Abstract
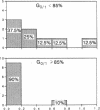
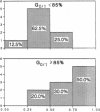
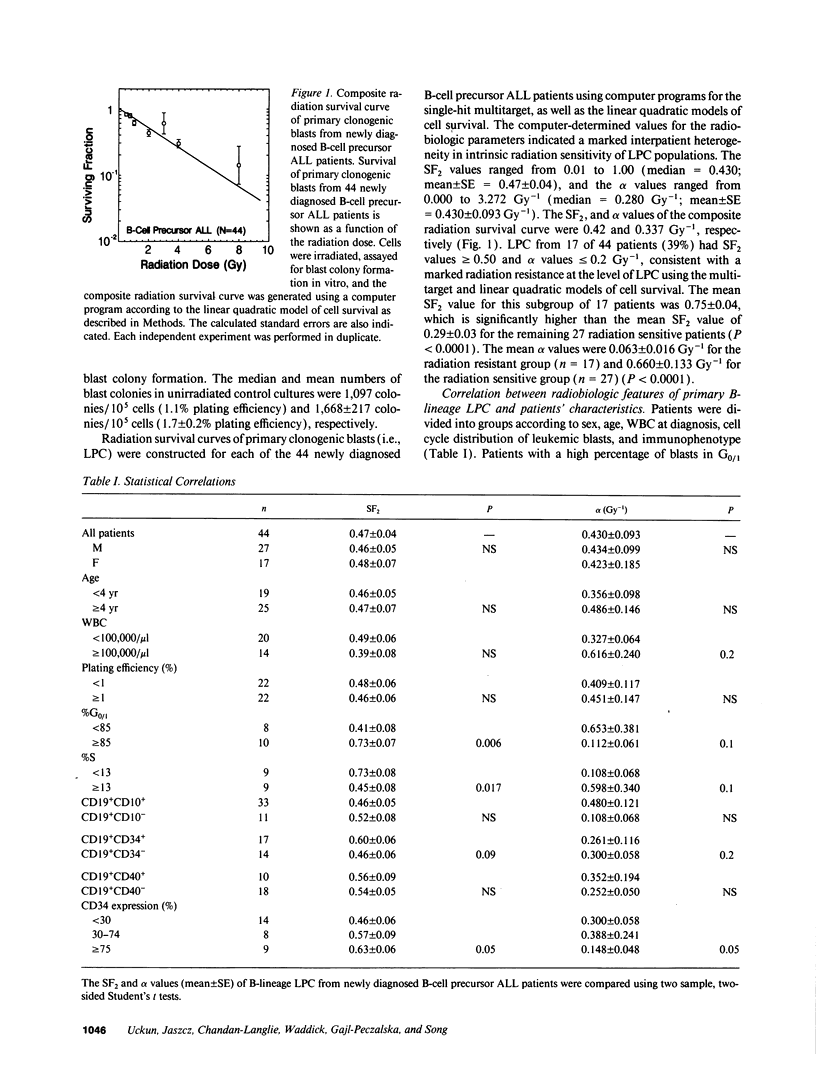
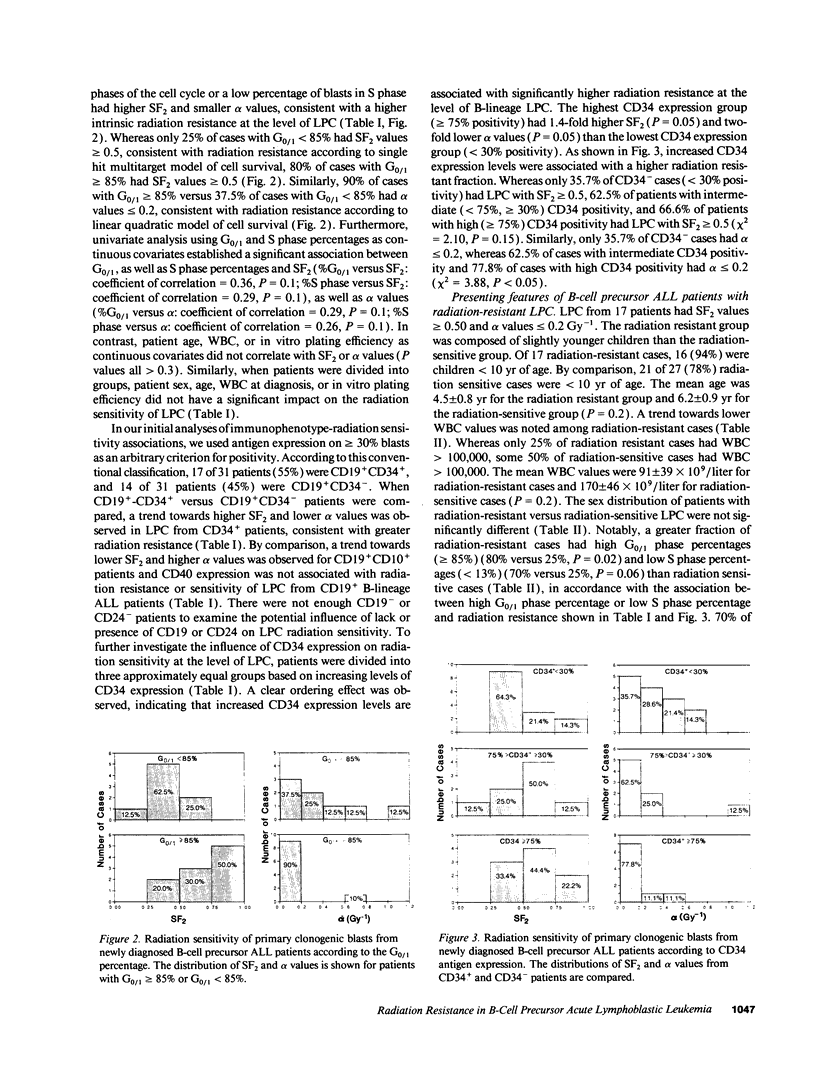
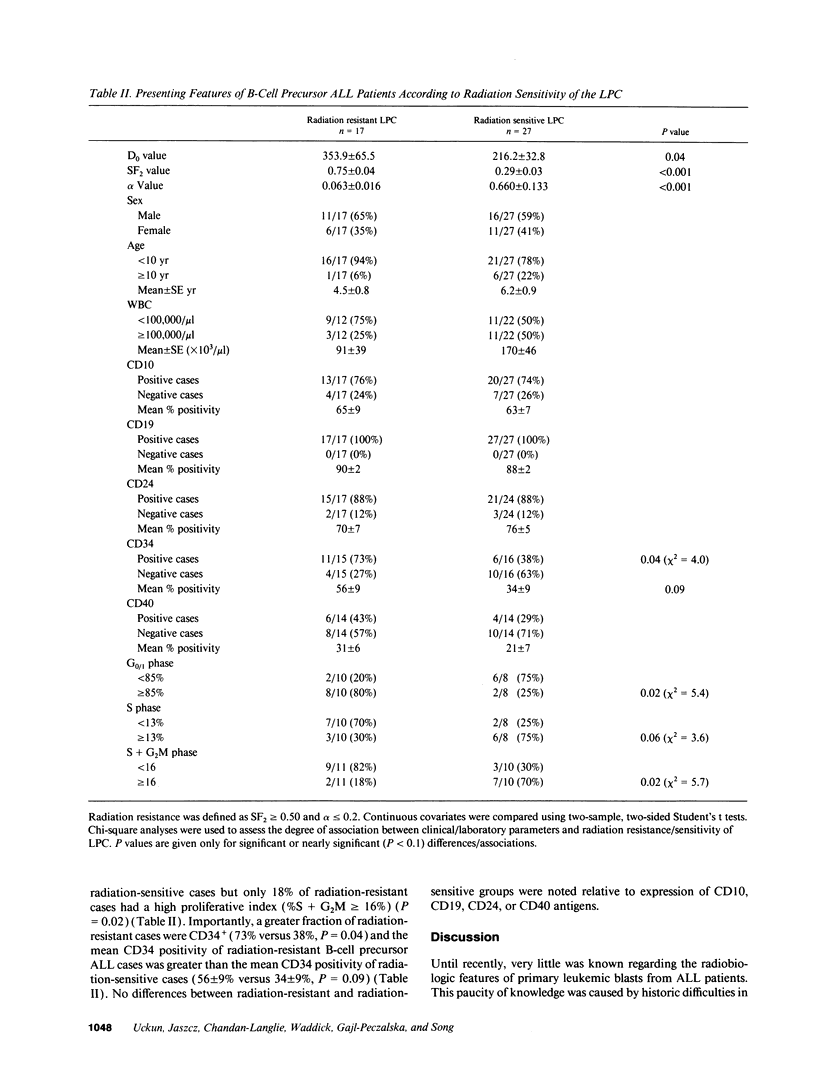
The radiation sensitivity of primary clonogenic blasts from 44 children with newly diagnosed B-cell precursor acute lymphoblastic leukemia (ALL) was analyzed using leukemic progenitor cell (LPC) colony assays. The derived values for SF2 (surviving fraction at 200 cGy) and alpha (initial slope of radiation survival curves constructed according to the linear quadratic model) indicated a marked interpatient heterogeneity in intrinsic radiation sensitivity of LPC populations. The SF2 values ranged from 0.01 to 1.00 (median = 0.430; mean +/- SE = 0.47 +/- 0.04), and the alpha values ranged from 0.000 to 3.272 Gy-1 (median = 0.280 Gy-1; mean +/- SE = 0.430 +/- 0.093 Gy-1). When CD19+ CD34+ versus CD19+ CD34- immunophenotypes were compared, a trend toward higher SF2 and lower alpha values were observed in LPC from CD34+ patients, consistent with greater radiation resistance. When patients were divided into three approximately equal groups based on increasing levels of CD34 expression, a clear ordering effect was observed indicating that increased CD34 expression levels are associated with significantly higher radiation resistance at the level of B-lineage LPC. The highest CD34 expression group (> or = 75% positivity) had 1.4-fold higher SF2 (P = 0.05) and twofold lower alpha values (P = 0.06) than the lowest group (< 30% positivity). Furthermore, the CD34 positivity of radiation resistant (alpha < or = 0.2 and SF2 > or = 0.5) B-cell precursor ALL cases was greater than the CD34 positivity of radiation sensitive (alpha > 0.2 and/or SF2 < 0.5) cases (56 +/- 9% versus 34 +/- 9%, P = 0.09). Whereas only 6 of 16 (38%) of radiation sensitive cases were CD34+, 11 of 15 (73%) of radiation resistant cases expressed CD34 (P = 0.04). Our results offer new insights into the inherent and/or acquired radiation resistance of primary clonogenic blasts from B-cell precursor ALL patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright N. Computer programs for the analysis of cellular survival data. Radiat Res. 1987 Nov;112(2):331–340. [PubMed] [Google Scholar]
- Azuma E., Umemoto M., Kubo M., Ohta Y., Zhang S. L., Komada Y., Ito M., Sakurai M. CD34 antigen expression in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 1991 Mar 15;67(6):1565–1569. doi: 10.1002/1097-0142(19910315)67:6<1565::aid-cncr2820670618>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Barendsen G. W., Roelse H., Hermens A. F., Madhuizen H. T., van Peperzeel H. A., Rutgers D. H. Clonogenic capacity of proliferating and nonproliferating cells of a transplantable rat rhabdomyosarcoma in relation to its radiosensitivity. J Natl Cancer Inst. 1973 Nov;51(5):1521–1526. doi: 10.1093/jnci/51.5.1521. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Horowitz M. M., Gale R. P., Biggs J. C., Camitta B. M., Dicke K. A., Gluckman E., Good R. A., Herzig R. H., Lee M. B. Marrow transplantation for acute lymphoblastic leukemia: factors affecting relapse and survival. Blood. 1989 Aug 1;74(2):862–871. [PubMed] [Google Scholar]
- Brach M. A., Hass R., Sherman M. L., Gunji H., Weichselbaum R., Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991 Aug;88(2):691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champlin R., Gale R. P. Acute lymphoblastic leukemia: recent advances in biology and therapy. Blood. 1989 Jun;73(8):2051–2066. [PubMed] [Google Scholar]
- Chao N. J., Forman S. J., Schmidt G. M., Snyder D. S., Amylon M. D., Konrad P. N., Nademanee A. P., O'Donnell M. R., Parker P. M., Stein A. S. Allogeneic bone marrow transplantation for high-risk acute lymphoblastic leukemia during first complete remission. Blood. 1991 Oct 15;78(8):1923–1927. [PubMed] [Google Scholar]
- Coccia P. F., Strandjord S. E., Warkentin P. I., Cheung N. K., Gordon E. M., Novak L. J., Shina D. C., Herzig R. H. High-dose cytosine arabinoside and fractionated total-body irradiation: an improved preparative regimen for bone marrow transplantation of children with acute lymphoblastic leukemia in remission. Blood. 1988 Apr;71(4):888–893. [PubMed] [Google Scholar]
- Crissman H. A., Darzynkiewicz Z., Tobey R. A., Steinkamp J. A. Correlated measurements of DNA, RNA, and protein in individual cells by flow cytometry. Science. 1985 Jun 14;228(4705):1321–1324. doi: 10.1126/science.2408339. [DOI] [PubMed] [Google Scholar]
- Deschavanne P. J., Malaise E. P. The relevance of alpha/beta ratios determined in vitro for human cell lines to the understanding of in vivo values. Int J Radiat Biol. 1989 Nov;56(5):539–542. doi: 10.1080/09553008914551701. [DOI] [PubMed] [Google Scholar]
- Dicke K. A., Spitzer G. Clinical studies of autografting in acute lymphocytic leukaemia. Clin Haematol. 1986 Feb;15(1):85–103. doi: 10.1016/s0308-2261(86)80007-8. [DOI] [PubMed] [Google Scholar]
- Doney K., Buckner C. D., Kopecky K. J., Sanders J. E., Appelbaum F. R., Clift R., Sullivan K., Witherspoon R., Storb R., Thomas E. D. Marrow transplantation for patients with acute lymphoblastic leukemia in first marrow remission. Bone Marrow Transplant. 1987 Dec;2(4):355–363. [PubMed] [Google Scholar]
- Fackler M. J., Civin C. I., Sutherland D. R., Baker M. A., May W. S. Activated protein kinase C directly phosphorylates the CD34 antigen on hematopoietic cells. J Biol Chem. 1990 Jul 5;265(19):11056–11061. [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981 May;7(5):621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys. 1985 Sep;11(9):1699–1707. doi: 10.1016/0360-3016(85)90223-8. [DOI] [PubMed] [Google Scholar]
- FitzGerald T. J., McKenna M., Kase K., Daugherty C., Rothstein L., Greenberger J. S. Effect of X-irradiation dose rate on the clonagenic survival of human and experimental animal hematopoietic tumor cell lines: evidence for heterogeneity. Int J Radiat Oncol Biol Phys. 1986 Jan;12(1):69–73. doi: 10.1016/0360-3016(86)90417-7. [DOI] [PubMed] [Google Scholar]
- FitzGerald T. J., McKenna M., Rothstein L., Daugherty C., Kase K., Greenberger J. S. Radiosensitivity of human bone marrow granulocyte-macrophage progenitor cells and stromal colony-forming cells: effect of dose rate. Radiat Res. 1986 Aug;107(2):205–215. [PubMed] [Google Scholar]
- Geller R. B., Zahurak M., Hurwitz C. A., Burke P. J., Karp J. E., Piantadosi S., Civin C. I. Prognostic importance of immunophenotyping in adults with acute myelocytic leukaemia: the significance of the stem-cell glycoprotein CD34 (My10) Br J Haematol. 1990 Nov;76(3):340–347. doi: 10.1111/j.1365-2141.1990.tb06365.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown J., Molgaard H. V., Spurr N. K., Robertson D., Delia D., Sutherland D. R. Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia. 1992;6 (Suppl 1):31–36. [PubMed] [Google Scholar]
- Guinot M., Sanz G. F., Sempere A., Arilla M. J., Jarque I., Gomis F., Sanz M. A. Prognostic value of CD34 expression in de novo acute myeloblastic leukaemia. Br J Haematol. 1991 Nov;79(3):533–534. doi: 10.1111/j.1365-2141.1991.tb08075.x. [DOI] [PubMed] [Google Scholar]
- Howell S. M., Molgaard H. V., Greaves M. F., Spurr N. K. Localisation of the gene coding for the haemopoietic stem cell antigen CD34 to chromosome 1q32. Hum Genet. 1991 Sep;87(5):625–627. doi: 10.1007/BF00209027. [DOI] [PubMed] [Google Scholar]
- Kersey J. H., Weisdorf D., Nesbit M. E., LeBien T. W., Woods W. G., McGlave P. B., Kim T., Vallera D. A., Goldman A. I., Bostrom B. Comparison of autologous and allogeneic bone marrow transplantation for treatment of high-risk refractory acute lymphoblastic leukemia. N Engl J Med. 1987 Aug 20;317(8):461–467. doi: 10.1056/NEJM198708203170801. [DOI] [PubMed] [Google Scholar]
- Kimler B. F., Henderson S. D. Cyclic responses of cultured 9L cells to radiation. Radiat Res. 1982 Jul;91(1):155–168. [PubMed] [Google Scholar]
- Kimler B. F., Park C. H., Yakar D., Mies R. M. Radiation response of human normal and leukemic hemopoietic cells assayed by in vitro colony formation. Int J Radiat Oncol Biol Phys. 1985 Apr;11(4):809–816. doi: 10.1016/0360-3016(85)90315-3. [DOI] [PubMed] [Google Scholar]
- Little J. B. Repair of sub-lethal and potentially lethal radiation damage in plateau phase cultures of human cells. Nature. 1969 Nov 22;224(5221):804–806. doi: 10.1038/224804a0. [DOI] [PubMed] [Google Scholar]
- MADOC-JONES H. VARIATIONS IN RADIOSENSITIVITY OF A MAMMALIAN CELL LINE WITH PHASE OF THE GROWTH CYCLE. Nature. 1964 Aug 29;203:983–984. doi: 10.1038/203983a0. [DOI] [PubMed] [Google Scholar]
- Malaise E. P., Fertil B., Deschavanne P. J., Chavaudra N., Brock W. A. Initial slope of radiation survival curves is characteristic of the origin of primary and established cultures of human tumor cells and fibroblasts. Radiat Res. 1987 Aug;111(2):319–333. [PubMed] [Google Scholar]
- Mendonca M. S., Rodriguez A., Alpen E. L. Quiescence in 9L cells and correlation with radiosensitivity and PLD repair. Radiat Res. 1989 Mar;117(3):433–447. [PubMed] [Google Scholar]
- O'Reilly R. J. Allogenic bone marrow transplantation: current status and future directions. Blood. 1983 Nov;62(5):941–964. [PubMed] [Google Scholar]
- Potmesil M., Goldfeder A. Cell kinetics of irradiated experimental tumors: cell transition from the non-proliferating to the proliferating pool. Cell Tissue Kinet. 1980 Sep;13(5):563–570. doi: 10.1111/j.1365-2184.1980.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Potmesil M., Ludwig D., Goldfeder A. Cell kinetics of irradiated experimental tumors: relationship between the proliferating and the nonproliferating pool. Cell Tissue Kinet. 1975 Jul;8(4):369–385. doi: 10.1111/j.1365-2184.1975.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Ramsay N., LeBien T., Nesbit M., McGlave P., Weisdorf D., Kenyon P., Hurd D., Goldman A., Kim T., Kersey J. Autologous bone marrow transplantation for patients with acute lymphoblastic leukemia in second or subsequent remission: results of bone marrow treated with monoclonal antibodies BA-1, BA-2, and BA-3 plus complement. Blood. 1985 Sep;66(3):508–513. [PubMed] [Google Scholar]
- Rofstad E. K., Wahl A., Brustad T. Radiation sensitivity in vitro of cells isolated from human tumor surgical specimens. Cancer Res. 1987 Jan 1;47(1):106–110. [PubMed] [Google Scholar]
- Santos G. W., Kaizer H. Bone marrow transplantation in acute leukemia. Semin Hematol. 1982 Jul;19(3):227–239. [PubMed] [Google Scholar]
- Simmons D. L., Satterthwaite A. B., Tenen D. G., Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol. 1992 Jan 1;148(1):267–271. [PubMed] [Google Scholar]
- Song C. W., Kim T. H., Khan F. M., Kersey J. H., Levitt S. H. Radiobiological basis of total body irradiation with different dose rate and fractionation: repair capacity of hemopoietic cells. Int J Radiat Oncol Biol Phys. 1981 Dec;7(12):1695–1701. doi: 10.1016/0360-3016(81)90195-4. [DOI] [PubMed] [Google Scholar]
- Steel G. G., McMillan T. J., Peacock J. H. The radiobiology of human cells and tissues. In vitro radiosensitivity. The picture has changed in the 1980s. Int J Radiat Biol. 1989 Nov;56(5):525–537. doi: 10.1080/09553008914551691. [DOI] [PubMed] [Google Scholar]
- Tenen D. G., Satterthwaite A. B., Borson R., Simmons D., Eddy R. L., Shows T. B. Chromosome 1 localization of the gene for CD34, a surface antigen of human stem cells. Cytogenet Cell Genet. 1990;53(1):55–57. doi: 10.1159/000132894. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Fauci A. S., Heerema N. A., Song C. W., Mehta S. R., Gajl-Peczalska K., Chandan M., Ambrus J. L. B-cell growth factor receptor expression and B-cell growth factor response of leukemic B cell precursors and B lineage lymphoid progenitor cells. Blood. 1987 Oct;70(4):1020–1034. [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K. J., Kersey J. H., Houston L. L., Vallera D. A. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986 Feb 1;163(2):347–368. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K., Meyers D. E., Ramsay N. C., Kersey J. H., Colvin M., Vallera D. A. Marrow purging in autologous bone marrow transplantation for T-lineage acute lymphoblastic leukemia: efficacy of ex vivo treatment with immunotoxins and 4-hydroperoxycyclophosphamide against fresh leukemic marrow progenitor cells. Blood. 1987 Jan;69(1):361–366. [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K., Myers D. E., Jaszcz W., Haissig S., Ledbetter J. A. Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgkin's lymphoma cells. Blood. 1990 Dec 15;76(12):2449–2456. [PubMed] [Google Scholar]
- Uckun F. M., Gillis S., Souza L., Song C. W. Effects of recombinant growth factors on radiation survival of human bone marrow progenitor cells. Int J Radiat Oncol Biol Phys. 1989 Feb;16(2):415–435. doi: 10.1016/0360-3016(89)90338-6. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Kersey J. H., Gajl-Peczalska K. J., Heerema N. A., Provisor A. J., Haag D., Gilchrist G., Song C. W., Arthur D. C., Roloff J. Heterogeneity of cultured leukemic lymphoid progenitor cells from B cell precursor acute lymphoblastic leukemia (ALL) patients. J Clin Invest. 1987 Sep;80(3):639–646. doi: 10.1172/JCI113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Kersey J. H., Haake R., Weisdorf D., Ramsay N. K. Autologous bone marrow transplantation in high-risk remission B-lineage acute lymphoblastic leukemia using a cocktail of three monoclonal antibodies (BA-1/CD24, BA-2/CD9, and BA-3/CD10) plus complement and 4-hydroperoxycyclophosphamide for ex vivo bone marrow purging. Blood. 1992 Feb 15;79(4):1094–1104. [PubMed] [Google Scholar]
- Uckun F. M., Kersey J. H., Vallera D. A., Ledbetter J. A., Weisdorf D., Myers D. E., Haake R., Ramsay N. K. Autologous bone marrow transplantation in high-risk remission T-lineage acute lymphoblastic leukemia using immunotoxins plus 4-hydroperoxycyclophosphamide for marrow purging. Blood. 1990 Nov 1;76(9):1723–1733. [PubMed] [Google Scholar]
- Uckun F. M., Ledbetter J. A. Immunobiologic differences between normal and leukemic human B-cell precursors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8603–8607. doi: 10.1073/pnas.85.22.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Mitchell J. B., Obuz V., Park C. H., Waddick K., Friedman N., Oubaha L., Min W. S., Song C. W. Radiation sensitivity of human B-lineage lymphoid precursor cells. Int J Radiat Oncol Biol Phys. 1991 Nov;21(6):1553–1560. doi: 10.1016/0360-3016(91)90332-x. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Muraguchi A., Ledbetter J. A., Kishimoto T., O'Brien R. T., Roloff J. S., Gajl-Peczalska K., Provisor A., Koller B. Biphenotypic leukemic lymphocyte precursors in CD2+CD19+ acute lymphoblastic leukemia and their putative normal counterparts in human fetal hematopoietic tissues. Blood. 1989 Mar;73(4):1000–1015. [PubMed] [Google Scholar]
- Uckun F. M., Myers D. E., Ledbetter J. A., Swaim S. E., Gajl-Peczalska K. J., Vallera D. A. Use of colony assays and anti-T cell immunotoxins to elucidate the immunobiologic features of leukemic progenitor cells in T-lineage acute lymphoblastic leukemia. J Immunol. 1988 Mar 15;140(6):2103–2111. [PubMed] [Google Scholar]
- Uckun F. M., Ramsay N. K., Waddick K. G., Jaszcz W., Chandan-Langlie M., Obuz V., Haake R., Gajl-Peczalska K., Kersey J. H., Song C. W. In vitro and in vivo radiation resistance associated with CD3 surface antigen expression in T-lineage acute lymphoblastic leukemia. Blood. 1991 Dec 1;78(11):2945–2955. [PubMed] [Google Scholar]
- Uckun F. M. Regulation of human B-cell ontogeny. Blood. 1990 Nov 15;76(10):1908–1923. [PubMed] [Google Scholar]
- Uckun F. M., Song C. W., Nesbit M., Kersey J. H., Ramsay N. K. Immunophenotype predicts radiation resistance in T-lineage acute lymphoblastic leukemia and T-lineage non-Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 1992;24(4):705–712. doi: 10.1016/0360-3016(92)90718-w. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Song C. W. Radiobiological features of fresh leukemic bone marrow progenitor cells in acute lymphoblastic leukemia. Cancer Res. 1988 Oct 15;48(20):5788–5795. [PubMed] [Google Scholar]
- Uckun F. M., Song C. W. Radiobiological features of human pluripotent bone marrow progenitor cells (CFU-GEMM). Int J Radiat Oncol Biol Phys. 1989 Nov;17(5):1021–1025. doi: 10.1016/0360-3016(89)90150-8. [DOI] [PubMed] [Google Scholar]
- Vaughan W. P., Civin C. I., Weisenburger D. D., Karp J. E., Graham M. L., Sanger W. G., Grierson H. L., Joshi S. S., Burke P. J. Acute leukemia expressing the normal human hematopoietic stem cell membrane glycoprotein CD34 (MY10). Leukemia. 1988 Oct;2(10):661–666. [PubMed] [Google Scholar]
- Wallen C. A., Ridinger D. N., Dethlefsen L. A. Heterogeneity of X-ray cytotoxicity in proliferating and quiescent murine mammary carcinoma cells. Cancer Res. 1985 Jul;45(7):3064–3069. [PubMed] [Google Scholar]
- Weichselbaum R. R., Dahlberg W., Little J. B. Inherently radioresistant cells exist in some human tumors. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4732–4735. doi: 10.1073/pnas.82.14.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum R. R., Greenberger J. S., Schmidt A., Karpas A., Moloney W. C., Little J. B. In vitro radiosensitivity of human leukemia cell lines. Radiology. 1981 May;139(2):485–487. doi: 10.1148/radiology.139.2.6939019. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R. Radioresistant and repair proficient cells may determine radiocurability in human tumors. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):637–639. doi: 10.1016/0360-3016(86)90073-8. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R. Radioresistant and repair proficient cells may determine radiocurability in human tumors. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):637–639. doi: 10.1016/0360-3016(86)90073-8. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Rotmensch J., Ahmed-Swan S., Beckett M. A. Radiobiological characterization of 53 human tumor cell lines. Int J Radiat Biol. 1989 Nov;56(5):553–560. doi: 10.1080/09553008914551731. [DOI] [PubMed] [Google Scholar]