Abstract
In an ovine model of persistent pulmonary hypertension of the newborn (PPHN), endothelin-1 (ET-1) expression is increased, while endothelial nitric oxide synthase (eNOS) expression is decreased. However, the molecular mechanisms by which ET-1 attenuates eNOS expression in endothelial cells are not completely understood. Thus, the goal of this study was to determine if the overexpression of ET-1 decreases eNOS expression in pulmonary arterial endothelial cells isolated from fetal lambs. To increase the ET-1 expression, cells were transfected with a plasmid coding for Prepro-ET-1, a precursor of ET-1. After overexpression of Prepro-ET-1, ET-1 levels in the culture medium were significantly increased (control = 805.3 ± 69.8; Prepro-ET-1 overexpression = 1351 ± 127.9). eNOS promoter activity, protein levels, and NO generation were all significantly decreased by the overexpression of Prepro-ET-1. The decrease in transcription correlated with increased activity of protein kinase Cδ (PKCδ) and STAT3. Further, DNA binding activity of STAT3 was also increased by Prepro-ET-1 overexpression. The increase in STAT3 activity and decrease in eNOS promoter activity were inhibited by the overexpression of dominant negative mutants of PKCδ or STAT3. Further, a 2 bp mutation in the STAT3 binding site in the eNOS promoter inhibited STAT3 binding and led to enhanced promoter activity in the presence of Prepro-ET-1 overexpression. In conclusion, ET-1 secretion is increased by Prepro-ET-1 overexpression. This results in activation of PKCδ, which phosphorylates STAT3, increasing its binding to the eNOS promoter. This in turn decreases eNOS promoter activity, protein levels, and NO production. Thus, ET-1 can reduce eNOS expression and NO generation in fetal pulmonary artery endothelial cells through PKCδ-mediated activation of STAT3.
Introduction
Endothelin-1 (ET-1) plays an important role in the regulation of pulmonary vascular tone in the perinatal period. It is synthesized as Prepro-ET-1, a 203-amino-acid peptide, which is cleaved into big ET-1, a 38-amino-acid peptide. Big ET-1 is in turn cleaved into its functional form (ET-1) by endothelin converting enzyme-1 (Ohnaka et al., 1993; Chiou et al., 1994; Xu et al., 1994). Although ET-1 produces systemic vasoconstriction, its effects on the pulmonary circulation vary with age and vascular tone (Cassin et al., 1991; Wong et al., 1993, 1994). These hemodynamic effects are mediated through two receptor subtypes: endothelin receptor subtype A (ETA) receptors, located on vascular smooth muscle cells that are responsible for the vasoconstricting effects of ET-1, and endothelin receptor subtype B (ETB) receptors, located on vascular endothelial cells and are responsible for the vasodilating effects of ET-1 (Luscher et al., 1990; Sakurai et al., 1990; Arai et al., 1993). A subpopulation of ETB receptors are also found on SMC, whose function is still unclear.
Pulmonary vascular resistance decreases and pulmonary blood flow increases with the initiation of ventilation and oxygenation after birth (Fineman et al., 1995). Increased endothelial nitric oxide synthase (eNOS) gene expression, eNOS activity, and NO production have been shown to contribute to these changes (Abman et al., 1990; Moore et al., 1992; Shaul et al., 1993; Black et al., 1997; Villamor et al., 1997). However, in a number of clinical conditions, there is failure of the pulmonary circulation to undergo this normal transition to postnatal life, resulting in persistent pulmonary hypertension of the newborn (PPHN) (Sakurai et al., 1990; Fineman et al., 1995). Prolonged compression or ligation of the ductus arteriosus in utero in the lamb produces fetal and neonatal pulmonary hypertension (Wild et al., 1989). Similar to newborns who die with PPHN, these lambs have an increase in the thickness of the smooth muscle of the small pulmonary arteries, complete muscularization of normally partially muscularized pulmonary arteries, and extension of muscle to nonmuscularized arteries (Murphy et al., 1981). These lambs demonstrate altered hemodynamic responses to and changes in the production and/or concentration of NO. It has been previously shown that fetal pulmonary hypertension after ligation of the ductus arteriosus in utero is associated with increased expression of PreproET-1 mRNA and elevated levels of ET-1 (Black et al., 1998b). There is also a decrease in the expression of eNOS mRNA and protein in lung tissue (Shaul et al., 1993; Villamor et al., 1997). However, the molecular mechanisms by which increased ET-1 levels reduce eNOS expression and NO production have not been completely elucidated. In this study, we focus on the signaling pathway by which elevated ET-1 levels lead to decreased NO production in endothelial cells. We show that ET-1 stimulates protein kinase Cδ (PKCδ) activity, which in turn attenuates eNOS transcription and NOS signaling through the activation of STAT3.
Materials and Methods
Cell culture
Primary cultures of ovine fetal pulmonary artery endothelial cells (FPAECs) were isolated as described previously (Wedgwood et al., 2003). Cells were maintained in DMEM containing phenol red supplemented with 10% fetal calf serum (Hyclone, Logan, UT), antibiotics, and antimycotics (MediaTech, Herndon, VA) at 37°C in a humidified atmosphere with 5% CO2–95% air. Cells were between passages 3 and 10, seeded at ∼50% confluence, and utilized when fully confluent.
Western blot analysis
Ovine FPAECs were solubilized with a lysis buffer containing 1% Triton X-100, 20 mM Tris pH 7.4, 100 mM NaCl, 1 mM EDTA, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitor cocktail (Pierce, Rockford, IL). Insoluble proteins were precipitated by centrifugation at 13,000 rpm for 10 min at 4°C, and the supernatants were then subjected to sodium dodecyl sulfate–PAGE on 4–12% polyacrylamide gels and transferred to a nitrocellulose membrane (Biorad, Hercules, CA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween (TBST). The primary antibodies used for immunoblotting were anti-PKCδ, anti-phospho tyr311 PKCδ, anti-Stat3, anti-phospho ser727 Stat3, and anti-eNOS (1:1000; Cell Signaling Technology, Beverly, MA). The membrane was then washed with TBST thrice for 10 min, incubated with secondary antibodies coupled to horseradish peroxidase, and washed again with TBST as described above, and the protein bands were observed with ECL reagent (Pierce, Rockford, IL).
Overexpression of Prepro-ET-1, DN PKCδ, and S727A STAT3
Ovine FPAECs were transfected with pEGFP-N1-Prepro-ET-1, encoding ovine Prepro-ET-1, or the parental vector as a control. Overexpression of DN PKCδ and S727A STAT3 mutant construct (Addgene, Cambridge, MA) in which ser 727 is mutated to alanine was performed as described previously (Sud et al., 2008).
Quantitation of ET-1 levels in the culture supernatant
The ET-1 levels in the cell culture supernatant were quantitated using QuantiGlo Chemiluminescent ELISA kit (R&D Systems) for ET-1 according to manufacturer's instructions. Briefly, cell supernatant was added to wells of microplate precoated with monoclonal antibody specific for ET-1. After washing away any unbound substances, an enzyme-linked antibody specific for ET-1 was added to the wells. After a wash to remove any unbound antibody–enzyme reagent, an enhanced luminal/peroxide substrate solution was added, and the light emitted is measured by luminometer. A standard curve using known concentration of ET-1 was prepared to quantify ET-1 in the cell supernatant.
eNOS promoter analysis
eNOS transcription was analyzed using a 1600 bp promoter fragment fused to a luciferase reporter gene as described previously (Wedgwood et al., 2003). Ovine FPAECs were cotransfected with the 1.6 kb eNOS promoter-luciferase and β-galactosidase construct (to normalize for transfection efficiency). To analyze the effect of PKCδ or STAT3, the cells were cotransfected with either DN PKCδ of S727A STAT3 mutant. Transfected cells were serum starved overnight, and then incubated with either bryostatin (10 ng/mL, 30 min) before shear. Luciferase activity was determined using the Luciferase assay kit (Promega, Madison, WI) and a Fluoroskan Ascent FL luminometer (Thermo Electron Corporation, Waltham, MA). In addition, a mutant eNOS promoter construct was prepared in which the STAT3 binding site of eNOS (TTTCTTT) was mutated by 2 bp to (TTTCccT) to eliminate the binding of STAT3 (Isomoto et al., 2005).
Detection of NOx
NOx generated by FPAECs was measured using an NO-sensitive electrode with a 2-mm-diameter tip (ISO-NOP sensor; WPI) connected to an NO meter (ISO-NO Mark II; WPI, Sarasota, FL) as described previously (Sud et al., 2007).
DNA binding analysis
Nuclear extracts were prepared using the NE-PER kit (Pierce) as specified by the manufacturer. To quantify the STAT3-specific binding activity of nuclear extract, Transfactor kit (Clonetech, Mountain View, CA) was used according to the manufacturer's protocol. The wells of streptavidin-coated plates were blocked for 15 min with 1× TransFactor/Blocking buffer and incubated for 1 h at room temperature. Nuclear extract was diluted with 1× TransFactor/Blocking buffer and mixed with poly dIdC and either wild-type biotinylated double-stranded oligonucleotide (5′CTGAGCTTCCGTTTCTTTCTTAAACTTTCTCTCAGTC3′) corresponding to Stat3 binding site at −1024 in eNOS gene or double-stranded mutant oligonucleotide (5′CTGAGCTTCCGTTTCccTCTTAAACTTTCTCTCAGTC3′), with a 2 bp mutation in STAT3 binding site at −1024 and incubated on ice for 15 min. This was then added to the wells of the plate and incubated at RT for 60 min. Microtiter wells were then washed three times, and anti-STAT3 was added per well and incubated further at room temperature for an hour. After extensive washing, diluted secondary antibody conjugated with horseradish peroxidase was added to each well and further incubated at room temperature for 30 min. After repeated washing, 100 μL of tetramethylbenzidine substrate solution was added to each well in the dark for the color development at room temperature for 10 min, and binding intensity was measured as absorbance at 655 nm using a microtiter plate reader.
Statistical analysis
Statistical calculations were performed using the GraphPad Prism V. 4.01 software. The mean ± SD was calculated for all samples and significance determined either by the unpaired t-test or ANOVA. For ANOVA Neuman–Keuls post hoc testing was also utilized. A value of p < 0.05 was considered significant.
Results
Overexpression of Prepro-ET-1 increases ET-1 release in ovine FPAECs
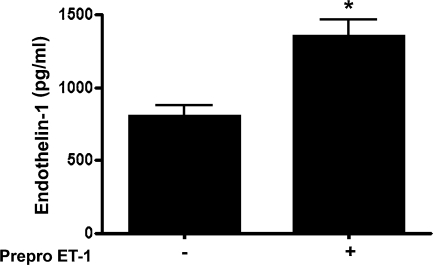
Ovine FPAECs were transfected with pEGFP-N1-ET-1 encoding a full-length ovine Prepro-ET-1 cDNA or a control vector. After 24 h the cell culture supernatant was assayed for ET-1 levels. We found that after overexpression of Prepro-ET-1, ET-1 levels in the culture medium were significantly increased (control = 805.3 ± 69.8 pg/mL; Prepro-ET-1 overexpression = 1351 ± 127.9 pg/mL; Fig. 1, p < 0.05).
FIG. 1.
Prepro-endothelin (ET)-1 overexpression increases ET-1 release in ovine fetal pulmonary arterial endothelial cells (FPAECs). Ovine fetal FPAECs were transfected with an expression plasmid for Prepro-ET-1 (pEGFP-N1-Prepro-ET-1) or the parental plasmid as a control. After 24 h, ET-1 levels in the culture medium was determined by ELISA. ET-1 levels are significantly higher in cells overexpressing Prepro-ET-1. Data are mean ± SD. *p < 0.05 versus control, n = 3.
Increased ET-1 levels reduce eNOS promoter activity, eNOS protein levels, and NO generation in ovine FPAECs
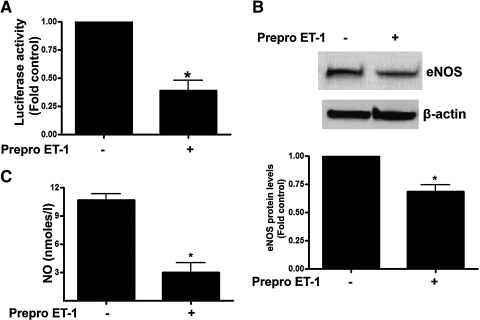
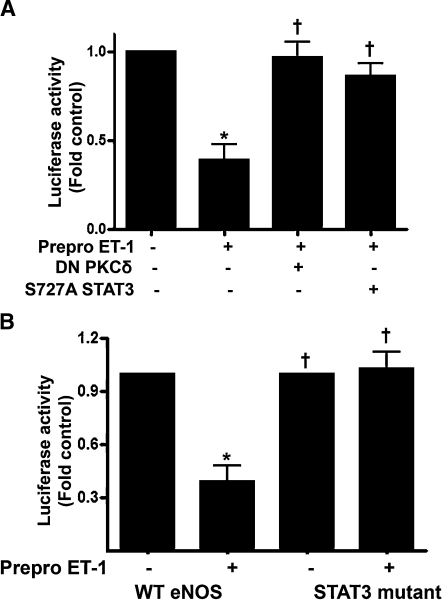
To determine the effect of Prepro-ET-1 overexpression on eNOS transcription, we cotransfected FPAECs with a 1.6 kb eNOS promoter construct linked to a luciferase reporter gene and either pEGFP-N1-ET-1 or the control vector. We then analyzed eNOS promoter activity after 24 h. Our data indicate that Prepro-ET-1 overexpression significantly reduced eNOS promoter activity (Fig. 2A, p < 0.05). Analysis of eNOS protein levels by immunoblotting revealed that eNOS protein levels were also significantly decreased in Prepro-ET-1 overexpressing cells (Fig. 2B). Further Prepro-ET-1 overexpression significantly reduced NO generation (Fig. 2C).
FIG. 2.
The overexpression of Prepro-ET-1 attenuates endothelial nitric oxide synthase (eNOS) expression and NO signaling in ovine FPAECs. Ovine FPAECs were cotransfected with pEGFP-N1-Prepro-ET-1 or the parental plasmid in combination with a 1.6 kb eNOS promoter–luciferase reporter construct. All cells were also trasnfected with a plasmid containing β-galactosidase as a transfection control. After 24 h, luciferase activity was measured. Prepro-ET-1 significantly decreases eNOS promoter activity (A). In addition, the decrease in eNOS promoter activity in PrePro-ET-1 overexpressing cells leads to a significant decrease in both eNOS protein (B) and NO generation (C). Data are mean ± SD. *p < 0.05 versus control, n = 3.
Increased ET-1 signaling stimulates PKCδ activity in ovine FPAECs
We next analyzed the effect of Prepro-ET-1 overexpression on PKCδ activity using phospho tyr 311 PKCδ levels as a measure of activity as we have previously described (Sud et al., 2008). Prepro-ET-1 overexpression increased phospho tyr 311 PKCδ levels (Fig. 3). Further, the increase in phospho tyr 311 PKCδ levels induced by Prepro-ET-1 overexpression was reversed by overexpressing a dominant negative mutant of PKCδ (DN PKCδ, Fig. 3). As a previous study has suggested that PKC lambda can also be activated by ET-1 (Ramzy et al., 2006), we evaluated its expression in our PAECs. However, we did not detect its expression (data not shown). Together, these data indicate that PKCδ is activated by ET-1.
FIG. 3.
Prepro-ET-1 overexpression increases protein kinase Cδ (PKCδ) activation in ovine FPAECs. Ovine FPAECs were transfected with pEGFP-N1-Prepro-ET-1 or the parental plasmid in the presence and absence of a dominant negative mutant of PKCδ (DN PKCδ). After 24 h, total protein extracts (20 μg) were subjected to Western blot analysis using either a PKCδ antibody or a phospho-specific antibody recognizing Tyr311 (the activation site of PKCδ). The overexpression of Prepro-ET-1 increases phopsho-Tyr311 levels, indicating that PKCδ activity is increased by ET-1. Data are mean ± SD. *p < 0.05 versus control, †p < 0.05 versus Prepro-ET-1 alone, n = 3.
Increased ET-1 signaling stimulates STAT3 activity in ovine FPAECs
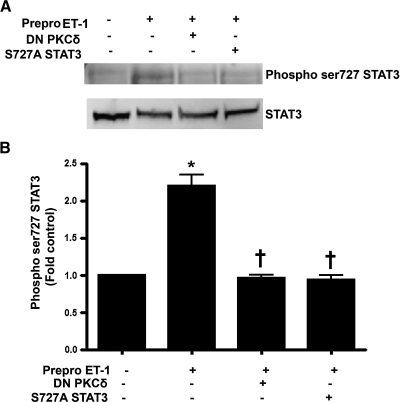
Previous studies have shown that PKCδ can increase STAT3 activity (Jain et al., 1999; Gartsbein et al., 2006). Thus, we next determined the effect of Prepro-ET-1 overexpression on STAT3 activity using STAT3 phosphorylation at serine 727 as a measure of activity. We found that Prepro-ET-1 overexpression significantly increased phospho-ser 727 STAT3 levels, indicating increased STAT3 activity (Fig. 4). To confirm our data, a dominant negative STAT3 mutant deficient in its ability to be phosphorylated at ser727, S727A STAT3, was cotransfected with our Prepro-ET-1 expression plasmid. We found that the phosphorylation of STAT3 at ser727 induced by Prepro-ET-1 overexpression was inhibited by the S727A STAT3 mutant (Fig. 4). The increase in phospho-ser 727 STAT3 induced by ET-1 was also attenuated by the overexpression of DN PKCδ (Fig. 4). These data imply that ET-1 increases STAT3 activity through PKCδ signaling.
FIG. 4.
Prepro-ET-1 overexpression increases STAT3 activity in ovine FPAECs in a PKCδ-dependent manner. Ovine PAECs were cotransfected with pEGFP-N1-Prepro-ET-1 and either DN PKCδ or S727A STAT3. After 24 h, total protein extracts (20 μg) were subjected to Western blot analysis using an anti-phospho ser727 STAT3 antibody. Prepro-ET-1 overexpression increases STAT3 activity as determined by an increase in ser 727 phosphorylation. The increase in ser727 phosphorylation of STAT3 is attenuated by cotransfection with either DN PKCδ or S727A STAT3. Data are mean ± SD. *p < 0.05 versus control, †p < 0.05 versus Prepro-ET-1 alone, n = 3.
Increased ET-1 signaling enhances STAT3 DNA binding activity in ovine FPAECs
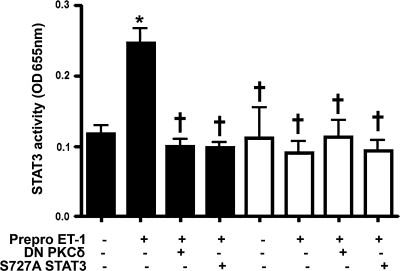
We next sought to determine the functional consequences of STAT3 activation on its DNA binding activity. Of the four consensus sequences for STAT3 binding in the eNOS promoter, the site located at −1024 has been reported to inhibit eNOS promoter activity (Saura et al., 2006). We therefore analyzed STAT3 DNA binding activity using either an oligonucleotide spanning the STAT3 binding site at the position −1024 of eNOS gene or a mutant oligonucleotide in which this STAT3 binding site was disrupted by 2 bp mutation using site-directed mutagenesis. We found that Prepro-ET-1 overexpression significantly enhanced STAT3 binding to the STAT3 sequence of the eNOS promoter (Fig. 5). However, the overexpression of either PKCδ or S727A STAT3 abrogated ET-1–mediated increase in STAT3 binding (Fig. 5). In addition, when the STAT3 DNA binding assay was carried out in the presence of an oligonucleotide containing a mutation in the STAT3 sequence, the DNA binding activity was dramatically reduced in all cases (Fig. 5).
FIG. 5.
Prepro-ET-1 overexpression increases STAT3 activity in a PKCδ-dependent manner in ovine FPAECs. FPAECs were transfected with pEGFP-N1-Prepro-ET-1 in the presence and absence of DN PKCδ or S727A STAT3. After 24 h, nuclear fractions were prepared and STAT3 DNA binding activity was then determined using an oligonucleotide spanning STAT3 binding site at position −1024 of eNOS promoter or a mutant oligonucleotide with a 2 bp mutation in this STAT3 binding site. Prepro-ET-1 overexpression increases STAT3 binding to the wild-type oligonucleotide (black bars). Cotransfection with either DN PKCδ or S727A STAT3 attenuates the ET-1–mediated activation of STAT3 binding. Using the mutant oligonucleotide (white bars), STAT3 binding to eNOS promoter is not affected by either pEGFP-N1-Prepro-ET-1 or DN PKCδ or S727A STAT3. Data are mean ± SD. *p < 0.05 versus control, †p < 0.05 versus Prepro-ET-1 alone, n = 5.
Increased ET-1 signaling reduces eNOS promoter activity via a PKCδ-mediated activation of STAT3 in ovine FPAECs
We next determined whether PKCδ and STAT3 are involved in ET-1–mediated inhibition of eNOS promoter activity. When FPAECs were cotransfected with 1.6 kb eNOS promoter–luciferase construct and Prepro-ET-1, a decrease in eNOS promoter activity was detected (Fig. 6A). This effect of Prepro-ET-1 was reversed by overexpression of either DN PKCδ or S727A STAT3 (Fig. 6A). Further, we examined the effect of mutating the STAT3 binding site located at −1024 of the human eNOS promoter and which has previously been shown to mediate STAT3-induced inhibition of eNOS expression (Saura et al., 2006). To accomplish this, we transfected FPAECs with an eNOS–luciferase construct bearing a 2 bp mutation in STAT3 binding site at position −1024. Our data indicate that the Prepro-ET-1 overexpression–mediated inhibition of eNOS promoter activity is significantly attenuated in cells transfected with mutant promoter (Fig. 6B). Together, these data demonstrate that PKCδ exerts its negative effect on eNOS promoter activity through the stimulation of STAT3 activity and an increase in SAT3 binding to the eNOS promoter.
FIG. 6.
Inhibition of PKCδ or STAT3 signaling attenuates the ET-1–mediated decrease in eNOS promoter activity in ovine FPAECs. FPAECs were cotransfected with pEGFP-N1-Prepro-ET-1 and a 1.6 kb eNOS promoter–luciferase reporter construct in the presence and absence of DN PKCδ or S727A STAT3. All cells were also transfected with a plasmid containing β-galactosidase as a transfection control. After 24 h, luciferase activity was measured. The ET-1–mediated decrease in eNOS promoter activity is attenuated by inhibiting either PKCδ or STAT3 activity (A). FPAECs were also transfected with 1.6 kb eNOS promoter–luciferase reporter construct in which the STAT3 binding element at −1024 was mutated. The overexpression of Prepro-ET-1 fails to inhibit eNOS promoter activity in cells transfected with mutant eNOS promoter (B). Values are mean ± SD. *p < 0.05 versus control, †p < 0.05 versus Prepro-ET-1 alone, n = 4.
Inhibiting PKCδ or STAT3 signaling attenuates the ET-1–mediated decrease in NO signaling in ovine FPAECs
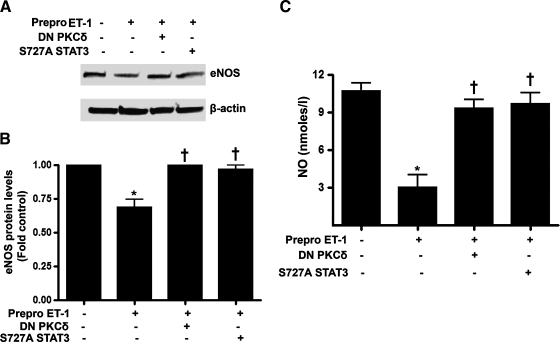
Finally, we determined whether inhibiting PKCδ or STAT3 activity could mitigate the decrease in NO signaling induced by increased ET-1 signaling. Cells were cotransfected with Prepro-ET-1 and either DN PKCδ or S727A STAT3 and after 24 h, eNOS protein and NOx levels were analyzed. Our data indicate that the overexpression of either DN PKCδ or S727A STAT3 attenuated the decrease in eNOS protein levels (Fig. 7A) and NOx levels (Fig. 7B).
FIG. 7.
Inhibition of PKCδ or STAT3 signaling attenuates the ET-1–mediated decrease in NO signaling in ovine FPAECs. Ovine PAECs were cotransfected with pEGFP-N1-Prepro-ET-1 and either DN PKCδ or S727A STAT3. Cellular extracts (20 μg) were then subjected to Western blot analysis using a specific antibody raised against eNOS; a representative image is shown (A). The ET-1–mediated decrease in eNOS protein levels are significantly attenuated by DN PKCδ and S727A STAT3 (B). Similarly, DN PKCδ and S727A STAT3 reverses the ET-1–mediated inhibition of NO generation (C). Values are mean ± SD. *p < 0.05 versus control, †p < 0.05 versus Prepro-ET-1 alone, n = 4.
Discussion
PPHN is a disease characterized by a failure of the normal pulmonary vascular transition at birth leading to increased pulmonary vascular resistance, decreased pulmonary blood flow, and severe hypoxemia (Fineman et al., 2001). PPHN affects between 2 and 6/1000 live born term infants and continues to have significant morbidity and mortality despite recent advances in therapy (Fineman et al., 2001). Ligation of the ductus arteriosus in utero produces fetal and neonatal pulmonary hypertension and alterations in the hemodynamic responses to NO and ET-1 in fetal and newborn lambs that mimics the changes seen in children born with PPHN. We have previously established that in the ductal ligation lamb model of PPHN there is abnormal regulation of the ET-1 and NO signaling cascades such that ET-1 levels are elevated and eNOS expression is decreased (Black et al., 1998a). It is thought that these alterations are responsible for the maintenance of the fetal pulmonary vascular resistance, contributing to the development of pulmonary hypertension after birth. ET-1 has also been identified as one of the important vasoactive substances associated with pathophysiological conditions such as hypertension, ischemic heart disease, congestive heart failure, transplant coronary disease, and the like (Badimon et al., 1992; Nava and Luscher, 1995; Zeiher et al., 1995). Recent studies have revealed that ET-1 modulates eNOS expression as well as NO production (Chen et al., 2003; Dong et al., 2005). Low levels of ET-1 are known to increase NO production; however, recent evidence suggests that elevated levels of ET-1 (as seen after heart failure and ischemia-reperfusion) may impair NO production (Watschinger et al., 1995; Dong et al., 2005). This apparent coordinated regulation between NO and ET-1 signaling is complicated and data in vitro and in vivo are at odds. For example, we have previously shown that the exposure of PAECs isolated from juvenile lambs to exogenous NO leads to a decrease in secreted ET-1. This decrease occurs in the absence of alterations in ECE-1 expression but is associated with a decrease in ET-1 gene expression. The decrease in ET-1 secretion appears to be related to activation of sGC (Kelly et al., 2004). Previous studies utilizing primary cultures of systemic vascular endothelial cells isolated from adult animals have also shown that stimulating endogenous NO production with agonists will decrease secreted ET-1 (Boulanger and Luscher, 1990). It has also been previously demonstrated in human umbilical venous endothelial cells that blocking NO production increases ET-1 release (Morawietz et al., 2000). This increased ET-1 release was associated with an increase in ET-1 transcription (Morawietz et al., 2000). Conversely, treatment with the NO donor molsidomine reduced the hypoxia-induced increase in ET-1 release in the lungs of rats (Blumberg et al., 2001). However, we have shown in vivo that the exposure of juvenile lambs to 40 ppm inhaled NO lead to a twofold increase in circulating immunoreactive ET-1 levels after 24 h (McMullan et al., 2001). In addition, in another model of pulmonary hypertension induced by increased pulmonary blood flow we have found that there are increases both in circulating levels of ET-1 and increased eNOS expression (Black et al., 1998a). Thus, the interactions of NO and ET-1 are clearly complex.
To evaluate the role of high levels of ET-1 in impairing NO, we overexpressed the precursor of ET-1, that is, Prepro-ET-1 in endothelial cells. This resulted in a significant increase in ET-1 levels in the culture medium. This mimicked the condition of higher levels of ET-1 as observed in PPHN (Giaid et al., 1993; Wedgwood and Black, 2005). Our data indicate that overexpression of Prepro-ET-1 attenuated eNOS promoter activity, eNOS protein levels, and NO generation in endothelial cells. This is consistent with previous studies that found higher levels of ET-1 correlate with decreased eNOS expression both in animal models and in children born with PPHN. Other studies support the observation of elevated levels of ET-1 paralleling a decrease in eNOS expression, including acute renal failure (Yanagisawa et al., 2002), hypercholestrolemia, and atherosclerosis (Lerman et al., 1991). Cocaine-exposed human aortic endothelial cells also have been shown to exhibit increased ET-1 expression and decreased eNOS expression and NO production (Pradhan et al., 2008). In this study, we found that the overexpression of Prepro-ET-1 increased PKCδ activation as assessed by an increase in phosphorylation at tyr 311. This ET-1–mediated increase in phospho tyr 311 was abrogated by the concomitant overexpression of a dominant negative mutant of PKCδ. Interestingly, we found that inhibiting PKCδ activity abolished the inhibitory effect of ET-1 on eNOS promoter activity, eNOS protein, and NO generation. These data support the hypothesis that ET-1–induced decrease in NO production is through the activation of PKCδ. However, it should be noted that our data are in contrast with a previous study that found that ET-1 decreases NO production through the inhibition of PKC lambda (Ramzy et al., 2006). Thus, it is possible that ET-1 exerts its effect through different PKC isoforms as different cell types were utilized. Our study was carried out using PAECs isolated from the fetal lamb, whereas the previous study was performed in human saphenous vein endothelial cells. Further, our data indicate that there can be differences in PKC isoform expression between the two cell types, as we could detect PKCδ but not PKC lambda in our cells. Hence, further studies will be required to determine if PKCδ and PKC lambda activate common downstream pathways to inhibit eNOS transcription. Together, these two studies highlight the fact that the regulation of eNOS by PKC is complex and not completely understood. In fact, we have recently demonstrated that, in ovine PAECS isolated from the fetal lamb, activation of PKCδ signaling inhibits the shear stress–mediated increases in eNOS transcription via STAT3 (Sud et al., 2009), while under basal conditions PKCδ activity is essential to maintain eNOS expression (Sud et al., 2008). Likewise, some studies have shown that PKC activates eNOS (Li et al., 1994; Wedgwood et al., 2001), whereas others show that it inhibits eNOS (Mukherjee et al., 2001; Yakubu et al., 2004). The role of ET-1 in regulating eNOS through PKC is also controversial. Our data show that it decreases eNOS expression by a pathway involving PKC, which is supported by another recent study (Yakubu et al., 2004), whereas it is contradicted by another study that found that ET-1 increases eNOS expression through a PKC-dependent pathway (Marsen et al., 1999). Again highlighting the complexity of the interactions between ET-1 and NO.
Previous studies have shown that PKCδ can increase STAT3 activity (Jain et al., 1999; Gartsbein et al., 2006). We therefore investigated the effect of ET-1 on STAT3 activity. Our results indicate that ET-1 increases STAT3 activity as measured by an increase in ser727 phosphorylation and increased DNA binding activity. We also found that the ET-1–mediated increase in ser 727 phosphorylation was attenuated by simultaneous overexpression of either DN PKCδ or a S727A STAT3 mutant, which cannot be phosphorylated at ser727 and is thus inactive. Together, our results suggest that PKCδ mediates ET-1–induced increase in STAT3 activation through increased ser727 phosphorylation. Our results are in agreement with a prior study that found that PKCδ activation regulates STAT3 serine phosphorylation in keratinocytes (Jain et al., 1999; Gartsbein et al., 2006).
It should be noted that the functional consequences of ser727 phosphorylation of STAT3 are still controversial as some studies have shown it to be required for the DNA binding of STAT3 (Xu et al., 1994; Black et al., 1997; Sengupta et al., 1998), while others show that it inhibits DNA binding (Jain et al., 1998, 1999; Woetmann et al., 1999). Thus, to further evaluate the role of STAT3 signaling in regulating eNOS expression we focused on the eNOS promoter. Of the four consensus sequences for STAT3 binding in the eNOS promoter at positions −1520, −61024, −840, and −540 the sequence at −1024 has been reported to be involved in the inhibition of eNOS promoter activity (Saura et al., 2006). Thus, we determined if there was a correlation between STAT3 ser727 phosphorylation and its binding ability to eNOS promoter. To test this, we evaluated STAT3 DNA binding activity using a DNA binding assay in which either a biotinylated double-stranded oligonucleotide encompassing the STAT3 binding element of the eNOS gene at position −1024 or a mutant oligonucleotide with a 2 bp mutation at −1024 was used. Our data demonstrate that ET-1 induced an increase in STAT3 DNA binding, which was reversed by the overexpression of dominant negative mutant proteins for either PKCδ or STAT3. These data suggest that PKCδ is involved in regulating STAT3 activity and that the decrease in eNOS transcription induced by increased ET-1 involves an increase in STAT3 activity mediated through the activation of PKCδ signaling.
We also evaluated wild-type eNOS promoter and STAT3 mutant eNOS promoter luciferase constructs cotransfected into FPAECs with Prepro-ET-1. Our data indicate that the decrease in eNOS promoter activity in response to ET-1 is significantly abolished when the STAT3 binding sequence located at −1024 is mutated. Together, these data highlight the important role of STAT3 inhibition in the downregulation of eNOS expression–induced ET-1. It is also worth noting that the mechanisms by which ET-1 leads to a reduction in eNOS expression are likely to be multifactorial. Indeed, we have previously shown that ET-1 can decrease eNOS promoter activity in FPAECs when in coculture with fetal pulmonary arterial smooth muscle cells (FPASMC) (Wedgwood and Black, 2005). This appears to involve an ETA-mediated increase in H2O2 levels as the effect could be attenuated by the addition of catalase (Wedgwood and Black, 2005). In addition, the direct addition of H2O2 also led to a decrease in eNOS transcription through the attenuation of both AP-1 (Kumar et al., 2008a) and Sp1 (Kumar et al., 2008b) transcription factor activity. However, we found that the direct addition of ET-1 as a bolus dose did not lead to a decrease in eNOS transcription (Kumar et al., 2008b). This discrepancy is likely because of the fact that in this study the presence of the Prepro-ET-1 expression plasmid leads to a sustained increase in ET-1 levels allowing constitutive activation of PKCδ that might not be achieved with the single dose of ET-1 used in our previous study. Further studies will be required to characterize the differences in PKCδ activation between overexpression of Prepro-ET-1 and the bolus addition of ET-1.
Several studies have utilized the strategy of increasing ET-1 levels in vivo to understand ET system on a cellular level in vivo. Renal cell-type-specific ET-1–NO interactions on the promoter level in vivo were investigated by establishing a transgenic mouse model carrying a lacZ reporter gene construct under control of the human Prepro-ET-1 gene promoter sequence using 8 kb of 5′ sequence (Slowinski et al., 2007). Another study utilized adenovirus-mediated gene transfer to overexpress human Prepro-ET-1 cDNA in vivo in rats. Plasma ET-1 levels were increased sixfold over the control animals, and remained elevated at these levels 72–120 h after administration of the virus (Niranjan et al., 1996). Overall, these studies demonstrated that endogenous overexpression of PreproET-1 is accompanied by an elevation of plasma ET-1 concentrations to the levels seen in pathophysiological states. In this study, we have utilized an in vitro approach to investigate the mechanism of regulation of eNOS by ET by overexpression of Prepro-ET-1 in PAECs isolated from the fetal lamb. We have achieved a significant increase above basal levels in the secretion of ET-1 in culture supernatant. Levels increased ∼1.5-fold (805.3 ± 69.8 pg/mL to 1351 ± 127.9 pg/mL with Prepro-ET-1 overexpression). Although the overall levels appear to be much higher than that have been observed in previous in vivo studies, the overall fold change in ET-1 levels is consistent with that seen in infants with PPHN compared to normal children. Also, it should be noted that previous studies are also variable in the levels of ET-1 detected. For example, two studies have shown that mean plasma immunoreactive ET-1 concentrations are significantly elevated in neonates with PPHN compared to those of normal term infants. In one, the ET-1 levels were reported as to 2.04 ± 0.30 versus 1.04 ± 0.29 pg/mL (Kumar et al., 1996), while in the other the levels were 28 versus 11 pM (equivalent to 69.7 pg/mL vs. 27.4 pg/mL) (Christou et al., 1997). It is also worth noting that there may be species differences in ET-1 levels. Increased ET-1 plasma concentrations have been identified in calves derived from somatic cell clone technology and a high percentage suffer from PPHN (Wilkins et al., 2005). Fetal fluid ET-1 concentrations in clone calves at term delivery are >1400 pg/mL, threefold higher than that found in normal calves.
In conclusion, although extrapolation from cell culture studies to the situation in vivo is limited to a certain extent, the findings of the present study elucidate a novel pathway by which increased levels of ET-1 impairs endothelial NO production via PKCδ-mediated activation of STAT3. These results add to our knowledge regarding the signaling mechanisms by which elevated ET-1 impairs vascular function and suggests that PKCδ or STAT3 antagonism may provide a novel therapeutic strategy to improve vascular homeostasis in diseases that are associated with increased ET-1 levels such as PPHN. Further, in future studies the introduction of other cell types found in the vessel wall such as smooth muscle cell could lead to the identification of new signaling pathways that regulate ET-1 signaling through the cell–cell communication that likely occurs in the vessel wall.
Acknowledgments
This research was supported in part by Grants HL60190 (to S.M.B.), HL67841 (to S.M.B.), HL72123 (to S.M.B.), and HL70061 (to S.M.B.), all from the National Institutes of Health, and by a grant from the Fondation Leducq (to S.M.B.). Neetu Sud is supported in part by a postdoctoral fellowship award from the AHA Southwest Affiliates.
Disclosure Statement
No competing financial interests exist.
References
- Abman S.H. Chatfield B.A. Hall S.L. McMurtry I.F. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- Arai H. Nakao K. Takaya K. Hosoda K. Ogawa Y. Nakanishi S. Imura H. The human endothelin-B receptor gene. Structural organization and chromosomal assignment. J Biol Chem. 1993;268:3463–3470. [PubMed] [Google Scholar]
- Badimon L. Badimon J.J. Penny W. Webster M.W. Chesebro J.H. Fuster V. Endothelium and atherosclerosis. J Hypertens Suppl. 1992;10:S43–S50. [PubMed] [Google Scholar]
- Black S.M. Fineman J.R. Steinhorn R.H. Bristow J. Soifer S.J. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol. 1998a;275:H1643–H1651. doi: 10.1152/ajpheart.1998.275.5.H1643. [DOI] [PubMed] [Google Scholar]
- Black S.M. Johengen M.J. Ma Z.D. Bristow J. Soifer S.J. Ventilation and oxygenation induce endothelial nitric oxide synthase gene expression in the lungs of fetal lambs. J Clin Invest. 1997;100:1448–1458. doi: 10.1172/JCI119665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S.M. Johengen M.J. Soifer S.J. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res. 1998b;44:821–830. doi: 10.1203/00006450-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Blumberg F. Wolf K. Sandner P. Lorenz C. Riegger G.A. Pfeifer M. The NO donor molsidomine reduces endothelin-1 gene expression in chronic hypoxic rat lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L258–L263. doi: 10.1152/ajplung.2001.280.2.L258. [DOI] [PubMed] [Google Scholar]
- Boulanger C. Luscher T.F. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin S. Kristova V. Davis T. Kadowitz P. Gause G. Tone-dependent responses to endothelin in the isolated perfused fetal sheep pulmonary circulation in situ. J Appl Physiol. 1991;70:1228–1234. doi: 10.1152/jappl.1991.70.3.1228. [DOI] [PubMed] [Google Scholar]
- Chen Y. McCarron R.M. Golech S. Bembry J. Ford B. Lenz F.A. Azzam N. Spatz M. ET-1- and NO-mediated signal transduction pathway in human brain capillary endothelial cells. Am J Physiol Cell Physiol. 2003;284:C243–C249. doi: 10.1152/ajpcell.00305.2002. [DOI] [PubMed] [Google Scholar]
- Chiou W.J. Shiosaki K. Tasker A.S. Wu-Wong J.R. Characterization of two endothelin converting enzymes and their preference for big endothelin-1 and −2 as substrates. Life Sci. 1994;54:1613–1619. doi: 10.1016/0024-3205(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Christou H. Adatia I. van Marter L.J. Kane J.W. Thompson J.E. Stark A.R. Wessel D.L. Kourembanas S. Effect of inhaled nitric oxide on endothelin-1 and cyclic guanosine 5′-monophosphate plasma concentrations in newborn infants with persistent pulmonary hypertension. J Pediatr. 1997;130:603–611. doi: 10.1016/s0022-3476(97)70245-2. [DOI] [PubMed] [Google Scholar]
- Dong F. Zhang X. Wold L.E. Ren Q. Zhang Z. Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineman J. Heymann M. Morin F.I. Pulmonary Hypertension. In: Emmanouilides A.H., editor; Riemenschneider T.A., editor; Gutgesell H.P., editor. Moss and Adams, Heart Disease in Infants, Children, and Adolescents. Williams & Wilkens; Baltimore, MD: 2001. pp. 41–53. [Google Scholar]
- Fineman J.R. Soifer S.J. Heymann M.A. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134. doi: 10.1146/annurev.ph.57.030195.000555. [DOI] [PubMed] [Google Scholar]
- Gartsbein M. Alt A. Hashimoto K. Nakajima K. Kuroki T. Tennenbaum T. The role of protein kinase C delta activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 2006;119:470–481. doi: 10.1242/jcs.02744. [DOI] [PubMed] [Google Scholar]
- Giaid A. Yanagisawa M. Langleben D. Michel R.P. Levy R. Shennib H. Kimura S. Masaki T. Duguid W.P. Stewart D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- Isomoto H. Kobayashi S. Werneburg N.W. Bronk S.F. Guicciardi M.E. Frank D.A. Gores G.J. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329–1338. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- Jain N. Zhang T. Fong S.L. Lim C.P. Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK) Oncogene. 1998;17:3157–3167. doi: 10.1038/sj.onc.1202238. [DOI] [PubMed] [Google Scholar]
- Jain N. Zhang T. Kee W.H. Li W. Cao X. Protein kinase C delta associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem. 1999;274:24392–24400. doi: 10.1074/jbc.274.34.24392. [DOI] [PubMed] [Google Scholar]
- Kelly L.K. Wedgwood S. Steinhorn R.H. Black S.M. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L984–L991. doi: 10.1152/ajplung.00224.2003. [DOI] [PubMed] [Google Scholar]
- Kumar P. Kazzi N.J. Shankaran S. Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. Am J Perinatol. 1996;13:335–341. doi: 10.1055/s-2007-994352. [DOI] [PubMed] [Google Scholar]
- Kumar S. Sun X. Wedgwood S. Black S.M. Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of AP-1 activity. Am J Physiol Lung Cell Mol Physiol. 2008a;295:L756–L766. doi: 10.1152/ajplung.90205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Sun X. Wiseman D.A. Tian J. Siddaramappa U. Verin A.D. Black S.M. Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of Sp1 activity. DNA Cell Biol. 2008b;28:119–129. doi: 10.1089/dna.2008.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman A. Edwards B.S. Hallett J.W. Heublein D.M. Sandberg S.M. Burnett J.C., Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Li W. Mischak H. Yu J.C. Wang L.M. Mushinski J.F. Heidaran M.A. Pierce J.H. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- Luscher T.F. Yang Z. Tschudi M. von Segesser L. Stulz P. Boulanger C. Siebenmann R. Turina M. Buhler F.R. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ Res. 1990;66:1088–1094. doi: 10.1161/01.res.66.4.1088. [DOI] [PubMed] [Google Scholar]
- Marsen T.A. Egink G. Suckau G. Baldamus C.A. Tyrosine-kinase-dependent regulation of the nitric oxide synthase gene by endothelin-1 in human endothelial cells. Pflugers Arch. 1999;438:538–544. doi: 10.1007/s004249900079. [DOI] [PubMed] [Google Scholar]
- McMullan D.M. Bekker J.M. Johengen M.J. Hendricks-Munoz K. Gerrets R. Black S.M. Fineman J.R. Inhaled nitric oxide-induced rebound pulmonary hypertension: role for endothelin-1. Am J Physiol Heart Circ Physiol. 2001;280:H777–H785. doi: 10.1152/ajpheart.2001.280.2.H777. [DOI] [PubMed] [Google Scholar]
- Moore P. Velvis H. Fineman J.R. Soifer S.J. Heymann M.A. EDRF inhibition attenuates the increase in pulmonary blood flow due to oxygen ventilation in fetal lambs. J Appl Physiol. 1992;73:2151–2157. doi: 10.1152/jappl.1992.73.5.2151. [DOI] [PubMed] [Google Scholar]
- Morawietz H. Talanow R. Szibor M. Rueckschloss U. Schubert A. Bartling B. Darmer D. Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol. 2000;525(Pt 3):761–770. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S. Coaxum S.D. Maleque M. Das S.K. Effects of oxidized low density lipoprotein on nitric oxide synthetase and protein kinase C activities in bovine endothelial cells. Cell Mol Biol (Noisy-le-grand) 2001;47:1051–1058. [PubMed] [Google Scholar]
- Murphy J.D. Rabinovitch M. Goldstein J.D. Reid L.M. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981;98:962–967. doi: 10.1016/s0022-3476(81)80605-1. [DOI] [PubMed] [Google Scholar]
- Nava E. Luscher T.F. Endothelium-derived vasoactive factors in hypertension: nitric oxide and endothelin. J Hypertens Suppl. 1995;13:S39–S48. doi: 10.1097/00004872-199508001-00007. [DOI] [PubMed] [Google Scholar]
- Niranjan V. Télémaque S. deWit D. Gerard R.D. Yanagisawa M. Systemic hypertension induced by hepatic overexpression of human preproendothelin-1 in rats. J Clin Invest. 1996;98:2364–2372. doi: 10.1172/JCI119049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnaka K. Takayanagi R. Nishikawa M. Haji M. Nawata H. Purification and characterization of a phosphoramidon-sensitive endothelin-converting enzyme in porcine aortic endothelium. Off J Biol Chem. 1993;268:26759–26766. [PubMed] [Google Scholar]
- Pradhan L. Mondal D. Chandra S. Ali M. Agrawal K.C. Molecular analysis of cocaine-induced endothelial dysfunction: role of endothelin-1 and nitric oxide. Cardiovasc Toxicol. 2008;8:161–171. doi: 10.1007/s12012-008-9025-z. [DOI] [PubMed] [Google Scholar]
- Ramzy D. Rao V. Tumiati L.C. Xu N. Sheshgiri R. Miriuka S. Delgado D.H. Ross H.J. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation. 2006;114:I319–I326. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Yanagisawa M. Takuwa Y. Miyazaki H. Kimura S. Goto K. Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Saura M. Zaragoza C. Bao C. Herranz B. Rodriguez-Puyol M. Lowenstein C.J. Stat3 mediates interleukin-6 [correction of interleukin-6] inhibition of human endothelial nitric-oxide synthase expression. J Biol Chem. 2006;281:30057–30062. doi: 10.1074/jbc.M606279200. [DOI] [PubMed] [Google Scholar]
- Sengupta T.K. Talbot E.S. Scherle P.A. Ivashkiv L.B. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1998;95:11107–11112. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul P.W. Farrar M.A. Magness R.R. Pulmonary endothelial nitric oxide production is developmentally regulated in the fetus and newborn. Am J Physiol. 1993;265:H1056–H1063. doi: 10.1152/ajpheart.1993.265.4.H1056. [DOI] [PubMed] [Google Scholar]
- Slowinski T. Kalk P. Christian M. Schmager F. Relle K. Godes M. Funke-Kaiser H. Neumayer H.H. Bauer C. Theuring F. Hocher B. Cell-type specific interaction of endothelin and the nitric oxide system: pattern of prepro-ET-1 expression in kidneys of L-NAME treated prepro-ET-1 promoter-lacZ-transgenic mice. J Physiol. 2007;581:1173–1181. doi: 10.1113/jphysiol.2007.131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud N. Kumar S. Wedgwood S. Black S.M. Modulation of PKCdelta signaling alters the shear stress-mediated increases in endothelial nitric oxide synthase transcription: role of STAT3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L519–L526. doi: 10.1152/ajplung.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud N. Sharma S. Wiseman D.A. Harmon C. Kumar S. Venema R.C. Fineman J.R. Black S.M. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–L1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- Sud N. Wedgwood S. Black S.M. Protein kinase Cdelta regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L582–L591. doi: 10.1152/ajplung.00353.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Villamor E. Le Cras T.D. Horan M.P. Halbower A.C. Tuder R.M. Abman S.H. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol. 1997;272:L1013–L1020. doi: 10.1152/ajplung.1997.272.5.L1013. [DOI] [PubMed] [Google Scholar]
- Watschinger B. Sayegh M.H. Hancock W.W. Russell M.E. Up-regulation of endothelin-1 mRNA and peptide expression in rat cardiac allografts with rejection and arteriosclerosis. Am J Pathol. 1995;146:1065–1072. [PMC free article] [PubMed] [Google Scholar]
- Wedgwood S. Bekker J.M. Black S.M. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol. 2001;281:L490–L498. doi: 10.1152/ajplung.2001.281.2.L490. [DOI] [PubMed] [Google Scholar]
- Wedgwood S. Black S.M. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2005;288:L480–L487. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- Wedgwood S. Mitchell C.J. Fineman J.R. Black S.M. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol. 2003;284:L650–L662. doi: 10.1152/ajplung.00252.2002. [DOI] [PubMed] [Google Scholar]
- Wild L.M. Nickerson P.A. Morin F.C., 3rd Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res. 1989;25:251–257. doi: 10.1203/00006450-198903000-00006. [DOI] [PubMed] [Google Scholar]
- Wilkins P.A. Boston R. Palmer J.E. Armstead W.M. Endothelin-1 concentrations in clone calves, their surrogate dams, and fetal fluids at birth: association with oxygen treatment. J Vet Intern Med. 2005;19:594–598. doi: 10.1892/0891-6640(2005)19[594:ecicct]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Woetmann A. Nielsen M. Christensen S.T. Brockdorff J. Kaltoft K. Engel A.M. Skov S. Brender C. Geisler C. Svejgaard A. Rygaard J. Leick V. Odum N. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci USA. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. Vanderford P.A. Fineman J.R. Chang R. Soifer S.J. Endothelin-1 produces pulmonary vasodilation in the intact newborn lamb. Am J Physiol. 1993;265:H1318–H1325. doi: 10.1152/ajpheart.1993.265.4.H1318. [DOI] [PubMed] [Google Scholar]
- Wong J. Vanderford P.A. Fineman J.R. Soifer S.J. Developmental effects of endothelin-1 on the pulmonary circulation in sheep. Pediatr Res. 1994;36:394–401. doi: 10.1203/00006450-199409000-00021. [DOI] [PubMed] [Google Scholar]
- Xu D. Emoto N. Giaid A. Slaughter C. Kaw S. deWit D. Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yakubu M.A. Sofola O.A. Igbo I. Oyekan A.O. Link between free radicals and protein kinase C in glucose-induced alteration of vascular dilation. Life Sci. 2004;75:2921–2932. doi: 10.1016/j.lfs.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H. Nodera M. Sato M. Moridaira K. Sato G. Wada O. Decreased expression of brain nitric oxide synthase in macula densa cells and glomerular epithelial cells of rats with mercury chloride-induced acute renal failure. Toxicol Appl Pharmacol. 2002;184:165–171. doi: 10.1006/taap.2002.9510. [DOI] [PubMed] [Google Scholar]
- Zeiher A.M. Goebel H. Schachinger V. Ihling C. Tissue endothelin-1 immunoreactivity in the active coronary atherosclerotic plaque. A clue to the mechanism of increased vasoreactivity of the culprit lesion in unstable angina. Circulation. 1995;91:941–947. doi: 10.1161/01.cir.91.4.941. [DOI] [PubMed] [Google Scholar]