf expansion in tobacco leaves.
Leaf growth in plants is initially facilitated by active cell divisions in the leaf lamina, which is replaced by extensive cell expansion in later growth phases (Sloan et al., 2009). Given that herbivory and/or wounding reduce leaf growth (Yan et al., 2007; Zhang and Turner, 2008; this report), both cell division and cell expansion could be possible targets of JA and ET action in preventing additional growth, putatively enhancing the accumulation of various defense metabolites used against herbivores. It was shown previously that JA and ET inhibited the growth of isolated cells, seedlings, and mature plants (Pierik et al., 2006; Wasternack, 2007). In addition, the root growth inhibition assays are routinely used to screen seedlings for JA insensitivity (Staswick et al., 1992; Ellis et al., 2002), although the mechanism(s) of this inhibition is not well understood.
As a potential explanation, Swiatek et al. (2002) showed that exogenously applied JA inhibits tobacco BY-2 cell proliferation by arresting the cells in the G1 phase of their cell cycle. Recently, Zhang and Turner (2008) demonstrated that increasing endogenous JA levels in vivo caused by continuous wounding of Arabidopsis plants reduced their growth by suppressing mitosis in the young leaves. However, the leaf growth inhibition described in our study is associated with a smaller leaf size as well as reduced fresh mass, but not dry mass, of leaves, suggesting that a decrease in cell expansion might be responsible for the observed growth differences. In a closer comparison of the two studies, 10 leaves on each plant were wounded in the Arabidopsis experiment, one leaf per day over a period of 10 d, while we treated, once per day, the same N. attenuata leaf with W+OS at days 0, 1, and 2 (Fig. 4). Therefore, while the very young leaves in Arabidopsis were influenced by systemically accumulated JA, our locally treated leaves were already in the mid stage of development that associates with cell expansion rather than with cell division (Sloan et al., 2009), which was then less affected by simulated herbivory treatments on the local leaves.
Restriction of Growth by Increasing Rigidity of Plant Cell Walls during Herbivory
Cell expansion in plants is normally counteracted by the existence of rigid cell walls, composed of complex polysaccharide network and structural proteins (Cosgrove, 2005). However, a complete lack of flexibility would be detrimental to plants living in extremely variable conditions; for example, rice seedlings avoid submergence by rapid coleoptile elongation (Vriezen et al., 2003), a response to high water levels that would be constrained by rigid cell walls. Therefore, plants evolved specific mechanisms that allow rapid and reversible modifications of cell walls in response to specific environmental changes (Cosgrove, 2005).
Because the cells around wounds in mETR1asLOX3 plants significantly expanded (Fig. 2C), we proposed that cell wall-loosening mechanisms, negatively regulated by JA and ET, could be involved in this unusual ectopic response in mETR1asLOX3 leaves. Indeed, a comparison of global transcript profiles of asLOX3, mETR1, and mETR1asLOX3 leaves with those of wild-type plants in response to W+OS elicitation revealed that ectopic cell expansion in mETR1asLOX3 is associated with a preferential accumulation of transcripts related to cell wall loosening, including expansin, extensin, endo-β-1,4-glucanase, and several pectin methylesterases (Supplemental Table S1; Cosgrove, 2005; Irshad et al., 2008). In particular, expansins are well-known primary cell wall-loosening agents that disrupt noncovalent bonds of polysaccharides, making cell walls more flexible and expandable (McQueen-Mason and Cosgrove, 1995; Cho and Kende, 1997; Cosgrove et al., 2002; Cosgrove, 2005). Importantly, a strong correlation between transcript accumulation of expansins and growth responses has already been demonstrated in plants (Cosgrove, 2000), supporting the idea that expansin's transcriptional changes observed in mETR1asLOX3 leaves (Fig. 6) could be directly associated with the ectopic cell expansion in these plants.
IAA Levels Are Controlled by JA-ET Cross Talk after W+OS Elicitation
While the potential mechanisms involved in cell expansion in mETR1asLOX3 leaves have been revealed by the microarrays, the actual signal(s) mediating cell expansion after wounding in mETR1asLOX3 remained elusive. When we examined IAA content as a potential mediator of cell and leaf expansion in mETR1asLOX3 leaves, higher IAA levels associated with the late growth phase of W+OS-treated mETR1asLOX3 leaves were found. In Arabidopsis, wounding has been shown to down-regulate a number of genes that are positively associated with response to the auxin (Cheong et al., 2002), which suggests a suppressive effect of wound-induced signals, like ET and JA, on the auxin signal transduction pathway after wounding. In addition, suppression of the basipetal IAA transport (Shi et al., 2006; Wang et al., 2008) and application of auxin to the wounds (Baldwin, 1989; Baldwin et al., 1997) have been shown to increase and decrease nicotine accumulation in tobacco leaves, respectively, indicative of a general negative effect of IAA on induced plant defenses against herbivores.
ET is known to interact with IAA at multiple levels, including IAA biosynthesis, transport, and perception (Woodward and Bartel, 2005); however, in most of these interactions, ET exerts synergistic effects on IAA function and/or biosynthesis, usually examined in the roots and young seedlings and most frequently in hypocotyls (Visser et al., 1996; Vandenbussche et al., 2003; Stepanova et al., 2005, 2007; Ivanchenko et al., 2008). In contrast, mETR1asLOX3 plants showed increased auxin levels and increased cell expansion in the leaves, indicative of JA-ET cross talk antagonizing the accumulation of IAA in wounded tissues (Thornburg and Li, 1991). It suggests that synergistic effects of ET on auxin may be tissue specific, limited to roots and seedlings, while the antagonistic effect of ET on IAA in leaves may be similar to that found during abscission of fruits and flowers (Brown, 1997).
Recently, Trp conjugates with JA (JA-Trp) have been shown to function as endogenous auxin function inhibitors in Arabidopsis, offering an alternative model for the antagonism of JA on auxin signal transduction after wounding (Staswick, 2009). However, the amounts of JA-Trp found in wounded Arabidopsis leaves are relatively small, making JA-Trp conjugate unlikely to be the strong negative regulator of IAA-mediated responses in these plants. Despite our attempts, JA-Trp has not been identified in W+OS-elicited N. attenuata leaves (Wang et al., 2007).
JA, ET, and auxin cross talk in mETRasLOX3 plants is not limited to changes in IAA concentrations after W+OS elicitation. A simultaneous treatment of mETR1 leaves with exogenous IAA produced significantly larger expansion zones around the wounds (Fig. 7, B and C), which were not observed in the two other genotypes, asLOX3 and the wild type (Fig. 7C). This suggests that in addition to contributing to increased IAA levels in mETR1asLOX3 leaves, ET insensitivity may also play an important role in the regulation of cell sensitivity to IAA. Alternatively, IAA could have reduced the perception or production of JA in wounded mETR1 leaves. Previously, small molecules have been shown to influence hormone perception in other systems. For example, a 22-amino acid peptide flg22 derived from bacterial flagellin protein induced the microRNA-mediated degradation of several F-box proteins belonging to the auxin receptor family in Arabidopsis (Navarro et al., 2006), pointing to small RNAs as regulators of hormone perception in plants.
Recently, a short-lived ET and auxin-regulated tomato transcriptional regulator, S. lycopersicum IAA3 (Sl-IAA3), a member of the auxin/IAA gene family, has been shown to connect auxin and ET signal transduction pathways. An antisense-mediated down-regulation of Sl-IAA3 transcription resulted in auxin- and ET-related phenotypes, including altered apical dominance, lower auxin sensitivity, exaggerated apical hook curvature in the dark, and reduced petiole epinasty in the light (Chaabouni et al., 2009). Similarly, ERF1, an ethylene-responsive element binding family transcription factor, and ORA59, an AP2/ERF domain transcription factor, have been proposed to integrate JA and ET signaling pathways in plants (Lorenzo et al., 2003; Pre et al., 2008). Functional analysis of these proteins and the quantification of other JA conjugates, including JA-Trp, in the wounded tobacco leaves should help to unravel the molecular basis of ectopic cell and leaf expansion found in mETR1asLOX3 leaves and to elucidate the mechanisms of cross talk between defense- and growth-related signals (Fig. 8).
Natural Occurrence of the mETR1asLOX3 Phenotype
A response similar to the W+OS-elicited mETR1asLOX3 leaf phenotype was identified in other plants. For example, young leaves of Brassica oleracea geminifera “Rosella” plants respond to wounding by the formation of a large transparent zone around wounds, followed by visible cell enlargement (Supplemental Fig. S2). It remains to be determined whether these plants are deficient in ET perception; however, the relatively low levels of JA (20–40 ng g−1 fresh mass) have been reported in B. oleracea after 12 to 30 caterpillars were allowed to feed on these plants for 24 h (Bruinsma et al., 2009), which suggests that induced JA responses in this species are strongly down-regulated. For comparison, JA levels of more than 1,000 ng g−1 fresh mass are commonly measured in N. attenuata leaf tissues within a 2-cm feeding zone of M. sexta caterpillars after 22 h of feeding (Skibbe et al., 2008). It would be interesting to see if exogenously increasing JA and/or ET levels could prevent the local growth responses in wounded B. oleracea leaves, resulting in a no-local-expansion N. attenuata-like phenotype in these plants.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type and transformed lines of Nicotiana attenuata were germinated on Gamborg's B5 medium (Duchefa; http://www.duchefa.com) as described previously by Krügel et al. (2002). The transformed plants used were 35s-etr1a, harboring a mutated form of the Arabidopsis (Arabidopsis thaliana) ET receptor etr1-1 as described by von Dahl et al. (2007; mETR), antisense (as) LOX3 as described by Halitschke and Baldwin (2003; asLOX3), and the genetic cross between 35s-etr1a and asLOX3, prepared in this study (mETR1asLOX3). Seeds were germinated and maintained in a 26°C/16-h, 155 μmol m−2 s−1 light:24°C/8-h dark cycle (Percival) for 10 d, and young seedlings were planted individually in soil in Teku plastic pots (Pöppelmann). Ten days later, early rosette plants were transferred to soil in 1-L pots and grown in the glasshouse with a day/night cycle of 16 (26°C–28°C)/8 (22°C–24°C) h under supplemental light from Master Sun-T PIA Agro 400 or Master Sun-T PIA Plus 600W high-pressure sodium lamps (Philips). Rosette-stage plants were approximately 4 weeks old when used for the experiments.
JA and ET Measurements and Plant Treatments
JA was measured by the 1200L triple quadrupole tandem mass spectrometry system (Varian; http://www.varianinc.com) as described by Diezel et al. (2009). ET emissions were measured with a photoacoustic spectrometer (INVIVO; https://www.invivo-gmbh.de) as described by Wu et al. (2007). For plant treatments, a source-sink transition rosette leaf from each plant was wounded with a fabric pattern wheel to produce three puncture rows on each side of the midvein. Fresh wounds were immediately treated with either 20 μL of water or 20 μL of 1:5 (v/v) water-diluted OS from Manduca sexta. Control plants remained untreated. M. sexta larval OS were collected after larvae were reared on N. attenuata wild-type plants until the third to fifth instar; OS were collected after regurgitation through a Teflon tube connected to vacuum and stored under argon at −20°C.
For leaf expansion measurements, a single transition rosette leaf from 10 independent plants of the wild type and each of the transformed genotypes was wounded with a fabric pattern wheel to produce one row of wounds on each side of the midvein. Fresh wounds were immediately treated with 20 μL of 1:5 (v/v) water-diluted OS from M. sexta. The same treatment was repeated on days 1 and 2, producing one additional row of wounds each time. Control leaves remained untreated. Width and length of the leaves were determined until 7 d after the first treatment. The leaves were then dissected from the plants, and their leaf area (SigmaPlot; http://www.sigmaplot.com), fresh mass, and dry mass were determined.
For treatments of leaves with exogenous IAA, transition leaves of rosette-stage wild-type, mETR1, asLOX3, and mETR1asLOX3 plants were treated with W+OS, immediately followed by the application of 1 μg mL−1 IAA water solution. The IAA solution (50 μL) was evenly distributed by pipette over the wounds. The same amount of IAA was reapplied to leaves every 2nd d; control leaves were W+OS elicited, and 50 μL of water was applied instead of IAA at the same time intervals.
Leaf Microdissection and Light Microscopy
N. attenuata wild-type and transgenic leaves were wounded with a fabric pattern wheel to produce two puncture rows on each side of the midvein. Freshly wounded leaves were treated with OS as described above, and leaves were harvested at 1, 3, 5, and 7 d after treatment. Each row of puncture wounds was observed with a light microscope (Leica Microsystem; http://www.leica-microsystems.com), and individual puncture wound images were stored with LM Image Manager (Leica Microsystem; http://www.leica-microsystems.com). The diameter of puncture wounds and the size of the transparent zone around each puncture were measured with Adobe Photoshop CS version 8.0 software (http://adobe.com); at least 20 punctures distributed across the whole leaf lamina were examined per genotype and treatment.
Wounded parts of the leaves were fixed and embedded in hydroxyethylmethacrylate resin (Technovit 7100; Heraeus Kulzer; http://www.Kulzer-Technik.de) as recommended by the manufacturer. Embedded samples were dissected using a rotation microtome (Micro International) and stained with toluidine blue to increase image contrast. Stained sections were observed with a Zeiss Stereo Discovery V.8 microscope (Carl Zeiss; http://www.zeiss.de), and images were created and stored with AxioVision 4.5 software.
1-MCP Treatment
To inhibit ET perception, leaves were exposed to 1-MCP, an ET receptor antagonist: 250 mg of SmartFresh (3.3% 1-MCP [AgroFresh; Rohm and Haas]) was dissolved in 25 mL of alkaline solution (0.75% KOH + NaOH in a 1:1 ratio) to release the active substance, 1-MCP. A total of 500 μL of activated solution of 1-MCP was infiltrated into a cotton bud and immediately placed inside of the clip cages attached to the leaves. Leaves were preexposed overnight to 1-MCP before wounding with a pattern wheel and immediately applying 1:5 diluted (v/v) M. sexta OS. Leaves were continuously maintained in the clip cages with 1-MCP, which was replaced every 2 d. Control leaves were kept in the identical clip cages supplied with an equivalent amount of the solvent alkaline solution. Tissues from wounded areas were fixed, embedded, and sectioned as described above.
In Vitro Cultivation of Leaf Discs
Five mature leaves from wild-type and transformed lines were sterilized with 2% (w/v) dichloroisocyanuric acid (Sigma-Aldrich; http://www.sigmaaldrich.com) and washed three times in sterile water. Four discs per leaf were punched out from the middle part of the leaves, avoiding tip and leaf bases that differed substantially in their morphogenetic potential. Leaf discs were placed on Murashige and Skoog medium (Duchefa; http://www.duchefa.com) supplied with 3% Suc and 0, 5, or 50 μm methyl jasmonate (Sigma-Aldrich; http://www.sigmaaldrich.com). Plates with leaf discs were incubated in a 26°C/16-h, 155 μmol m−2 s−1 light:24°C/8-h dark cycle (Percival) for 10 d before taking images of the leaf discs.
IAA Measurements
Three transition leaves from three individual plants of each wild-type and transformed genotype were treated with OS as described for the hormone analysis and pooled for each measurement. Leaves were harvested before and 3, 5, and 7 d after treatment, snap frozen in liquid nitrogen, and kept at −80°C until analyzed. Approximately 1 g of ground leaf material was extracted overnight by diffusion in the dark at −20°C using 10 mL of 100% methanol supplemented with 25 mm antioxidant (diethyldithocarbamic acid; Sigma-Aldrich; http://www.sigmaaldrich.com) and 50 ng of internal standard (99% phenyl-13C6-indole-3-acetic acid; Cambridge Isotope Laboratories; http://www.isotope.com). Samples were centrifuged at 3,000g and 4°C for 30 min, and supernatants were collected before reextracting the pellets with 5 mL of 100% methanol/25 mm diethyldithocarbamic acid on ice for 30 min. Samples were centrifuged as before, and supernatants were combined in single tubes. Water was added to adjust the final concentration of methanol in each sample to 50% (v/v) methanol, and diluted samples were purified using preconditioned Supelclean LC-18 SPE columns (Supelco; http://www.sigmaaldrich). Flow-through fractions were immediately applied to activated DEAE Sephadex A25 columns (Amersham Pharmacia Biotech; http://www.gelifesciences.com) equilibrated with 50% methanol, and IAA was absorbed to the resin by gravity flow. Columns were additionally rinsed with 50 mL of 50% (v/v) methanol, and new Supelclean LC-18 SPE columns were connected bellow the DEAE columns. IAA was eluted with 6% (v/v) formic acid (Riedel-de Haen; http://www.sigmaalrich.com) and trapped on SPE columns. IAA retentate was subsequently eluted from the SPE columns with 5 mL of pure diethyl ether (Fluka; http://www.sigmaaldrich). The acidic water phase was quickly removed by pipetting, and samples were immediately dried under a stream of nitrogen. Dry samples were quantitatively redissolved in 1.5 mL of 100% methanol, transferred to Eppendorf tubes, and dried under vacuum in Eppendorf Concentrator 5301 (http://www.eppendorf.com).
Before measurements, samples were redissolved in 150 μL of 70% (v/v) methanol, centrifuged at 16,000g and 4°C for 30 min, and supernatants were applied to the 1200L quadrupole tandem mass spectrometry system (Varian; http://www.varianinc.com). A total of 10 μL was injected onto a Prodigy column (150 × 2 mm, 3 μm diameter; Phenomenex) attached to a C18 precolumn (4 × 2 mm; Phenomenex). The mobile phase comprised solvent A (0.05% acetic acid) and solvent B (acetonitrile) used in a gradient mode time/concentration as follows (min/%B): 1.5/20, 6/97, 17/97, 18/20, and 25/20. IAA was detected as negative ions in multiple reaction monitoring mode: molecular ions M-H (−) at mass-to-charge ratio (m/z) 174 generated from endogenous auxin and from its internal standard [molecular ion M-H (−) at m/z 180] were fragmented under 35 V collision energy. The product ions of auxin and its internal standard were m/z 130 and 136, respectively. The ratio of ion intensities of the product ions was normalized to the fresh mass of the samples and used to directly quantify auxin.
cDNA Microarrays
To analyze transcriptional changes, wild-type and transformed plants were treated with W+OS as described above, and leaf material from 15 individual plants in each transgenic genotype and 45 corresponding wild-type plants were harvested at 60 h after induction. For each microarray experiment, five leaves of five independent plants were pooled to obtain three biological replicates before total RNA was extracted from the leaves. mRNA was isolated, reverse transcribed, and labeled as described previously (Halitschke et al., 2003). To monitor relative transcriptional changes in each genotype, treated samples from transgenic lines were always hybridized against identically treated wild-type samples.
We used a custom-made 16K tobacco microarray spotted in-house (VersArray; Bio-Rad; http://www.bio-rad.com) with PCR-amplified tobacco cDNA clones that were a gift from the Plant Science Center RIKEN Institute and described previously by Galis et al. (2006). Spotted arrays were hybridized and washed as described by Halitschke et al. (2003). Spot intensities for Cy3 (transgenic lines) and Cy5 (wild-type) signals were analyzed with AIDA software (Raytest; http://www.raytest.com), and global background-subtracted signal intensities (Supplemental Tables S2–S4) were Lowess normalized using MIDAS software (http://www.tm4.org). Significance analysis of microarrays (two class-paired test; Tusher et al. 2001) and Student's t test were used to determine significantly regulated genes with normalized signal ratios of Cy3/Cy5 ≥ 1.5 (up-regulated) and Cy3/Cy5 ≤ 0.66 (down-regulated) between transgenic genotypes and the wild type (false discovery rate [FDR] ≤ 5%; P ≤ 0.05; Supplemental Table S1). Selected up-regulated genes from mETR1asLOX3 plants were validated with qRT-PCR at 60 h and three additional time points (0, 6, 20 h) after W+OS treatment.
qRT-PCR Analysis
Total RNA was isolated from approximately 150 mg of leaf tissue using Trizol reagent following the manufacturer's protocol (Invitrogen; http://www.invitrogen.com). Crude RNA samples were treated with RQ1 DNase (Promega; http://www.promega.com), followed by phenol-chloroform extraction and ethanol precipitation. DNA-free RNA samples were reverse transcribed using oligo(dT)18 primer and SuperScript II enzyme (Invitrogen; http://www.invitrogen.com) following the manufacturer's recommendations. qRT-PCR of the candidate genes selected from the microarray study was performed with a Stratagene MX3005P instrument (http://www.stratagene.com) as recommended by the manufacturer. For normalization purposes, primers specific for the elongation factor-1α gene from tobacco (accession no. D63396) were used as an internal standard to adjust concentrations of cDNA in the samples. Specific primers in the 5′ to 3′ direction used for SYBR Green-based analyses of up-regulated genes were as follows: PME3-F, 5′-CCCCGGAGTGTTCAGCTTGAAC-3′; PME3-R, 5′-ATGCCTAGTAGGAGCGTCAACAAG-3′; EXP-F, 5′-CATCGCTCTTCTACTTCATGGA-3′; EXP-R, 5′-CTCTATTCTTCCCCTCCATTCAC-3′; RSI-F, 5′-CCACAAACAAACATGCCCTTGC-3′; RSI-R, 5′-CCGGTACGACAACTCATCTTAGAC-3′.
Statistical Analysis
Data were analyzed with StatView software, and statistical significance is displayed where relevant to the measurements.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BP131988, BP531140, BP530317, BP533791, BP131199, and D63396.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Leaf cyclin B1 expression shows no major differences between wild-type and mETR1asLOX3 plants after treatment with W+OS.
Supplemental Figure S2. Mechanically wounded B. oleracea leaves respond to wounding as N. attenuata mETR1asLOX3 leaves.
Supplemental Table S1. List of up- and down-regulated genes in W+OS-treated mETR1asLOX3/wild-type, asLOX3/wild-type, and mETR1/wild-type leaves (60 h post elicitation; fold change ≥ 1.5; P ≤ 0.05; FDR ≤ 5%; n = 3).
Supplemental Table S2. Raw and MIDAS-normalized microarray data values from W+OS-treated mETR1asLOX3 and wild-type leaves, 60 h post elicitation.
Supplemental Table S3. Raw and MIDAS-normalized microarray data values from W+OS-treated asLOX3 and wild-type leaves, 60 h post elicitation.
Supplemental Table S4. Raw and MIDAS-normalized microarray data values from W+OS-treated mETR1 and wild-type leaves, 60 h post elicitation.
Supplementary Material
Acknowledgments
We thank A. Weber and A. Schünzel for taking care of the plants in the glasshouse, Dr. M. Schöttner for analytical and technical support, Dr. K. Gase for assistance with microarray spotting and gene cloning, W. Kröber for help with microarray processing, and E. Wheeler for editorial assistance. We acknowledge Rohm and Haas for providing the ET receptor antagonist SmartFresh 3.3%.
References
- Alonso JM, Ecker JR. (2001) The ethylene pathway: a paradigm for plant hormone signaling and interaction. Sci STKE 2001: re1. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. (1989) Mechanism of damage-induced alkaloid production in wild tobacco. J Chem Ecol 15: 1661–1680 [DOI] [PubMed] [Google Scholar]
- Baldwin IT. (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA 95: 8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Zhang ZP, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. (1997) Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201: 397–404 [Google Scholar]
- Binder BM, O'Malley RC, Wang WY, Moore JM, Parks BM, Spalding EP, Bleecker AB. (2004) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bostock RM. (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43: 545–580 [DOI] [PubMed] [Google Scholar]
- Brown KM. (1997) Ethylene and abscission. Physiol Plant 100: 567–576 [Google Scholar]
- Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, van Loon JJA, Dicke M. (2009) Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. J Exp Bot 60: 2575–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaabouni S, Jones B, Delalande C, Wang H, Li ZG, Mila I, Frasse P, Latche A, Pech JC, Bouzayen M. (2009) Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J Exp Bot 60: 1349–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. (1993) Arabidopsis ethylene-response gene Etr1: similarity of product to 2-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chen JG. (2001) Dual auxin signalling pathways control cell elongation and division. J Plant Growth Regul 20: 255–264 [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. (2005) Ethylene signal transduction. Ann Bot (Lond) 95: 901–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JJ, Lee GH, Kwon HB. (1999) Expression and regulation of the RSI-1 gene during lateral root initiation. J Plant Biol 42: 259–265 [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S. (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129: 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Kende H. (1997) Expression of expansin genes is correlated with growth in deepwater rice. Plant Cell 9: 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D. (2002) The growing world of expansins. Plant Cell Physiol 43: 1436–1444 [DOI] [PubMed] [Google Scholar]
- Cui ML, Takada K, Ma B, Ezura H. (2004) Overexpression of a mutated melon ethylene receptor gene Cm-ETR1/H69A confers reduced ethylene sensitivity in a heterologous plant, Nemesia strumosa. Plant Sci 167: 253–258 [Google Scholar]
- Delker C, Stenzel I, Hause B, Miersch O, Feussner I, Wasternack C. (2006) Jasmonate biosynthesis in Arabidopsis thaliana: enzymes, products, regulation. Plant Biol 8: 297–306 [DOI] [PubMed] [Google Scholar]
- Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. (2009) Different lepidopteran elicitors account for cross talk in herbivory-induced phytohormone signaling. Plant Physiol 150: 1576–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugardeyn J, Van der Straeten D. (2008) Ethylene: fine-tuning plant growth and development by stimulation and inhibition of elongation. Plant Sci 175: 59–70 [Google Scholar]
- Ecker JR. (1995) The ethylene signal-transduction pathway in plants. Science 268: 667–675 [DOI] [PubMed] [Google Scholar]
- Ehrlich P, Raven PH. (1964) Butterflies and plants: a study in coevolution. Evolution 18: 586–608 [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. (2002) The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis I, Simek P, Narisawa T, Sasaki M, Horiguchi T, Fukuda H, Matsuoka K. (2006) A novel R2R3 MYB transcription factor NtMYBJS1 is a methyl jasmonate-dependent regulator of phenylpropanoid-conjugate biosynthesis in tobacco. Plant J 46: 573–592 [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao MR, Li F, Guo QF, Xing SC, Wang W. (2008) Expansins and coleoptile elongation in wheat. Protoplasma 233: 73–81 [DOI] [PubMed] [Google Scholar]
- Grbic V, Bleecker AB. (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8: 595–602 [Google Scholar]
- Halitschke R, Baldwin IT. (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36: 794–807 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui D, Schmidt DD, Baldwin IT. (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol 131: 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-herbivore-specific plant responses. Plant Physiol 125: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hudgins JW, Franceschi VR. (2004) Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol 135: 2134–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad M, Canut H, Borderies G, Pont-Lezica R, Jamet E. (2008) A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: confirmed actors and newcomers. BMC Plant Biol 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55: 335–347 [DOI] [PubMed] [Google Scholar]
- Ito M. (2000) Factors controlling cyclin B expression. Plant Mol Biol 43: 677–690 [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, Baldwin IT. (2000) Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210: 336–342 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. (1997) Induced defense and evolution of induced resistance. Thompson JN, , Induced Responses to Herbivory. University of Chicago Press, Chicago, pp 167–223 [Google Scholar]
- Kareiva P. (1999) Coevolutionary arms races: is victory possible? Proc Natl Acad Sci USA 96: 8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. (1993) Ethylene biosynthesis. Annu Rev Plant Physiol 44: 283–307 [Google Scholar]
- Kessler A, Baldwin IT. (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis encodes a member of the RAF family of protein-kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Knoester M, Linthorst HJM, Bol JF, vanLoon LC. (1997) Modulation of stress-inducible ethylene biosynthesis by sense and antisense gene expression in tobacco. Plant Sci 126: 173–183 [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12: 177–183 [Google Scholar]
- Kwon HB, Lee GH, Cheong JJ. (1999) Expression of the RSI-1 gene during development of roots and reproductive organs in tomato. J Plant Biol 42: 266–272 [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15: 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, Boller T, Jones JDG, Romeis T. (2005) Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc Natl Acad Sci USA 102: 10736–10741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J. (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Demura T, Galis I, Horiguchi T, Sasaki M, Tashiro G, Fukuda H. (2004) A comprehensive gene expression analysis toward the understanding of growth and differentiation of tobacco BY-2 cells. Plant Cell Physiol 45: 1280–1289 [DOI] [PubMed] [Google Scholar]
- McKey D. (1974) Adaptive patterns in alkaloid physiology. Am Nat 108: 305–320 [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. (1995) Expansin mode of action on cell-walls: analysis of wall hydrolysis, stress-relaxation, and binding. Plant Physiol 107: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG. (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312: 436–439 [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917 [DOI] [PubMed] [Google Scholar]
- Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A. (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98: 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek L. (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Pre M, Atallah M, Champion A, De Vos M, Pieterse CMJ, Memelink J. (2008) The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol 147: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purrington CB. (2000) Costs of resistance. Curr Opin Plant Biol 3: 305–308 [DOI] [PubMed] [Google Scholar]
- Redman AM, Cipollini DF, Schultz JC. (2001) Fitness costs of jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia 126: 380–385 [DOI] [PubMed] [Google Scholar]
- Shi QM, Li CJ, Zhang FS. (2006) Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin. J Exp Bot 57: 2899–2907 [DOI] [PubMed] [Google Scholar]
- Sisler EC, Serek M. (1997) Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant 100: 577–582 [Google Scholar]
- Skibbe M, Qu N, Galis I, Baldwin IT. (2008) Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 20: 1984–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Tsui C. (1948) Chemical control of growth and bud formation in tobacco stem segments and callus cultured in vitro. Am J Bot 35: 782–787 [Google Scholar]
- Sloan J, Backhaus A, Malinowski R, McQueen-Mason S, Fleming AJ. (2009) Phased control of expansin activity during leaf development identifies a sensitivity window for expansin-mediated induction of leaf growth. Plant Physiol 151: 1844–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE. (2009) The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol 150: 1310–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Su WP, Howell SH. (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89: 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Alonso JM. (2009) Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12: 548–555 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Pittendrigh BR, Kroymann J, Weniger K, Fritsche J, Bauke A, Mitchell-Olds T. (2000) Induced plant defense responses against chewing insects: ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol 124: 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Parry G, Graham N, Allen T, Bennett M. (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49: 411–426 [DOI] [PubMed] [Google Scholar]
- Swiatek A, Lenjou M, Van Bockstaele D, Inze D, Van Onckelen H. (2002) Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol 128: 201–211 [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7: 847–859 [DOI] [PubMed] [Google Scholar]
- Thornburg RW, Li X. (1991) Wounding Nicotiana tabacum leaves causes a decline in endogenous indole-3-acetic acid. Plant Physiol 96: 802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Smalle J, Le J, Saibo NJM, De Paepe A, Chaerle L, Tietz O, Smets R, Laarhoven LJJ, Harren FJM, et al. (2003) The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiol 131: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Cohen JD, Barendse GWM, Blom C, Voesenek L. (1996) An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol 112: 1687–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dahl CC, Winz RA, Halitschke R, Kuhnemann F, Gase K, Baldwin IT. (2007) Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J 51: 293–307 [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Zhou ZY, Van der Straeten D. (2003) Regulation of submergence-induced enhanced shoot elongation in Oryza sativa L. Ann Bot 91: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. (2007) Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 226: 159–167 [DOI] [PubMed] [Google Scholar]
- Wang SS, Shi QM, Li WQ, Niu JF, Li CJ, Zhang FS. (2008) Nicotine concentration in leaves of flue-cured tobacco plants as affected by removal of the shoot apex and lateral buds. J Integr Plant Biol 50: 958–964 [DOI] [PubMed] [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annu Bot 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H. (2002) Fatty acid-derived signals in plants. Trends Plant Sci 7: 217–224 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15: 444–447 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action, and interaction. Ann Bot 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19: 1096–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YX, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT. (2004) Fitness benefits of trypsin proteinase inhibitor expression in Nicotiana attenuata are greater than their costs when plants are attacked. BMC Ecol 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui DQ, Baldwin IT. (2004) Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol 134: 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Turner JG. (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3: e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Zhang HW, Quan RD, Wang XC, Huang RF. (2009) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150: 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.