Abstract
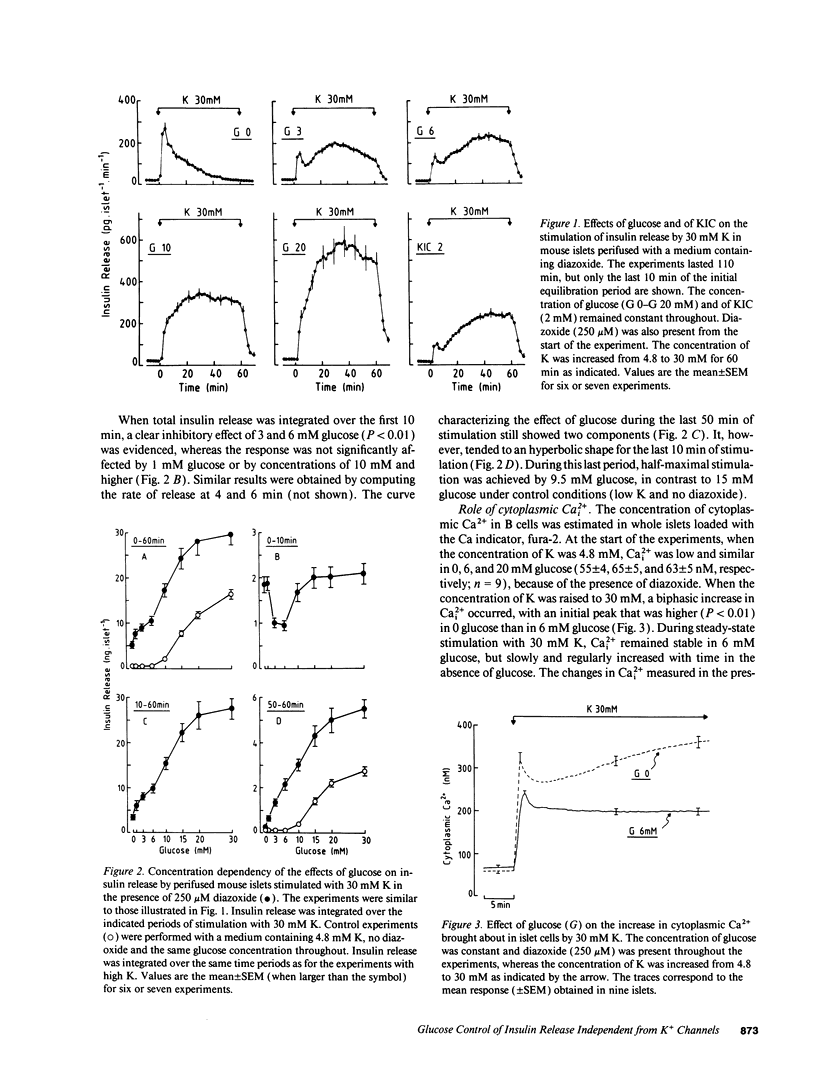
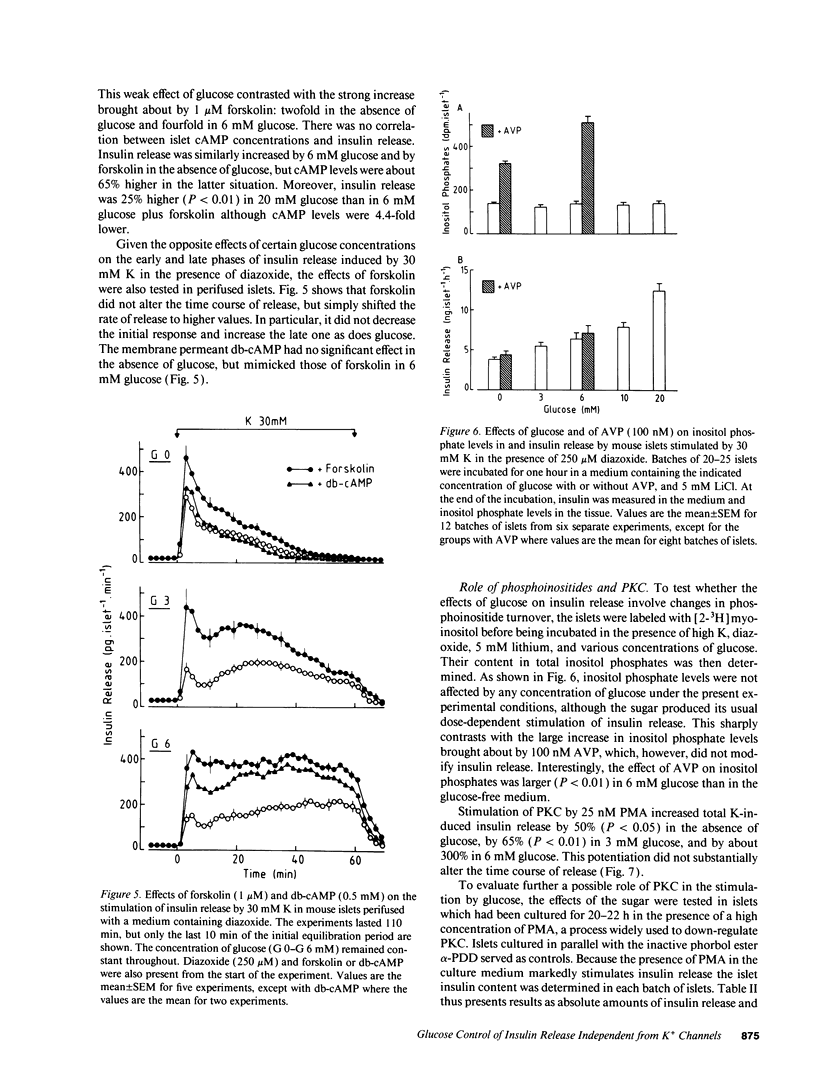
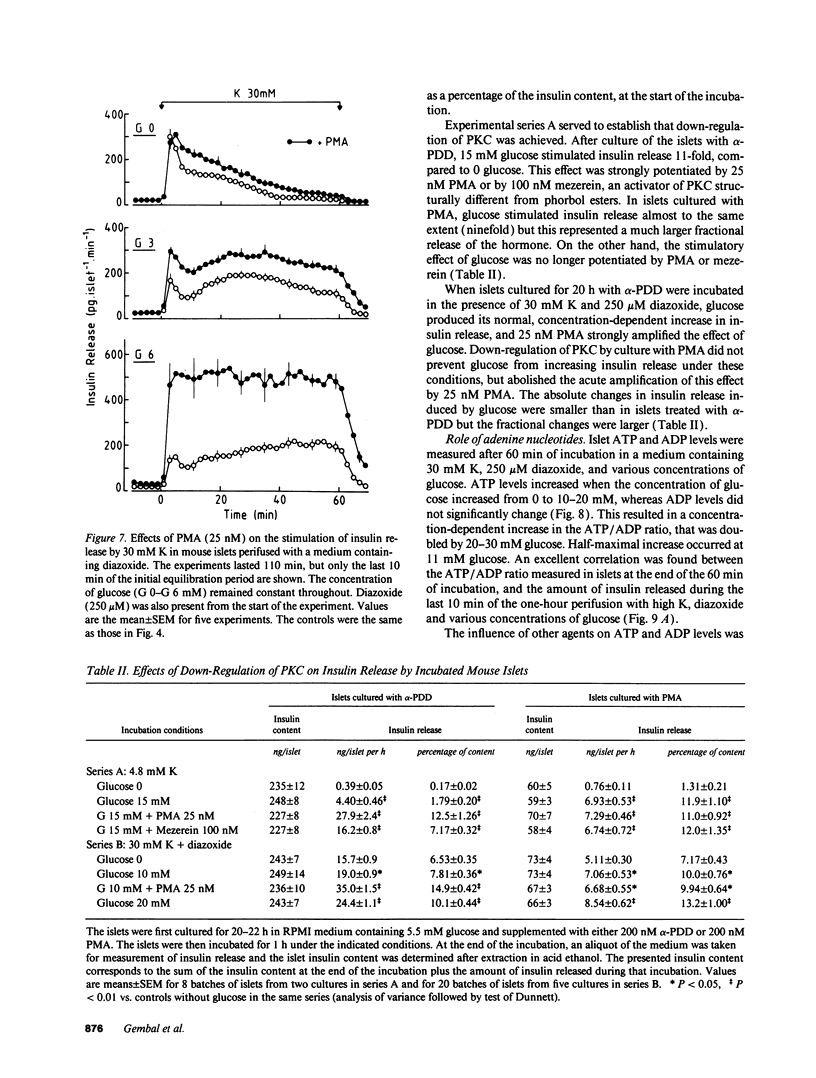
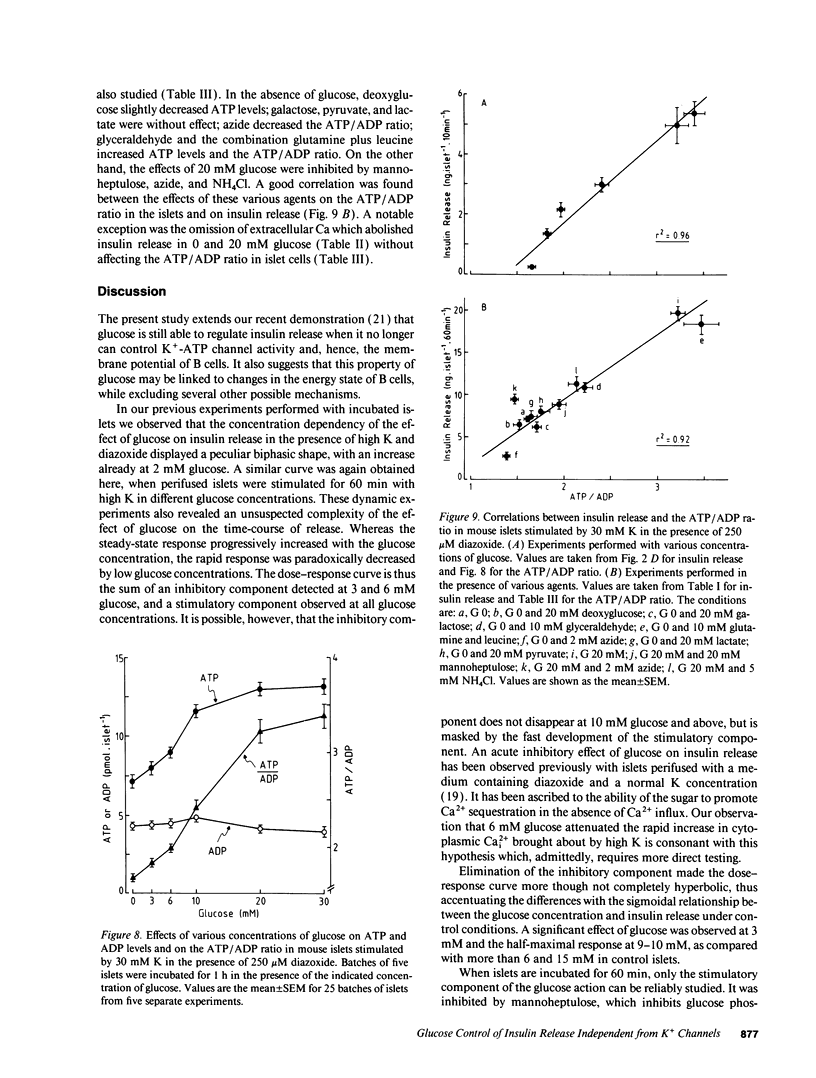
Glucose stimulation of insulin release involves closure of ATP-sensitive K+ channels (K(+)-ATP channels), depolarization, and Ca2+ influx in B cells. However, by using diazoxide to open K(+)-ATP channels, and 30 mM K to depolarize the membrane, we could demonstrate that another mechanism exists, by which glucose can control insulin release independently from changes in K(+)-ATP channel activity and in membrane potential (Gembal et al. 1992. J. Clin. Invest. 89:1288-1295). A similar approach was followed here to investigate, with mouse islets, the nature of this newly identified mechanism. The membrane potential-independent increase in insulin release produced by glucose required metabolism of the sugar and was mimicked by other metabolized secretagogues. It also required elevated levels of cytoplasmic Cai2+, but was not due to further changes in Cai2+. It could not be ascribed to acceleration of phosphoinositide metabolism, or to activation of protein kinases A or C. Thus, glucose did not increase inositol phosphate levels and hardly affected cAMP levels. Moreover, increasing inositol phosphates by vasopressin or cAMP by forskolin, and activating protein kinase C by phorbol esters did not mimic the action of glucose on release, and down-regulation of protein kinase C did not prevent these effects. On the other hand, it correlated with an increase in the ATP/ADP ratio in islet cells. We suggest that the membrane potential-independent control of insulin release exerted by glucose involves changes in the energy state of B cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten P., Gylfe E., Wesslén N., Hellman B. Diazoxide unmasks glucose inhibition of insulin release by counteracting entry of Ca2+. Am J Physiol. 1988 Oct;255(4 Pt 1):E422–E427. doi: 10.1152/ajpendo.1988.255.4.E422. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Draznin B. Intracellular calcium, insulin secretion, and action. Am J Med. 1988 Nov 28;85(5A):44–58. doi: 10.1016/0002-9343(88)90397-x. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Calle R., Zawalich K., Smallwood J. I., Zawalich W. S., Rasmussen H. Glucose-induced translocation of protein kinase C in rat pancreatic islets. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9893–9897. doi: 10.1073/pnas.87.24.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z. Y., Drews G., Nenquin M., Plant T. D., Henquin J. C. Mechanisms of the stimulation of insulin release by arginine-vasopressin in normal mouse islets. J Biol Chem. 1990 Sep 15;265(26):15724–15730. [PubMed] [Google Scholar]
- Gembal M., Gilon P., Henquin J. C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992 Apr;89(4):1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Ronner P., Cheong E., Khalid P., Matschinsky F. M. The role of ATP and free ADP in metabolic coupling during fuel-stimulated insulin release from islet beta-cells in the isolated perfused rat pancreas. J Biol Chem. 1991 Dec 5;266(34):22887–22892. [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Danielsson A. Adenosine triphosphate levels of mammalian pancreatic B cells after stimulation with glucose and hypoglycemic sulfonylureas. Diabetes. 1969 Aug;18(8):509–516. doi: 10.2337/diab.18.8.509. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Effects of trifluoperazine and pimozide on stimulus-secretion coupling in pancreatic B-cells. Suggestion for a role of calmodulin? Biochem J. 1981 Jun 15;196(3):771–780. doi: 10.1042/bj1960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. The interplay between cyclic AMP and ions in the stimulus-secretion coupling in pancreatic B-cells. Arch Int Physiol Biochim. 1985 May;93(1):37–48. doi: 10.3109/13813458509104514. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Protein kinase C and the regulation of insulin secretion from pancreatic B cells. J Mol Endocrinol. 1991 Apr;6(2):121–127. doi: 10.1677/jme.0.0060121. [DOI] [PubMed] [Google Scholar]
- Lindström P., Sehlin J. Effect of intracellular alkalinization on pancreatic islet calcium uptake and insulin secretion. Biochem J. 1986 Oct 1;239(1):199–204. doi: 10.1042/bj2390199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J. Branched-chain amino and keto acid metabolism in pancreatic islets. Adv Enzyme Regul. 1986;25:203–217. doi: 10.1016/0065-2571(86)90015-4. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A. Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim Biophys Acta. 1987 Feb 18;927(2):190–195. doi: 10.1016/0167-4889(87)90134-0. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Nelson J., Nelson D., Erecinska M. Bioenergetic response of pancreatic islets to stimulation by fuel molecules. Metabolism. 1989 Dec;38(12):1188–1195. doi: 10.1016/0026-0495(89)90158-3. [DOI] [PubMed] [Google Scholar]
- Metz S. A. Perspectives in diabetes. Is protein kinase C required for physiologic insulin release? Diabetes. 1988 Jan;37(1):3–7. doi: 10.2337/diab.37.1.3. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Rajan A. S., Aguilar-Bryan L., Nelson D. A., Yaney G. C., Hsu W. H., Kunze D. L., Boyd A. E., 3rd Ion channels and insulin secretion. Diabetes Care. 1990 Mar;13(3):340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Zawalich K. C., Ganesan S., Calle R., Zawalich W. S. Physiology and pathophysiology of insulin secretion. Diabetes Care. 1990 Jun;13(6):655–666. doi: 10.2337/diacare.13.6.655. [DOI] [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Colca J. R., Turk J., Florholmen J., McDaniel M. L. Regulation of Ca2+ homeostasis by islet endoplasmic reticulum and its role in insulin secretion. Am J Physiol. 1988 Feb;254(2 Pt 1):E121–E136. doi: 10.1152/ajpendo.1988.254.2.E121. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Easom R. A., McDaniel M. L., Turk J. Diacylglycerol synthesis de novo from glucose by pancreatic islets isolated from rats and humans. J Clin Invest. 1990 Feb;85(2):482–490. doi: 10.1172/JCI114463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–333. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Regazzi R. Protein kinase C in insulin releasing cells. Putative role in stimulus secretion coupling. FEBS Lett. 1990 Aug 1;268(2):376–380. doi: 10.1016/0014-5793(90)81289-z. [DOI] [PubMed] [Google Scholar]
- Zawalich W. S. Intermediary metabolism and insulin secretion from isolated rat islets of Langerhans. Diabetes. 1979 Mar;28(3):252–262. doi: 10.2337/diab.28.3.252. [DOI] [PubMed] [Google Scholar]


