Abstract
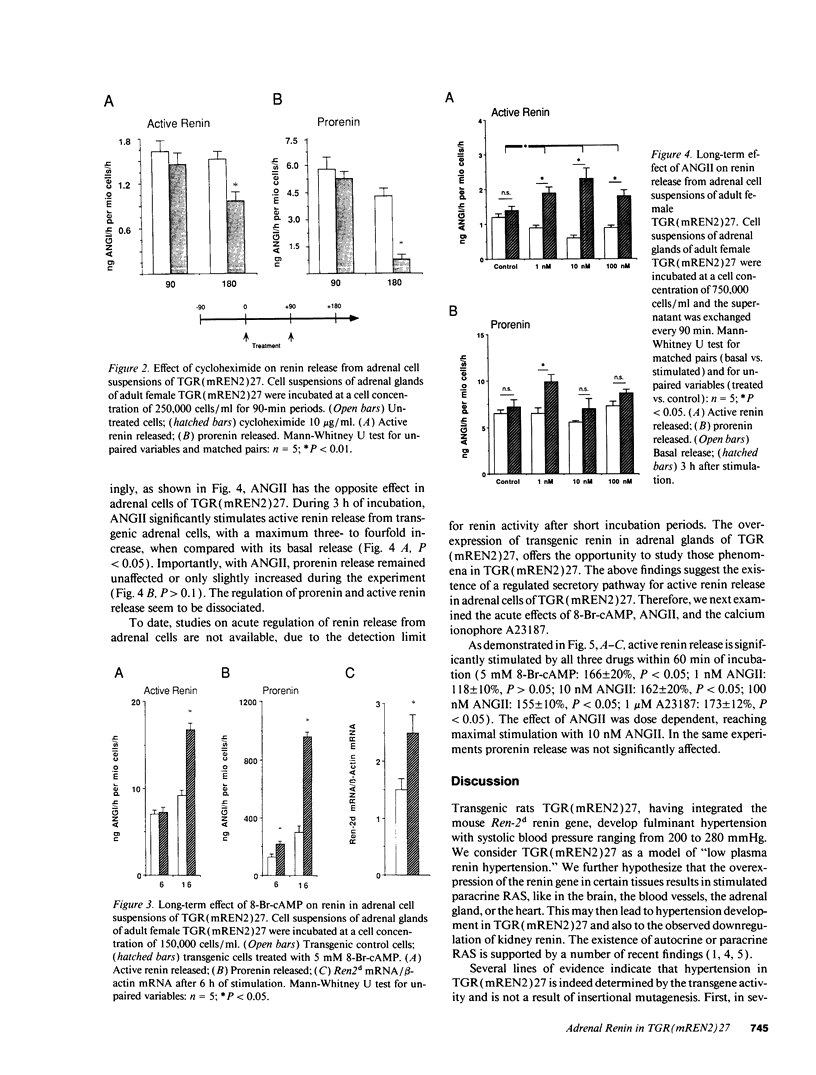
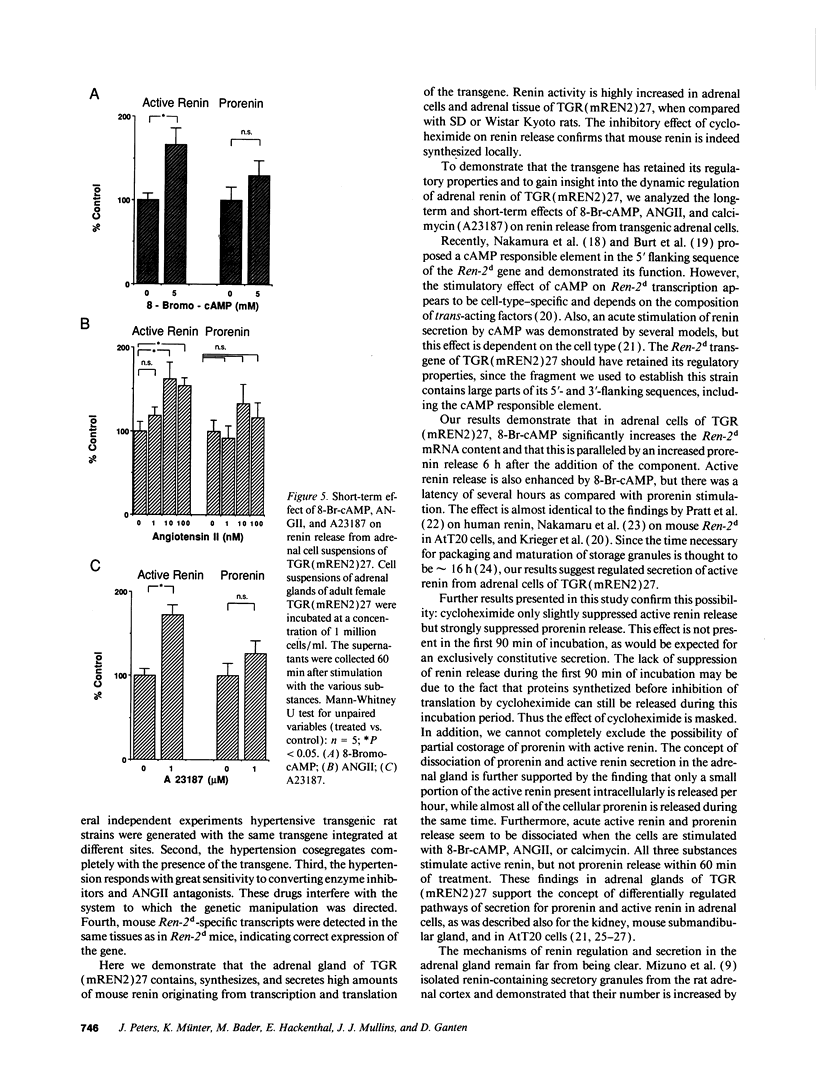
The newly established rat strain TGR(mREN2)27 is a monogenetic model in hypertension research. Microinjecting the mouse Ren-2d renin gene caused it to become a stable part of the genome. The rats are characterized by fulminant hypertension, low plasma active renin, suppressed kidney renin, high plasma inactive renin, and high extrarenal transgene expression, most prominently in the adrenal cortex. Additionally, they exhibit significantly enhanced excretion of corticosteroids. Here we demonstrate that part of the plasma renin and most of the adrenal renin are transgene determined and that the adrenal renin is strongly activated. TGR(mREN2)27 adrenal cells may serve as a new tool to investigate the regulation and processing of Ren-2d-derived renin and its significance in hypertension and steroid metabolism. Adrenal renin in TGR(mREN2)27 is stimulated by 8-bromo-cAMP (8-Br-cAMP), angiotensin II (ANGII), and calcium. 8-Br-cAMP significantly stimulates active renin and prorenin release, as well as Ren-2d mRNA. Interestingly, within 60 min 8-Br-cAMP, ANGII, and calcimycin stimulate active renin, but not prorenin release. This indicates different intracellular pathways. An activated adrenal renin-angiotensin system in TGR (mREN2)27 as well as the lack of negative feedback on renin secretion by ANGII may be of pathophysiological significance in this hypertensive model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann S., Peters J., Engler E., Ganten D., Mullins J. Transgenic rats carrying the mouse renin gene--morphological characterization of a low-renin hypertension model. Kidney Int. 1992 Jan;41(1):24–36. doi: 10.1038/ki.1992.4. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Nakamura N., Kelley P., Dzau V. J. Identification of negative and positive regulatory elements in the human renin gene. J Biol Chem. 1989 May 5;264(13):7357–7362. [PubMed] [Google Scholar]
- Ganten D., Ganten U., Kubo S., Granger P., Nowaczynski W., Boucher R., Genest J. Influence of sodium, potassium, and pituitary hormones on iso-renin in rat adrenal glands. Am J Physiol. 1974 Jul;227(1):224–229. doi: 10.1152/ajplegacy.1974.227.1.224. [DOI] [PubMed] [Google Scholar]
- Ganten D., Hermann K., Unger T., Lang R. E. The tissue renin-angiotensin systems: focus on brain angiotensin, adrenal gland and arterial wall. Clin Exp Hypertens A. 1983;5(7-8):1099–1118. doi: 10.3109/10641968309048844. [DOI] [PubMed] [Google Scholar]
- Ganten D., Hutchinson J. S., Schelling P., Ganten U., Fischer H. The iso-renin angiotensin systems in extrarenal tissue. Clin Exp Pharmacol Physiol. 1976 Mar-Apr;3(2):103–126. doi: 10.1111/j.1440-1681.1976.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Glorioso N., Madeddu P., Dessi'-Fulgheri P., Fois G., Meloni F., Bandiera F., Tonolo G., Rappelli A. Trypsin-activatable inactive renin in rat plasma. Clin Sci (Lond) 1983 Feb;64(2):137–140. doi: 10.1042/cs0640137. [DOI] [PubMed] [Google Scholar]
- Hackenthal E., Paul M., Ganten D., Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990 Oct;70(4):1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- Hermann K., Ganten D., Unger T., Bayer C., Lang R. E. Measurement and characterization of angiotensin peptides in plasma. Clin Chem. 1988 Jun;34(6):1046–1051. [PubMed] [Google Scholar]
- Jin M., Wilhelm M. J., Lang R. E., Unger T., Lindpaintner K., Ganten D. Endogenous tissue renin-angiotensin systems. From molecular biology to therapy. Am J Med. 1988 Mar 11;84(3A):28–36. doi: 10.1016/0002-9343(88)90202-1. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Hoffman L. H., McKenzie J. C., Inagami T. Presence of renin secretory granules in rat adrenal gland and stimulation of renin secretion by angiotensin II but not by adrenocorticotropin. J Clin Invest. 1988 Sep;82(3):1007–1016. doi: 10.1172/JCI113657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. J., Peters J., Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990 Apr 5;344(6266):541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Burt D. W., Paul M., Dzau V. J. Negative control elements and cAMP responsive sequences in the tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):56–59. doi: 10.1073/pnas.86.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M., Nakamura N., Pratt R. E., Burt D. W., Dzau V. J. Cell-dependent posttranslational processing and secretion of recombinant mouse renin-2. Am J Physiol. 1992 Feb;262(2 Pt 1):E224–E229. doi: 10.1152/ajpendo.1992.262.2.E224. [DOI] [PubMed] [Google Scholar]
- Pratt R. E., Carleton J. E., Richie J. P., Heusser C., Dzau V. J. Human renin biosynthesis and secretion in normal and ischemic kidneys. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7837–7840. doi: 10.1073/pnas.84.22.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt R. E., Carleton J. E., Roth T. P., Dzau V. J. Evidence for two cellular pathways of renin secretion by the mouse submandibular gland. Endocrinology. 1988 Oct;123(4):1721–1727. doi: 10.1210/endo-123-4-1721. [DOI] [PubMed] [Google Scholar]
- Pratt R. E., Flynn J. A., Hobart P. M., Paul M., Dzau V. J. Different secretory pathways of renin from mouse cells transfected with the human renin gene. J Biol Chem. 1988 Mar 5;263(7):3137–3141. [PubMed] [Google Scholar]
- Pratt R. E., Ouellette A. J., Dzau V. J. Biosynthesis of renin: multiplicity of active and intermediate forms. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6809–6813. doi: 10.1073/pnas.80.22.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. W. Renin-like enzyme in the adrenal gland. Science. 1967 Dec 22;158(3808):1589–1590. doi: 10.1126/science.158.3808.1589. [DOI] [PubMed] [Google Scholar]
- Sander M., Bader M., Djavidani B., Maser-Gluth C., Vecsei P., Mullins J., Ganten D., Peters J. The role of the adrenal gland in hypertensive transgenic rat TGR(mREN2)27. Endocrinology. 1992 Aug;131(2):807–814. doi: 10.1210/endo.131.2.1322284. [DOI] [PubMed] [Google Scholar]
- Schelling P., Ganten U., Sponer G., Unger T., Ganten D. Components of the renin-angiotensin system in the cerebrospinal fluid of rats and dogs with special consideration of the origin and the fate of angiotensin II. Neuroendocrinology. 1980 Nov;31(5):297–308. doi: 10.1159/000123092. [DOI] [PubMed] [Google Scholar]
- Taugner R., Kim S. J., Murakami K., Waldherr R. The fate of prorenin during granulopoiesis in epithelioid cells. Immunocytochemical experiments with antisera against renin and different portions of the renin prosegment. Histochemistry. 1987;86(3):249–253. doi: 10.1007/BF00490255. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. RNA isolation: a mini-prep method. Nucleic Acids Res. 1988 Nov 25;16(22):10933–10933. doi: 10.1093/nar/16.22.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Franco-Saenz R., Mulrow P. J. Effect of angiotensin II on renin production by rat adrenal glomerulosa cells in culture. Hypertension. 1992 Mar;19(3):263–269. doi: 10.1161/01.hyp.19.3.263. [DOI] [PubMed] [Google Scholar]






