Abstract
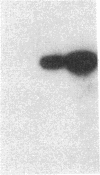
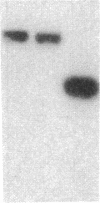
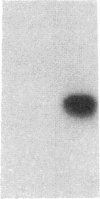
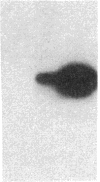
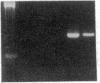
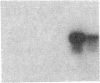
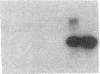
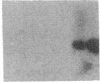
Endothelium-derived relaxing factor (EDRF) has profound effects on the renal vasculature, the glomerular mesangium, and also affects renal salt excretion. EDRF stimulates guanylyl cyclases, which are thought to be heterodimers comprised of alpha and beta subunits. Two alpha and two beta isoforms have been identified thus far. However, the molecular composition of in vivo guanylyl cyclase-linked EDRF receptors is unknown. We used polymerase chain reaction to clone a portion of the rat alpha 2 subunit. Guanylyl cyclase-linked EDRF receptor mRNA was detected in microdissected renal structures using a reverse transcription/polymerase chain reaction assay. The interlobular artery/afferent arteriole contained mRNA for the alpha 1, alpha 2, and beta 1 subunits; a faint beta 2 band was found in 29% of experiments. In contrast, the cortical collecting duct contained mRNA only for alpha 1 and beta 2 subunits. We conclude that guanylyl cyclase-linked EDRF receptor subunit isoforms are independently and heterogeneously expressed in the renal vasculature and cortical collecting duct, suggesting that several different EDRF receptors exist in vivo. These data suggest that the tubule receptor is composed of alpha 1/beta 2. The vasculature may contain at least two different EDRF receptors (alpha 1/beta 1 and alpha 2/beta 1). Some beta 2 may also be expressed, allowing for even greater heterogeneity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez A., Martin B., Murphy H. R., Uchida S., Murer H., Cowley B. D., Jr, Handler J. S., Burg M. B. Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem. 1989 Oct 5;264(28):16815–16821. [PubMed] [Google Scholar]
- Harteneck C., Koesling D., Söling A., Schultz G., Böhme E. Expression of soluble guanylyl cyclase. Catalytic activity requires two enzyme subunits. FEBS Lett. 1990 Oct 15;272(1-2):221–223. doi: 10.1016/0014-5793(90)80489-6. [DOI] [PubMed] [Google Scholar]
- Harteneck C., Wedel B., Koesling D., Malkewitz J., Böhme E., Schultz G. Molecular cloning and expression of a new alpha-subunit of soluble guanylyl cyclase. Interchangeability of the alpha-subunits of the enzyme. FEBS Lett. 1991 Nov 4;292(1-2):217–222. doi: 10.1016/0014-5793(91)80871-y. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Adams J. B., Horwitz P. M., Wood K. S. Activation of soluble guanylate cyclase by NO-hemoproteins involves NO-heme exchange. Comparison of heme-containing and heme-deficient enzyme forms. J Biol Chem. 1986 Apr 15;261(11):4997–5002. [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- King A. J., Brenner B. M. Endothelium-derived vasoactive factors and the renal vasculature. Am J Physiol. 1991 Apr;260(4 Pt 2):R653–R662. doi: 10.1152/ajpregu.1991.260.4.R653. [DOI] [PubMed] [Google Scholar]
- Koesling D., Harteneck C., Humbert P., Bosserhoff A., Frank R., Schultz G., Böhme E. The primary structure of the larger subunit of soluble guanylyl cyclase from bovine lung. Homology between the two subunits of the enzyme. FEBS Lett. 1990 Jun 18;266(1-2):128–132. doi: 10.1016/0014-5793(90)81523-q. [DOI] [PubMed] [Google Scholar]
- Koesling D., Herz J., Gausepohl H., Niroomand F., Hinsch K. D., Mülsch A., Böhme E., Schultz G., Frank R. The primary structure of the 70 kDa subunit of bovine soluble guanylate cyclase. FEBS Lett. 1988 Oct 24;239(1):29–34. doi: 10.1016/0014-5793(88)80539-8. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Fiksen-Olsen M. J., Romero J. C. Mediatory role of endothelium-derived nitric oxide in renal vasodilatory and excretory effects of bradykinin. Am J Hypertens. 1991 Mar;4(3 Pt 1):260–262. doi: 10.1093/ajh/4.3.260. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Miranda-Guardiola F., Moncada S., Romero J. C. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol. 1991 Dec;261(6 Pt 2):F1033–F1037. doi: 10.1152/ajprenal.1991.261.6.F1033. [DOI] [PubMed] [Google Scholar]
- Lowe T., Sharefkin J., Yang S. Q., Dieffenbach C. W. A computer program for selection of oligonucleotide primers for polymerase chain reactions. Nucleic Acids Res. 1990 Apr 11;18(7):1757–1761. doi: 10.1093/nar/18.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T., Murphy H. R., Martin B. M., Garcia-Perez A. Detection of specific mRNAs in single nephron segments by use of the polymerase chain reaction. Am J Physiol. 1990 May;258(5 Pt 2):F1470–F1474. doi: 10.1152/ajprenal.1990.258.5.F1470. [DOI] [PubMed] [Google Scholar]
- Nakane M., Arai K., Saheki S., Kuno T., Buechler W., Murad F. Molecular cloning and expression of cDNAs coding for soluble guanylate cyclase from rat lung. J Biol Chem. 1990 Oct 5;265(28):16841–16845. [PubMed] [Google Scholar]
- Nakane M., Saheki S., Kuno T., Ishii K., Murad F. Molecular cloning of a cDNA coding for 70 kilodalton subunit of soluble guanylate cyclase from rat lung. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1139–1147. doi: 10.1016/s0006-291x(88)80992-6. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Knepper M. A., Manganiello V. C. Effects of atrial natriuretic factor on cyclic guanosine monophosphate and cyclic adenosine monophosphate accumulation in microdissected nephron segments from rats. J Clin Invest. 1987 Feb;79(2):500–507. doi: 10.1172/JCI112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. C., Lahera V., Salom M. G., Biondi M. L. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol. 1992 Mar;2(9):1371–1387. doi: 10.1681/ASN.V291371. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Spencer W. J., Rhoads R. E. Optimization of the annealing temperature for DNA amplification in vitro. Nucleic Acids Res. 1990 Nov 11;18(21):6409–6412. doi: 10.1093/nar/18.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Star R. A., Nonoguchi H., Balaban R., Knepper M. A. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest. 1988 Jun;81(6):1879–1888. doi: 10.1172/JCI113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoos B. A., Carretero O. A., Farhy R. D., Scicli G., Garvin J. L. Endothelium-derived relaxing factor inhibits transport and increases cGMP content in cultured mouse cortical collecting duct cells. J Clin Invest. 1992 Mar;89(3):761–765. doi: 10.1172/JCI115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y., Moriyama T., Martin B. M., Knepper M. A., Garcia-Perez A. RT-PCR microlocalization of mRNA for guanylyl cyclase-coupled ANF receptor in rat kidney. Am J Physiol. 1991 Dec;261(6 Pt 2):F1080–F1087. doi: 10.1152/ajprenal.1991.261.6.F1080. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tomita K., Nonoguchi H., Marumo F. Polymerase chain reaction localization of constitutive nitric oxide synthase and soluble guanylate cyclase messenger RNAs in microdissected rat nephron segments. J Clin Invest. 1992 Aug;90(2):659–665. doi: 10.1172/JCI115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujiie K., Nonoguchi H., Tomita K., Marumo F. Effects of ANF on cGMP synthesis in inner medullary collecting duct subsegments of rats. Am J Physiol. 1990 Sep;259(3 Pt 2):F535–F538. doi: 10.1152/ajprenal.1990.259.3.F535. [DOI] [PubMed] [Google Scholar]
- Yuen P. S., Garbers D. L. Guanylyl cyclase-linked receptors. Annu Rev Neurosci. 1992;15:193–225. doi: 10.1146/annurev.ne.15.030192.001205. [DOI] [PubMed] [Google Scholar]
- Yuen P. S., Potter L. R., Garbers D. L. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry. 1990 Dec 11;29(49):10872–10878. doi: 10.1021/bi00501a002. [DOI] [PubMed] [Google Scholar]