Abstract
Human adult mesenchymal stem cells (hMSC) are under active investigation as cellular carriers for gene therapy. hMSC possess natural tropism toward tumors, however, the targeting of hMSC to specific cell populations within tumors is unexplored. In the case of glioblastoma multiforme (GBM), at least half of the tumors express EGFRvIII on the cell surface, an ideal target for antibody-mediated gene/drug delivery. In this study, we investigated the feasibility of genetically modifying hMSC to express a single-chain antibody (scFv) to EGFRvIII on their surface. Nucleofection was used to transfect hMSC with cDNA encoding scFv EGFRvIII fused with PDGFR or human B7-1 transmembrane domains. The expression of scFv EGFRvIII on the cell surface was assessed by FACS. A stable population of scFv EGFRvIII-expressing hMSC was selected based on antibiotic resistance and enriched using FACS. We found that nucleofection allows the efficient expression of scFv EGFRvIII on the cell surface of hMSC. hMSCs transfected with the construct encoding scFv EGFRvIII as a fusion with PDGFRtm showed scFv EGFRvIII expression in up to 86% of cells. Most importantly, human MSC expressing scFv against EGFRvIII demonstrated enhanced binding to U87-EGFRvIII cells in vitro and at least 7-fold increased retention in human U87-EGFRvIII expressing tumors in vivo. In summary, we provide the first conclusive evidence of genetic modification of hMSC with a single-chain antibody against an antigen expressed on the surface of tumor cells, thereby opening up a new venue for enhanced delivery of gene therapy applications in the context of malignant brain cancer.
Keywords: mesenchymal stem cells, single-chain antibody, surface, targeting, brain tumors, EGFRvIII
1. Introduction
Human adult mesenchymal stem cells (hMSC) are under intense investigation as cellular vehicles for delivery of anti-tumor agents to solid tumors. They are relatively easy to purify, characterize, and expand in vitro. In addition, hMSC possess the plasticity and ability to differentiate into multiple cell types under appropriate cell culture conditions (Pittenger et al., 1999; Pittenger and Martin 2004). The genetic modification of MSC has been performed using various approaches, and each of these methods has advantages and drawbacks. hMSC have been shown to be prone to viral infection, and as such, adenoviruses encoding a protein of interest have been used for genetic engineering of MSC to express therapeutic proteins. However, such an approach does not allow efficient and stable incorporation of a gene of interest in the host genome and over time, expression is lost (Harui et al., 1999). Retro- and lentiviruses, on another hand, are able to stably integrate a gene of interest into the genome of hMSC, but the cells expressing these viral proteins could be immunogenic in animals and humans (Cherry et al., 2000). In contrast, non-viral methods, such as lipofection, have been shown to be inefficient at transducing hMSC (Gheisari et al., 2008). Additionally, electroporation requires the use high concentration of DNA and is not favorable for cell viability (Helledie et al., 2008). Recently developed nucleofection technology overcomes these limitations and allows the introduction of a gene directly in the nucleus of difficult to transfect primary cells and produce reasonable viability and efficiency of transfection (Aluigi et al., 2006).
To date, multiple attempts to utilize MSC as cellular vehicles to deliver therapeutic molecules to tumors have been described. Our group and others have demonstrated the ability of hMSC to deliver viral loads (Sonabend et al., 2008), interferon-β (Nakamizo et al., 2005), IL-12 (Eliopoulos et al., 2008), IL-2 (Nakamura et al., 2004), cytosine deaminase (Kucerova et al., 2008), and NK4, an antagonist of hepatocyte growth factor (Kanehira et al., 2007) to tumors. It has been demonstrated that hMSC may persist for prolonged time within the tumor environment, contribute to the stroma of tumors (Studeny et al., 2002), and engraft in neovasculature of the tumor (Beckermann et al., 2008; Bexell et al., 2009). However, the targeting of drugs/genes to a specific cell population remains a challenging task.
Antibody mediated targeting of drugs and genes to specific cells population has been a long-term interest, and there has been some success in the targeting of tumors with antibodies or engineered derivatives (Kioi et al., 2008; Modjtahedi et al., 2003). However, the fast clearance of small antibody fragments or poor penetration of antibodies through the tumor requires multiple injections and limits their therapeutic potential. The use of therapeutic scFv by engineering tumor cells or by modification of hMSC might overcome this problem (Compte et al., 2008).
Previously, several attempts were made to express scFv on the surface of tumor cells. The anti-CD3 scFv was expressed on the cell surface of colon cancer cells and able to induce cytotoxic lymphocytes (Liao et al., 2000). The anti-4-1BB scFv was successfully expressed on hepatoma cells and shown to mediate immune activity and anti-tumor effect (Liu et al., 2008). Radiometal chelates binding single-chain antibodies were also expressed on the surface of U87 human glioma cells as a reporter system for PET imaging (Wei et al., 2008). In addition, the scFv display on the surface of mammalian cells was proposed as a method for selection of high affinity antibodies (Ho and Pastan 2009).
In this study we sought to investigate the feasibility of genetically engineering hMSC to express a single-chain antibody (scFv) on the cell surface and test for their targeting properties in vitro and in vivo. We chose the scFv which specifically recognizes EGFRvIII, a mutation of EGFR that is not observed in normal tissues but has a high prevalence in patients with GBM (Emrich et al., 2002; Frederick et al., 2000). This scFv was previously well characterized (Lorimer et al., 1996) and shown to specifically target EGFRvIII expressing U87 glioma cells in vivo (Kuan et al., 1999).
It is possible that the combination of the natural tropism of hMSC to the tumor and the ability of targeting hMSC to specific cell population within tumor bed could be advantageous in anti-cancer therapies using MSC as cellular vehicles. To the best of our knowledge, this is the first report to document successful expression of scFv antibody on the surface of hMSC demonstrating specific targeting to glioma cells expressing EGFRvIII in vivo.
2. Materials and Methods
2.1. Reagents
The pDisplay vector was purchased from Invitrogen (Carlsbad, CA). Monoclonal antibodies to HA and Fc fragment specific goat anti-mouse conjugated to FITC, 4′, 6 –Diamino-2-phenylindole dihydrochloride (DAPI), poly-D-lysin hydrobromide, bovine serum albumin (BSA) were obtained from Sigma (St. Louis, MO). Hybridoma producing mAb to c-myc (clone 9E10) was purchased from ATCC (Rockville, MD). The SfiI AccI, BsmI and NotI restriction enzymes were obtained from New England Biolab (Ipswich, MA). DNA gel purification and maxi DNA purification kit were obtained from Qiagen (Valencia, CA). In-fusion Advantage cloning kit and Advantage HD polymerase were purchased from Clontech (Mountin View, CA). Top10 competent cells were obtained from Invitrogen (Carlsbad, CA). The cDNA (Gene Bank accession number U76382.1) encoding scFv EGFRvIII (MR1) was provided by Dr. Lorimer (Ottawa Health Research Institute, Canada). Retroviruses encoding green fluorescent protein (GFP) and red fluorescent protein (RFP) were a kind gift from Dr. David Curiel (University of Alabama at Birmingham). The U87 cells expressing EGFR or EGFRvIII were kindly provided by Dr. Furnary (University of California, San Diego). The cDNA encoding hB7-1 transmembrane domain was kindly provided by Dr. Freeman (Dana-Farber Cancer Institute, Harvard Medical School). The Advantage HD polymerase (Clontech, Mountain View, CA) was used in all PCR reactions.
2.2. Cloning
The scFv EGFRvIII was previously obtained using a phage–display library. Using in-Fuse cloning system, we subsequently re-cloned scFv EGFRvIII into the pDisplay vector encoding PDGFR transmembrane domain (PDGFRtm). Briefly, scFv EGFRvIII was amplified for 18 cycles by PCR at an annealing temperature of 55°C using the following pair of primers: forward 5′-caggtgaaactgcagcagtct g-3′ and reverse 5′-tttgatttccagcttggtgccatcaccgaa-3′. The PCR product was further amplified using the pair of In-Fusion primers suitable for sub-cloning scFvEGFRvIII into SfiI and AccI digested pDisplay vector: forward 5′-attatgctggggcccagccggcccaggtgaaactg-3′ and reverse 5′-agtttttgttcgtcgactttgatttccag -3′. The pDisplay vector was digested with SfiI and AccI restriction enzymes and run on 1% agarose gel. DNA band corresponding to digested pDisplay vector was cut and purified using the gel purification kit. The scFv EGFRvIII was sub-cloned in pDisplay vector using in-fusion cloning kit according to manufacturer’s recommendations. In order to generate a vector encoding hB7-1 transmembrane domain, the gene encoding hB7-1 was amplified using the pair of following primers: forward 5′-ctgctcccatcctgggccatt-3′ and reverse 5′-ttatacagggcgtacactttc-3′. The PCR product was further amplified using the pair of In-Fusion primers suitable for sub-cloning hB7-1 in BsmI and NotI digested pDisplay vector: forward 5′-tcagaagaggatctgaatgctctgctcccatcc-3′ and reverse 5′-tgatctcgagcggccgcttatacagggcgtac-3′. The scFv EGFRvIII was sub-cloned into pDisplay vector encoding hB7-1 as described above.
2.3. Cell Culture
Human MSC were grown in MEM alpha media (Invitrogen, Carlsbad, CA) supplemented with antibiotic-antimycotic (Invitrogen, Carlsbad, CA) and 10% premium select FBS (Atlanta Biologicals, Lawrenceville, GA). Media was replaced every 48 hours. Cells were re-plated using 0.25% trypsin-EDTA after they reached 80% of confluency. HEK 293 cells were grown in MEM media (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and antibiotic-antimycotic. U87 glioma cells expressing EGFR and EGFRvIII were grown in MEM media supplemented with 10% heat inactivated FBS and 200 μg/ml of G418.
2.4. Nucleofection
Nucleofection of hMSC was performed according to manufacturer’s recommendations (AMAXA Biosystem, Cologne, Germany). Briefly, hMSC were collected using 0.25% trypsin-EDTA and counted. Cells were span at 300g for 5 minutes and 5×105 cells were resuspended in 100 μl of nucleofection reagent (human MSC kit, Amaxa Biosystem, Cologne, Germany) and 2 μg of plasmid DNA, and nucleofected using U-23 protocol Nucleofector II device (Amaxa Biosystem, Cologne, Germany), after which the hMSC were plated immediately in warm culture medium in T25 culture flasks. Culture medium was replaced next morning to remove dead cells. In 48 hours, the cells were taken for the analysis of transient expression of scFv EGFRvIII on the cells surface by flow cytometry. Remaining cells were plated again in T25 flasks in the presence of antibiotic G418 (600 μg/ml) for stable selection of transfected cells.
2.5. Transfection using lipofectamine method
We used conventional lipofectamine method to transfect HEK 293 cells. In brief, lipofectamine 2000 (Invitrogen, Carlsbad, CA) was mixed with plasmid DNAs in Opti-MEM media (Invitrogen, Carlsbad, CA) according to manufacturer’s recommendation. The HEK 293 cells were incubated in Opti-MEM media with DNA-lipofectamine complexes for 6 hours after which the transfection media was replaced with complete media containing FBS. At 48 hours after transfection, HEK 293 cells were taken for flow analysis of the scFvEGFRvIII expression on the cell surface.
2.6. Retroviral infection
The U87 glioma cells expressing EGFR or EGFRvIII, and hMSC-vector or hMSC-scFvEGFRvIII were infected overnight with retroviruses expressing either GFP or RFP in the presence of polybrene (Sigma-Aldrich, St. Louis, MO) at 4 μg/ml. Next day, the media was replaced and 1ug/ml puromycin (Sigma-Aldrich, St. Louis, MO) was added for positive selection of GFP or RFP expressing cells. After the selection. GFP or RFP expression in cells was confirmed by flow cytometry and fluorescent microscopy.
2.7. Cell surface staining
The expression of scFvEGFRvIII was evaluated by FACS analysis. Briefly, cells were collected with 0.25% trypsin-EDTA and washed once with phosphate buffered saline (PBS). Anti-HA mAb at concentration 2 μg/ml or anti-myc mAb hybridoma supernatant (clone 9E10, 1:10 dilution) were used to capture the expression scFv EGFRvIII on the cell surface, followed by Fc specific anti-mouse antibody conjugated with FITC for detection. In limited set of experiments, MSC were fixed and permeabilized using BD cytofix/cytoperm fixation/permeabilization kit (BD Biosciences Pharmigen, San Diego, CA) before the staining with anti-HA or anti-myc antibodies.
2.8. Immunocytochemistry
The MSC stably expressing scFv EGFRvIII were evaluated for surface and intracellular expression of scFv EGFRvIII. Briefly, hMSC were plated on glass cover slips covered with 10 μg/ml poly-D-lysin hydrobromide and grown in complete media. In 48 hours, cells were washed once with PBS and fixed with 2% paraformaldehyde (PFA) for the detection scFv on the cell surface, and with 4% PFA followed by treatment with 0.3% Triton X100 for intracellular detection of the scFv. Non-specific antibody binding was blocked with 2% BSA and cells were stained with anti-HA mAb or isotype specific mIgG at concentration 2 μg/ml followed by detection with Fc specific anti-mouse IgG-FITC diluted 1:200. After repeated washing to remove unbound antibodies with PBS containing 0.05% Tween 20, cover slips were rinsed once in water and mounted in fluoromount-G (Southern Biotech, Birmingham, Alabama) containing 1μg/ml DAPI. MSC were analyzed for surface and intracellular expression of scFv EGFRvIII using confocal microscope Leica SP2.
2.9. FACS sorting
Following the selection of a stable population of hMSC expressing scFvEGFRvIII on the cell surface, we enriched the population of scFvEGFRvIII positive cells by FACS cell sorting (FACS Aria, BD). hMSC were stained as described above and the FITC-positive cells, and the FITC positive cells were collected. Immediately after the sorting cells were centrifuged at 300g for 5 min and plated in fresh culture medium for expansion.
2.10. Western Blotting
Stable populations of hMSC nucleofected with empty vector or vector encoding scFv EGFRvIII were lysed using the M-Per mammalian protein extraction buffer (Thermo Scientific, Rockford, IL) containing Halt protease inhibitors cocktail (Pierce, Rockford, IL). Samples were applied to 10% Tris-HCl gel (Bio-Rad, Hercules, CA) at 40 μg/lane and resolved under reducing conditions. After the transfer of proteins to PVDF membrane (Bio-Rad, Hercules, CA) and blocking with 2% non-fat dry milk, membrane was stained anti-myc hybridoma supernatant (diluted 1:10), followed by goat anti-mouse antibody conjugated with peroxidase (Chemicon Intl., Temicula, CA). ImmunStar WesternC (Bio-Rad, Hercules, CA) was used to develop the reaction. Images were captured using the ChemiDoc imaging system (Bio-Rad, Hercules, CA).
2.11. Targeting of hMSC-scFvEGFRvIII to EGFRvIII expressing tumor cells in vitro
GFP positive U87 cells expressing EGFR or EGFRVIII were grown until confluency in 6 well plates. RFP positive control hMSC or hMSC-scFvEGFRvIII (1×105 cells per well) were added to U87 cells and incubated at 37°C. After one hour, the cells were washed three times with PBS to remove unbound cells and fixed with 4% PFA for 20 minutes. Cells were analyzed for GFP and RFP expressing cells using Olympus IX70 inverted microscope and MetaMorph software. The number of RFP positive cells was counted per each of five fields. In order to show the specificity of interaction, the data were calculated as ratio of hMSC attached to U87 EGFRvIII expressing cells and U87 cells expressing wild type EGFR. Data from three separate experiments was analyzed and is summarized in the presented figure.
2.12. Animal studies and tissues preparation
All experiments with animals were performed in accordance with protocols approved by the University of Chicago Animal Care Committee. U87-EGFRvIII cells expressing GFP (1×106 cells per animal) were injected subcutaneously in the right flank of 6–8 weeks male athymic mice obtained from the Charles River (Willmington, MA). After two weeks, control hMSC or hMSC- scFvEGFRvIII expressing RFP (3×103 cells per animal) were injected into the growing tumor. Tumor tissues were flash frozen in dry iced 2-methylbutane at day 5 and day 15 after injection of hMSC cells. Cryosections were prepared at 20μm and analyzed for GFP and RFP expressing cells using Olympus IX70 inverted microscope and MetaMorph software. The number of RFP positive cells was counted in each field at 10× magnification.
2.13. Statistical analysis
Statistical difference between samples was estimated using single factor ANOVA. p values below 0.05 were considered to be statistically significant.
3. Results
3.1. scFv EGFRvIII expression on the cell surface of hMSC
cDNA encoding the scFv EGFRvIII was amplified by PCR reaction from pMR1ENV1 (Lorimer et al., 1996) using scFv EGFRvIII specific primers and subsequently re-cloned into a vector encoding either PDGFRtm or hB7-1tm. The cDNA for hB7-1tm was obtained by PCR reaction from the template and cloned in pDisplay vector by substitution of PDGFRtm. The resulting plasmid encoded mouse origin immunoglobulin kappa chain leader, an HA epitope followed by the scFvEGFRvIII, a myc epitope and the PDGFR or hB7-1tm. While the immunoglobulin kappa leader ensures that scFv traffics to the cell surface, the PDGFR and hB7-1 transmembrane domains allow the scFv to be anchored in the cell membrane.
We used the nucleofection method developed by AMAXA Biosystems to transfect hMSC with (i) control plasmid encoding GFP, (ii) an empty vectors encoding the PDGFRtm or the hB7-1tm, and (iii) plasmid encoding fusion the scFvEGFRvIII with PDGFRtm or hB7-1tm. Human MSC were analyzed by flow cytometry 48 hours after nucleofection to assess the efficiency of nucleofection. On average, 50% of cells were GFP positive and the level of GPP expression varied significantly between individual cells (Fig 1A). No specific signal was present in hMSC nucleofected with empty vectors (data not shown). The expression of scFv on the surface of hMSC was detected in 20.3±2.8% and 8.7±1.9 % (p=0.039) of cells nucleofected with plasmid encoding scFvEGFRvIII PDGFRtm and scFvEGFRvIII hB7-1tm fusion proteins respectively (Fig 1B–D). Although, we observed a more that 2-fold higher percentage of positive cells expressing scFvEGFRvIII PDGFRtm fusions than hB7-1 tm fusions, the mean specific fluorescence (MFI) of positive cells did not differ significantly. It exceeded the MFI of isotype matched control 2.8±1.3 and 2.3±0.6 times for hMSC expressing scFvEGFRvIII PDGFRtm and scFvEGFRvIII hB7-1tm fusion proteins, respectively. The expression of scFv EGFRvIII in hMSC was further confirmed by the detection of the protein using anti-myc antibodies by immunoblotting technique (Fig 1E).
Figure 1. Transient expression of scFvEGFRvIII on the surface hMSC.
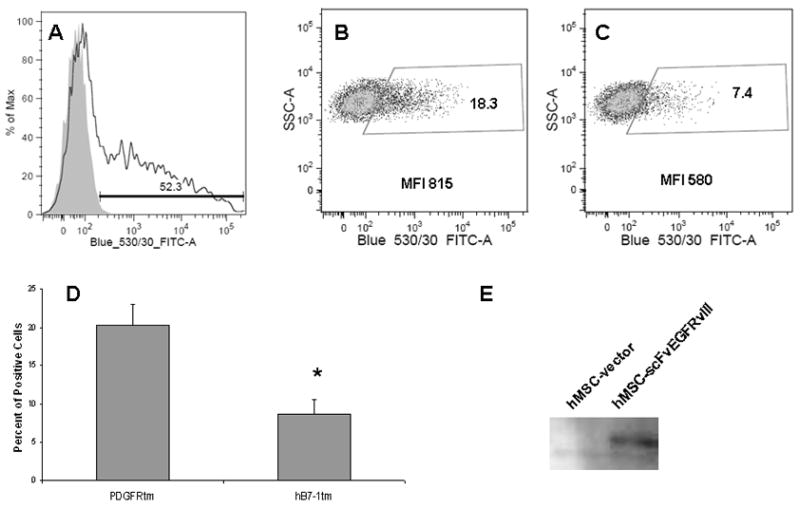
Flow cytometric analysis of scFvEGFRvIII expression in hMSC after nucleofection with plasmids encoding A-GFP; B- scFvEGFRvIII PDGFRtm fusion; C- scFvEGFRvIII hB7-1tm fusion. Data shown from a representative experiment. D. Comparison of the surface expression of scFv EGFRvIII PDGFRtm or hB7-1tm fusions expressed as percent of positive cells. Summary of two independent experiments. Data are shown as mean ± SD, * p< 0.05. E. Expression of the scFvEGFRvIII in MSC was confirmed by Western Blot.
Previous studies have demonstrated variable expression of fusion proteins based on the transmembrane domain utilized (Chou et al., 1999; Liao et al., 2000). In order to determine whether the impact of transmembrane construct for nucleofection was cell type specific, we examined the level of expression of scFvEGFRvIII as a fusion with PDGFRtm or hB7-1tm in the fibroblast cell line. HEK 293 cells were transfected using the conventional lipofectamine method. In transiently transfected HEK 293 cells, the number of positive cells was 1.5-fold higher for scFvEGFRvIII-PDGFRtm as compared with that for scFvEGFRvIII-hB7-1tm. The MFI of positive scFvEGFRvIII-PDFRtm cells also was 1.76-fold higher than that of scFv EGFRvIII-hB7-1tm (Fig 3E, F). Thus, the pattern of expression for scFv on the cell surface was similar in hMSC and HEK 293. For future experiments, PDGFRtm became preferred over hB7-1 due to the higher level expression scFv on the cells surface.
Figure 3. Expression of scFv EGFRvIII in stably selected hMSC cultured at various temperatures and in HEK cells.
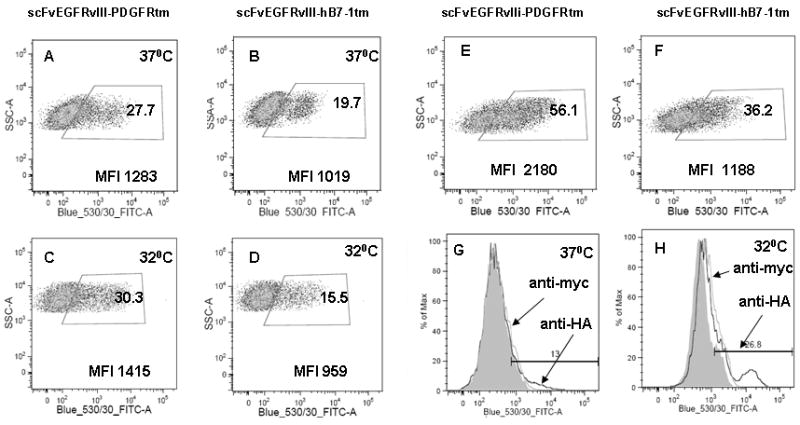
Comparison of surface expression of the scFv EGFRvIII PDGFRtm and hB7-1tm fusion in hMSC cultured at 37°C (A and B) and 32°C (C and D). Expression the scFv EGFRvIII PDGFRtm (E) and hB7-1tm fusion (F) in HEK cells cultured at 37°C 48 hours after transfection. hMSC grown at 37°C for a month show decreased scFvEGFRvIII surface expression (G), which can be “boosted” by transferring cells to 32°C (H) as detected by anti-HA antibodies by flow cytometry.
3.2. Generation of a stable population of hMSC expressing scFv on the cell surface
Having identified scFv EGFRvIII as the better transmembrane domain for hMSC, we sought to generate a stable hMSC cell line. First, hMSC were nucleofected with an empty vector, a vector encoding GFP, or a vector encoding scFv EGFRvIII. Transfected hMSC were selected on the basis of antibiotic resistance. Figure 2 shows the phase-contrast microphotograph taken on day 5 after the antibiotic G418 was added to the cells (magnification 10 ×). The cells nucleofected with vector encoding GFP were mostly dead in the presence of G418, whereas hMSC nucleofected with vector encoding the scFvEGFRvIII PDGFRtm were viable. Cells nucleofected in the absence of DNA were viable in the absence of antibiotic, thus showing that the smaller fraction of hMSC was indeed transfected and selected. Interestingly, even after the selection with antibiotic only a small fraction (up to 30%) of antibiotic-resistant cells had scFv EGFRvIII expressed on the cell surface, which was similar to the cells transiently expressing scFvEGFRvIII on the cell surface (see Fig 1B and Fig 3A). In the stable population of hMSC selected with antibiotic, about 28% of cells displayed the scFv EGFRvIII-PDGFRtm fusion and 20% of cells were positive for scFv EGFRvIII-hB7-1tm fusion (Fig 3A and 3B). The mean fluorescence of the positive populations was 3.9- and 3.1-fold higher of that for the isotype matching control mIgG for the scFv EGFRvIII-PDGFRtm and scFv EGFRvIII-hB7-1tm fusion proteins, respectively and differed from each other only by 25% (Fig 3A and 3B).
Figure 2. Stable selection of hMSC with antibiotic.

hMSC were nucleofected without plasmid DNA (A), in the presence of GFP encoding plasmid (no gene of resistance to G418 in the expression cassette) (B), and in the presence scFv EGFRvIII PDGFRtm encoding plasmids (with gene of resistance to G418 in the expression cassette) (C). Photos taken at day 5 after G418 was added at a concentration of 600 μg/ml. (D). Data calculated as number of live cells per field and presented as mean ± SD, * p< 0.05.
Since the expression of recombinant proteins can be influenced by temperature, we therefore varied the temperature of our hMSC cultures. The incubation of cells for one week at a decreased temperature of 32°C did not improve the expression of the scFv on the cell surface either for the scFv EGFRvIII-PDGFRtm or the scFv EGFRvIII-hB7-1tm fusions (Fig 3C,D). Although we noticed that hMSCs maintained for several weeks at 37°C after the stable selection lost their expression of scFvEGFRvIII on the cell surface. The expression of the scFvEGFRvIII in those cells was restored by incubation at 32°C (Fig 3G, H), whereas the transfer of cells back to the 37°C incubator reversed the expression to the initial level (data not shown).
3.3. Enrichment of cells expressing scFv on the cell surface by cell sorting
Taking into consideration that expression of scFvEGFRvIII on the surface of hMSC did not exceed 30%, we used FACS sorting in order to enrich the population of the positive cells. After sorting the FITC negative population retained less than 1% of positive cells (Fig 4A), whereas in the positive sorted population up to 86% of the cells were FITC positive, and therefore expressed scFv EGFRvIII on the cell surface (Fig 4B). The MFI of the positive cells exceeded the background of the isotype control by more than 75-fold. We observed a decrease in scFvEGFRvIII expression on the cell surface (about 50%) in hMSC growing for a month in cell culture (data not shown).
Figure 4. Sorting of hMSC expressing scFvEGFRvIII.
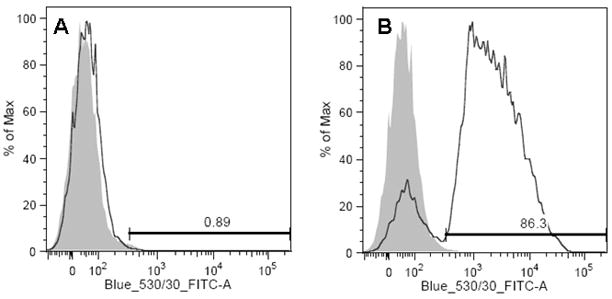
Human MSC were stained with anti-HA antibodies following by goat anti-mouse Fc fragment specific antibodies conjugated with FITC and were sorted for FITC positive cells The negative control cells were used to set the positive gate. After the sorting, cells were analyzed by flow cytometry for positive staining in FITC negative (A) and FITC positive population (B).
3.4. Determining the best epitope for detection of scFv on the cell surface
As we noted above, the fusion of scFv EGFRvIII with PDGFRtm or hB7-1 has two epitopes suitable for detection of the scFvEGFRvIII on the cell surface. The fusion proteins have an HA epitope exposed on N-terminal part of the protein and a myc epitope localized between the C-terminal part of scFv EGFRvIII and transmembrane domain. Initially, we used an anti-myc antibody (clone 9E10) for detection of scFv EGFRvIII on the cell surface of HEK 293 cells or hMSC cells. However, we found that the detection of the scFv with anti-myc antibodies was 3.4 folds better in the cells which were fixed and permeabilized prior to the staining procedure (Fig. 5, pane A-b and A-d). Interestingly, staining the cells expressing scFv EGFRvIII with anti-HA antibody (i) was twice higher than with anti-myc antibodies (Fig 5A-a and -b), and (ii) differed between surface and permeabilized cells to a lesser extent (2.1 folds) than seen for anti-myc mAb (Fig 5A- a and -c). We concluded that the HA epitope on N-terminal part of the fusion protein is more suitable for the detection of scFv on the cell surface and should be utilized for monitoring expression on hMSC.
Figure 5. Detection of scFv EGFRvIII on the cell surface using HA- or Myc-tag epitopes.
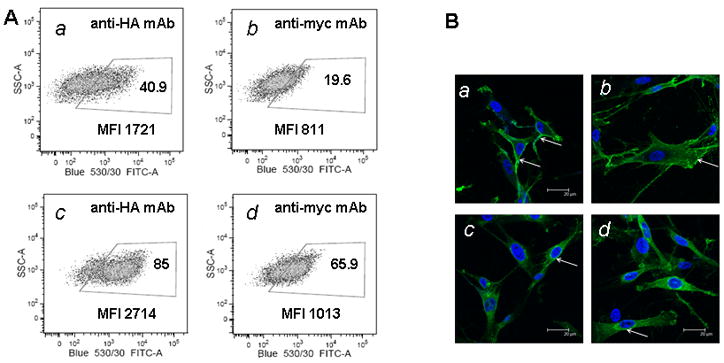
One week after sorting, hMSC were stained either with anti-HA or anti-myc tag antibodies and analyzed by flow cytometry (panel A) for scFv EGFRvIII expression in live cells (a–b), and in fixed and permeabilized cells (c–d). Panel B shows analysis of surface (a–b) and intracellular (c–d) expression of scFv EGFRvIII by using anti HA antibody and confocal microscopy. White arrows show sites of increased expression scFv EGFRvIII either on the cell surface or intracellularly.
In addition, scFv EGFRvIII can be detected by its HA and myc epitopes not only on the surface but intracellularly with flow cytometry. We confirmed this finding by staining hMSC for scFv EGFRvIII expression on the cell surface and intracellularly and analyzed cells using confocal microscopy. Figure 5B shows membrane localization of the scFvEGFRvIII (a and b) which is especially apparent at the periphery of the cells, whereas in permeabilized cells (c and d) the localization is more pronounced in perinuclear area and also detected throughout the cytoplasm.
3.5. Targeting of hMSC expressing scFv EGFRvIII in vitro
In order to demonstrate that hMSC expressing scFv EGFRvIII on the cell surface possess targeting properties, U87 glioma cells expressing either wild type EGFR or EGFRvIII were co-incubated with control or modified hMSC. Figure 6A shows microscopy images of bound hMSC-RFP to the GFP positive U87 cells from a representative experiment. Control hMSC encoding vector alone were bound equally (presented as ratio of bound cells, 0.95±0.05) to U87 expressing EGFR or EGFRvIII, whereas hMSC-scFvEGFRvIII showed on average 1.7 times better (1.7±0.23, p <0.05) binding to U87 EGFRvIII cells (Fig 6B).
Figure 6. Interaction of hMSC modified with scFv EGFRvIII with U87 cells expressing EGFRvIII in vitro.
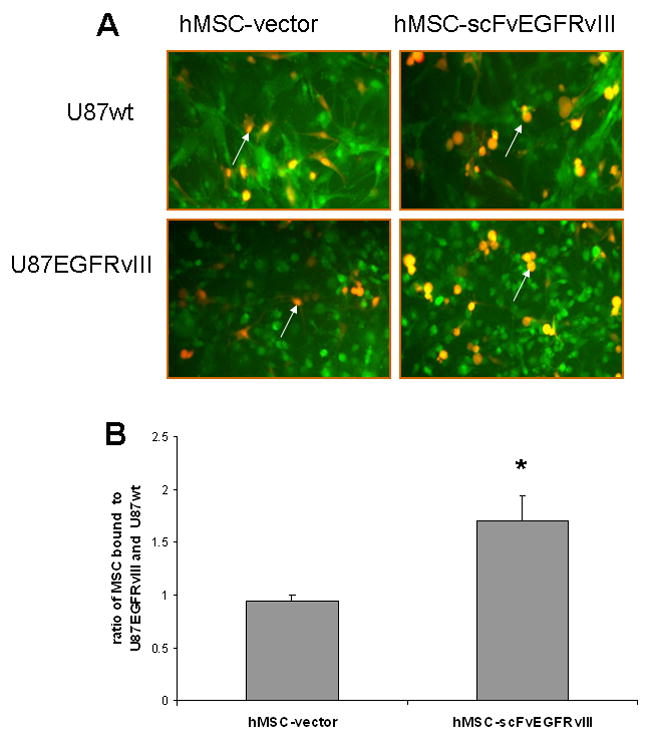
U87wt and U87-EGFRvIII cells expressing GFP were incubated either with hMSC-vector or hMSC-scFvEGFRvIII expressing RFP at 37°C. (A) Overlay images of hMSC and U87 cells from representative experiments. Arrows show hMSC. Original magnification 10×. (B) Summary of four independent experiments. * p< 0.005 vs hMSC-vector.
3.6. Targeting of hMSC expressing scFv EGFRvIII in vivo
In order to confirm the retargeted properties of modified hMSC in vivo, cells expressing vector alone or scFvEGFRvIII on the cells surface were injected in the flank tumor of U87 cells expressing EGFRvIII. Tumor cryo-sections were analyzed for the presence of RFP expressing hMSC in the tumor bed. Figures 7A–C show overlay images of GFP positive tumor cells and RFP positive hMSC from the tissue sections at day 5 and day 15 after hMSC injection. We found that the number of hMSC inside of the tumor decreased from day 5 to day 15. However, the presence of hMSC expressing scFv EGFRvIII.in tumor was significantly enhanced at both day 5 and day 15 after injection vs control hMSC (Fig. 7A–B, E)
Figure 7. Imaging of hMSC-scFv EGFRvIII in flank U87 tumor expressing EGFRvIII.
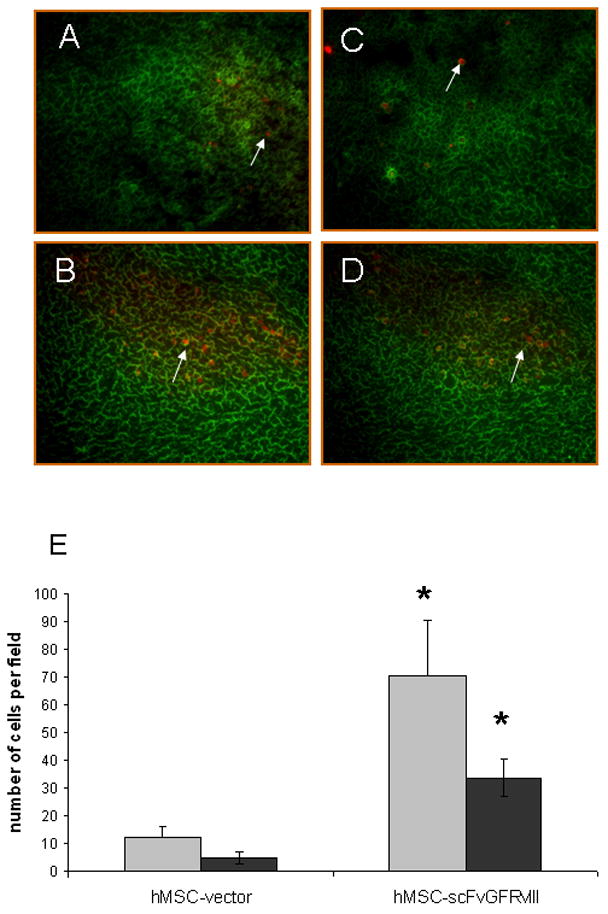
hMSC-vector and hMSC-scFvEGFRvIII expressing RFP were injected in the right flank of U87-EGFRvIII GFP expressing tumor. Tumor tissues were frozen on day 5 and 15 after injection of hMSC. Cryosections (20 microns) were analyzed using Olympus IX70 inverted microscope and MetaMorph software. Original magnification 10×. (A) and (C) show hMSC-vector within the tumor on day 5 and day 15, respectively, after the stem cells injection. (B) and (D) show hMSC-scFvEGFRvIII within the tumor on day 5 and day 15, respectively. Panel E shows the summary of the quantification of hMSC in tumors sections. Gray and black bars show the data at day 5 and 15, respectively. * p< 0.005 vs. hMSC-vector.
4. Discussion
In this study, we investigated the feasibility of expressing scFv EGFRvIII on the cell surface of hMSC using nucleofection, in order to specifically target the cells expressing EGFRvIII within the tumor. Using the Amaxa Biosystem’s nucleofection U-023 protocol, we found that the efficiency of transfection of hMSC with plasmid encoding GFP was about 50%, in agreement with a previous study (Aluigi et al., 2006). In hMSC transiently nucleofected with a plasmid encoding scFv EGFRvIII, an average of 20% of the cells were positive as detected by FACS analysis. Various factors might account for the difference in the expression of the protein we observed in cells nucleofected with GFP and scFvEGFRvIII gene encoding plasmids such as (i) the size of the plasmid and methods of the purification; (ii) the nature of the protein; and (iii) stability of the foreign protein expressed in hMSC. Interestingly, the level of GFP expression in hMSC varied and showed about 1000 times difference between the transfected cells with the lowest and the highest expression. Therefore, hMSC which received the low number of copies of the vector encoding scFv EGFRvIII might not express the scFv on the cells surface in the amount sufficient for detection. It might explain in part the fact that even after the stable selection of the cells with G418, we were not able to detect more then 30% of positive cells.
The expression of scFv on the cell surface may depend on the transmembrane domain and cytosolic tail to which it is fused. Previous studies suggested that PDGFRtm is not the best choice of transmembrane domain for the surface expression of proteins, rather B7-1 might be the better option (Chou et al., 1999; Liao et al., 2000). We found it to be different in our experiments using hMSC. The percent of positive cells transiently expressing the scFv EGFRvIII fused with PDGFRtm was on average 2.3-fold higher than that of the scFvEGFRvIII-hB7-1 fusion, although the difference in mean fluorescent intensity was not that apparent. This difference was not greater than 1.4-fold in stably transfected cells. In the study published by Liao and colleagues (Liao et al., 2000), the B7- tm was of murine origin, whereas we used B7-1 tm of human origin for consistency of comparison with human PDGFRtm and for purpose of transfection in human MSC. Moreover, it appeared that in the previously published studies by Chou and co-authors (Chou et al., 1999) and Liao and co-authors (Liao et al., 2000), which compared PDGFRtm to mB7-1, these two constructs were not identical. A myc epitope was present between the scFv and PDGFRtm, but was absent in the construct encoding mB7-1. Thus, the conclusion that B7-1tm is the better choice of the transmembrane domain over PDGFR is not accurate. Our study shows that PDGFRtm and hB7-1tm provide comparable expression scFvEGFRvIII fusion on the surface hMSC and HEK cells.
It was previously reported that temperature manipulation modulates the expression of some recombinant proteins (Fox et al., 2004; Schatz et al., 2003). It could be mediated in part by improved folding, and therefore, stability of scFv cultivated at lower temperatures. This approach provides one of the methods of increasing and standardizing the production of recombinant proteins, which is especially important for industrial large scale preparation (Andersen and Reilly 2004; Chartrain and Chu 2008). However, the effect of temperature could be host and cell line specific. In our experimental setting, shifting the temperature from 37°C to 32°C did not improve the expression of either protein, suggesting that the scFv EGFRvIII is relatively stable, such that the stability of scFv was not the contributing factor to the low number of positive cells expressing on the cells surface. In that regard, this particular scFvEGFRvIII originally named as MR1 was deemed as a stable in previous studies. It was obtained by the phage-display technique which allows the selection of stable species of the scFv presented on the phage surface. In addition it was found that scFv MR1 possesses a longer CDR3 domain, which improves the stability of this scFv (Kuan et al., 1999). However, we noticed that hMSC expressing scFv on the cells surface which were cultured for prolonged time at 37°C showed lower expression, and this expression could be boosted by shifting the temperature to 32 °C, thus suggesting that the stability of the surface expression scFv EGFRvIII could be compromised in long-term cultures.
In this study we used cell sorting as mean to enrich the population of scFvEGFRvIII expressing cells. After sorting, our purity was greater than 85% positive. However, continued culturing of the hMSC resulted in a steady decrease of scFv EGFRvIII expression on the cell surface. This decrease could be due to the fact that 15% of scFv-negative cells slowly overgrew the scFv-positive cells. The decreased stability of scFv EGFRvIII during cultivation for prolonged time could be also the contributing factor. To overcome this caveat in the future, we recommend freezing multiple aliquots of cells after the sorting for the future experiments using hMSC expressing scFvEGFRvIII on the cell surface.
We also addressed the method of detection of scFv EGFRvIII on the cell surface. In our final construct, the myc epitope is positioned between the C-terminus of the scFvEGFRvIII and transmembrane domain, whereas HA epitope is between signal peptide and N-terminal part of the scFvEGFRvIII. In this study, it appears that the HA epitope is the better choice for the detection of scFv on the surface of the live cells rather than the myc epitope. We found that fixation and permeabilization of the cells does not affect the detection scFv EGFRvIII with anti-HA antibodies to the extent seen when anti-myc mAb (clone 9E10). This may be related to the fact that the myc epitope is positioned in very close proximity to transmembrane domain and buried under the scFv globule and therefore not readily accessible for anti-myc antibodies on live cells. The fixation and permeabilization of cells might change the conformation of the scFv, thus making the myc epitope more accessible. However, we found that the increase in detection of both HA and myc epitopes of the scFv EGFRvIII in fixed and permeabilized cells was not simply due to conformational changes. Analysis of hMSC for the expression scFv EGFRvIII by confocal microscopy confirmed the plasma membrane and intracellular localization of recombinant protein as detected by its HA epitope.
We compared hMSC expressing scFv EGFRvIII on the cell surface with control hMSC for their targeting properties in vitro and in vivo. It appeared that non-modified hMSC are no different in terms of their binding to U87 cells expressing wild type EGFR or EGFRvIII. In contrast, modified hMSC showed preference in their binding to U87 EGFRvIII expressing glioma cells. This difference in the binding between two types of cells could be significantly masked due to the other receptor-ligand interactions existing between hMSC and glioma cells. We hypothesized that modified MSC will demonstrate improved retention in EGFRvIII expressing tumors. Previous studies have shown that the number of hMSC injected in EGFRvIII expressing tumors steadily decreases over two week time period (Sasportas et al., 2009). Similarly we observed the decrease in the presence hMSC from day 5 to day 15 after intratumoral injection. However, the retention of hMSC modified to express scFvEGFRvIII on the cells surface in EGFRvIII expressing flank glial tumor was significantly improved over the unmodified MSC at both day 5 and day 15 after injection. Such improvement in retention of hMSC-scFvEGFRvIII offers a significant advantage in the therapy of EGFRvIII expressing tumors injected with hMSC loaded with therapeutic proteins specifically acting on glioma or immune cells. Of note, while there is clear heterogeneity in EGFRvIII expression in brain tumors, the enhanced benefit of targeting modified hMSC should result in a bystander effect to cells which do not express EGFRvIII. The prolonged association of these MSC with the tumor may translate in the prolonged release of secreted therapeutics in the tumor environment.
Conclusions
To our knowledge this study is the first report on the genetic modification of MSC to express scFv EGFRvIII on the cell surface and to investigate the targeting properties of these MSC in vitro and in vivo. MSCs modified to express scFv EGFRvIII on the cell surface may represent a very valuable reagent for specifically targeting GBM or other tumors expressing EGFRvIII and the delivery of therapeutic molecules or viruses in vivo.
Acknowledgments
We thank Dr. Cleo Rolle for critical review of the manuscript.
Grant Support: This work was supported by the National Cancer Institute (R01-CA122930), the National Institute of Neurological Disorders and Stroke (K08-NS046430), The Alliance for Cancer Gene Therapy Young Investigator Award, and the American Cancer Society (RSG-07-276-01-MGO).
Footnotes
Disclosure of Potential Conflict of Interest
The authors indicate no potential conflict of interest.
References
- Aluigi M, Fogli M, Curti A, et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(2):454–61. doi: 10.1634/stemcells.2005-0198. [DOI] [PubMed] [Google Scholar]
- Andersen DC, Reilly DE. Production technologies for monoclonal antibodies and their fragments. Curr Opin Biotechnol. 2004;15(5):456–62. doi: 10.1016/j.copbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Beckermann BM, Kallifatidis G, Groth A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99(4):622–31. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexell D, Gunnarsson S, Tormin A, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17(1):183–90. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrain M, Chu L. Development and production of commercial therapeutic monoclonal antibodies in Mammalian cell expression systems: an overview of the current upstream technologies. Curr Pharm Biotechnol. 2008;9(6):447–67. doi: 10.2174/138920108786786367. [DOI] [PubMed] [Google Scholar]
- Cherry SR, Biniszkiewicz D, van Parijs L, et al. Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol Cell Biol. 2000;20(20):7419–26. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Liao KW, Lo YC, et al. Expression of chimeric monomer and dimer proteins on the plasma membrane of mammalian cells. Biotechnol Bioeng. 1999;65(2):160–9. doi: 10.1002/(sici)1097-0290(19991020)65:2<160::aid-bit5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Compte M, Cuesta AM, Sanchez-Martin D, et al. Tumor Immunotherapy Using Gene-Modified Human Mesenchymal Stem Cells Loaded into Synthetic Extracellular Matrix Scaffolds. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos N, Francois M, Boivin MN, et al. Neo-organoid of marrow mesenchymal stromal cells secreting interleukin-12 for breast cancer therapy. Cancer Res. 2008;68(12):4810–8. doi: 10.1158/0008-5472.CAN-08-0160. [DOI] [PubMed] [Google Scholar]
- Emrich JG, Brady LW, Quang TS, et al. Radioiodinated (I-125) monoclonal antibody 425 in the treatment of high grade glioma patients: ten-year synopsis of a novel treatment. Am J Clin Oncol. 2002;25(6):541–6. doi: 10.1097/00000421-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Fox SR, Patel UA, Yap MG, et al. Maximizing interferon-gamma production by Chinese hamster ovary cells through temperature shift optimization: experimental and modeling. Biotechnol Bioeng. 2004;85(2):177–84. doi: 10.1002/bit.10861. [DOI] [PubMed] [Google Scholar]
- Frederick L, Eley G, Wang XY, et al. Analysis of genomic rearrangements associated with EGRFvIII expression suggests involvement of Alu repeat elements. Neuro Oncol. 2000;2(3):159–63. doi: 10.1093/neuonc/2.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheisari Y, Soleimani M, Azadmanesh K, et al. Multipotent mesenchymal stromal cells: optimization and comparison of five cationic polymer-based gene delivery methods. Cytotherapy. 2008;10(8):815–23. doi: 10.1080/14653240802474307. [DOI] [PubMed] [Google Scholar]
- Harui A, Suzuki S, Kochanek S, et al. Frequency and stability of chromosomal integration of adenovirus vectors. J Virol. 1999;73(7):6141–6. doi: 10.1128/jvi.73.7.6141-6146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helledie T, Nurcombe V, Cool SM. A simple and reliable electroporation method for human bone marrow mesenchymal stem cells. Stem Cells Dev. 2008;17(4):837–48. doi: 10.1089/scd.2007.0209. [DOI] [PubMed] [Google Scholar]
- Ho M, Pastan I. Mammalian cell display for antibody engineering. Methods Mol Biol. 2009;525:337–52. xiv. doi: 10.1007/978-1-59745-554-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehira M, Xin H, Hoshino K, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14(11):894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- Kioi M, Seetharam S, Puri RK. Targeting IL-13Ralpha2-positive cancer with a novel recombinant immunotoxin composed of a single-chain antibody and mutated Pseudomonas exotoxin. Mol Cancer Ther. 2008;7(6):1579–87. doi: 10.1158/1535-7163.MCT-07-2131. [DOI] [PubMed] [Google Scholar]
- Kuan CT, Reist CJ, Foulon CF, et al. 125I-labeled anti-epidermal growth factor receptor-vIII single-chain Fv exhibits specific and high-level targeting of glioma xenografts. Clin Cancer Res. 1999;5(6):1539–49. [PubMed] [Google Scholar]
- Kucerova L, Matuskova M, Pastorakova A, et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;10(10):1071–82. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- Liao KW, Lo YC, Roffler SR. Activation of lymphocytes by anti-CD3 single-chain antibody dimers expressed on the plasma membrane of tumor cells. Gene Ther. 2000;7(4):339–47. doi: 10.1038/sj.gt.3301080. [DOI] [PubMed] [Google Scholar]
- Liu F, Lu Q, Ye X, et al. Cancer gene therapy of adenovirus-mediated anti-4-1BB scFv in immunocompetent mice. Cancer Biol Ther. 2008;7(3):448–53. doi: 10.4161/cbt.7.3.5425. [DOI] [PubMed] [Google Scholar]
- Lorimer IA, Keppler-Hafkemeyer A, Beers RA, et al. Recombinant immunotoxins specific for a mutant epidermal growth factor receptor: targeting with a single chain antibody variable domain isolated by phage display. Proc Natl Acad Sci U S A. 1996;93(25):14815–20. doi: 10.1073/pnas.93.25.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjtahedi H, Moscatello DK, Box G, et al. Targeting of cells expressing wild-type EGFR and type-III mutant EGFR (EGFRvIII) by anti-EGFR MAb ICR62: a two-pronged attack for tumour therapy. Int J Cancer. 2003;105(2):273–80. doi: 10.1002/ijc.11055. [DOI] [PubMed] [Google Scholar]
- Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11(14):1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106(12):4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz SM, Kerschbaumer RJ, Gerstenbauer G, et al. Higher expression of Fab antibody fragments in a CHO cell line at reduced temperature. Biotechnol Bioeng. 2003;84(4):433–8. doi: 10.1002/bit.10793. [DOI] [PubMed] [Google Scholar]
- Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26(3):831–41. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–8. [PubMed] [Google Scholar]
- Wei LH, Olafsen T, Radu C, et al. Engineered antibody fragments with infinite affinity as reporter genes for PET imaging. J Nucl Med. 2008;49(11):1828–35. doi: 10.2967/jnumed.108.054452. [DOI] [PubMed] [Google Scholar]


