Abstract
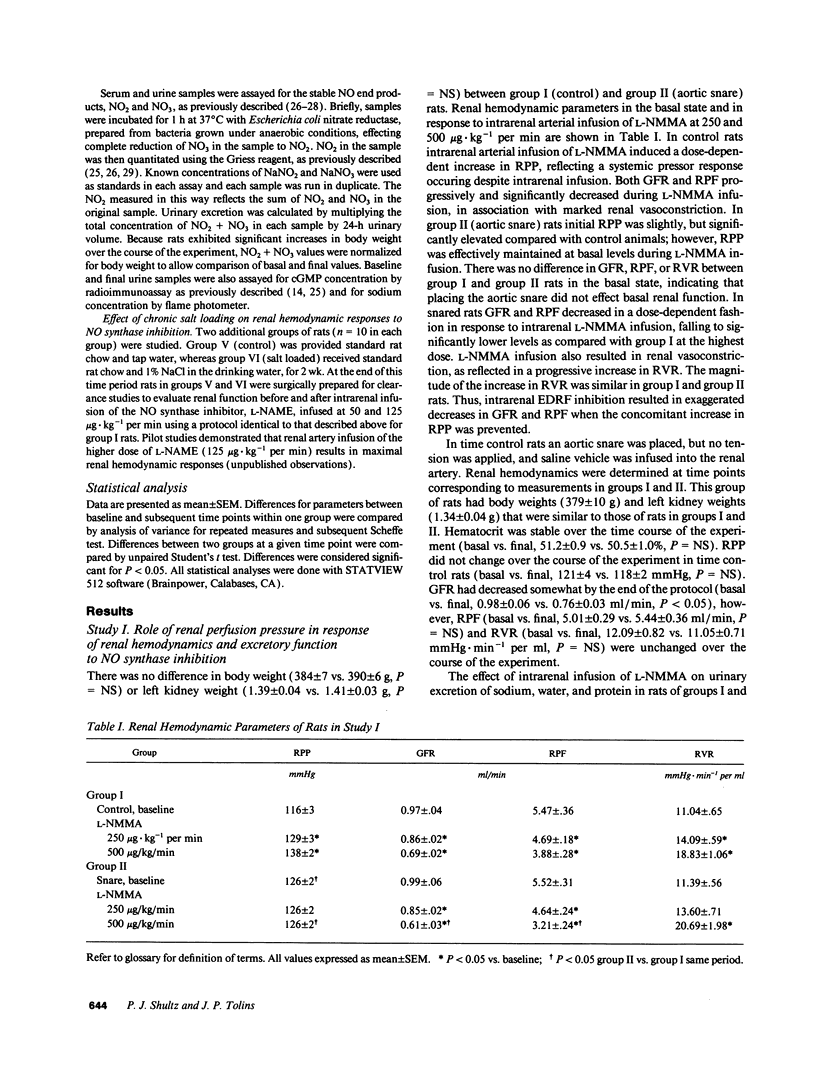
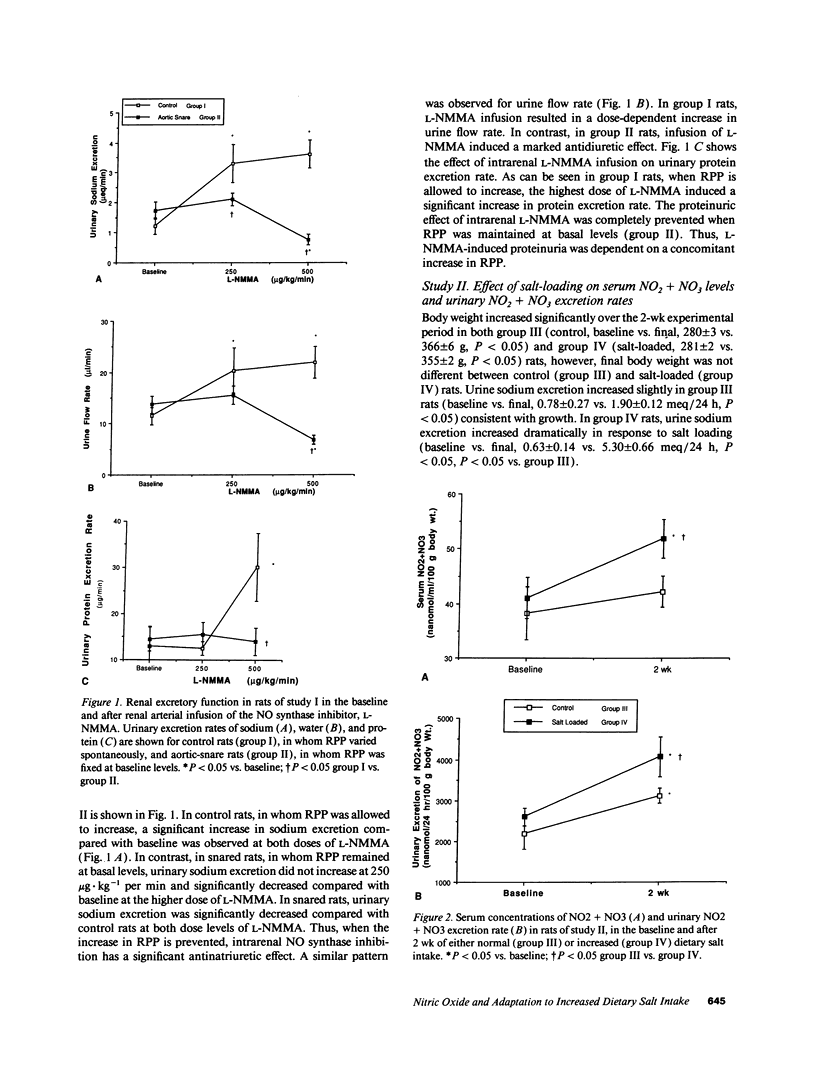
Previous studies have suggested that nitric oxide (NO) plays a role in regulation of renal vascular tone and sodium handling. We questioned whether the effects of NO synthase inhibition on renal function are direct or due to increased renal perfusion pressure (RPP) and whether stimulation of endogenous NO activity plays a role in adaptation to increased dietary salt intake. Intrarenal arterial infusion of the NO synthase inhibitor NG-monomethyl-L-arginine (L-NMMA) in control rats resulted in decreased glomerular filtration rate, renal vasoconstriction, natriuresis, and proteinuria. When RPP was held at basal levels with suprarenal aortic snare, L-NMMA had similar hemodynamic effects but decreased sodium excretion and did not induce proteinuria. Exposure of rats to high salt intake (1% NaCl drinking water) for 2 wk induced increased serum concentration and urinary excretion of the NO decomposition products, NO2 + NO3. Urinary NO2 + NO3 and sodium excretion were significantly correlated. Compared with controls, chronically salt-loaded rats also demonstrated enhanced renal hemodynamic responses to NO synthase inhibition. We conclude that the endogenous NO system directly modulates renal hemodynamics and sodium handling and participates in the renal adaptation to increased dietary salt intake. Enhanced NO synthesis in response to increased salt intake may facilitate sodium excretion and allow maintenance of normal blood pressure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberola A., Pinilla J. M., Quesada T., Romero J. C., Salom M. G., Salazar F. J. Role of nitric oxide in mediating renal response to volume expansion. Hypertension. 1992 Jun;19(6 Pt 2):780–784. doi: 10.1161/01.hyp.19.6.780. [DOI] [PubMed] [Google Scholar]
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer P. G., Navar L. G., Guyton A. C. Renal autoregulation, filtration rate, and electrolyte excretion during vasodilatation. Am J Physiol. 1970 Sep;219(3):619–625. doi: 10.1152/ajplegacy.1970.219.3.619. [DOI] [PubMed] [Google Scholar]
- Bartholomew B. A rapid method for the assay of nitrate in urine using the nitrate reductase enzyme of Escherichia coli. Food Chem Toxicol. 1984 Jul;22(7):541–543. doi: 10.1016/0278-6915(84)90224-2. [DOI] [PubMed] [Google Scholar]
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Baylis C., Harton P., Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol. 1990 Dec;1(6):875–881. doi: 10.1681/ASN.V16875. [DOI] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer M. P., Deen W. M., Robertson C. R., Brenner B. M. Mechanism of angiotensin II-induced proteinuria in the rat. Am J Physiol. 1977 Jul;233(1):F13–F21. doi: 10.1152/ajprenal.1977.233.1.F13. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. Y., Sanders P. W. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991 Nov;88(5):1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nicola L., Blantz R. C., Gabbai F. B. Nitric oxide and angiotensin II. Glomerular and tubular interaction in the rat. J Clin Invest. 1992 Apr;89(4):1248–1256. doi: 10.1172/JCI115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Bohrer M. P., Brenner B. M. Macromolecule transport across glomerular capillaries: application of pore theory. Kidney Int. 1979 Sep;16(3):353–365. doi: 10.1038/ki.1979.138. [DOI] [PubMed] [Google Scholar]
- Dubbin P. N., Zambetis M., Dusting G. J. Inhibition of endothelial nitric oxide biosynthesis by N-nitro-L-arginine. Clin Exp Pharmacol Physiol. 1990 Apr;17(4):281–286. doi: 10.1111/j.1440-1681.1990.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Earley L. E., Friedler R. M. Studies on the mechanism of natriuresis accompanying increased renal blood flow and its role in the renal response to extracellular volume expansion. J Clin Invest. 1965 Nov;44(11):1857–1865. doi: 10.1172/JCI105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Witzgall H., Tremblay J., Gutkowska J., Hamet P. Rapid increase in plasma and urinary cyclic GMP after bolus injection of atrial natriuretic factor in man. J Clin Endocrinol Metab. 1985 Dec;61(6):1217–1219. doi: 10.1210/jcem-61-6-1217. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson P. J., Palmer R. M., Moncada S. Comparative pharmacology of EDRF and nitric oxide on vascular strips. Eur J Pharmacol. 1987 Sep 23;141(3):445–451. doi: 10.1016/0014-2999(87)90563-2. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Freeman R. H. Pressure natriuresis in rats during blockade of the L-arginine/nitric oxide pathway. Hypertension. 1992 Apr;19(4):333–338. doi: 10.1161/01.hyp.19.4.333. [DOI] [PubMed] [Google Scholar]
- King A. J., Troy J. L., Anderson S., Neuringer J. R., Gunning M., Brenner B. M. Nitric oxide: a potential mediator of amino acid-induced renal hyperemia and hyperfiltration. J Am Soc Nephrol. 1991 Jun;1(12):1271–1277. doi: 10.1681/ASN.V1121271. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Fiksen-Olsen M. J., Raij L., Romero J. C. Effects of NG-monomethyl-L-arginine and L-arginine on acetylcholine renal response. Hypertension. 1990 Jun;15(6 Pt 1):659–663. doi: 10.1161/01.hyp.15.6.659. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Miranda-Guardiola F., Moncada S., Romero J. C. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol. 1991 Dec;261(6 Pt 2):F1033–F1037. doi: 10.1152/ajprenal.1991.261.6.F1033. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Raij L., Vanhoutte P. M. Endothelium-dependent vascular responses in normotensive and hypertensive Dahl rats. Hypertension. 1987 Feb;9(2):157–163. doi: 10.1161/01.hyp.9.2.157. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F. The endothelium. Target and promoter of hypertension? Hypertension. 1990 May;15(5):482–485. doi: 10.1161/01.hyp.15.5.482. [DOI] [PubMed] [Google Scholar]
- Majid D. S., Navar L. G. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol. 1992 Jan;262(1 Pt 2):F40–F46. doi: 10.1152/ajprenal.1992.262.1.F40. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Radermacher J., Förstermann U., Frölich J. C. Endothelium-derived relaxing factor influences renal vascular resistance. Am J Physiol. 1990 Jul;259(1 Pt 2):F9–17. doi: 10.1152/ajprenal.1990.259.1.F9. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. C., Bentley M. D., Vanhoutte P. M., Knox F. G. Intrarenal mechanisms that regulate sodium excretion in relationship to changes in blood pressure. Mayo Clin Proc. 1989 Nov;64(11):1406–1424. doi: 10.1016/s0025-6196(12)65383-x. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., Raij L. Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J Clin Invest. 1992 Nov;90(5):1718–1725. doi: 10.1172/JCI116045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz P. J., Schorer A. E., Raij L. Effects of endothelium-derived relaxing factor and nitric oxide on rat mesangial cells. Am J Physiol. 1990 Jan;258(1 Pt 2):F162–F167. doi: 10.1152/ajprenal.1990.258.1.F162. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., Tayeh M. A., Marletta M. A., Raij L. Synthesis and action of nitric oxide in rat glomerular mesangial cells. Am J Physiol. 1991 Oct;261(4 Pt 2):F600–F606. doi: 10.1152/ajprenal.1991.261.4.F600. [DOI] [PubMed] [Google Scholar]
- Tolins J. P., Palmer R. M., Moncada S., Raij L. Role of endothelium-derived relaxing factor in regulation of renal hemodynamic responses. Am J Physiol. 1990 Mar;258(3 Pt 2):H655–H662. doi: 10.1152/ajpheart.1990.258.3.H655. [DOI] [PubMed] [Google Scholar]
- Tolins J. P., Raij L. Effects of amino acid infusion on renal hemodynamics. Role of endothelium-derived relaxing factor. Hypertension. 1991 Jun;17(6 Pt 2):1045–1051. doi: 10.1161/01.hyp.17.6.1045. [DOI] [PubMed] [Google Scholar]
- Tolins J. P., Shultz P. J., Raij L. Role of endothelium-derived relaxing factor in regulation of vascular tone and remodeling. Update on humoral regulation of vascular tone. Hypertension. 1991 Jun;17(6 Pt 2):909–916. doi: 10.1161/01.hyp.17.6.909. [DOI] [PubMed] [Google Scholar]
- Zatz R., de Nucci G. Effects of acute nitric oxide inhibition on rat glomerular microcirculation. Am J Physiol. 1991 Aug;261(2 Pt 2):F360–F363. doi: 10.1152/ajprenal.1991.261.2.F360. [DOI] [PubMed] [Google Scholar]