Abstract
Mammalian sterile20-like kinases (MST1/2) are involved in stress-induced apoptosis signalling. MST2 is inhibited by Raf-1 binding, and its activation requires dissociation from Raf-1 and binding to the RASSF1A tumour suppressor protein. Here, we have investigated the regulation of MST2 by the pro-survival phosphoinositide-3 kinase (PI3K)-Akt pathway. Akt phosphorylates MST2 in response to mitogens, oncogenic Ras expression or depletion of the tumour suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN). We identified two Akt phosphorylation sites (T117 and T384) in MST2. Mutation of these sites individually reduced phosphorylation, while the double mutation abolished it. These mutations, especially the double mutation, inhibited MST2 interactions with Raf-1, but enhanced binding to RASSF1A resulting in higher activation of downstream stress signalling pathways (JNK and p38 MAPK) and apoptosis. Biochemical and in situ FLIM experiments revealed a dual mechanism of inhibition. Akt phosphorylation of MST2 (i) blocks binding to RASSF1A and promotes sequestration into the inhibitory complex with Raf-1; and (ii) prevents MST2 homo-dimerisation which is essential for MST2 activation. Our results further show that the dissociation of the Raf-1-MST2 complex is part of mitogenic signalling, thereby linking induction of proliferation with the risk of apoptosis. Results with Ras effector domain mutants that selectively couple to either PI3K or Raf-1 show that Akt activation is necessary to abrogate MST2 activation in response to mitogenic stimulation. Thus, MST2 serves as a hub to integrate the biological outputs of the Raf-1 and Akt pathways.
Keywords: MST2, Raf-1, Akt, crosstalk, apoptosis
Introduction
Apoptosis is a safeguard against unlicensed growth of aberrant cells. Thus, pro-apoptotic signalling pathways are often silenced in tumours. MST1/2 are involved in pro-apoptotic signalling in response to stress signals (1). MST1 induces JNK and caspase activation in response to stress and Fas (2, 3). During apoptosis MST kinases are cleaved by caspases releasing a constitutively active kinase domain that translocates to the nucleus and phosphorylates Histone2B eventually inducing DNA fragmentation (4). In neuronal cells MST1 mediates oxidative stress induced apoptosis by phosphorylating and activating FOXO3 transcription factors (5). In cancer cells MST2 is activated by RASSF1A and mediates apoptosis via induction of the proapoptotic BH3 family gene puma (6) and activation of the Bax binding protein MAP-1/MOAP-1(7, 8). Thus, in mammalian cells MST kinases are bona fide apoptosis inducers in response to stress and tumour suppressor genes.
RASSF1A is a prominent tumour suppressor that is frequently inactivated in many different cancers usually by gene silencing (9-11). Binding of MST2 by RASSF1A induces MST2 activation by promoting dimerisation and transphosphorylation of T180. The Raf-1 proto-oncogene binds to MST2 thereby inhibiting RASSF1A association and dimerisation (12). Downregulation of Raf-1 can substitute for the loss of RASSF1A expression (6) suggesting that Raf-1 binding to MST2 is critical for preventing RASSF1A mediated MST2 activation and apoptosis. However, mitogenic stimulation and activated Ras also can disrupt the MST2-Raf-1 complex without causing MST2 activation (13, 14). This suggests that MST2 release is part of normal mitogenic regulation, and that mitogens must suppress the pro-apoptotic function of MST2.
Recent work showed that Akt phosphorylates MST1 on S387, blocking MST1 cleavage by caspases and its ability to phosphorylate FOXO3 (15). Akt is a main downstream effector of PI3K, whose pleiotropic functions include the inhibition of apoptosis (16-18). The Akt pathway is often deregulated in cancer due to mutations in Akt or p110 PI3K, or more commonly because of mutations, silencing or deletion of PTEN (19). PTEN is a tumour suppressor that dephosphorylates phosphatidyl-inositol-3,4,5 triphosphate (PIP3), the product of PI3K (20). Here, we show that MST2 is phosphorylated by Akt, which inhibits its pro-apoptotic activity, however by a completely different mechanism than MST1. Akt-induced phosphorylation of MST2 (i) promotes MST2 interaction with Raf-1, (ii) blocks MST2 recruitment by RASSF1A and (iii) directly inhibits MST2 kinase activity.
Materials and Methods
Cells, reagents and plasmids
MCF7 and Hela cells were grown in DMEM, HCC1937 cells were grown in RPMI, supplemented with 10% foetal calf serum (Gibco-BRL) and 2mM L-Glutamine (Gibco-BRL). Human recombinant IGF-1 was from PromoCell. Cells were transfected using LipofectAMINE2000 (Invitrogen) following manufacturer's protocol. For each experiment, LY294002 and Akt inhibitor IV were from Calbiochem (Merck). Protein G-sepharose and anti-Flag-M2 agarose conjugated were from Sigma. Antibodies were from commercial sources: mouse monoclonal anti-Raf-1 (BD transduction laboratories); mouse monoclonal anti-phospho(Ser73) JNK (Upstate); goat polyclonal anti-Krs1/MST2, mouse monoclonal anti-PTEN A2B1, mouse monoclonal anti-RASSF1 3F3, rabbit polyclonal anti-JNK-1 C19 (Santa Cruz); rabbit polyclonal anti-phospho-p38 and total p38, anti-phospho-Ser473-Akt and total Akt, anti-phospho-Ser21-GSK3β and total GSK3β, anti-phospho-Ser/Thr-substrate of Akt, anti-phospho-Ser-14-3-3 binding motif (Cell Signalling, NEB); anti-HA-HRP3F10 (Roche); anti-Flag-M2-HRP, mouse monoclonal anti-phospho-ERK1/2 and total ERK1/2 (Sigma); rabbit monoclonal anti-MST2 (Epitomics, Insight Biotech.); mouse monoclonal anti-RASSF1A (eBioscience). Scramble (control), Raf-1, MST2 and Akt1/2 siRNAs were from Dharmacon and described previously (6). pcDNA3.1-HA-RASSF1A and pSG5-gag-Akt were previously described (21). pcDNA3.1-PTEN and pcDNA3.1-PTENC124S were a kind gift from Nick Leslie (Dundee, UK). pCEFL-AU5-HRasV12 and the domain specific mutants (S35 and C40) were generously provided by Piero Crespo (Santander, Spain). pME18S-Flag-MST2 (12) was used to make point mutants using the Quickchange kit (Stratagene). Myc-MST2 was a kind gift from J. Chernoff. MST2 wild type and T117/384AA double mutant were cloned into pEGFP-C3 (Clontech) to make MST2-GFP. Raf-1 was cloned into pcDNA 3.1 (Invitrogen) that was modified by the addition of mRFP1 to make mRFP1-Raf-1.
Co-immunoprecipitations, immunoblottings and in gel kinase assays
Cells were serum starved overnight and lysed in 20mM HEPES pH7.5, NaCl 150mM, 1% NP-40, 2mM NaF, 10mM β-glycerophosphate, 2mM Na4P2O4 and protease and phosphatase inhibitors. Immunoprecipitates were washed three times in lysis buffer, separated by SDS-PAGE and analysed by Western Blotting. Alternatively, in gel kinase assays were performed using MST2 immunoprecipitates as previously described (12).
FRET determination by multiphoton FLIM measurements
Time-domain FLIM and analysis of the data were performed as described previously (22).
Apoptosis assays
Attached and floating cells were harvested, washed with PBS, resuspended in 0.25% trypsin, fixed in 70% ethanol, incubated with RNase (250μg/ml, Qiagen), and stained with propidium iodide (10μg/ml, Sigma) prior to analysis with a FACScalibur flow cytometer (Becton-Dickinson) to determine DNA fragmentation shown as fold increase in the SubG1 population.
In vitro kinase and binding assays
100ng purified Akt and MST2 (Proqinase) were incubated for 5 minutes in 100μl kinase buffer (50mM TrisHCl pH7.4, 0.1mM EGTA, 10mM MgCl2, 0.1% β-mercaptoethanol plus/minus 0.5mM ATP). 2μl of each kinase reaction was Western blotted with the anti-phospho-Ser/Thr-substrate of Akt antibody to assess phosphorylation. The remainder was split into two aliquots and incubated with RASSF1A or Raf-1 immunoprecipitates, washed and analysed by Western blotting with the anti-phospho-Ser/Thr-substrate, MST2 and Akt antibodies. The immunoprecipitates were prepared from HEK293 cells transfected with Flag-tagged Raf-1 or RASSF1A. Cells were lysed in RIPA buffer, and anti-Flag-M2 agarose immunoprecipitates were washed 5 times in RIPA buffer and once in kinase buffer.
Results
MST2-Raf-1 interaction is promoted by PI3K/Akt
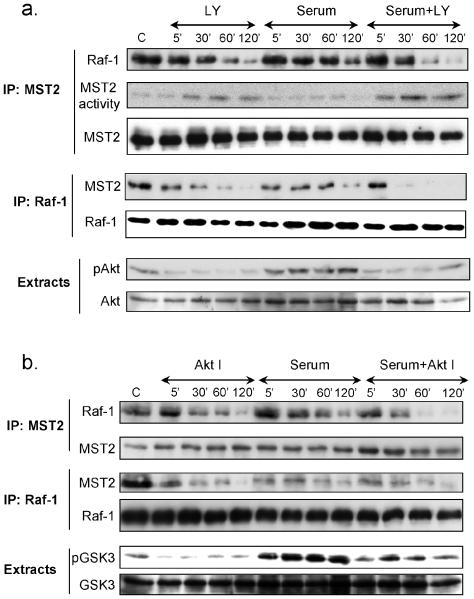
We examined whether the PI3K/Akt pathway regulates the MST2-Raf-1 complex. LY294002 (LY), a PI3K inhibitor, induced a time dependent decrease of Raf-1 co-immunoprecipitation with MST2 and vice versa (Figs.1a & S1a). As observed previously (12), serum also decreased Raf-1 and MST2 co-immunoprecipitations, but with a lower efficiency and a longer delay than LY treatment. LY enhanced and accelerated the serum-induced disruption of MST2-Raf-1 (Figs.1a & S1a). These results were confirmed using AktI, a specific Akt inhibitor (Figs.1b & S1b). The efficacy of the inhibitors was ascertained by showing that LY blocked Akt phosphorylation (Fig. 1a), and AktI inhibited phosphorylation of the Akt substrate GSK-3 (Fig. 1b). Importantly, LY increased MST2 kinase activity both in untreated and serum stimulated cells (Figs.1a & S1a). Treatment with IGF-1 produced similar results (Fig. S1c), suggesting that the PI3K/Akt pathway promotes the MST2-Raf-1 interaction and negatively regulates MST2 activity.
Figure 1. PI3K/Akt regulates MST2-Raf1 interaction and MST2 activity.
Serum starved HeLa cells (c) were treated with (a) LY294002 (LY, 5μM) or (b) Akt inhibitor (Akt I, 10μM) in the presence or absence of serum 10% (serum+LY or serum+Akt I) for the indicated periods of time. In the serum+LY and serum +Akt I conditions, cells were pre-incubated 30min with LY or Akt I, respectively, before stimulation with 10% serum. MST2 or Raf1 immunoprecipitates (IP) were analyzed by Western blots using antibodies for the indicated proteins. MST2 kinase activity was determined by in gel kinase assays as described in Materials and Methods. 10μg of cellular extracts were analyzed by Western blotting using phospho-S473Akt or phospho-S21GSK3β antibodies, and then re-probed with antibodies against total Akt and total GSK3β, respectively.
MST2 is a substrate for Akt
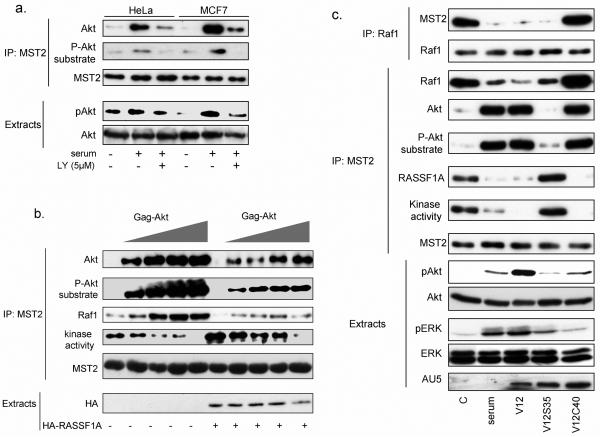
Serum induced co-immunoprecipitation of Akt with MST2 (Figs.2a & S2a), which was reduced by LY. An antibody specific for Akt substrate phosphorylation motifs revealed a PI3K-dependent increase in Akt-induced phosphorylation of MST2 in immunoprecipitates (Figs.2a & S2a). Overexpression of a constitutively active form of Akt, gag-Akt, induced a concomitant increase of co-immunoprecipitated Akt with MST2 and hyperphosphorylation of MST2 on Akt motifs. Also, Raf-1 co-immunoprecipitated with MST2 increased, while MST2 kinase activity decreased proportionally (Figs.2b & S2b). Re-expression of RASSF1A counteracted the Akt effects decreasing both the co-immunoprecipitation of Akt with MST2 and phosphorylation of MST2, while elevating MST2 kinase activity (Figs.2b & S2b).
Figure 2. MST2 interacts with Akt and a target for Akt induced phosphorylation.
(a) Serum starved HeLa and MCF7 cells were treated with serum 10% for 2hours in the presence or absence of LY294002 (LY; pre-incubated for 30min). MST2 immunoprecipitates (IP) were analyzed by Western blotting using phospho-serine/threonine substrate of Akt (P-Akt substrate) and total Akt antibodies. 10μg cellular extracts were Western blotted using a phospho-S473Akt antibody, and then re-probed with a total Akt antibody. (b) MCF7 cells were co-transfected with increasing amounts of gag-Akt (0, 0.1μg, 0.25μg, 0.5μg and 1μg) and HA-RASSF1A where indicated. MST2 IPs were analyzed by Western blotting with the indicated antibodies. MST2 kinase activity was measured by in gel kinase assays. 10μg of cellular extracts were analyzed by Western blot using HA antibody. (c) HeLa cells were transfected with HRasV12 (V12), HRasV12S35 (V12S35) or HRasV12C40 (V12C40). Raf-1 and MST2 IPs were analyzed by Western blot using antibodies as stated. MST2 kinase activity was determined as above. 10μg of cellular extracts were analyzed by Western blot using antibodies against the indicated proteins.
Akt inhibits MST2 by a dual mechanism
To clarify whether Akt phosphorylation inhibits MST2 kinase activation only by promoting Raf-1 binding or also directly, we used H-RasV12 effector domain mutants (Fig.2c). H-RasV12S35 and H-RasV12C40 selectively stimulate Raf-1 and PI3K, respectively (23). Serum stimulation, expression of H-RasV12 or H-RasV12S35 decreased Raf-1 co-immunoprecipitated with MST2. In contrast, serum stimulation, H-RasV12 and the specific PI3K activator mutant H-RasV12C40 increased both Akt co-immunoprecipitation with MST2 and Akt-induced phosphorylation of MST2. Importantly, serum stimulation, H-RasV12, and H-RasV12C40, but not H-RasV12S35, inhibited RASSF1A binding to MST2 and MST2 kinase activity. By contrast, H-RasV12S35 disrupted the MST2-Raf-1 complex and increased RASSF1A recruitment and MST2 kinase activity. These results indicate that Akt exerts dual control over MST2 by preventing binding to RASSF1A while promoting interaction with Raf-1, and by directly inhibiting MST2 activity. Both processes require Akt kinase activity.
Akt regulates MST2 in cancer cells
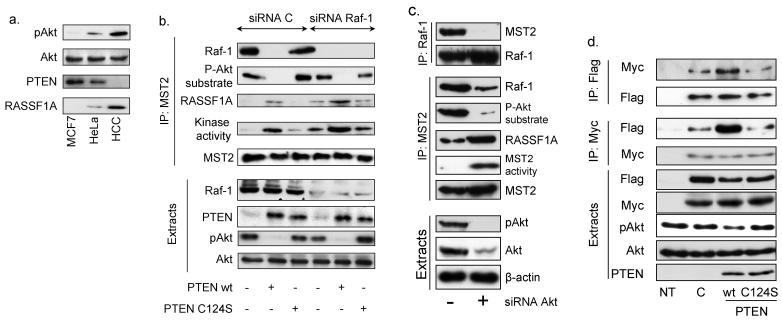
PTEN loss is frequent in cancer, resulting in the hyperactivation of PI3K/Akt signalling (24). In the PTEN−/− breast cancer cell line HCC1937 endogenous Akt was highly activated (Fig.3a). Despite the retention of RASSF1A expression MST2 had little kinase activity, efficiently co-immunoprecipitated with Raf-1 and was phosphorylated on Akt consensus sites. Re-expression of wild-type PTEN reversed all these parameters, whereas the phosphatase dead C124S mutant was without effect (Figs. 3b & S3a).
Figure 3. Regulation of endogenous MST2 activation by Akt, PTEN and Raf-1.
(a) 10μg cellular extracts from MCF7, HeLa and HCC1937 cells were analyzed for Akt activation (phospho-S473Akt), expression of Akt, RASSF1A and PTEN. (b) HCC1937 cells were co-transfected with the indicated combinations of wild type PTEN, phosphatase dead PTEN C124S, and small interference RNAs for Raf-1 (siRNA Raf-1) or a control (siRNA C). MST2 immunoprecipitates were analyzed by Western blotting using antibodies as stated. MST2 kinase activity was tested by in gel kinase assays. 10μg cellular extracts were analyzed by Western blotting using the indicated antibodies. (c) Akt was downregulated by siRNA. Cell extracts, Raf-1 and MST2 IPs were analyzed by Western blotting with the indicated antibodies. (d) Cells were co-transfected with Myc- and Flag-tagged MST2 and either wild type PTEN (wt) or phosphatase-dead mutant PTEN (C124S). IP and Western blot analysis were performed as described above.
As Akt can bind Raf-1 (25, 26) we tested whether Raf-1 promotes the phosphorylation of MST2 by Akt. Raf-1 downregulation increased the MST2 and RASSF1A interaction and MST2 kinase activity even in absence of a functional PTEN. Interestingly, Raf-1 downregulation only slightly decreased MST2 phosphorylation by Akt (Figs. 3b & S3a) suggesting that Raf-1 is not required for the Akt-induced phosphorylation of MST2.
However, Akt is essential as Akt downregulation by siRNA completely reversed the effects of PTEN loss, decreasing MST2-Raf-1 association and Akt phosphorylation of MST2, while increasing MST2 kinase activity. In addition, RASSF1A now could engage MST2 (Fig.3c). We then tested whether Akt affected MST2 homo-dimerisation, an early event required for MST2 activation, by assessing co-immunoprecipitation of Flag- and Myc-tagged MST2. In PTEN−/− HCC1937 cells, re-expression of wild type PTEN increased MST2 homo-dimerisation, while PTEN C124S had no effect (Fig. 3d). In MCF7 cells the expression of gag-Akt decreased MST2 homo-dimerisation, and this effect was counteracted by re-expressing RASSF1A (Fig. S3b).
Taken together, these data suggest that Akt is a physiological regulator of MST2 that cooperates with Raf-1 to prevent MST homo-dimerisation and activation in cancer cells.
Akt induces MST2 phosphorylation on threonines 117 and 384
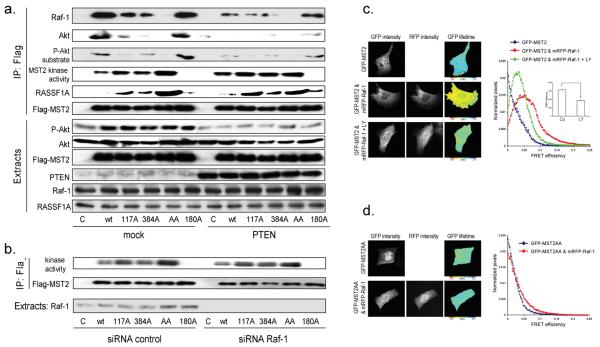
MST2 contains two Akt consensus phosphorylation motifs, T117 and T384 (Fig. S4a). To assess the role of these sites we generated single (T117A, T384A) and double (T117/384AA) Flag-tagged MST2 mutants. When expressed in PTEN−/− HCC1937 cells, both single mutants showed reduced interaction with Raf-1 and Akt, and reduced phosphorylation on Akt consensus sites (Fig. 4a). The double TT117/384AA mutant (AA) was completely devoid of binding to Raf-1 or Akt and Akt phosphorylation. MST2 single mutants co-immunoprecipitated more efficiently with RASSF1A and had enhanced kinase activity, which was further enhanced by the double mutation. PTEN expression increased the interaction of RASSF1A with MST2 and MST kinase activity, whereas the MST2 mutants were largely unaffected. Mutation of the activating autophosphorylation site T180 had no effect on any of these parameters (Figs.4a & S4b). These results suggest that Akt phosphorylation of MST2 at T117 and T384 inhibits binding to RASSF1A and concomitant MST2 activation, while facilitating binding to Raf-1 while. Therefore, PTEN loss contributes to the shutdown of MST2 signalling in cancer cells.
Figure 4. MST2 phosphorylation by Akt at T117 and T384 regulates MST2 kinase activity and Raf-1 interaction.
(a) HCC1937 cells were transfected with empty vector (c), Flag-tagged MST2 wild type (wt), single mutants T117A and T384A (117A, 384A), the double mutant T117/384AA, (AA) or the inactive mutant T180A (180A). PTEN was co-expressed where indicated. Flag- IPs were analyzed by Western blot using specific antibodies as stated. MST2 kinase activity was determined by in gel kinase assays. (b) Cells were co-transfected with Flag-tagged MST2 mutants and control (siRNA control) or Raf-1 specific (siRNA Raf-1) siRNAs. MST2 IPs were analyzed for kinase activity. (c) Cells were co-transfected with GFP-MST2 or the GFP-MST2 double mutant T117/384AA and mRFP1-Raf1. The interaction between MST2 and Raf-1 was imaged by FLIM in cells treated with or without LY292002 (5μM, 2 hours). FRET between GFP and mRFP1 results in shortening of the fluorescent lifetime (τ) of GFP. The graph shows FRET efficiency calculated using the control lifetime (GFP alone) and effect of LY292002 (inset, 11 cells per group). Statistical analysis was performed using Student's t-test. (d) FLIM of cells co-expressing the GFP-MST2 double mutant T117/384AA and mRFP1-Raf1.
Does the Akt-mediated regulation of MST2 depend on Raf-1? Downregulation of Raf-1 in PTEN−/− cells only slightly affected the kinase activities of wild type and mutant MST2 (Figs.4b & S4c), suggesting that the phosphorylation by Akt exerts a dual effect on MST2. First, it prevents RASSF1A binding and promotes sequestration of MST2 into the inhibitory complex with Raf-1. Second, it has a direct inhibitory effect on the catalytic activity of MST2 which does not require MST2 interaction with Raf-1. Thus, these data independently confirm the results from the Ras effector domain mutants experiments.
To confirm the biochemical interactions by in situ imaging we performed Fluorescence Lifetime Imaging Microscopy (FLIM). GFP-MST2 and mRFP1-Raf-1 expressed in HCC1937 cells interacted as indicated by a shortening of FLI in comparison to GFP-MST2 alone, and this interaction was significantly decreased when PI3K was inhibited with LY294002 (Fig. 4c). Mutation of the two Akt phosphorylation sites (GFP-MST2-TT117/384AA) inhibited the interaction with Raf-1 (Fig.4d), confirming the biochemical observations that Akt-induced phosphorylation of MST2 is critical for the MST2-Raf1 interaction.
Akt phosphorylates MST2 directly and inhibits binding to RASSF1A
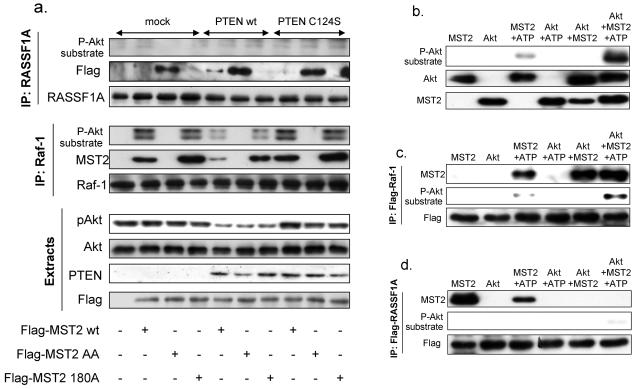
To assess the distribution of MST2 between the two competing binding partners, we analysed Raf-1 and RASSF1A immunoprecipitates for the presence and phosphorylation status of MST2 in HCC1937 cells (Fig. 5a). Wildtype MST2 preferentially co-immunoprecipitated with Raf-1. PTEN expression diminished MST2 association with Raf-1, but enhanced binding to RASSF1A. While MST2 was phosphorylated on the Akt sites in Raf-1 immunoprecipitates, RASSF1A immunoprecipitates were devoid of phosphorylated MST2. By contrast, MST2-TT117/384AA showed the opposite behaviour, readily associating with RASSF1A but not with Raf-1.
Figure 5. Akt phosphorylates Akt directly and prevents binding to RASSF1A.
(a) HCC1937 cells were transfected with empty vector, Flag-tagged wild type MST2 (wt), the double mutant T117/384AA (AA) or the inactive mutant T180A (180A). PTEN wild type (wt) or the phosphatase-dead mutant C124S were co-expressed as indicated. RASSF1A and Raf-1 immunoprecipitates and 10μg cellular extracts were analyzed by Western blotting using the indicated antibodies. (b) Purified MST2 was incubated with purified Akt plus/minus ATP in a total volume of 100μl. 2μl aliquots were immunoblotted with the indicated antibodies. The remaining samples were divided in two and incubated with (c) Flag-Raf-1 or (d) Flag-RASSF1A immunoprecipitates. After washing MST2 proteins bound to the immunoprecipitates were examined with the indicated antibodies.
In vitro kinase assays with purified Akt and MST2 proteins showed that MST2 was a direct Akt substrate, although some background phosphorylation of the Akt sites was observable when MST2 was incubated with ATP alone (Fig. 5b). The in vitro kinase reactions were subsequently incubated with Raf-1 or RASSF1A immobilised on beads (Figs. 5c & 5d). Phosphorylated MST2 bound to Raf-1 but not to RASSF1A. Interestingly, MST2 binding to Raf-1 was enhanced by the presence of Akt raising the possibility that Akt can facilitate this interaction. However, in cells Akt can be recruited to MST2 while Raf-1 dissociates (Fig. 2c) suggesting this adaptor role of Akt is further regulated in cells or an in vitro artefact. However, RASSF1A consistently only bound non-phosphorylated MST2 in vitro and in cells. These data suggest that MST2 is a direct Akt substrate and that this phosphorylation precludes MST2 binding to RASSF1A, but enhances binding to Raf-1.
Akt regulates MST2 pro-apoptotic function
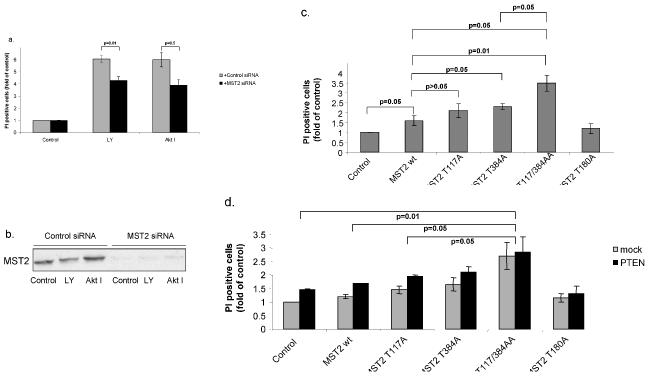
Inhibiting MST2 expression by siRNA in Hela cells reduced both LY294002- and AktI inhibitor-induced apoptosis, as assayed by DNA fragmentation, to a modest but significant extent (Figs. 6a & 6b). In order to examine the role of Akt-mediated MST2 phosphorylation we overexpressed MST2 and the phosphorylation site mutants. Wildtype MST2 caused a small but significant increase of apoptosis, which was further increased when the single or double MST2 mutants were expressed. The inactive MST2 mutant T180A was unable to trigger apoptosis (Fig. 6c). Consistent results were obtained using caspase activation to monitor apoptosis (Fig. S5). Similarly, in the PTEN−/− HCC1937 cells, overexpression of wildtype MST2 and the single mutants T117A and T384A stimulated apoptosis (Fig.6d). Moreover, PTEN re-expression slightly enhanced apoptosis (Fig.6d). As in HeLa cells, the MST2 double mutant displayed the strongest pro-apoptotic activity, and this effect was resistant to PTEN expression.
Figure 6. Akt protection against apoptosis involves MST2 inhibition.
(a) HeLa cells were transfected with control or MST2 siRNA and treated or not (control) for 15hours with 5μM LY294002 (LY) or 5μM Akt inhibitor (Akt I). Apoptosis was quantified by assaying DNA fragmentation as described in Materials and Methods. Statistical analysis was performed using the Student's t-test (n=4). (b) 10μg cellular extracts were analyzed for full length MST2 expression by Western blot. (c) HeLa cells were transfected with empty vector (control), Flag-tagged wild type MST2 (wt), MST2 single mutants T117A and T384A (117A, 384A), the double mutant T117/384AA (AA), or the inactive mutant T180A (180A). Apoptosis was measured as above. Statistical analysis was performed using Student's t-test (n=5). There were no statistically significant differences between control and the T180A mutant, and wt MST2 and T180A. (d) HCC1937 cells were co-transfected with MST2 constructs as described above with or without (mock) PTEN. Apoptosis was measured as above.
In order to test the effects of MST2 mutations on other downstream pathways we examined activation of JNK and p38 (Fig. S6), as they have been implicated in pro-apoptotic MST signalling (27-29). Expression of the MST2 single mutants and especially the double mutant induced JNK and, to a lesser extent, p38 activation. These effects were enhanced by the re-expression of PTEN. The kinase inactive MST2 T180A mutant did not stimulate p38 and JNK under any condition. These results further confirm that Akt is critical in inhibiting MST2 and downstream signalling into different pathways.
Discussion
Our results provide evidence that MST2 is a hub that integrates the output activities of three pathways, i.e. the Raf-1, RASSF1A and Akt pathways. This conclusion is based on the following evidence.
We show that MST2 is a physiological substrate of Akt. Endogenous MST2 is phosphorylated by Akt in both serum-stimulated and PTEN−/− cells. In quiescent cells, we have previously described that Raf-1 interacts with MST2 to inhibit its pro-apoptotic activity (6, 12). Interestingly, we have previously also observed that mitogens can disrupt the MST2-Raf-1 interaction without leading to activation of MST2 (12). Our current results explain this conundrum suggesting the following model. In quiescent cells, MST2 is inhibited by being sequestered in a complex with Raf-1. Pro-apoptotic stimuli, such as Fas activation, staurosporine or RASSF1A, break up this complex and induce MST2 activation by promoting RASSF1A binding and homo-dimerisation (6, 12). It is not entirely clear how pro-apoptotic stimuli dissociate the MST2-Raf-1 complex. The Raf-1 and RASSF1A interaction sites in MST2 overlap and these proteins compete for MST2 binding {Matallanas, 2007 #4, suggesting that pro-apoptotic stimuli which suppress Akt activity and MST2 phosphorylation will shift MST2 affinity from Raf-1 to RASSF1A. Mitogen stimulation and oncogenic Ras also dissociate the MST2-Raf-1 complex, however due to the simultaneous activation of Akt and Akt mediated MST2 phosphorylation, MST2 remains inhibited. This latter mechanism is especially relevant in PTEN−/− cells which display high Akt activity and hence efficient MST2 inhibition. PTEN is one of the most frequently altered tumour suppressor genes in human cancer (19). As loss of PTEN expression or function will interfere with MST2 activation and pro-apoptotic activity, MST2 may have a role as effector of the PTEN tumour suppressor to prevent unlicensed cell proliferation in normal cells and tissues.
It seems that the basal level of Akt activity is sufficient to phosphorylate MST2, and is at least in part is responsible for MST2 binding to Raf-1 under non-stimulated conditions. The interaction of endogenous MST2 with Raf-1 is diminished when the basal levels of active Akt in quiescent cells are reduced by re-expressing PTEN, pharmacological inhibition of the PI3K-Akt pathway, specific downregulation of Akt expression or mutation of the Akt phosphorylation sites. Interestingly, Akt-induced phosphorylation of MST2 is not dependent on Raf-1, suggesting that Akt phosphorylation of MST2 takes place prior Raf-1 binding and promotes Raf-1-MST2 complex formation.
Previous work with the related MST1 kinase showed that Akt phosphorylation occurs only at the C-terminal site (15). Based on mutational analysis, our study clearly shows that in MST2 both N- and C-terminal Akt motifs are phosphorylated. Indeed, the single MST2 mutants remained competent for interaction with Raf-1 and Akt, albeit to a lower extent than wild type MST2. These single mutants also were more active and showed stronger interaction with RASSF1A than wild type MST2, but to a lower extent than the double mutant T117/384AA. The double mutant showed a higher kinase activity and was more pro-apoptotic than the single mutants. These data show that Akt phosphorylation of both N- and C-terminal sites is necessary for full regulation of MST2 by Akt, whereas phosphorylation of the C-terminal site is sufficient for regulation of MST1. Importantly, the regulation of MST1 and MST2 by Akt differs fundamentally. Akt phosphorylation prevents MST1 activation by caspase cleavage and selectively inhibits MST1 kinase activity towards FOXO-3, but not histone H2B (15). In contrast, as our data show Akt inhibits MST2 by a dual mechanism, i.e. shifting its binding from RASSF1A to Raf-1, and by directly inhibiting MST2 kinase activity independent of proteolytic cleavage. The relevant mechanism is inhibition of MST2 homo-dimerisation, which is an early activation step that precedes activation by proteolytic cleavage.
Akt also has been reported to phosphorylate and inhibit YAP (18), a transcription factor that functions downstream of MST2 (30). Thus, Akt could regulate the pathway at several levels. Most importantly, our data suggest that MST2 is a point of coordination between the Raf, RASSF1A and Akt pathways, with successful proliferation in response to Raf-1 stimulation requiring concomitant Akt activation. MST2 release is part of normal mitogenic regulation, and maybe a safeguard against unlicensed proliferation by coupling mitogenic signalling with priming of an apoptotic signal. Intriguingly, Raf-1 and Akt can be differentially recruited by Ras protein isoforms. H-Ras is more effective in activating the PI3K-Akt pathway, whereas N-Ras and K-Ras are more potent in activating ERK (31). Therefore, upon mitogen stimulation, MST2 release from Raf-1 should be triggered by Ras isoforms that preferentially bind Raf-1 and signal to ERK, whereas PI3K would be recruited by distinct Ras isoforms to activate Akt. Such coordination would ensure that Raf-1 and Akt activation proceed in parallel by avoiding competition between Raf-1 and PI3K for binding to the same Ras effector sites. In summary, these data suggest that MST2 is a point of convergence for Ras signalling that regulates the balance between proliferation and apoptosis by serving as common regulation target for the Raf-1 and Akt pathways.
Supplementary Material
Acknowledgments
This work was supported by the European Union FP6 project “Growthstop” (LSHC-CT-2006-037731) and Cancer Research UK.
References
- 1.Taylor LK, Wang HC, Erikson RL. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc Natl Acad Sci U S A. 1996;93:10099–104. doi: 10.1073/pnas.93.19.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ura S, Masuyama N, Graves JD, Gotoh Y. MST1-JNK promotes apoptosis via caspase-dependent and independent pathways. Genes Cells. 2001;6:519–30. doi: 10.1046/j.1365-2443.2001.00439.x. [DOI] [PubMed] [Google Scholar]
- 3.Ura S, Nishina H, Gotoh Y, Katada T. Activation of the c-Jun N-terminal kinase pathway by MST1 is essential and sufficient for the induction of chromatin condensation during apoptosis. Mol Cell Biol. 2007;27:5514–22. doi: 10.1128/MCB.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ura S, Masuyama N, Graves JD, Gotoh Y. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc Natl Acad Sci U S A. 2001;98:10148–53. doi: 10.1073/pnas.181161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Matallanas D, Romano D, Yee K, et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–75. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baksh S, Tommasi S, Fenton S, et al. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell. 2005;18:637–50. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Vos MD, Dallol A, Eckfeld K, et al. The RASSF1A tumor suppressor activates Bax via MOAP-1. J Biol Chem. 2006;281:4557–63. doi: 10.1074/jbc.M512128200. [DOI] [PubMed] [Google Scholar]
- 9.Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005;65:3497–508. doi: 10.1158/0008-5472.CAN-04-4088. [DOI] [PubMed] [Google Scholar]
- 10.Avruch J, Xavier R, Bardeesy N, et al. Rassf family of tumor suppressor polypeptides. J Biol Chem. 2009;284:11001–5. doi: 10.1074/jbc.R800073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donninger H, Vos MD, Clark GJ. The RASSF1A tumor suppressor. J Cell Sci. 2007;120:3163–72. doi: 10.1242/jcs.010389. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill E, Rushworth L, Baccarini M, Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science. 2004;306:2267–70. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill E, Kolch W. Taming the Hippo: Raf-1 controls apoptosis by suppressing MST2/Hippo. Cell Cycle. 2005;4:365–7. doi: 10.4161/cc.4.3.1531. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signalling pathway. Br J Cancer. 2004;90:283–8. doi: 10.1038/sj.bjc.6601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang SW, Yang SJ, Srinivasan S, Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem. 2007;282:30836–44. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- 16.Franke TF. Intracellular signaling by Akt: bound to be specific. Sci Signal. 2008;1:pe29. doi: 10.1126/scisignal.124pe29. [DOI] [PubMed] [Google Scholar]
- 17.Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20-related kinases. Cell Signal. 2008;20:1237–47. doi: 10.1016/j.cellsig.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 19.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–98. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 20.Myers MP, Pass I, Batty IH, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–8. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz-Vega S, Khokhlatchev A, Nedwidek M, et al. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene. 2002;21:1381–90. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- 22.Peter M, Ameer-Beg SM, Hughes MK, et al. Multiphoton-FLIM quantification of the EGFP-mRFP1 FRET pair for localization of membrane receptor-kinase interactions. Biophys J. 2005;88:1224–37. doi: 10.1529/biophysj.104.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joneson T, White MA, Wigler MH, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–2. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 24.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia R, Grindlay J, Rath O, Fee F, Kolch W. Regulation of human myoblast differentiation by PEBP4. EMBO Rep. 2009 doi: 10.1038/embor.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rommel C, Clarke BA, Zimmermann S, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–41. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 27.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal. 2008;20:892–906. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graves JD, Gotoh Y, Draves KE, et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. Embo J. 1998;17:2224–34. doi: 10.1093/emboj/17.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watabe M, Kakeya H, Osada H. Requirement of protein kinase (Krs/MST) activation for MT-21-induced apoptosis. Oncogene. 1999;18:5211–20. doi: 10.1038/sj.onc.1202901. [DOI] [PubMed] [Google Scholar]
- 30.Wang K, Degerny C, Xu M, Yang XJ. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009;87:77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Zhu T, Guan KL. Transformation potential of Ras isoforms correlates with activation of phosphatidylinositol 3-kinase but not ERK. J Biol Chem. 2004;279:37398–406. doi: 10.1074/jbc.M405730200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.