Abstract
The adverse effects of aging of other organs (ovaries at menopause) on the skeleton are well known, but ironically little is known of skeletal aging itself. Evidence indicates that age-related changes, such as oxidative stress, are fundamental mechanisms of the decline of bone mass and strength. Unlike the short-lived osteoclasts and osteoblasts, osteocytes— former osteoblasts entombed in the mineralized matrix— live as long as 50 years, and their death is dependent on skeletal age. Osteocyte death is a major contributor to the decline of bone strength with age, and the likely mechanisms are oxidative stress, autophagy failure, and nuclear pore “leakiness.” Unraveling these mechanisms should improve understanding of the age-related increase in fractures and suggest novel targets for its prevention.
Skeletal aging
After death, mammalian skeletons can remain intact for millions of years, but during life they undergo a process of continuous regeneration (remodeling) during which old bone is removed and replaced with new. With advancing age, the balance between the amounts of old bone removed and new bone formed during the remodeling process becomes negative. In addition, bone strength declines disproportionally to the decline of bone mass. Together the decrease of bone mass and the decline of strength lead to the bone fragility syndrome known as osteoporosis— the most common metabolic disorder of old age in humans. During the last sixty years, skeletal involution with advancing age was thought primarily to be the result of age-related changes in other organs, and in particular from the decline of ovarian function in women at menopause [1,2]. Because of this “estrogen-centric” view, the so-called “post-menopausal” form of the disease and its therapy with estrogens or selective estrogen receptor modulators (SERMs) has held central stage.
Nonetheless, emerging epidemiologic evidence in humans and mechanistic studies in cell and animal models, reviewed recently in greater detail elsewhere [3], provide a paradigm shift from the “estrogen-centric” account to one in which age-related mechanisms (such as oxidative stress) occurring within bone itself are protagonists, and age-related changes in other organs and tissues, such as ovaries and the adrenals, are contributory (Box 1). In addition, mounting evidence from the study of other degenerative disorders of old age suggests that osteoporosis is not an isolated disease entity resulting from its own unique mechanisms but rather a co-morbidity with other degenerative disorders such as atherosclerosis, myocardial hypertrophy, sarcopenia, hyperlipidemia, insulin resistance, and Alzheimer's disease, which inexorably share pathogenetic mechanisms resulting from the aging of the respective tissues [4]. This fresh perspective helps to explain exciting new discoveries that ligands of a class of nutrient-sensing deacetylases, known as sirtuins, may be effective in treating more than one age-associated disease, for example, insulin resistance and osteoporosis [5].
This article reviews what is known about the effects of the aging process on bone itself and on the cellular constituents of bone, and discusses how the intrinsic aging of bone cells, as opposed to aging of other organs which indirectly affect the skeleton, contributes to the detrimental consequence of osteoporosis, namely bone fractures.
Bone remodeling
During remodeling, old bone is removed by teams of osteoclasts— highly specialized multi-nucleated cells derived from hematopoietic precursors— and replaced with new bone by teams of osteoblasts— a progeny of the mesenchymal stem cell lineage— which are responsible for the production and mineralization of the bone matrix [1]. The teams of osteoclasts and osteoblasts constitute distinct anatomical structures, called basic multi-cellular units (BMUs), which move in tandem, with osteoclasts always in the front of the advancing BMU and osteoblasts following in the rear. At any given time several millions of BMUs carry out turnover at discrete sites of the skeleton. In healthy adult humans, the remodeling cycle lasts 6–9 months [1,3]. A representative value for the average turnover (volume replacement) of bone is 10% per year, corresponding to a mean lifespan of about 10 years and a mean age of about 5 years, but there are large differences in turnover rate and mean age between different regions of the skeleton [6].
Osteocytes – choreographers and master regulators of skeletal homeostasis
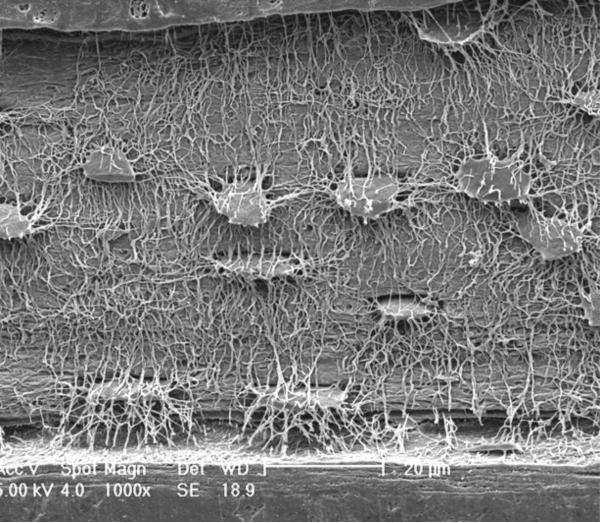
All osteoclasts and 60–80% of osteoblasts die via apoptosis [7]. The remaining osteoblasts become either lining cells that cover quiescent surfaces or are entombed individually in lacunae of the mineralized matrix and become osteocytes. In the process of their entombment, osteocytes undergo a dramatic morphologic transformation which includes the development of an average of fifty slender cytoplasmic processes that radiate from the cell body and give osteocytes the resemblance of neuronal cells (Figure 1) [8]. The processes run, like buried cable, along narrow canaliculi and are linked with the processes of neighboring osteocytes, as well as with cells present on the bone surface, including the lining cells, cellular elements of the bone marrow, and endothelial cells of the bone marrow vasculature, by gap junctions. In contrast to osteoclasts and osteoblasts, which are relatively short-lived and transiently present only on a small fraction of the bone surface, osteocytes are deployed throughout the skeleton, are long-lived, and are far more abundant than either osteoclasts (one thousand times) or osteoblasts (ten times). During the last few years, it has become evident that osteocytes are the choreographers of the remodeling process on the bone surface by virtue of their ability to: a) sense effete bone and direct the homing of osteoclasts (and perhaps osteoblasts) to the site that is in need of remodeling [9], b) produce factors that influence osteoblast and osteoclast generation as well as mineral homeostasis, c) mediate the homeostatic adaptation of bone to mechanical loading, and d) control and modify mineralization of the matrix produced by osteoblasts [10,11].
Figure 1.
The osteocyte-canalicular network by scanning electron microscopy. Reliefs of the osteocytes and their canalicular network were obtained following acid-etching of murine bone sections to remove the mineral (Courtesy of Lilian Plotkin and Teresita Bellido, Indiana University, and Lynda Bonewald, University of Missouri-Kansas City School of Dentistry).
Bone vasculature, hydration, and strength
Besides specialized cells and mineralized matrix, there are spaces in bone that include not only the bone marrow cavity, but also the lacunae and canaliculi which surround the osteocyte cell bodies and cytoplasmic processes, respectively; these form an elaborate system of tubes and vascular canals that deliver nutrients and oxygen to the cellular constituents of bone. In addition, bone contains water, which represents at least 25% of its wet weight and provides much of its unique strength and resilience [12,13]. Of the total volume of water, approximately 90% resides in the lacunar-canalicular system, bone vasculature, and bone marrow [14]. The remaining 10% is incorporated within collagen fibrils and hydroxyapatite crystals, and in submicroscopic channels that are widely distributed and allow rapid percolation of water soluble substances through apparently solid bone [15].
Bone formation almost always occurs in close proximity to capillaries [16]. Moreover, mesenchymal stem cell progenitors of osteoblasts reside on the outer surface of blood vessels and are critical for the formation of blood vessels as well [16,17]. In addition, one or more capillaries are always in close proximity to the teams of osteoclasts and osteoblasts that remodel bone, and are most likely the conduits by which both osteoclast and osteoblast precursors reach the site that is targeted for remodeling [18,19]. In cancellous bone, blood vessels are juxtaposed to a canopy of flat cells which separates the BMU from the bone marrow [20]. Low oxygen tension, developed as bone accumulates and the distance of osteocytes from the surface and the capillaries increases, stimulates the expression of endothelial growth factors by osteoblasts and initiates a neovasculogenic program [21–23].
Osteocytes and their dendritic processes are surrounded by a gel-like matrix which is in continuity with the peripheral circulation [24]. Transport of solutes through the lacunar-canalicular system is accommodated in part by hydraulic vascular pressure and in part by diffusion and convection induced by mechanical forces. Transmission of fluid shear stresses to the cytoskeleton by the matrix is essential for the ability of osteocytes to sense mechanical loading and choreograph the adaptation of bone to prevailing mechanical forces.
Osteoporosis is one of many factors responsible for the decrease of bone strength with age
Osteoporosis, the Greek word for porous bones, is the term used to define decreased bone mass per unit volume of anatomical bone. However, decreased bone mass is evidently only one of many factors responsible for the increased risk of fracture in old age, since at the same bone mineral density (BMD), the risk of fracture increases 4-fold with a 20-year increase in age [25]. While decline in neuromuscular function and increased incidence of falls is undoubtedly a factor for the age-related increase in fracture risk, several age-related changes in the bone itself contribute to the increase in fracture risk for the same BMD. For example,the age-related decline in cortical bone tensile strength [26] may be caused by deterioration of type I collagen, resulting from loss of cross-linking between the component chains [27], and accumulation of advanced glycation end products [28] — another general feature of the aging process. Further, a fracture at any site increases the risk of a subsequent fracture at any other site [29], highlighting the importance of non-mass factors. Likewise, only a small part of the decreased fracture incidence in patients receiving anticatabolic therapies for osteoporosis can be explained by the increase in bone mass [30], and many of the genetic effects on bone strength are mediated by factors other than bone mass [31,32]. Small bone size [33], disrupted bone architecture [34], changes in bone mineral and matrix [27], delayed repair of fatigue microdamage [35], and excessive turnover [36], are some additional non-mass factors. But the most important is loss of osteocyte viability with age. [37].
What cells are old in the aging skeleton?
Different components of the body grow old at different rates and die after different periods of time [38]. Osteoclasts live only for a few days or weeks and osteoblasts (while they are making bone) live only for a few months [1]; the only long-lived cells in bone are the stem cells from which osteoclasts or osteoblasts are derived, cells of the periosteum about which very little is known [39], and the lining cell-osteocyte syncytium consisting of terminally differentiated osteoblasts, which are the resident cells of bone [40] (Table 1). The bulk of bone substance consists largely of an extracellular matrix, which changes with age in ways previously summarized that are partly the result of changes in the resident cells and partly intrinsic to its structure and composition [9,41].
Table 1.
Life spans of bone cell nuclei (approximate ranges)
| Osteoclasts | 1–25 days |
| Osteoblasts | 1–200 days |
| Lining cells | 1–10 years |
| Osteocytes | 1–50 years |
The fate of lining cells, which separate all endosteal surfaces of bone from adjacent soft tissue, is unclear. At the start of a new episode of remodeling, osteoclasts could destroy lining cells as well as the underlying bone, but recent evidence indicates that lining cells can persist as a canopy over the bone remodeling compartment [20]. However, when new lining cells arise after osteoblasts stop making bone, it seems likely that the old lining cells die, presumably by apoptosis. In either case, the lifespan of lining cells would approximate the time interval between successive remodeling cycles at the same location, which is usually between one and ten years [42]. The fate of osteocytes, permanently immured within the lacunar-canalicular system, is more complex but better understood. In many regions of the skeleton, osteocytes persist until the bone in which they lie is resorbed, so that their lifespan is the same as of that bone. However, in regions where turnover is very low, osteocytes may die by apoptosis, leaving lacunae which appear empty in histologic sections [43]. Seemingly empty lacunae may contain the remnants of the osteocyte which have not undergone phagocytosis [44], but are removed during section preparation.
Aging and osteocyte death
The notion that osteocytes have a lifespan that is both relevant to pathophysiology and amenable to study was first conceived by Harold Frost almost fifty years ago [45]. In rib cortical bone, he noted that osteocyte death, inferred from the presence of an empty lacuna, increased in prevalence with age and was followed by hypermineralization of perilacunar bone and later by filling of canaliculae with mineralized connective tissue. Such changes, collectively referred to as micropetrosis, probably lead to increased brittleness of bone [46]. From these and other data, Frost estimated the natural lifespan of osteocytes to be about 25 years [47]. It is generally believed that only in cortical bone would osteocytes be left undisturbed by remodeling long enough for this intrinsic lifespan to be exceeded, but this view disregarded the effect of distance from the surface on the probability of remodeling, and hence mean bone age [42]. In human cancellous bone, this effect is minor in bone less than 50 μm upon the surface, but as the depth increases this effect increases rapidly, so that bone more than 75 μm from the surface is essentially isolated from surface remodeling and will be at least as old as the duration since skeletal maturity [42]. Since trabeculae increase in thickness by remodeling with positive balance during growth, the age of such bone could be even greater [48].
Even in normal human iliac cancellous bone, a substantial proportion is more than 20 years old [42], so it is not surprising that osteocyte death (inferred from an empty lacuna) has been demonstrated. In superficial bone (<25 μm from the surface) obtained from healthy women, the density (number/unit area) of total lacunae, osteocytes and empty lacunae does not change with age [43], because bone in which some osteocytes have died is replaced by bone containing a full complement of osteocytes [49]. The data suggest that rather than having a fixed lifespan, osteocytes die by a stochastic process occurring at a fractional rate of about 2.5% per year. Consequently, in deep bone (more than 45 μm from the surface) that is rarely or never remodeled, osteocyte density declines exponentially with age, approaching an asymptotic value which at age 75 is about 40% of the value at age 20 [49]. Evidently osteocyte death is dependent on the age of the bone, not on the age of the subject. In deep bone, total lacunar density is lower than in superficial bone and falls substantially with age, so that micropetrosis, the only process that could lead to obliteration of lacunae, occurs in cancellous as well as in cortical bone [43]. There is a close spatial relationship between empty lacunae and microscopic fatigue damage [50] and while both are the expected consequences of excessive bone age, it is unclear which occurs first [9].
Contribution of osteocytes to bone strength
The described age related decline in osteocyte number is accompanied by reduced bone strength, both in patients with vertebral fracture [51] and in healthy mice [52]. Moreover, ablation of osteocytes rapidly leads to decreased bone strength, microfractures, and osteoporosis in young mice [53]. Several mechanisms likely contribute to this relationship. First, osteocytes release one or more factors that inhibit bone remodeling [54], so that reduced osteocyte density in central cancellous bone is associated with increased surface remodeling [49] which is an independent contributor to bone fragility [36]. Second, there is disruption of several signals, likely including chemoattractants, proinflammatory cytokines, prostaglandins, sclerostin, receptor activator for NF-kB ligand, and macrophage-colony stimulating factor, that are necessary for microdamage repair [55]; these signals may be released during or shortly after osteocyte apoptosis, but if the osteocyte is already dead, such signals cannot be generated. Third, and probably most important, osteocyte death leads to a decline in bone vascularity and hydration, which reduces bone strength by mechanisms not yet fully understood, but which probably include changes in crystallinity and promotion of micropetrosis [37,46]. Conversely, protection of osteocytes from the adverse affect of aging on their apoptosis maintains bone crystallinity, vasculature volume, circulation of interstitial fluid, and strength [37].
Mechanisms of osteocyte death with advancing age
Aging, changes in systemic hormones levels such as sex hormone deficiency or glucocorticoid excess, and loss of mechanical strains all promote osteocyte death [56]. Thus, the prevalence of osteocyte apoptosis increases progressively with age in mice [52]. An increase in osteocyte apoptosis occurs in sexually mature mice, rabbits, and humans following loss of ovarian or testicular function [3]. Both in aging and acute sex steroid deficiency, the seminal mechanism leading to increased osteocyte apoptosis is evidently oxidative stress (Figure 2). The increase in osteocyte apoptosis is prevented in ovariectomised mice treated with anti-oxidants [52]. Increased osteocyte apoptosis has been also documented in animals and humans treated with glucocorticoids [57]. A change in the prevalence of osteocyte apoptosis is thought to account for the rapid increase of fracture incidence in patients receiving these drugs and the rapid return of fracture incidence to normal following the cessation of treatment, long before any loss or gain of bone mass [40,58]. Furthermore, the age related increase in endogenous glucocorticoid levels in mice contributes to age related loss of both osteocyte liability and strength [37]. Increased osteocyte apoptosis also ensues shortly following immobilization in mice [59]. Both glucocorticoid excess and loss of mechanical strain increase osteocyte apoptosis by interfering with focal adhesion kinase-mediated survival signals, thereby leading to anoikis [60]. Hence, oxidative stress, estrogen deficiency, endogenous hyperglucocorticoidism, and reduced physical activity all contribute to osteocyte death with advancing age.
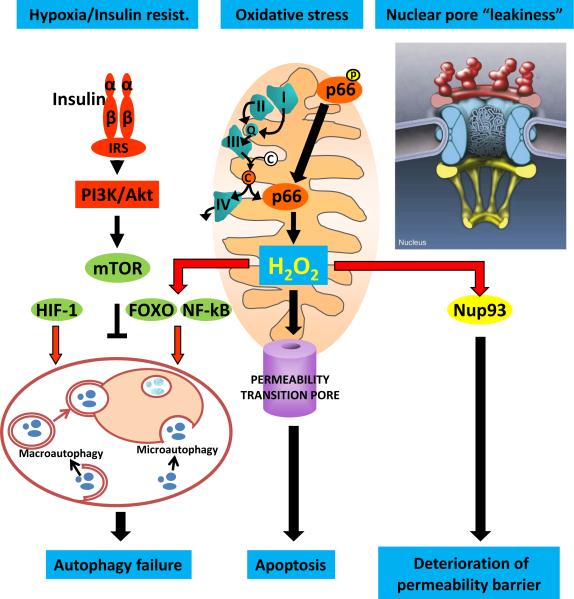
Figure 2.
Putative mechanisms of decreased osteocyte number by the age-associated increase in oxidative stress (OS). The production of H2O2 and several other reactive oxygen species, generated during aerobic metabolism within the mitochondria, increases with advancing age. This increase is amplified by the phosphorylation of p66, which oxidizes reduced cytochrome c (C) released from the electron transfer chain, and opens the permeability transition pore leading to apoptosis. Cells attempt to counteract the adverse consequences of OS by several mechanisms, including an increase in different types of autophagy, stimulated by the activation of transcription factors such as FoxOs and NF-kB. Failure of autophagy with age further contributes to the cell demise. Additionally, with advancing age, long lived post-mitotic cells fail to maintain the nuclear pore diffusion barrier as a result of leakiness caused by the oxidation of nucleoporins, such as Nup-93.
Autophagy failure and nuclear pore leakiness
Although not directly demonstrated, we strongly suspect that two mechanisms, both resulting from long-term increases in oxidative stress, are likely culprits in the increased death of long-lived osteocytes with advancing age: autophagy failure and nuclear pore leakiness (Figure 2). Autophagy is a highly conserved endocytic process that is mediated by lysosomes and critical for an efficient cell defense to stressful stimuli. Autophagy is responsible for the recycling of cellular materials, such as unwanted or damaged proteins, and the generation of amino acids during periods of starvation, as well as adaptive immunity. Many factors influencing aging/longevity such as SIRTs, mTOR, FoxOs, NF-kB, and p-53 exert their effects by modulating autophagy, and autophagy is stimulated by hypoxia [61] and caloric restriction [62]. Failure of autophagy, on the other hand, is responsible for the ultimate demise of long-lived post-mitotic cells in many organs, including brain, heart, muscle, and kidney [63]. Moreover, autophagy failure has been linked to degenerative diseases of the nervous system, like Alzheimer's and Parkinson's disease as well as heart failure and cancer. It is therefore very likely that osteocytes are not immune to autophagy failure, and that this mechanism plays a role in the increased rate of osteocyte death with age.
Recent evidence also suggests that aging and age-related pathologies are associated with the deterioration of nuclear pore structures [64,65]. Nuclear pore complexes (NPCs), aqueous channels made by multiple copies of over 30 different types of proteins known as nucleoporins, serve as physical barriers within the nuclear envelope by restricting the accessibility of cytoplasmic proteins to DNA and allowing the selective export of the newly synthesized mRNA from the nucleus to the cytoplasm and the ribosomes. NPCs disassemble during mitosis and reassemble in the nuclei of newly formed cells. Once formed, however, their proteins are extremely stable and persist for the entire lifespan of postmitotic cells. Exciting new evidence indicates that NPCs deteriorate with age in postmitotic cells as a result of oxidative damage of nucleoporins, like Nup93 [65]. In view of this finding, the exceptionally long lifespan of osteocytes, and the evidence that oxidative stress promotes osteocyte death, it is possible that nuclear pore leakage is also involved in the age-dependent depletion of osteocytes from bone and/or the malfunction of these cells with age.
Future studies utilizing genetic manipulation of these pathways in osteoblasts progenitors and osteocytes should establish the importance of age-dependent mechanisms of cell death and functional deterioration to the age-dependent involution of bone mass and strength.
Box 1. A paradigm shift from the “estrogen-centric” account of osteoporosis.
In contrast to traditional thinking that loss of estrogens at menopause is the seminal mechanism of osteoporosis, bone loss begins as soon as the early part of the third decade in both women and men — long before any decline in sex steroid production [66,67]. Consistent with this, an increase in oxidative stress (OS) is a fundamental pathogenetic mechanism of age-related bone loss strength in sex steroid sufficient female or male mice, leading to, among other changes, a decrease in osteoblast lifespan and bone formation [3,52]. Constant defense against OS is indispensable for bone mass homeostasis at all ages as deletion of FoxO transcription factors— an important defense mechanism against OS— for only 5 weeks in young (3 month old) mice, recapitulates the adverse effects of aging in bone, including increases in OS, osteoblast and osteocyte apoptosis, and osteoporosis; conversely, over-expression of FoxO3 in osteoblasts increases bone mass [68]. In support of the view that reactive oxygen species (ROS) are major regulators of bone homeostasis, mitochondria biogenesis and ROS are critical for the generation and survival of osteoclasts, osteoblasts, and osteocytes [3]. Loss of sex steroids accelerates the effects of aging on bone. Indeed, loss of estrogens or androgens decreases defense against OS, and this mechanism accounts for the increased bone resorption associated with acute loss of these hormones. The same mechanism accounts for the suppressive effects of either class of sex steroids on osteoclastogenesis, as well as the stimulatory effects of estrogens and androgens on osteoclast apoptosis and their attenuating effects on osteoblast apoptosis. Further, age-associated OS diverts multipotential mesenchymal progenitors into the adipocyte lineage at the expense of osteoblasts, by diverting ß-catenin, a co-activator of Wnt signaling that promotes osteoblastogenesis and suppresses adipogensis, from Wnt- to FoxO-mediated transcription of anti-aging/anti-oxidant gene programs [69]. The same mechanism is implicated in the pathogenesis of type 1 and 2 diabetes and the adverse effects of diabetes on bone formation and may also provide an explanation for strong epidemiologic evidence that atherosclerosis and aortic calcification (processes caused by lipid oxidation) are inversely related to bone mass and directly related to fractures [70,71]. Indeed, oxidized lipids promote anti-oxidant FoxO-mediated transcription but attenuate pro-osteogenic Wnt-signaling by acting themselves as oxidants as well as serving as PPARγ ligands [69]. Aging is also associated with increased endogenous glucocorticoid production and increased sensitivity of bone cells to glucocorticoids, resulting from increased production of 11β-HSD1, the enzyme that converts inactive into active glucocorticoids [37]. Importantly, endogenous hyperglucocorticoidism similar to exogenous glucocorticoid excess contributes to skeletal fragility with advancing age by decreasing the volume of the vasculature in bone, skeletal fluid flow, and thereby skeletal hydration [37]. The molecular mechanism behind these effects is decreased angiogenesis secondary to decreased VEGF production by osteoblasts/osteocytes as well as decreased VEGF action.
Acknowledgements
The authors wish to thank Robert Jilka, Maria Almeida, Charles O'Brien, and Robert Weinstein for critical reading of the manuscript prior to its submission to the journal and Beth Bailey for help with its preparation. SCM's research is supported by the National Institutes of Health (P01 AG13918); the Department of Veterans Affairs (Merit review grant); and Tobacco Settlement funds provided by the University of Arkansas for Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, et al. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 3.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. doi: 10.1210/er.2009-0024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007;21:2605–2614. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- 5.Pearson KJ, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002;30:807–809. doi: 10.1016/s8756-3282(02)00735-4. [DOI] [PubMed] [Google Scholar]
- 7.Jilka RL, et al. Apoptosis and Bone Cells. In: Bilezikian JP, et al., editors. Principles of Bone Biology. 3rd edn Academic Press; 2008. pp. 235–259. [Google Scholar]
- 8.Marotti G, et al. Structure-function relationships in the osteocyte. Ital. J. Min. Electro. Metab. 1990;4:93–106. [Google Scholar]
- 9.Parfitt AM. Skeletal heterogeneity and the purposes of bone remodeling: implications for the understanding of osteoporosis. In: Marcus R, et al., editors. Osteoporosis. 3rd edn Elsevier; 2007. [Google Scholar]
- 10.Manolagas SC. Perspective: Choreography from the tomb: an emerging role of dying osteocytes in the purposeful, and perhaps not so purposeful, targeting of bone remodeling. BoneKey-Osteovision. 2006;3:5–14. doi:10.1138/20060193. [Google Scholar]
- 11.Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann. N Y. Acad. Sci. 2007;1116:281–290. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 12.Ishijima H, et al. Water fraction of lumbar vertebral bone marrow estimated from chemical shift misregistration on MR imaging: normal variations with age and sex. AJR Am. J Roentgenol. 1996;167:355–358. doi: 10.2214/ajr.167.2.8686603. [DOI] [PubMed] [Google Scholar]
- 13.Wilson EE, et al. Three structural roles for water in bone observed by solid-state NMR. Biophys. J. 2006;90:3722–3731. doi: 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmins PA, Wall JC. Bone water. Calcif. Tissue Res. 1977;23:1–5. doi: 10.1007/BF02012759. [DOI] [PubMed] [Google Scholar]
- 15.Baud CA. Submicroscopic structure and functional aspects of the osteocyte. Clin. Orthop. Relat Res. 1968;56:227–236. [PubMed] [Google Scholar]
- 16.Brandi ML, Collin-Osdoby P. Vascular Biology and the Skeleton. J. Bone Miner. Res. 2006;21:183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 17.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Burkhardt R, et al. Changes in Trabecular Bone, Hematopoiesis and Bone Marrow Vessels in Aplastic Anemia, Primary Osteoporosis, and Old Age: A Comparative Histomorphometric Study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Parfitt AM. Misconceptions V-activation of osteoclasts is the first step in the bone remodeling cycle. Bone. 2006;39:1170–1172. doi: 10.1016/j.bone.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Andersen TL, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am. J Pathol. 2009;174:239–247. doi: 10.2353/ajpath.2009.080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, et al. The hypoxia-inducible factor {alpha} pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelzer E, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 23.Wan C, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad Sci U. S. A. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tate MLK, et al. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- 25.Hui SL, et al. Age and bone mass as predictors of fracture in a prospective study. J. Clin. Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall JC, et al. Age-related changes in the density and tensile strength of human femoral cortical bone. Calcif. Tissue Int. 1979;27:105–108. doi: 10.1007/BF02441170. [DOI] [PubMed] [Google Scholar]
- 27.Bailey AJ, Knott L. Molecular changes in bone collagen in osteoporosis and osteoarthritis in the elderly. Exp. Gerontol. 1999;34:337–351. doi: 10.1016/s0531-5565(99)00016-9. [DOI] [PubMed] [Google Scholar]
- 28.Vashishth D, et al. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 29.Klotzbuecher CM, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J. Bone Miner. Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 30.Cummings SR, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am. J. Med. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 31.Li X, et al. Genetic dissection of femur breaking strength in a large population (MRL/MpJ × SJL/J) of F2 Mice: single QTL effects, epistasis, and pleiotropy. Genomics. 2002;79:734–740. doi: 10.1006/geno.2002.6760. [DOI] [PubMed] [Google Scholar]
- 32.Wergedal JE, et al. Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone. 2005;36:111–122. doi: 10.1016/j.bone.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Duan Y, et al. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J. Bone Miner. Res. 1999;14:1796–1802. doi: 10.1359/jbmr.1999.14.10.1796. [DOI] [PubMed] [Google Scholar]
- 34.Kleerekoper M, et al. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif. Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 35.Burr DB, et al. Does microdamage accumulation affect the mechanical properties of bone? J. Biomech. 1998;31:337–345. doi: 10.1016/s0021-9290(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 36.Heaney RP. Is the paradigm shifting? Bone. 2003;33:457–465. doi: 10.1016/s8756-3282(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein RS, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00545.x. 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leblond CP. The Time Dimension in Histology. Am. J Anat. 1965;116:1–27. doi: 10.1002/aja.1001160102. [DOI] [PubMed] [Google Scholar]
- 39.Balena R, et al. Bone resorption and formation on the periosteal envelope of the ilium: A histomorphometric study in healthy women. J. Bone Miner. Res. 1992;7:1475–1482. doi: 10.1002/jbmr.5650071216. [DOI] [PubMed] [Google Scholar]
- 40.Manolagas SC. Corticosteroids and fractures: a close encounter of the third cell kind [editorial] J Bone Miner. Res. 2000;15:1001–1005. doi: 10.1359/jbmr.2000.15.6.1001. [DOI] [PubMed] [Google Scholar]
- 41.Viguet-Carrin S, et al. The role of collagen in bone strength. Osteoporos. Int. 2006;17:319–336. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 42.Parfitt AM, et al. Increased bone age: Mechanisms and consequences. In: Christianson C, et al., editors. Osteoporosis. Osteopress ApS; 1987. pp. 301–308. [Google Scholar]
- 43.Qiu S, et al. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002;31:313–318. doi: 10.1016/s8756-3282(02)00819-0. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein RS, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frost HM. In vivo osteocyte death. J. Bone Joint Surg. [Am. ] 1960;42:138–143. [PubMed] [Google Scholar]
- 46.Frost HM. Micropetrosis. J. Bone Joint Surg. [Am. ] 1960;42A:144–150. [PubMed] [Google Scholar]
- 47.Frost HM. In: Bone Remodeling Dynamics. Thomas CC, editor. 1963. [Google Scholar]
- 48.Parfitt AM, et al. Structural and cellular changes during bone growth in healthy children. Bone. 2000;27:487–494. doi: 10.1016/s8756-3282(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 49.Qiu S, et al. Relationships between osteocyte density and bone formation rate in human cancellous bone. Bone. 2002;31:709–711. doi: 10.1016/s8756-3282(02)00907-9. [DOI] [PubMed] [Google Scholar]
- 50.Qiu S, et al. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone. 2007:10–15. doi: 10.1016/j.bone.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Qiu S, et al. Reduced iliac cancellous osteocyte density in patients with osteoporotic vertebral fracture. J Bone Miner. Res. 2003;18:1657–1663. doi: 10.1359/jbmr.2003.18.9.1657. [DOI] [PubMed] [Google Scholar]
- 52.Almeida M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol. Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Yavropoulou MP, Yovos JG. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones. (Athens.) 2007;6:279–294. doi: 10.14310/horm.2002.1111024. [DOI] [PubMed] [Google Scholar]
- 55.Cardoso L, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24:597–605. doi: 10.1359/JBMR.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jilka RL, et al. Quantifying Osteoblast and Osteocyte Apoptosis: Challenges and Rewards. J Bone Miner Res. 2007;22:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 57.Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am. J. Med. 2000;108:153–164. doi: 10.1016/s0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 58.van Staa T, et al. Use of Oral Corticosteroids and Risk of Fractures. J. Bone Miner. Res. 2000 doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 59.Aguirre JI, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 60.Plotkin LI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am. J. Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 61.Pursiheimo JP, et al. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene. 2009;28:334–344. doi: 10.1038/onc.2008.392. [DOI] [PubMed] [Google Scholar]
- 62.Cuervo AM. Calorie restriction and aging: the ultimate “cleansing diet”. J Gerontol. A Biol. Sci. Med Sci. 2008;63:547–549. doi: 10.1093/gerona/63.6.547. [DOI] [PubMed] [Google Scholar]
- 63.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D'Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D'Angelo MA, et al. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Looker AC, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos. Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 67.Riggs BL, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ambrogini E, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2009 doi: 10.1016/j.cmet.2009.12.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almeida M, et al. Increased lipid oxidation causes oxidative stress, increased PPAR{gamma} expression and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol. Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulz E, et al. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol. Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 71.Farhat GN, et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J. Bone Miner. Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]