Abstract
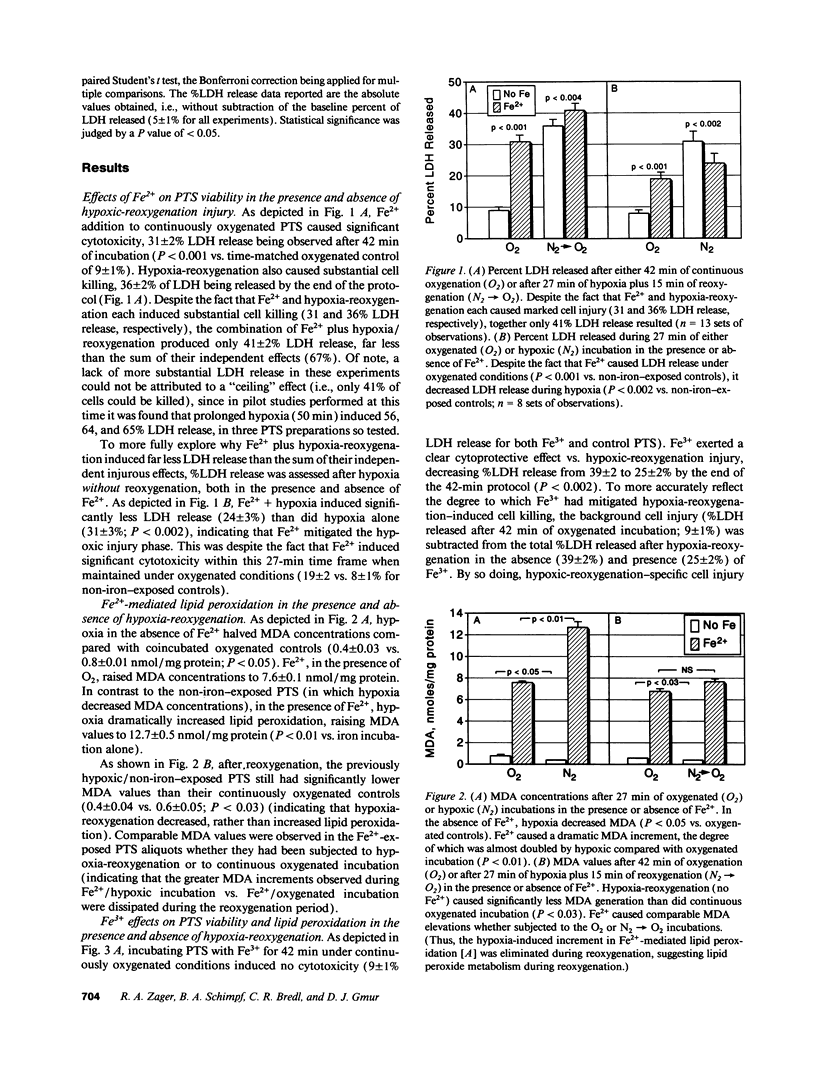
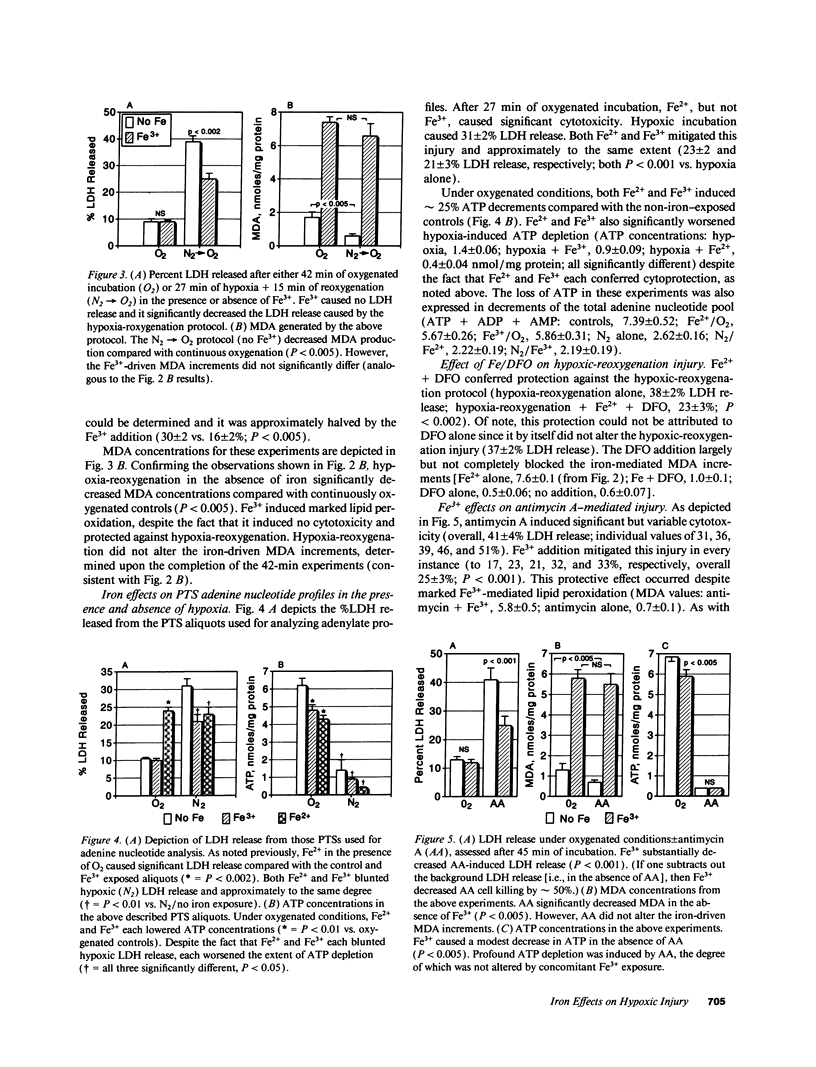
Iron-dependent free radical reactions and renal ischemia are believed to be critical mediators of myohemoglobinuric acute renal failure. Thus, this study assessed whether catalytic iron exacerbates O2 deprivation-induced proximal tubular injury, thereby providing an insight into this form of renal failure. Isolated rat proximal tubular segments (PTS) were subjected to either hypoxia/reoxygenation (H/R: 27:15 min), "chemical anoxia" (antimycin A; 7.5 microM x 45 min), or continuous oxygenated incubation +/- ferrous (Fe2+) or ferric (Fe3+) iron addition. Cell injury (% lactic dehydrogenase [LDH] release), lipid peroxidation (malondialdehyde, [MDA]), and ATP depletion were assessed. Under oxygenated conditions, Fe2+ and Fe3+ each raised MDA (approximately 7-10x) and decreased ATP (approximately 25%). Fe2+, but not Fe3+, caused LDH release (31 +/- 2%). During hypoxia, Fe2+ and Fe3+ worsened ATP depletion; however, each decreased LDH release (approximately 31 to approximately 22%; P < 0.01). Fe(2+)-mediated protection was negated during reoxygenation because Fe2+ exerted its intrinsic cytotoxic effect (LDH release: Fe2+ alone, 31 +/- 2%; H/R 36 +/- 2%; H/R + Fe2+, 41 +/- 2%). However, Fe(3+)-mediated protection persisted throughout reoxygenation because it induced no direct cytotoxicity (H/R, 39 +/- 2%; H/R + Fe3+, 25 +/- 2%; P < 0.002). Fe3+ also decreased antimycin toxicity (41 +/- 4 vs. 25 +/- 3%; P < 0.001) despite inducing marked lipid peroxidation and without affecting ATP. These results indicate that catalytic iron can mitigate, rather than exacerbate, O2 deprivation/reoxygenation PTS injury.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONN H. L., Jr, WOOD J. C., ROSE J. C. Circulatory and renal effects following transfusion of human blood and its components to dogs. Circ Res. 1956 Jan;4(1):18–24. doi: 10.1161/01.res.4.1.18. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Doctor R. B., Mandel L. J. Minimal role of xanthine oxidase and oxygen free radicals in rat renal tubular reoxygenation injury. J Am Soc Nephrol. 1991 Jan;1(7):959–969. doi: 10.1681/ASN.V17959. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Jaenike J. R. The renal lesion associated with hemoglobinemia: a study of the pathogenesis of the excretory defect in the rat. J Clin Invest. 1967 Mar;46(3):378–387. doi: 10.1172/JCI105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978 May;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Mitsos S. E., Kim D., Lucchesi B. R., Fantone J. C. Modulation of myoglobin-H2O2-mediated peroxidation reactions by sulfhydryl compounds. Lab Invest. 1988 Dec;59(6):824–830. [PubMed] [Google Scholar]
- Odeh M. The role of reperfusion-induced injury in the pathogenesis of the crush syndrome. N Engl J Med. 1991 May 16;324(20):1417–1422. doi: 10.1056/NEJM199105163242007. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hedlund B. E. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988 Oct;34(4):474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988 Sep;255(3 Pt 2):F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush J. D., Koppenol W. H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986 May 25;261(15):6730–6733. [PubMed] [Google Scholar]
- Shah S. V., Walker P. D. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988 Sep;255(3 Pt 2):F438–F443. doi: 10.1152/ajprenal.1988.255.3.F438. [DOI] [PubMed] [Google Scholar]
- Smith J. K., Carden D. L., Grisham M. B., Granger D. N., Korthuis R. J. Role of iron in postischemic microvascular injury. Am J Physiol. 1989 May;256(5 Pt 2):H1472–H1477. doi: 10.1152/ajpheart.1989.256.5.H1472. [DOI] [PubMed] [Google Scholar]
- Stocchi V., Cucchiarini L., Canestrari F., Piacentini M. P., Fornaini G. A very fast ion-pair reversed-phase HPLC method for the separation of the most significant nucleotides and their degradation products in human red blood cells. Anal Biochem. 1987 Nov 15;167(1):181–190. doi: 10.1016/0003-2697(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., O'Neal F. O., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation. Photochem Photobiol. 1978 Oct-Nov;28(4-5):803–809. doi: 10.1111/j.1751-1097.1978.tb07022.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M., Davis J. A., Abarzua M., Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987 Nov;80(5):1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. E., Zweier J. L., Flaherty J. T. Treatment with deferoxamine during ischemia improves functional and metabolic recovery and reduces reperfusion-induced oxygen radical generation in rabbit hearts. Circulation. 1991 Mar;83(3):1006–1014. doi: 10.1161/01.cir.83.3.1006. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J Clin Invest. 1992 Sep;90(3):711–719. doi: 10.1172/JCI115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager R. A., Foerder C. A. Effects of inorganic iron and myoglobin on in vitro proximal tubular lipid peroxidation and cytotoxicity. J Clin Invest. 1992 Mar;89(3):989–995. doi: 10.1172/JCI115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager R. A., Foerder C., Bredl C. The influence of mannitol on myoglobinuric acute renal failure: functional, biochemical, and morphological assessments. J Am Soc Nephrol. 1991 Oct;2(4):848–855. doi: 10.1681/ASN.V24848. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Gamelin L. M. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol. 1989 Mar;256(3 Pt 2):F446–F455. doi: 10.1152/ajprenal.1989.256.3.F446. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Gmur D. J., Bredl C. R., Eng M. J. Temperature effects on ischemic and hypoxic renal proximal tubular injury. Lab Invest. 1991 Jun;64(6):766–776. [PubMed] [Google Scholar]
- Zager R. A., Gmur D. J., Schimpf B. A., Bredl C. R., Foerder C. A. Evidence against increased hydroxyl radical production during oxygen deprivation-reoxygenation proximal tubular injury. J Am Soc Nephrol. 1992 May;2(11):1627–1633. doi: 10.1681/ASN.V2111627. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Mahan J., Merola A. J. Effects of mannitol on the postischemic kidney. Biochemical, functional, and morphologic assessments. Lab Invest. 1985 Oct;53(4):433–442. [PubMed] [Google Scholar]
- Zager R. A. Myoglobin depletes renal adenine nucleotide pools in the presence and absence of shock. Kidney Int. 1991 Jan;39(1):111–119. doi: 10.1038/ki.1991.14. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Schimpf B. A., Bredl C. R., Foerder C. A. Increased proximal tubular cell catalytic iron content: a result, not a mediator of, hypoxia-reoxygenation injury. J Am Soc Nephrol. 1992 Jul;3(1):116–118. doi: 10.1681/ASN.V31116. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab Invest. 1989 May;60(5):619–629. [PubMed] [Google Scholar]
- Zager R. A., Teubner E. J., Adler S. Low molecular weight proteinuria exacerbates experimental ischemic renal injury. Lab Invest. 1987 Feb;56(2):180–188. [PubMed] [Google Scholar]


