Abstract
Two hydroxyethylamine chroman derivatives, ammonificins A (1) and B (2), were isolated from the marine hydrothermal vent bacterium Thermovibrio ammonificans. The molecular structures of these compounds were determined using a combination of NMR, mass spectrometry, and CD analyses. Biological activities were determined using an antimicrobial assay and the patented ApopScreen cell-based screen for apoptosis induction and potential anticancer activity. To our knowledge, this is the first report of secondary metabolites from the marine hydrothermal vent bacterium T. ammonificans.
The oceans are Earth’s largest ecosystem and hold great, underexplored potential for drug discovery. Within this vast ecosystem, however, one area remains enigmatic: deep-sea hydrothermal vents, which are characterized by high concentrations of reduced sulfur compounds.1 Life is supported by the growth of chemolithoautotrophic bacteria, capable of oxidizing hydrogen sulfide, hydrogen, and other reduced inorganic compounds to provide energy that is used to fuel carbon dioxide fixation into macromolecules.
Chemoautotrophic organisms are known to produce chemical defenses against their consumers. Some chemically deterrent species are also known to harbor chemoautotropic endosymbiotic bacteria, and these microbial symbionts may produce metabolites that defend their host species.2 In this study we investigated the ability of chemolithoautotrophic bacteria to produce novel secondary metabolites.
Thermovibrio ammonificans, a thermophilic, anaerobic, chemolithoautotrophic bacterium, was isolated from the walls of an active deep-sea hydrothermal vent chimney on the East Pacific Rise at 9°50′ N. Cells of the organism were Gram-negative, motile rods that were about 1.0 μm in length and 0.6 μm in width. Growth occurred between 60 and 80 °C (optimum at 75 °C), 0.5 and 4.5% (w/v) NaCl (optimum at 2%), and pH 5 and 7 (optimum at 5.5). The generation time under optimal conditions was 1.57 h. Growth occurred under chemolithoautotrophic conditions in the presence of H2 and CO2, with nitrate or sulfur as the electron acceptor and with concomitant formation of ammonium or hydrogen sulfide, respectively.3
Forty grams wet weight of the organism was extracted in MeOH. One part of the MeOH soluble extract was dissolved in DMSO and was tested for apoptosis induction as assessed by the ApopScreen protocol.4–6 Screening should identify compounds that possess proapoptotic, and potentially anticancer, activity.
The extract induced apoptosis and therefore was fractionated, with subsequent purification by analytical RPHPLC. Using this strategy, two compounds were isolated. The chemical structures of these two compounds (1 and 2) were ascertained by standard spectroscopic techniques, as described below.
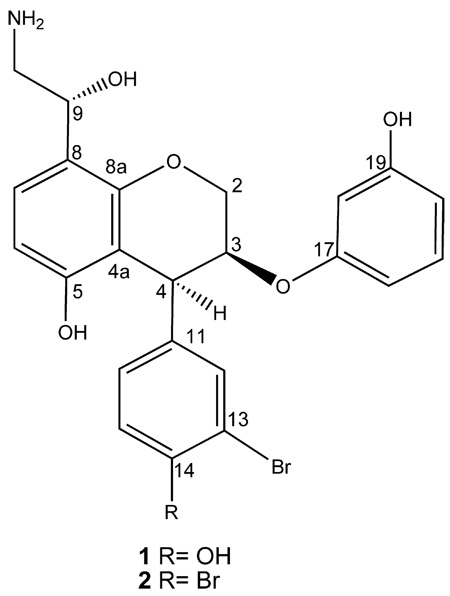
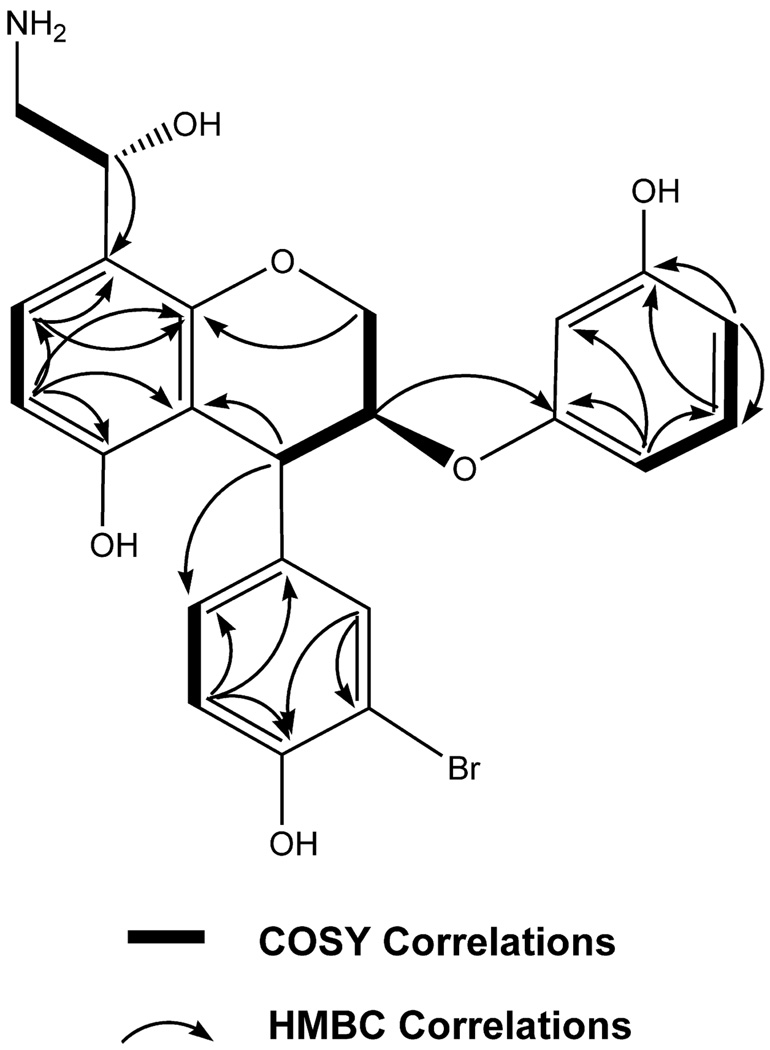
The LR ESIMS of ammonificin A (1) displayed ion clusters at m/z 488(100)/490(98), indicating the presence of one bromine. The molecular formula of 1 was established as C23H22BrNO6 on the basis of HR ESIMS [m/z 488.0701 (M + H)+]. The 1H spectrum of 1 indicated clearly the presence of nine aromatic ring signals: δH 6.67 [(d, J = 7.9 Hz), H-6], 7.09 [(d, J = 7.9 Hz), H-7], δH 7.41 [s, H-12], 6.77 [(d, J = 7.2 Hz), H-15], 7.26 [(d, J = 7.2 Hz), H-16], 6.65 [s, H-18], 6.74 [(d, J = 7.8 Hz), H-20], 7.01 [(dd, J = 7.8 Hz, 7.6 Hz), H-21], and 6.81 [(d, J = 7.6 Hz), H-22]. Their corresponding methine carbons were assigned from multiplicity edited HSQC: C-6 (δ 108.0), C-7 (δ 126.8), C-12 (δ 133.1), C-15 (δ 118.4), C-16 (δ 128.8), C-18 (δ 101.4), C-20 (δ 107.3), C-21 (δ 130.5), and C-22 (δ 106.8). Analysis of HMBC and multiplicity edited HSQC data suggested the presence of nine quaternary carbons characteristic of signals belonging to aromatic ring systems (δC 110.8, 116.1, 119.7, 137.8, 155.7, 156.9, 157.1, 157.9, and 158.9). Given the number of carbons belonging to the aromatic signals, ammonificin A (1) was found to possess three aromatic ring systems. Furthermore, three proton signals characteristic of hydroxy groups attached to aromatic ring systems were present in the 1H spectrum: δH 8.48 (br s), 9.26 (br s), and 9.47 (br s). Closer examination of the 1H–1H COSY along with the 1H NMR spectrum suggested the presence of signals characteristic of a dihydropyran moiety: δH 4.35 [(d, J = 5.6 Hz), H-4], 4.98 [m, H-3], and 4.45 [m, H-2]. HMBC correlations between H-6 and C-7 (δC 126.8), C-5 (δC 155.7), C-4a (δC 116.1); H-7 and C-8 (δC 119.7), C-8a (δC 157.9); H-4 and C-4a (δC 116.1); and H-2 and C-8a (δC 157.9) strongly suggested that 1 has a chroman moiety in its structure. Another interesting group resulting from the 1H–1H COSY analysis is a hydroxyethylamine moiety14 in 1: δH 4.70 [m, H-9] and 3.45 [m, H-10]. Moreover, this hydroxyethylamine moiety is found to be attached to C-8 according to the HMBC correlation between H-9 and C-8 (δC 119.7). The two remaining aromatic rings were established using 1H–1H COSY and HMBC correlations (Figure 1). The 1H–1H COSY correlation between H-15 and H-16, HMBC correlations between H-15 and C-11, C-13, C-14, C-16, and HMBC correlations between H-12 and C-13, C-14, C-16 define a trisubstituted aromatic ring system. The 1H-1H COSY correlations between H-21 and H-20, H-22 and HMBC correlations between H-20 and C-19, C-22 and between H-22 and C-17, C-18, C-20 generated a disubstituted aromatic ring system. The connectivity between the chroman moiety and the remaining two ring systems was established by HMBC correlations: H-4 to C-12, C-16 and H-3 to C-17. The chemical shifts of the quaternary carbons belonging to the aromatic ring systems played an important role in the assignment of the regiochemistry. For example, the chemical shift of the C-5 quaternary carbon (δC 155.7) indicated that hydroxy was attached, whereas the shift at the C-13 quaternary carbon (δC 110.8) indicated bromine was present. Similarly, the chemical shifts at C-14 (δC 157.1) and C-19 (δC 156.9) indicated that hydroxys were attached to these positions.
Figure 1.
Key HMBC and selected COSY correlations for ammonificin A (1).
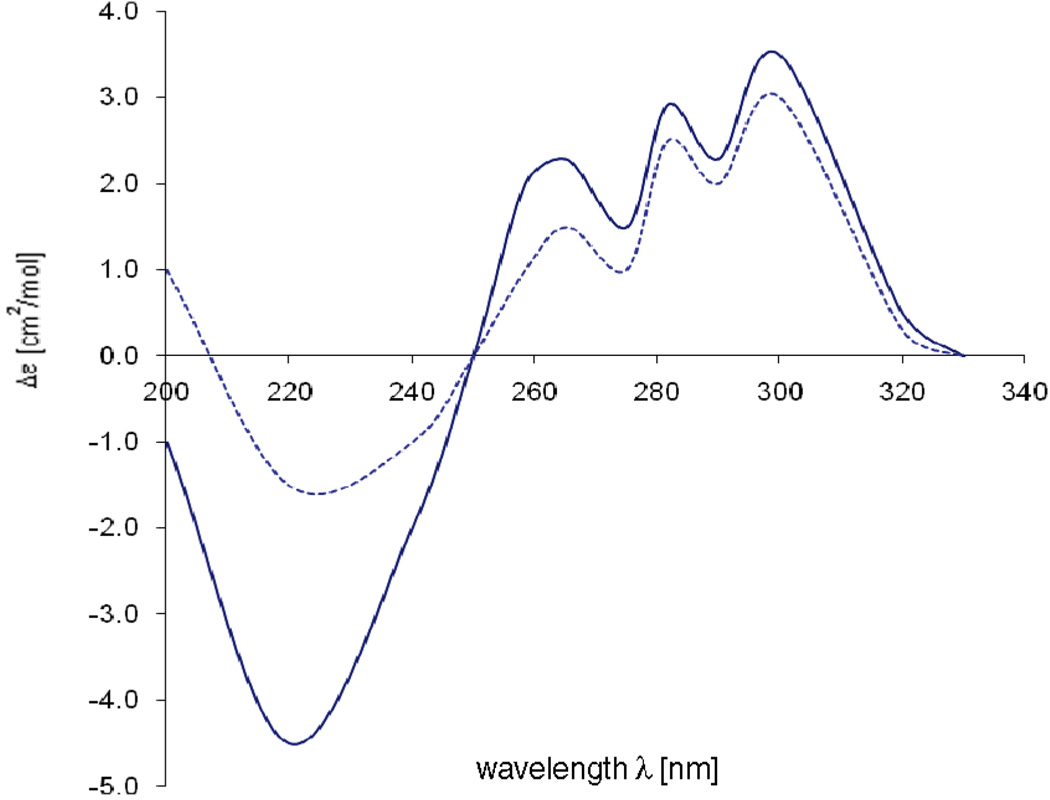
To determine the absolute configurations at C-3, C-4, and C-9, a circular dichroism (CD) spectrum of ammonificin A (1) was obtained. This experimental CD spectrum was then compared to the predicted CD spectra of all possible stereoisomers. Eight stereoisomers are possible for ammonificin A (1). The coupling constant between H-3 and H-4 (J = 5.6 Hz) suggested a cis relationship between these two protons (H-3 equatorial, H-4 axial), which indicated only four probable stereoisomers: (3S, 4S, 9R), (3S, 4S, 9S), (3R, 4R, 9R), (3R, 4R, 9S).
These four probable stereoisomers were submitted to geometry optimization by the DFT (BLYP/6-31G*) approach.7 For each minimized geometry a single CD spectrum was calculated using the TDDFT approach (B3LYP/TZVP).7 The overall CD spectra thus obtained were subsequently UV-corrected and compared with the experimental one of 1. An excellent agreement between the CD curve calculated for 3S, 4S, 9R and the experimental was found (Figure 2). This indicated that 1 has the configuration 3S, 4S, 9R, and the structure of 1 is established as shown.
Figure 2.
Comparison of the experimental CD spectrum (—) of 1 with the spectra calculated (•••) for 3S, 4S,9R.
The LR ESIMS of ammonificin B (2) displayed ion clusters at m/z 550(51)/552(100)/554(48), indicating the presence of two bromines. The molecular formula of 2 was established as C23H21Br2NO5 on the basis of HR ESIMS [m/z 549.9857 (M + H)+]. The molecular formula of 2 showed that it has one more bromine atom and one less hydrogen and oxygen atom compared to 1.
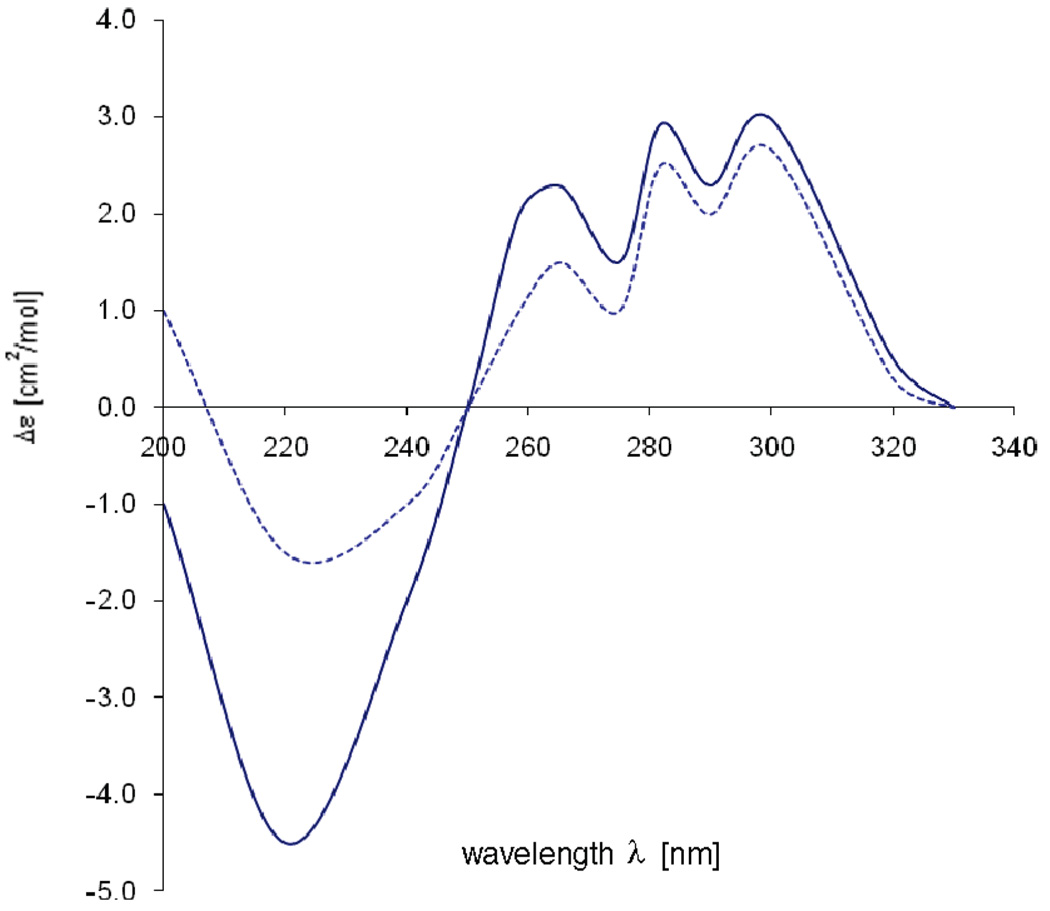
The strong similarity of its 1H NMR spectrum to that of ammonificin A (1) revealed that 1 and 2 share the same general structural features. Furthermore, only two proton signals characteristic of hydroxy groups attached to the aromatic ring system were present in the 1H spectrum of 2 (δH 9.27 (br s), 9.46 (br s)), suggesting that one hydroxy group was replaced by one bromine atom. HMBC correlations between H-16 and C-14 and also between H-12 and C-14 confirmed this suggestion. From the above analyses, it was concluded that the structure of 2 is similar to that of 1 except that the hydroxy group attached to C-14 was replaced by one bromine atom. The absolute configurations at C-3, C-4, and C-9 of ammonificin B (2) were ascertained by the same methods as described above. Although chroman derivatives are known structures, the co-occurrence of hydroxyethylamine and phenol in 1 or brominated phenol in 2 with chroman is unique.
The isolated compounds were evaluated in the apoptosis induction assay and with antimicrobial tests, but the results were inconclusive due to the presence of minor inseparable impurities. The original extract showed interesting activity; however pure compounds that correlated with the activity were not obtained. The minor components that could not be removed during the purification process probably have similar structure and polarity to 1 and 2. An effort to scale up the culture and reisolate ammonificins A and B as well as the minor components of the extract is still in progress. The compounds described herein represent new chemical structures and may have potential in future drug discovery efforts.
Experimental Section
General Experimental Procedures
Optical rotations were measured on a JASCO P 1010 polarimeter. UV and FT-IR spectra were obtained employing Hewlett-Packard 8452A and Nicolet 510 instruments, respectively. CD spectra were acquired on a JASCO J-720 spectropolarimeter. All NMR spectra were recorded on a Bruker Avance DRX400 spectrometer, Varian-400 instrument (400 MHz), and Varian-500 instrument (500 MHz). Spectra were referenced to residual solvent signals with resonances at δH/C 2.50/39.51 (DMSO-d6). ESIMS data were acquired on a Waters Micromass LCT Classic mass spectrometer and Varian 500-MS LC ion trap. HPLC separations were performed using Waters 510 HPLC pumps, a Waters 717 plus autosampler, and a Waters 996 photodiode array detector. All solvents were purchased as HPLC grade.
Extraction and Isolation Procedures
Cell Culturing
Thermovibrio ammonificans was routinely grown in modified SME medium as previously described.3 For the purpose of this study, bacterial cells were harvested from a total of 5 L of bacterial culture.
The material (40 g) was extracted three times with MeOH to give a polar crude organic extract (550 mg). A portion of this extract (20 mg) was tested for apoptosis induction. The crude organic extract was found active and subjected to fractionation using a solid-phase extraction cartridge (normal-phase silica) to give four fractions, F1 to F4, using hexane, hexane–EtOH, EtOH, and MeOH as an increasingly hydrophilic solvent system series. The fractions eluting with hexane–EtOH (F2) and MeOH (F4) had apoptosis induction activity. These fractions were further chromatographed on analytical RP HPLC (Phenomenex Luna C18, 250 × 4.60 mm) using isocratic elution with 50% MeOH and 50% H2O (flow rate 1 mL/min) to yield 3 mg of 1 (tR = 2.8 min) from F2, and 1.6 mg of 2 (tR = 4.4 min) from F4.
Computational Methods
Geometry optimization and UV and CD computations were undertaken using TDDFT with the B3LYP hybrid functional and a TZVP basis set, as included in the TURBOMOLE suite of programs with TmoleX, a graphical user interface to the Turbomole quantum chemistry program package.7 The corresponding oscillator and rotatory strengths thus obtained were summed and energetically weighted, following the Boltzmann statistics. Finally, the overall UV and CD spectra were simulated as sums of Gaussian functions centered at the wavelengths of the respective electronic transitions and multiplied by the corresponding oscillator or rotatory strengths, transformed into absorption and Δε values, respectively.8–11
Biological Evaluation: Apoptosis Induction
Apoptosis induction in the presence of compounds 1 and 2 was carried out as described in Andrianasolo et al. 2007 using the ApoScreen assay.5,15–18 In this assay viability of treated W2 (apoptosis competent) and D3 (apoptosis defective)12 cells is measured using a modification of the MTT assay.13 For this study, viability was measured at 0 and 48 h, and differential growth was calculated in the presence of the compounds staurosporine (an apoptosis inducer) as positive control and DMSO as negative controls.
Ammonificin A (1): [α]24D–150 (c 0.8, MeOH); UV (EtOH) λmax (log ε) 267 (2.90), 285 (3.68), 305 (3.70); CD (EtOH) see Figure 2; IR νmax (neat) 3350, 2950, 1620, 1460, 1380, 1230, 1160, 1120, 1090, 1020, 805 cm−1; 1H NMR and 13C NMR, see Table 2; HR ESIMS [m/z 488.0701 (M + H)+ (calcd for C23H23BrNO6, 488.0709)].
Table 2.
NMR Spectroscopic Data of Ammonificin B (2) (400 MHz, DMSO-d6)
| position | δC | δH | HMBCa |
|---|---|---|---|
| 2 | 70.2, CH2 | 4.45, m | 8a, 4 |
| 3 | 83.3, CH | 4.98, m | 17 |
| 4 | 27.5, CH | 4.35, d (5.6) | 4a, 12, 16 |
| 4a | 116.1, qC | ||
| 5 | 155.7, qC | ||
| 6 | 108.0, CH | 6.67, d (7.9) | 4a, 5, 7, 8a |
| 7 | 126.8, CH | 7.09, d (7.9) | 5, 8, 8a |
| 8 | 119.7, qC | ||
| 8a | 157.9, qC | ||
| 9 | 69.6, CH | 4.70, m | 7, 8 |
| 10 | 49.2, CH2 | 3.45, m | 8, 9 |
| 11 | 144.2, qC | ||
| 12 | 133.9, CH | 7.30, s | 11, 13, 14, 16 |
| 13 | 126.9, qC | ||
| 14 | 123.9, qC | ||
| 15 | 132.5, CH | 7.32, d (7.2) | 11, 13, 14, 16 |
| 16 | 129.6, CH | 7.28, d (7.2) | 11, 12, 14, 15 |
| 17 | 158.9, qC | ||
| 18 | 101.4, CH | 6.65, s | 17, 19, 20, 22 |
| 19 | 156.9, qC | ||
| 20 | 107.3, CH | 6.74, d (7.8) | 18, 19, 21, 22 |
| 21 | 130.5, CH | 7.01, dd (7.6,7.8) | 17, 19, 20, 22 |
| 22 | 106.8, CH | 6.81, d (7.6) | 17, 18, 20, 21 |
| OH on C-5 | 9.26, br s | ||
| OH on C-19 | 9.47, br s |
HMBC correlations, optimized for 8 Hz, are from proton(s) stated to the indicated carbon.
Ammonificin B (2): [α]24D –150 (c 0.8, MeOH); UV (EtOH) λmax (log ε) 267 (2.90), 285 (3.68), 305 (3.65); CD (EtOH) see Figure 3; IR νmax (neat) 3350, 2950, 1620, 1460, 1380, 1230, 1160, 1120, 1090, 1020, 805 cm−1; 1H NMR and 13C NMR, see Table 2; HR ESIMS [m/z 549.9857 (M + H)+ (calcd for C23H22Br2NO5, 549.9865)].
Figure 3.
Comparison of the experimental CD spectrum (—) of 2 with the spectra calculated (•••) for 3S, 4S,9R.
Supplementary Material
Table 1.
NMR Spectroscopic Data of Ammonificin A (1) (400 MHz, DMSO-d6)
| position | δC | δH | HMBCa |
|---|---|---|---|
| 2 | 70.2, CH2 | 4.45, m | 8a, 4 |
| 3 | 83.3, CH | 4.98, m | 17 |
| 4 | 27.5, CH | 4.35, d (5.6) | 4a, 12, 16 |
| 4a | 116.1, qC | ||
| 5 | 155.7, qC | ||
| 6 | 108.0, CH | 6.67, d (7.9) | 4a, 5, 7, 8a |
| 7 | 126.8, CH | 7.09, d (7.9) | 5, 8, 8a |
| 8 | 119.7, qC | ||
| 8a | 157.9, qC | ||
| 9 | 69.6, CH | 4.70, m | 7, 8 |
| 10 | 49.2, CH2 | 3.45, m | 8, 9 |
| 11 | 137.8, qC | ||
| 12 | 133.1, CH | 7.30, s | 11, 13, 14, 16 |
| 13 | 110.8, qC | ||
| 14 | 157.1, qC | ||
| 15 | 118.4, CH | 6.77, d (7.2) | 11, 13, 14, 16 |
| 16 | 128.8, CH | 7.26, d (7.2) | 11, 12, 14, 15 |
| 17 | 158.9, qC | ||
| 18 | 101.4, CH | 6.65, s | 17, 19, 20, 22 |
| 19 | 156.9, qC | ||
| 20 | 107.3, CH | 6.74, d (7.8) | 18, 19, 21, 22 |
| 21 | 130.5, CH | 7.01, dd (7.6,7.8) | 17, 19, 20, 22 |
| 22 | 106.8, CH | 6.81, d (7.6) | 17, 18, 20, 21 |
| OH on C-5 | 9.26, br s | ||
| OH on C-14 | 8.48, br s | ||
| OH on C-19 | 9.47, br s |
HMBC correlations, optimized for 8 Hz, are from proton(s) stated to the indicated carbon.
Acknowledgment
We thank K. McPhail and S. Kim for NMR data from NMR facilities at Oregon State University and Department of Chemistry at Rutgers University, respectively. We also thank H. Zheng for mass spectrometry analyses at the Center for Advanced Biotechnology and Medicine, Rutgers University. This research was funded by Rutgers University through an Academic Excellence award, by NSF grants OCE 03-27373 (R.A.L and C.V.) and MCB 04-56676 (C.V.), by the New Jersey Agricultural and Experiment Station (C.V.), and by NIH grant R37 CA53370 (E.W.).
Footnotes
Supporting Information Available: NMR and MS data of 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Gärtner A, Wiese J, Imhoff JF. Int. J. Syst. Evol. Microbiol. 2008;58:34–39. doi: 10.1099/ijs.0.65234-0. [DOI] [PubMed] [Google Scholar]
- 2.Kicklighter CE, Fisher CR, Hay ME. Mar. Ecol.: Prog. Ser. 2004;275:11–19. [Google Scholar]
- 3.Vetriani C, Speck MD, Ellor SV, Lutz RA, Starovoytov V. Int. J. Syst. Evol. Microbiol. 2004;54:175–181. doi: 10.1099/ijs.0.02781-0. [DOI] [PubMed] [Google Scholar]
- 4.Andrianasolo EH, Haramaty L, Degenhardt K, Mathew R, White E, Lutz R, Falkowski P. J. Nat. Prod. 2007;70:1551–1557. doi: 10.1021/np070088v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew R, Degenhart K, Haramaty L, Karp CM, White E. Methods Enzymol. 2008 doi: 10.1016/S0076-6879(08)01605-4. [DOI] [PubMed] [Google Scholar]
- 6.Karantza-Wadsworth V, White E. In: Progammed Cell Death. Cancer: Principles and Practice of Oncology. DeVita VT, Lawrence TS, Rosenberg SA, editors. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2008. in press. [Google Scholar]
- 7.Ahlrichs R, Furche F, Hättig C, Klopper W, Sierka M, Weigend F. TURBOMOLE 5.10. 2008 [Google Scholar]
- 8.Holscher D, Reichert M, Gorls H, Ohlenschlager O, Bringmann G, Schneider B. J. Nat. Prod. 2006;69:1614–1617. doi: 10.1021/np060192x. [DOI] [PubMed] [Google Scholar]
- 9.Pecul M, Ruud K, Helgaker T. Chem. Phys. Lett. 2004;388:110–119. [Google Scholar]
- 10.Diedrich C, Grimme S. J. Phys. Chem. 2003;107:2524–2539. [Google Scholar]
- 11.Antus S, Kurtan T, Juhász L, Kiss L, Hollósi M, Májer ZS. Chirality. 2001;13:493–506. doi: 10.1002/chir.1067. [DOI] [PubMed] [Google Scholar]
- 12.Degenhardt KSR, Chen G, Lindsten T, Thomson C, White E. J. Biol. Chem. 2002;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- 13.Denyer SP, Maillard J-Y. J. Immunol. Methods. 1983;65:55–63. [Google Scholar]
- 14.Fahy E, Potts BCM, Faulkner J. J. Nat. Prod. 1991;54:564–569. [Google Scholar]
- 15.Danial N, Korsmeyer S. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 16.Gelinas C, White E. Genes Dev. 2005;19:1263–1268. doi: 10.1101/gad.1326205. [DOI] [PubMed] [Google Scholar]
- 17.Degenhardt K, White E. Clin. Cancer Res. 2006;12:5274–5276. doi: 10.1158/1078-0432.CCR-06-0439. [DOI] [PubMed] [Google Scholar]
- 18.Fesik SW. Nat. Rev. Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.