Abstract
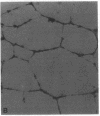
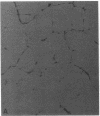
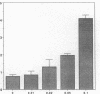
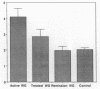
Angiogenesis is an important process in chronic inflammatory diseases. We observed that sera from patients with systemic vasculitis stimulated angiogenesis in an in vitro model using human umbilical vein endothelial cells cultured on a basement membrane (Matrigel) substrate. After 40% ammonium sulfate precipitation, angiogenic activity remained in the low molecular weight fraction and could be inactivated by heat. SDS-page of serum FPLC fractions exhibiting maximal angiogenic activity demonstrated two prominent species of 45 and 16-20 kD in patients' sera. These bands were much less apparent in sera obtained from control subjects. Amino-terminal sequencing of the 45-kD protein demonstrated that it was haptoglobin. Purified haptoglobin stimulated angiogenesis in a dose-dependent manner. The angiogenic activity of vasculitis patients' sera was partially inhibited by an antihaptoglobin antibody. Furthermore, serum haptoglobin levels in vasculitis patients correlated both with disease and angiogenic activity. Haptoglobin angiogenic activity was confirmed in two in vivo models using an implanted disc and a subcutaneous injection of basement membrane. Stimulation of angiogenesis is a newly recognized biological function of haptoglobin. The increased levels of haptoglobin found in chronic inflammatory conditions may play an important role in tissue repair. In systemic vasculitis, haptoglobin might also compensate for ischemia by promoting development of collateral vessels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostoni A., Vergani C., Stabilini R., Marasini B., Arcidiacono R., Sbaffi A., Binaghi P. C. Immunochemical quantitation of acute phase reactive proteins in myocardial infarction. Am Heart J. 1970 Sep;80(3):313–318. doi: 10.1016/0002-8703(70)90096-7. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P. C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989 Jan 2;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Chapelle J. P., Albert A., Smeets J. P., Heusghem C., Kulbertus H. E. Effect of the haptoglobin phenotype on the size of a myocardial infarct. N Engl J Med. 1982 Aug 19;307(8):457–463. doi: 10.1056/NEJM198208193070801. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo L. F., Kowalski J., Kwan H. H., Prionas S. D., Allison A. C. The disc angiogenesis system. Lab Invest. 1988 Jun;58(6):718–724. [PubMed] [Google Scholar]
- Fauci A. S., Haynes B., Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978 Nov;89(5 Pt 1):660–676. doi: 10.7326/0003-4819-89-5-660. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Form D. M., Pratt B. M., Madri J. A. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986 Nov;55(5):521–530. [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Hansen J. E., Iversen J., Lihme A., Bøg-Hansen T. C. Acute phase reaction, heterogeneity, and microheterogeneity of serum proteins as nonspecific tumor markers in lung cancer. Cancer. 1987 Oct 1;60(7):1630–1635. doi: 10.1002/1097-0142(19871001)60:7<1630::aid-cncr2820600735>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa F., Galbraith R. A., Sassa S. Effects of dimethyl sulphoxide on the synthesis of plasma proteins in the human hepatoma HepG2. Induction of an acute-phase-like reaction. Biochem J. 1988 Aug 1;253(3):927–930. doi: 10.1042/bj2530927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey M. C., Grant D. S., Kleinman H. K. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992 Nov 4;84(21):1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Kino K., Tsunoo H., Higa Y., Takami M., Hamaguchi H., Nakajima H. Hemoglobin-haptoglobin receptor in rat liver plasma membrane. J Biol Chem. 1980 Oct 25;255(20):9616–9620. [PubMed] [Google Scholar]
- Kino K., Tsunoo H., Higa Y., Takami M., Nakajima H. Kinetic aspects of hemoglobin.haptoglobin-receptor interaction in rat liver plasma membranes, isolated liver cells, and liver cells in primary culture. J Biol Chem. 1982 May 10;257(9):4828–4833. [PubMed] [Google Scholar]
- Kinsella J. L., Grant D. S., Weeks B. S., Kleinman H. K. Protein kinase C regulates endothelial cell tube formation on basement membrane matrix, Matrigel. Exp Cell Res. 1992 Mar;199(1):56–62. doi: 10.1016/0014-4827(92)90461-g. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhajda F. P., Katumuluwa A. I., Pasternack G. R. Expression of haptoglobin-related protein and its potential role as a tumor antigen. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1188–1192. doi: 10.1073/pnas.86.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhajda F. P., Piantadosi S., Pasternack G. R. Haptoglobin-related protein (Hpr) epitopes in breast cancer as a predictor of recurrence of the disease. N Engl J Med. 1989 Sep 7;321(10):636–641. doi: 10.1056/NEJM198909073211003. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M. Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem. 1986 Jan;34(1):85–91. doi: 10.1177/34.1.2416801. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Milland J., Tsykin A., Thomas T., Aldred A. R., Cole T., Schreiber G. Gene expression in regenerating and acute-phase rat liver. Am J Physiol. 1990 Sep;259(3 Pt 1):G340–G347. doi: 10.1152/ajpgi.1990.259.3.G340. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L., Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983 Nov;97(5 Pt 1):1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller W. K., Handschumacher R., Wade M. E. Serum haptoglobin in patients with ovarian malignancies. Obstet Gynecol. 1971 Sep;38(3):427–435. [PubMed] [Google Scholar]
- Oh S. K., Kim S. H., Walker J. E. Interference with immune response at the level of generating effector cells by tumor-associated haptoglobin. J Natl Cancer Inst. 1990 Jun 6;82(11):934–940. doi: 10.1093/jnci/82.11.934. [DOI] [PubMed] [Google Scholar]
- Oh S. K., Very D. L., Walker J., Raam S., Ju S. T. An analogy between fetal haptoglobin and a potent immunosuppressant in cancer. Cancer Res. 1987 Oct 1;47(19):5120–5126. [PubMed] [Google Scholar]
- Oliviero S., Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. 1989 Apr;8(4):1145–1151. doi: 10.1002/j.1460-2075.1989.tb03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Elling P., Elling H. Serological and immunohistochemical determination of von Willebrand factor antigen in serum and biopsy specimens from patients with arteritis temporalis and polymyalgia rheumatica. Clin Exp Rheumatol. 1990 Jan-Feb;8(1):55–58. [PubMed] [Google Scholar]
- Passaniti A., Taylor R. M., Pili R., Guo Y., Long P. V., Haney J. A., Pauly R. R., Grant D. S., Martin G. R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992 Oct;67(4):519–528. [PubMed] [Google Scholar]
- Raugei G., Bensi G., Colantuoni V., Romano V., Santoro C., Costanzo F., Cortese R. Sequence of human haptoglobin cDNA: evidence that the alpha and beta subunits are coded by the same mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5811–5819. doi: 10.1093/nar/11.17.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N., Iwahana M., Tanaka N. G., Osada Y. Inhibition of angiogenesis and tumor growth by a synthetic laminin peptide, CDPGYIGSR-NH2. Cancer Res. 1991 Feb 1;51(3):903–906. [PubMed] [Google Scholar]
- Snyder S., Ashwell G. Quantitation of specific serum glycoproteins in malignancy. Clin Chim Acta. 1971 Oct;34(3):449–455. doi: 10.1016/0009-8981(71)90100-8. [DOI] [PubMed] [Google Scholar]
- Thompson S., Stappenbeck R., Turner G. A. A multiwell lectin-binding assay using lotus tetragonolobus for measuring different glycosylated forms of haptoglobin. Clin Chim Acta. 1989 Apr 14;180(3):277–284. doi: 10.1016/0009-8981(89)90009-0. [DOI] [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Yang F., Brune J. L., Baldwin W. D., Barnett D. R., Bowman B. H. Identification and characterization of human haptoglobin cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5875–5879. doi: 10.1073/pnas.80.19.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vander Straten A., Herzog A., Jacobs P., Cabezón T., Bollen A. Molecular cloning of human haptoglobin cDNA: evidence for a single mRNA coding for alpha 2 and beta chains. EMBO J. 1983;2(6):1003–1007. doi: 10.1002/j.1460-2075.1983.tb01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]