Abstract
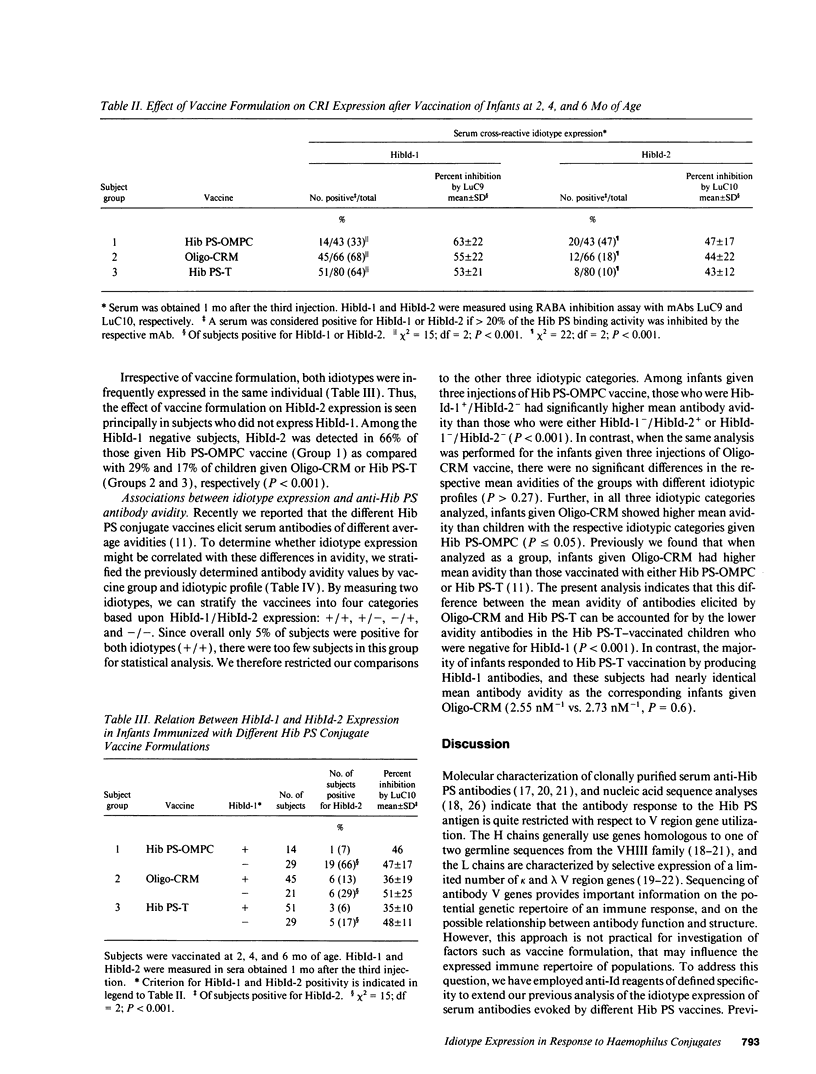
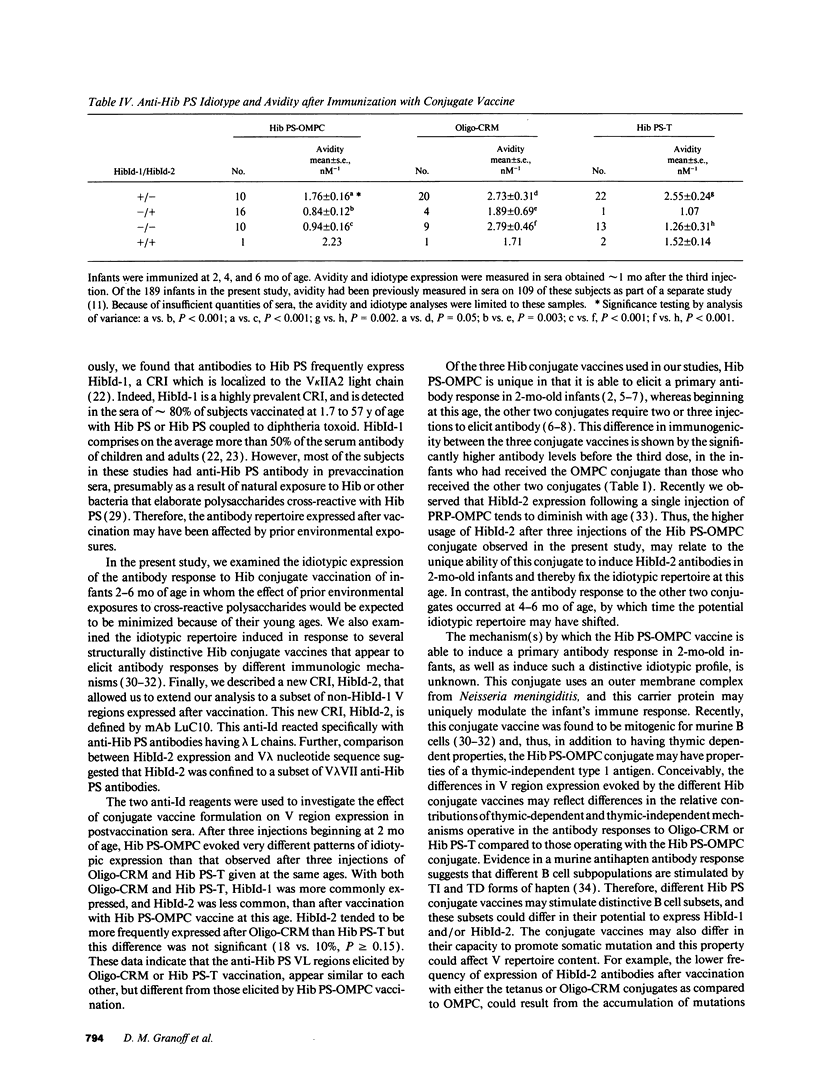
Haemophilus influenzae b polysaccharide (Hib PS)-protein conjugate vaccines differ chemically and immunologically. To determine whether anti-Hib PS variable region expression might differ according to vaccine formulation, infants were vaccinated at 2, 4, and 6 mo of age with Hib PS coupled to either meningococcal outer membrane protein complex (Hib PS-OMPC) or tetanus toxoid (Hib PS-T), or Hib PS oligomers coupled to a mutant diphtheria toxin (Oligo-CRM). Two anti-Hib PS idiotypes were measured in sera obtained after the third injection: HibId-1, expressed by anti-Hib PS antibodies having the kappa II-A2 variable region, and HibId-2, a newly defined cross-reactive idiotype associated with a subset of anti-Hib PS antibodies having lambda VII variable regions. HibId-1 was present in 33, 68, and 64% of infants given either Hib PS-OMPC, Oligo-CRM, or Hib PS-T, respectively (P < 0.001). The respective values for HibId-2 were 47, 18, and 10% (P = 0.001). Subjects who were vaccinated with Hib PS-OMPC or Hib PS-T and who produced detectable HibId-1-positive antibody, had significantly higher mean antibody avidity than subjects who did not produce HibId-1 positive antibodies. In contrast, Oligo-CRM evoked high avidity anti-Hib PS antibodies, irrespective of the idiotypic profile. These findings indicate fundamental differences in both variable region content and antibody quality elicited by different Hib PS conjugate vaccines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adderson E. E., Shackelford P. G., Insel R. A., Quinn A., Wilson P. M., Carroll W. L. Immunoglobulin light chain variable region gene sequences for human antibodies to Haemophilus influenzae type b capsular polysaccharide are dominated by a limited number of V kappa and V lambda segments and VJ combinations. J Clin Invest. 1992 Mar;89(3):729–738. doi: 10.1172/JCI115649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adderson E. E., Shackelford P. G., Quinn A., Carroll W. L. Restricted Ig H chain V gene usage in the human antibody response to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1991 Sep 1;147(5):1667–1674. [PubMed] [Google Scholar]
- Ambrosino D. M., Greif W., Thompson C., Siber G. R. Kappa and lambda light chain composition of antibody to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1990 May;161(5):922–925. doi: 10.1093/infdis/161.5.922. [DOI] [PubMed] [Google Scholar]
- Amir J., Liang X., Granoff D. M. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr Res. 1990 Apr;27(4 Pt 1):358–364. doi: 10.1203/00006450-199004000-00008. [DOI] [PubMed] [Google Scholar]
- Decker M. D., Edwards K. M., Bradley R., Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr. 1992 Feb;120(2 Pt 1):184–189. doi: 10.1016/s0022-3476(05)80424-x. [DOI] [PubMed] [Google Scholar]
- Donnelly J. J., Deck R. R., Liu M. A. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Immunol. 1990 Nov 1;145(9):3071–3079. [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Anderson E. L., Osterholm M. T., Holmes S. J., McHugh J. E., Belshe R. B., Medley F., Murphy T. V. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992 Aug;121(2):187–194. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Shackelford P. G., Pandey J. P., Boies E. G. Antibody responses to Haemophilus influenzae type b polysaccharide vaccine in relation to Km(1) and G2m(23) immunoglobulin allotypes. J Infect Dis. 1986 Aug;154(2):257–264. doi: 10.1093/infdis/154.2.257. [DOI] [PubMed] [Google Scholar]
- Griswold W. R., Lucas A. H., Bastian J. F., Garcia G. Functional affinity of antibody to the Haemophilus influenzae type b polysaccharide. J Infect Dis. 1989 Jun;159(6):1083–1087. doi: 10.1093/infdis/159.6.1083. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986 Feb 1;163(2):262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Kittelberger A., Anderson P. Isoelectric focusing of human antibody to the Haemophilus influenzae b capsular polysaccharide: restricted and identical spectrotypes in adults. J Immunol. 1985 Oct;135(4):2810–2816. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Lenoir A. A., Granoff P. D., Granoff D. M. Immunogenicity of Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer membrane protein conjugate vaccine in 2- to 6-month-old infants. Pediatrics. 1987 Aug;80(2):283–287. [PubMed] [Google Scholar]
- Liu M. A., Friedman A., Oliff A. I., Tai J., Martinez D., Deck R. R., Shieh J. T., Jenkins T. D., Donnelly J. J., Hawe L. A. A vaccine carrier derived from Neisseria meningitidis with mitogenic activity for lymphocytes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4633–4637. doi: 10.1073/pnas.89.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. H., Granoff D. M. A major crossreactive idiotype associated with human antibodies to the Haemophilus influenzae b polysaccharide. Expression in relation to age and immunoglobulin G subclass. J Clin Invest. 1990 Apr;85(4):1158–1166. doi: 10.1172/JCI114548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. H., Langley R. J., Granoff D. M., Nahm M. H., Kitamura M. Y., Scott M. G. An idiotypic marker associated with a germ-line encoded kappa light chain variable region that predominates the vaccine-induced human antibody response to the Haemophilus influenzae b polysaccharide. J Clin Invest. 1991 Dec;88(6):1811–1818. doi: 10.1172/JCI115502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore D. V., Johnson C. L., Phipps D. C., Popejoy L. A., Eby R., Smith D. H. Safety and immunologic response to Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in 1- to 6-month-old infants. Pediatrics. 1990 Mar;85(3):331–337. [PubMed] [Google Scholar]
- Mäkelä O., Mattila P., Rautonen N., Seppälä I., Eskola J., Käyhty H. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). J Immunol. 1987 Sep 15;139(6):1999–2004. [PubMed] [Google Scholar]
- Robbins J. B., Schneerson R., Glode M. P., Vann W., Schiffer M. S., Liu T. Y., Parke J. C., Jr, Huntley C. Cross-reactive antigens and immunity to diseases caused by encapsulated bacteria. J Allergy Clin Immunol. 1975 Aug;56(2):141–151. doi: 10.1016/0091-6749(75)90119-0. [DOI] [PubMed] [Google Scholar]
- Schlesinger Y., Granoff D. M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992 Mar 18;267(11):1489–1494. [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Chung G., Schäble K. F., Thiebe R., Quenzel E. M., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. IV. The less frequently expressed VL are heterogeneous. J Immunol. 1991 Dec 1;147(11):4007–4013. [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Zocher I., Thiebe R., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. III. A single VKII gene and one of several JK genes are joined by an invariant arginine to form the most common L chain V region. J Immunol. 1989 Dec 15;143(12):4110–4116. [PubMed] [Google Scholar]
- Scott M. G., Tarrand J. J., Crimmins D. L., McCourt D. W., Siegel N. R., Smith C. E., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. II. IgG antibodies contain VH genes from a single VH family and VL genes from at least four VL families. J Immunol. 1989 Jul 1;143(1):293–298. [PubMed] [Google Scholar]
- Shackelford P. G., Granoff D. M., Nelson S. J., Scott M. G., Smith D. S., Nahm M. H. Subclass distribution of human antibodies to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1987 Jan 15;138(2):587–592. [PubMed] [Google Scholar]
- Silverman G. J., Lucas A. H. Variable region diversity in human circulating antibodies specific for the capsular polysaccharide of Haemophilus influenzae type b. Preferential usage of two types of VH3 heavy chains. J Clin Invest. 1991 Sep;88(3):911–920. doi: 10.1172/JCI115394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992 Jun;165 (Suppl 1):S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- Tittle T. V., Rittenberg M. B. Distinct subpopulations of IgG memory B cells respond to different molecular forms of the same hapten. J Immunol. 1978 Sep;121(3):936–941. [PubMed] [Google Scholar]
- Vella P. P., Ellis R. W. Immunogenicity of Haemophilus influenzae type b conjugate vaccines in infant rhesus monkeys. Pediatr Res. 1991 Jan;29(1):10–13. doi: 10.1203/00006450-199101000-00003. [DOI] [PubMed] [Google Scholar]
- Weinberg G. A., Granoff D. M. Polysaccharide-protein conjugate vaccines for the prevention of Haemophilus influenzae type b disease. J Pediatr. 1988 Oct;113(4):621–631. doi: 10.1016/s0022-3476(88)80369-x. [DOI] [PubMed] [Google Scholar]


