Abstract
Brucella spp. establish an intracellular replicative niche in macrophages, while macrophages attempt to eliminate the bacteria by innate defense mechanisms. Brucella spp. possess similar genomes yet exhibit different macrophage infections. Few B. melitensis and B. neotomae enter macrophages with intracellular adaptation occurring over 4–8 hr. Conversely, B. ovis are readily ingested by macrophages and exhibit a persistent plateau of infection. Evaluating early macrophage interaction with Brucella spp. allows discovery of host entry and intracellular translocation mechanisms. Microarray analysis of macrophage transcriptional response following a 4 hr infection by different Brucella spp. revealed common macrophage genes altered in expression compared to uninfected macrophages. Macrophage infection with three different Brucella spp. provokes a common innate immune theme with increased transcript levels of chemokines and defense response genes and decreased transcript levels of GTPase signaling and cytoskeletal function that may affect trafficking of Brucella containing vesicles. For example, transcript levels of genes associated with chemotaxis (IL-1β, MIP-1α), cytokine regulation (Socs3) and defense (Fas, Tnf) were increased, while transcript levels of genes associated with vesicular trafficking (Rab3d) and lysosomal associated enzymes (prosaposin) were decreased. Genes with altered macrophage transcript levels among Brucella spp. infections may correlate with species specific host defenses and intracellular survival strategies. Depending on the infecting Brucella species, gene ontology categorization identified genes differentially involved in cell growth and maintenance, endopeptidase inhibitor activity and G-protein mediated signaling. Examples of decreased gene expression in B. melitensis infection but not other Brucella spp. were growth arrest (Gas2), immunoglobulin receptor (FcγrI) and chemokine receptor (Cxcr4) genes, suggesting opposing effects on intracellular functions.
Keywords: Brucella infection, macrophages, transcriptome, Brucella melitensis, Brucella ovis, Brucella neotomae
Introduction
Brucella species (spp.) are zoonotic pathogens able to infect humans and cause abortion in domestic animals. Human infection generally requires contact with a limited number of organisms (infectious dose estimated as less than 100 organisms) (1), progresses with inconsistent and persistent flu-like symptoms from 2–6 weeks post-inoculation and, if left untreated, develops into chronic brucellosis. Macrophages phagocytose Brucella spp. and initiate an innate immune response, while Brucella subvert the host antimicrobial defense mechanisms to establish an intracellular replicative niche (2). Once resident within the macrophage, Brucella avoid exposure and killing by the humoral immune response.
Host preference and virulence among species are unaccounted when comparing the few differences between genomic sequences of B. melitensis, B. abortus and B. suis (3). When comparing six historically identified Brucella spp., only 217 open reading frames present in B. melitensis were absent in the other species (4). Ultimately, Brucella research has revealed a limited number of factors that significantly alter host specificity by Brucella spp. Human infections with B. melitensis are severe in pathogenesis and are widely reported; conversely, neither B. ovis nor B. neotomae have been reported to cause human infection, and pathogenesis is at most limited. Investigating the murine macrophage response to highly similar Brucella spp. may provide additional understanding regarding the ability of Brucella spp. to establish and maintain infections.
Although transcriptional profiles of murine macrophages infected with B. abortus have been studied (7), no studies have compared host response among infections of differing Brucella spp. Altered host transcriptional response among Brucella spp. infections may identify not only common responses to infection, but also distinguish genes and pathways specific to each Brucella spp. infection. Identifying alterations in the macrophage transcriptome may provide greater understanding of host mechanisms involved in pathogen killing and bacterial regulation that limit damage to host cells during infection.
Murine macrophages are frequently used to investigate Brucella infection. The transcription profile after 4 hr of infection would evaluate general as well as specific response to different but genetically similar Brucella spp. Bacteria enter host cells and translocate to an endoplasmic reticulum containing a replicative niche within a few hours post infection; concurrently, a portion of the bacteria die by phagosome-lysosome fusion (8). The majority of host transcriptional response occurs during this early time (9). Examining an early time, such as 4 hr post infection, permits discovery of potential mechanisms of entry and intracellular translocation that take place before bacterial replication becomes evident after 8 hr (2, 10–12). The present microarray analyses evaluate macrophage response to Brucella spp. infection by testing 6 hypotheses and focusing on analogous and distinct transcriptional responses elicited by B. melitensis, B. neotomae and B. ovis.
Materials and Methods
Bacteria and Cell Lines
B. melitensis, B. neotomae and B. ovis were grown in 12- by 75-mm tubes on a shaker platform in BBL Brucella broth (BD Biosciences, Franklin Lakes, NJ) or on Brucella broth plates containing 1.5% agar. B. melitensis, B. neotomae and B. ovis were transformed with pBBR1MCS/GFPuv containing green fluorescent protein (gfpuv) under a constitutive Tac promoter and with chloramphenicol resistance (13). Brucella spp. for infections were grown in broth with or without chloramphenicol at 37°C for 1–2 days and colony forming units (CFUs) determined by plating on agar and incubating 3 days at 37°C with 5% CO2.
RAW 264.7 (TIB-71, ATCC) and J774A.1 (TIB-67, ATCC) mouse macrophage cell lines were maintained at 37°C with 5% CO2 in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 0.2 mM L-glutamine, antibiotic-antimycotic (100 U/mL penicillin G, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, Gibco), 1 mM sodium pyruvate (SAFC Biosciences, St. Louis, MO) and MEM amino acids (Hyclone, Logan, UT).
Intracellular Survival of Brucella spp. in Macrophages
Macrophages (0.5–1 × 106/well) were plated in 6-well plates 2–12 hr prior to infection in medium without antibiotics. Brucella spp. were grown to stationary phase in Brucella broth and then serially diluted and plated on Brucella agar to estimate CFU/mL. Macrophages were infected at a multiplicity of infection (MOI, bacteria to macrophage) of 1000:1 for 90 min at 37°C with 5% CO2. Extracellular bacteria were removed using three PBS washes followed by 30 μg/mL gentamicin (MP Biomedicals, Inc., Irvine, CA) in RPMI. After 30 min, macrophages were washed three times with PBS. RPMI supplemented with 2 μg/mL gentamicin was added to cultures after 2 hr. At 4 hr, cultures were washed, lysed with 0.1% Triton X-100, serially diluted and plated twice on Brucella agar to determine bacterial CFUs. Experiments were repeated independently a minimum of three times.
Flow Cytometry of Brucella-GFPuv Infected Macrophages
Macrophages (5 × 107/T25 flask) were plated 2–12 hr prior to infection in medium without antibiotics. Brucella spp. were grown to stationary phase, and macrophages were infected at a multiplicity of infection (MOI, bacteria to macrophage) of 1000:1 for 90 min at 37°C with 5% CO2 followed by gentamicin treatment to remove extracellular bacteria as described above. After 4 hr of incubation, cells were washed three times with PBS, fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hat-field, PA) for 30 min and observed by fluorescence microscopy (Carl Zeiss, Thornwood, NY). Using a FACScan (Becton Dickinson, Palo Alto, CA), ten thousand events were collected. Debris and dead cells were eliminated from the analysis on the basis of side scatter and forward scatter. Cells infected with B. melitensis, B. neotomae and B. ovis strains containing pBBR1MCS/GFPuv, were analyzed by flow cytometry to determine percentage of RAW 264.7 or J774A.1 cells infected at 4 hr.
Macrophage Infection for RNA Isolation
RAW 264.7 cells for microarray and RAW 264.7 and J774A.1 cells for RT-PCR were plated at 5 × 106 cells/T75 flask 12–24 hr prior to infection, in supplemented RPMI 1640 without antibiotics. Macrophages were infected with 1 mL of a stationary phase Brucella spp. culture (MOI 1000:1). Infected macrophages were incubated for 4 hr at 37°C with 5% CO2, washed once with PBS and then lysed for RNA collection (RNeasy, Qiagen, Germantown, MD).
Target Preparation for Microarray Hybridization
Total RNA was isolated from macrophage cultures (RNeasy, Qiagen) with lysate centrifugation to remove intact bacterial cells. DNase treated RNA from two independent infections was pooled for each target preparation. Target RNA was prepared according to the manufacturer’s protocols (Affymetrix, Santa Clara, CA). Briefly, RNA was converted to double stranded cDNA (Invitrogen) except that T-7-(dT)24 oligomer (Genset Corp., San Diego, CA) was used. Double stranded cDNA was isolated using GeneChip® Sample cleanup and in vitro synthesis of biotin-labeled cRNA was completed with Enzo BioArray High-Yield RNA Transcript Labeling (Affymetrix). Labeled cRNA mixed with fragmentation buffer was incubated at 94°C for 35 min and was confirmed by agarose gel electrophoresis. Final RNA concentration ranged from 0.5–1.1 μg/μL.
Microarray Hybridization and Analysis
Labeled cRNA was hybridized to GeneChip® Test3 arrays and Murine Genome U74Av2 microarrays (Affymetrix). Eleven MG_U74Av2 GeneChip® microarrays were independently hybridized with cRNA from uninfected macrophage samples (2 independent samples) and each of three Brucella spp. infected macrophage samples (3 independent samples for each Brucella species infection). GeneChip® washing, hybridization and scanning was performed by the University of WI Biotechnology Center, Gene Expression Center (University of WI-Madison) utilizing Affymetrix protocols and procedures. Affymetrix *.CHP, *.CEL and spreadsheets of signal output are available through NCBI Gene Expression Omnibus database at the time of publication, Series accession number GSE8385.
mRNA from uninfected RAW 246.7 macrophages was compared to macrophages infected for 4 hr with B. melitensis, B. ovis or B. neotomae. All genes were subjected to analysis by EBarrays (14, 15), a statistical analysis package in the comprehensive R archive network (http://cran.r-project.org/). Data conformity to the statistical assumptions was checked (coefficient of variation and log-normal normal model) and best fit models were used. Six biologically relevant hypotheses of altered transcript levels were tested (Mφ designates RAW 264.7 macrophages):
Infected and uninfected macrophages have similar transcription.
Brucella spp. infected macrophages express mRNA different from uninfected macrophages.
B. ovis and B. neotomae infected macrophages express mRNA different from B. melitensis or uninfected macrophages.
B. melitensis and B. neotomae infected macrophages express mRNA different from B. ovis or uninfected macrophages.
B. melitensis and B. ovis infected macrophages express mRNA different from B. neotomae or uninfected macrophages.
Each macrophage culture expresses distinct mRNA.
Empirical Bayesian statistics designated a posterior probability (EBarrays Probability) specific to each gene, evidence that a given gene follows the transcription pattern designated by each of the hypotheses. The probability of random gene assignment to any hypothesis was 0.167, while posterior probabilities ≥ 0.2 were considered significant. Genes were sorted within each experimental hypothesis, and each gene was allocated only to the hypothesis with the largest posterior probability. The signal logarithm ratio (SLR) was calculated as the logarithm, base 2, of the ratio between infected (experimental) signal and uninfected (control) signal. Thus, the experimental:control ratio was transformed to zero for no change between conditions and the equivalent of a two-fold increase or decrease becomes 1 or −1 SLR, respectively.
Additional data analysis was conducted utilizing TM4 microarray analysis tools ANOVA (16) and SAM (17, 18) contained within TIGR MultiExperiment Viewer (MeV) (http://www.tm4.org/) (19, 20). Genes were annotated utilizing Affymetrix’s NetAffx Analysis Center (http://www.affymetrix.com/analysis/index.affx) and DAVID Bio-informatics Resources (http://david.abcc.ncifcrf.gov/). Gene ontology (GO) categorization of genes identified with altered transcription between uninfected and Brucella spp. infected macrophages were completed with EASE (http://david.abcc.ncifcrf.gov/). Genes were iteratively subjected to GO biological processes categorization and, while genes may be categorized in several of the GO categories, each gene was listed once.
Real Time RT-PCR (RT-PCR)
Total RNA was isolated from macrophage cultures (RNeasy, Qiagen) according to manufacturer’s protocol with DNase treatment. Macrophage RNA (2–4 μg) was primed with oligo(dT)20, reverse transcribed with SuperScript™ II or III (Invitrogen), and resultant cDNA diluted 1:5. Primers (listed in Text S1) and double-dye oligonucleotide probes for PCR were developed to amplify mRNA fragments 80–130 basepairs in length using Primer3 (21). RT-PCR was performed on cDNA samples utilizing qPCR Mastermix (Eurogentec, San Diego, CA) or iQ supermix (Bio-Rad, Hercules, CA) with dual labeled probes and iQ SYBR® green Supermix (Bio-Rad), respectively, to quantify relative transcript levels. The PCR was optimized on the iCycler (Bio-Rad) with primer concentrations ranging from 300–500 nM and probe concentrations at 125 nM. Amplification cycles were 95°C for 15 sec followed by 60 sec at 60°C with fluorescence detected during the extension phase. Relative gene mRNA quantities were quantified by the standard curve method using a housekeeping gene as a reference gene. SLR was calculated with these normalized transcription values.
ELISA Measurement of TNF Activity
BMDM cells were flushed from the femurs and tibiae of 10–12 week-old wild-type C57BL/6 mice. Cells were grown for 5–8 days in RPMI 1640 (Gibco, Grand Island, NY), 10% FBS (Equitech-Bio Inc., Kerrville, TX), 25 μg/mL gentamicin (MP Biomedicals, Inc., Solon, OH) and 20–30 ng/mL M-CSF (R&D Systems, Minneapolis, MN). BMDM cells plated at 1 × 106 cells/well in 6-well plates were infected with B. melitensis at an MOI 100 and incubated at 37°C. Supernatants were collected at 12 hr from cells infected with B. melitensis or medium. Supernatants were filtered through a 0.22 micron PES Millipore filter (Millipore, Billerica, MA) and assayed for TNFα using a Ready-Set-Go ELISA kit (eBioscience, San Diego, CA).
Additional Calculations and Statistics
Error bars throughout indicate the standard error of the mean (SEM). A Student’s t test determined if there was significant difference between two independent samples using a pooled estimate of variation.
Results
Brucella spp. Infection of RAW 264.7 and J774A.1 Macrophages Assessed by Colony Forming Units (CFUs) and Flow Cytometry Analysis of Brucella-GFPuv
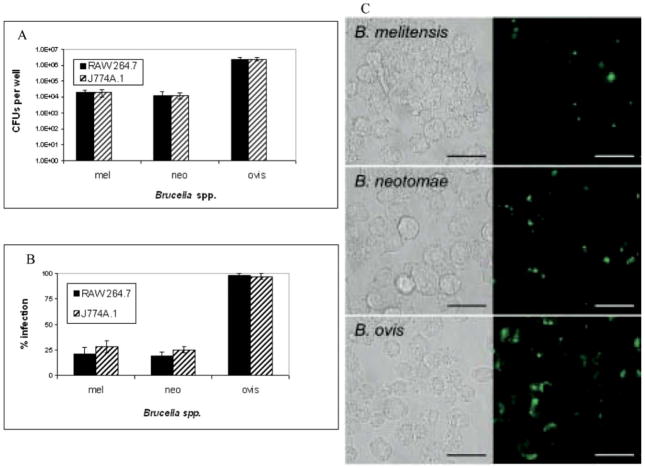
RAW 264.7 macrophage infections with B. melitensis and B. neotomae were similar, with low numbers of these bacteria phagocytosed compared to the number of bacteria introduced (Fig. 1, panel A). Macrophage uptake of B. ovis, at 4 hr in both RAW 264.7 and J774A.1 cells was nearly two logarithmic units higher than B. melitensis or B. neotomae, paralleling previous observations (6, 22).
Figure 1.
Intracellular survival of Brucella spp. in RAW 264.7 and J774A.1 macrophages. Panel A. Intracellular Brucella spp. were isolated from RAW 264.7 or J774A.1 macrophages and enumerated at 4 hr. Macrophages were infected with 1000:1 MOI of each Brucella spp. and allowed to infect for 90 min followed by gentamicin treatment for 30 min. At 4 hr, macrophages were washed then lysed with 0.1% Triton X-100. Intracellular Brucella were enumerated by plating serial dilutions on agar. Four independent experiments were conducted, and the number of Brucella spp. isolated per well was averaged with error bars representing the SEM. Comparing B. melitensis or B. neotomae to B. ovis infection is significantly different with P ≤ 0.005. Panel B. Flow cytometry analysis of Brucella-GFPuv infection in RAW 264.7 and J774A.1 macrophages at 4 hr. RAW 264.7 or J774A.1 macrophages were infected for 90 min with Brucella spp. containing GFPuv followed by gentamicin treatment to remove extracellular bacteria. After 4 hr, RAW 264.7 or J774A.1 cells were fixed in 4% paraformaldehyde, and the percent of infected cells determined by flow cytometry analysis. Comparing B. melitensis or B. neotomae to B. ovis infection is significantly different with P ≤ 0.005. Panel C. RAW 264.7 macrophages were infected continuously for 4 hr with Brucella spp. containing GFPuv. Cells were washed to remove extracellular Brucella and fixed in 4% paraformaldehyde. Matched bright field and fluorescence images were digitally captured at ×63 oil immersion magnification. Bar equals 20 μm. A color version of this figure is available in the online journal.
Similar to CFU results, flow cytometry analysis of RAW 264.7 macrophages infected for 4 hr with Brucella spp. expressing GFPuv had low levels of B. melitensis and B. neotomae, compared to B. ovis (Fig. 1, panel B). This observation was further supported by fluorescent microscopy (Fig. 1, panel C). At this early time, 20–30 percent of macrophages were infected with B. melitensis or B. neotomae, while greater than 95 percent of macrophages were infected with B. ovis (Fig. 1, panel B) consistent with uptake for smooth and rough Brucella (22). Interestingly, the numbers of Brucella phagocytosed at these levels did not correlate in a linear or proportional manner to levels of transcriptional response, as changes in macrophage mRNA amounts following B. ovis infection were often similar to B. melitensis and B. neotomae infections.
Because of the difference in macrophage infection by B. melitensis and B. neotomae compared to B. ovis, macrophage viability was examined to determine if infection levels would influence macrophage survival. RAW 264.7 and J774A.1 macrophages were continuously infected with Brucella spp. for 4 hr without antibiotics and viability determined. No significant macrophage death was observed (data not shown). Therefore, host transcription with Brucella spp. infection at 4 hr was not biased toward cell death pathways.
Microarray Analysis of RAW 264.7 Macrophages Infected with Each of Three Brucella spp
Genes Identified for Housekeeping Controls with No Transcriptome Change Across Three Brucella spp. Infection Conditions
A series of commonly used housekeeping genes were evaluated for potential as control genes in downstream analysis, and changes in transcript levels between Brucella spp. infected and uninfected macrophages were compared (Table 1). Genes with high posterior probabilities and low signal log ratio (SLR) with small variability appeared as the best control genes for Brucella spp. infections. Hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) and the TATA box binding protein (Tbp), which function in glycolysis and transcription, respectively (23), were transcribed at a moderate level (signal strength on microarray between 1 × 104 and 1 × 102) and had SLR near zero with small variability. Hprt1 and Tbp were unaltered by Brucella spp. infections and were therefore utilized as control housekeeping genes in downstream analysis of the transcriptome. The transcript levels of β-actin, β-glucuronidase, transferrin receptor and glyceraldehyde-3-phosphate dehydrogenase were minimally altered (−0.6 to 0.1 SLR) by infection, but each had a pronounced standard error and were considered not optimal for use as control genes.
Table 1.
RAW 264.7 Macrophage Transcription of Common Housekeeping Genes Following Brucella spp. Infection
| H0: Mφ = Mφ + B. melitensis = Mφ + B. neotomae = Mφ + B. ovis | ||||||
|---|---|---|---|---|---|---|
| Probe set | Entrez gene | Average signal | SLRa | EBarrays probability | Gene symbol | Gene product |
| 101213_at | 11837 | 5.7 × 104 | −0.2 ± 0.06 | 1.000 | Arbp | Acidic ribosomal phosphoprotein PO |
| 101578_f_at | 11461 | 2.2 × 104 | −0.6 ± 0.6 | 0.870 | Actb | β-actin |
| 101214_f_at 162210_r_at 97751_f_at | 14433 | 2.5 × 104 | 0.1 ± 0.2 | 0.894 | Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| 93346_at | 18655 | 2.0 × 104 | 0.2 ± 0.1 | 1.000 | Pgk1 | Phosphoglyerate kinase 1 |
| 93088_at | 12010 | 1.6 × 104 | 0.2 ± 0.2 | 1.000 | b2m | β 2 microglobulin |
| 97538_at | 110006 | 6.8 × 103 | −0.6 ± 0.2 | 0.999 | b-gus | β glucuronidase |
| 160107_at | 15452 | 6.5 × 103 | 0.07 ± 0.1 | 0.999 | Hprt1 | Hypoxanthine guanine phosphoribosyl transferase 1 |
| 103957_at 103958_g_at | 22042 | 3.4 × 103 | −0.5 ± 1.2 | 0.991 | Tfrc | Transferrin receptor |
| 99950_at | 21374 | 6.7 × 102 | −0.09 ± 0.2 | 0.996 | Tbp | TATA box binding protein |
Signal log ratio = LOG2 (experimental Brucella spp. infected signal/control uninfected signal) ± SE.
Genes Identified With Increased Transcription Following Brucella spp. Infections
Seventy-two genes were identified under H1 (transcription from macrophages infected by three Brucella spp. differs from uninfected macrophages) with SLR increases between 2.0 and 6.8 following infection with any Brucella spp. (Table 2). Defense and chemotactic response, both related to a common inflammatory response, encompass the largest groups of genes with increased transcript levels during infection. Interleukin-1β, tumor necrosis factor, macrophage inflammatory protein genes (MIP-1α, MIP-1β, MIP-2α), colony stimulating factor genes (Csf2 and Csf3) and Fas had increased transcription similar to reports with a variety of infectious agents (7, 24). Also, increased transcription was observed for cytokine regulation, anti-inflammatory and/or anti-apoptotic response genes (Socs3, Slfn2, IL-1rn, Gadd45b and Tnfaip3). These genes may prevent commitment to an inflammatory response pathway camouflaging immune recognition and providing a safe environment for bacterial survival. Although macrophages were infected with a greater number of B. ovis compared to B. melitensis and B. neotomae, Figure 1, no statistical difference was observed in the SLR between the 72 macrophage genes of Table 2 when infected with B. ovis compared to B. melitensis or B. neotomae.
Table 2.
Genes with Increased Transcription in Brucella spp. Infected as Compared to Uninfected RAW 264.7 Macrophages
| H1: Mφ ≠ Mφ + B. melitensis = Mφ + B. neotomae = Mφ + B. ovis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Probe set | Entrez gene | SLRa |
EBarrays probability | Gene symbol | Gene product | Additional statisticsc | |||
| Mb | Nb | Ob | Average ± SEM | ||||||
| Chemotaxis | |||||||||
| 102736_at | 20296 | 6.4 | 5.8 | 6.5 | 6.2 ± 0.5 | 0.996 | Ccl2 | Chemokine (C-C motif) ligand 2 (MCP-1) | a, b, c |
| 103486_at | 16176 | 7.5 | 6.1 | 6.7 | 6.8 ± 0.2 | 0.869 | Il1b | Interleukin 1 beta | a, b, c |
| 94761_at | 20306 | 5.6 | 4.6 | 6.0 | 5.4 ± 0.3 | 0.764 | Ccl7 | Chemokine (C-C motif) ligand 7 (MCP-3) | a, b, c |
| 98406_at | 20304 | 2.7 | 2.2 | 3.8 | 2.9 ± 0.6 | 0.658 | Ccl5 | Chemokine (C-C motif) ligand 5 (RANTES) | |
| 101160_at | 20310 | 4.8 | 4.4 | 4.6 | 4.6 ± 0.1 | 0.997 | Cxcl2 | Chemokine (C-X-C motif) ligand 2 (MIP-2α) | a, b, c |
| 94146_at | 20303 | 3.6 | 3.3 | 3.6 | 3.5 ± 0.2 | 0.999 | Ccl4 | Chemokine (C-C motif) ligand 4 (MIP-1β) | a, b, c |
| 98822_at | 53606 | 2.3 | 2.0 | 3.0 | 2.4 ± 0.3 | 0.891 | Isg15 | ISG15 ubiquitin-like modifier | |
| 102424_at | 20302 | 2.6 | 2.4 | 2.9 | 2.6 ± 0.2 | 0.905 | Ccl3 | Chemokine (C-C motif) ligand 3 (MIP-1α) | a, b |
| 104388_at | 20308 | 2.8 | 2.4 | 2.7 | 2.6 ± 0.2 | 0.932 | Ccl9 | Chemokine (C-C motif) ligand 9 (MIP-1γ) | a, b |
| 93858_at | 15945 | 1.7 | 2.1 | 2.2 | 2.0 ± 0.2 | 0.522 | Cxcl10 | Chemokine (C-X-C motif) ligand 10 | c |
| Defense response | |||||||||
| 92948_at | 12981 | 6.4 | 5.0 | 5.1 | 5.5 ± 0.4 | 0.723 | Csf2 | Colony stimulating factor 2 (granulocyte-macrophage) | a, b |
| 100981_at | 15957 | 4.6 | 4.2 | 5.6 | 4.8 ± 0.3 | 0.720 | Ifit1 | Interferon-induced protein with tetratricopeptide repeats 1 | a, b, c |
| 102629_at | 21926 | 4.3 | 4.1 | 4.2 | 4.2 ± 0.2 | 0.999 | Tnf | Tumor necrosis factor | a, b, c |
| 94142_at | 12985 | 4.7 | 3.7 | 4.6 | 4.3 ± 0.2 | 0.987 | Csf3 | Colony stimulating factor 3 (granulocyte) | a, b, c |
| 103639_at | 15958 | 2.8 | 3.2 | 3.7 | 3.2 ± 0.4 | 0.907 | Ifit2 | Interferon-induced protein with tetratricopeptide repeats 2 | |
| 93871_at | 16181 | 4.0 | 3.4 | 3.9 | 3.7 ± 0.2 | 0.996 | Il1rn | Interleukin 1 receptor antagonist | a, b, c |
| 102921_s_at | 14102 | 2.5 | 3.5 | 2.7 | 2.9 ± 0.4 | 0.914 | Fas | Fas (TNF receptor superfamily member) | c |
| 102712_at | 20210 | 3.4 | 2.3 | 2.9 | 2.9 ± 0.2 | 0.948 | Saa3 | Serum amyloid A 3 | c |
| 94928_at | 21938 | 2.5 | 2.0 | 2.5 | 2.4 ± 0.2 | 0.885 | Tnfrsf1b | Tumor necrosis factor receptor superfamily, member 1b | a, b, c |
| 92534_at | 14579 | 2.6 | 2.0 | 2.9 | 2.5 ± 0.2 | 0.839 | Gem | GTP binding protein (gene overexpressed in skeletal muscle) | |
| 98988_at | 80859 | 3.1 | 2.7 | 2.1 | 2.6 ± 0.2 | 0.94304859 | Nfkbiz | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta | |
| Protein-nucleus import | |||||||||
| 97238_at | 21335 | 2.8 | 3.2 | 2.9 | 3.0 ± 0.6 | 0.940 | Tacc3 | Transforming, acidic coiled-coil containing protein 3 | |
| 104149_at | 18035 | 2.6 | 2.1 | 1.9 | 2.2 ± 0.2 | 0.704 | Nfkbia | Nuclear factor of kappa light chain gene enhancer in B-cells inhibitor, alpha | a, b |
| 161135_f_at | 66406 | 2.3 | 1.2 | 2.6 | 2.0 ± 0.3 | 0.43736716 | Sac3d1 | SAC3 domain containing 1 | |
| Apoptosis | |||||||||
| 94186_at | 22029 | 5.0 | 4.4 | 4.6 | 4.7 ± 0.2 | 0.943 | Traf1 | Tnf receptor-associated factor 1 | a, b, c |
| 104712_at | 17869 | 3.7 | 4.0 | 3.0 | 3.6 ± 0.4 | 0.890 | Myc | Myelocytomatosis oncogene | c |
| 99392_at | 21929 | 2.6 | 2.3 | 2.0 | 2.3 ± 0.5 | 0.814 | Tnfaip3 | Tumor necrosis factor, alpha-induced protein 3 | c |
| 161666_f_at | 17873 | 3.8 | 3.4 | 3.6 | 3.6 ± 0.2 | 0.970 | Gadd45b | Growth arrest and DNA-damage-inducible 45 beta | a, b |
| 102779_at | 17873 | 2.7 | 2.4 | 2.7 | 2.6 ± 0.3 | 0.947 | Gadd45b | Growth arrest and DNA-damage-inducible 45 beta | c |
| Fatty acid biosynthesis | |||||||||
| 104647_at | 19225 | 5.6 | 4.7 | 4.4 | 4.9 ± 0.3 | 0.929 | Ptgs2 | Prostaglandin-endoperoxide synthase 2 | a, b, c |
| 94057_g_at | 20249 | 2.2 | 2.4 | 2.2 | 2.3 ± 0.2 | 0.849 | Scd1 | Stearoyl-coenzyme A desaturase 1 | |
| Cell surface receptor linked signal transduction | |||||||||
| 95344_at | 16165 | 3.0 | 3.3 | 3.0 | 3.1 ± 0.3 | 0.941 | Il13ra2 | Interleukin 13 receptor, alpha 2 | c |
| 97733_at | 11541 | 2.8 | 2.6 | 2.5 | 2.7 ± 0.2 | 0.931 | Adora2b | Adenosine A2b receptor | a, b |
| 104498_at | 26556 | 2.4 | 3.2 | 1.3 | 2.3 ± 0.3 | 0.421 | Homer1 | Homer homolog 1 (Drosophila) | b |
| 102663_at | 18793 | 2.7 | 2.2 | 2.7 | 2.5 ± 0.2 | 0.953 | Plaur | Urokinase plasminogen activator receptor | a, b, c |
| Regulation of biological process | |||||||||
| 92232_at | 12702 | 5.6 | 4.4 | 5.2 | 5.1 ± 0.2 | 0.912 | Socs3 | Suppressor of cytokine signaling 3 | a, b, c |
| 162206_f_at | 12702 | 3.9 | 3.1 | 3.4 | 3.5 ± 0.1 | 0.967 | Socs3 | Suppressor of cytokine signaling 3 | a, b |
| 92471_i_at | 20556 | 2.4 | 1.6 | 2.6 | 2.2 ± 0.3 | 0.595 | Slfn2 | Schlafen 2 | |
| Regulation of transcription, DNA-dependent | |||||||||
| 101415_i_at | 81845 | 2.5 | 2.9 | 1.9 | 2.4 ± 0.3 | 0.777 | Bat4 | HLA-B associated transcript 4 | |
| 102709_at | 15260 | 2.4 | 2.5 | 2.7 | 2.5 ± 0.1 | 0.915 | Hira | Histone cell cycle regulation defective homolog A (S. cerevisiae) | a, b |
| 103651_r_at | 68705 | 2.4 | 2.2 | 2.0 | 2.2 ± 0.2 | 0.808 | Gtf2f2 | General transcription factor IIF, polypeptide 2 | |
| 102882_at | 22704 | 2.4 | 2.1 | 2.5 | 2.3 ± 0.3 | 0.579 | Zfp46 | Zinc finger protein 46 | |
| Protein metabolism | |||||||||
| 160829_at | 21664 | 5.1 | 4.7 | 4.5 | 4.8 ± 0.4 | 0.958 | Phlda1 | Pleckstrin homology-like domain, family A, member 1 | a, b |
| 93352_at | 21817 | 2.2 | 1.8 | 2.0 | 2.0 ± 0.5 | 0.530 | Tgm2 | Transglutaminase 2, C polypeptide | |
| 102782_at | 71340 | 3.3 | 3.5 | 2.8 | 3.2 ± 0.2 | 0.926 | Riok1 | RIO kinase 1 | a, b |
| 97548_at | 328110 | 3.1 | 2.7 | 2.9 | 2.9 ± 0.2 | 0.950 | Prpf39 | PRP39 pre-mRNA processing factor 39 homolog (yeast) | |
| Cell growth and/or maintenance | |||||||||
| 104451_at | 18174 | 2.4 | 1.8 | 2.4 | 2.2 ± 0.1 | 0.805 | Slc11a2 | Solute carrier family 11 (proton-coupled divalent metal ion transporters), member 2 (Nramp2) | a, b |
| 94379_at | 16561 | 3.2 | 2.8 | 3.2 | 3.1 ± 0.1 | 0.945 | Kif1b | Kinesin family member 1B | a, b |
| 94384_at | 15937 | 2.8 | 2.6 | 2.2 | 2.5 ± 0.3 | 0.890 | Ier3 | Immediate early response 3 | b, c |
| 160729_f_at | 21884 | 3.3 | 2.1 | 3.4 | 2.9 ± 0.2 | 0.736 | Fabp9 | Fatty acid binding protein 9, testis | a, b |
| 161281_f_at | 15937 | 3.6 | 3.0 | 1.8 | 2.8 ± 0.3 | 0.44599685 | — | Immediate early response 3 | |
| Physiological process | |||||||||
| 160084_at | 546355 | 3.2 | 2.7 | 3.1 | 3.0 ± 0.3 | 0.995 | Odc | Similar to ornithine decarboxylase | b |
| AFFX-GapdhMur/M 32599_5_st | 14433 | 2.9 | 2.3 | 1.7 | 2.3 ± 0.4 | 0.761 | Gapdh | Similar to glyceraldehyde-3-phosphate dehydrogenase | |
| 102749_at | 12865 | 1.9 | 2.4 | 2.5 | 2.3 ± 0.4 | 0.672 | Cox7a1 | Cytochrome c oxidase, subunit VIIa 1 | |
| 94147_at | 18787 | 2.6 | 2.3 | 1.9 | 2.3 ± 0.3 | 0.813 | Serpine1 | Serine (or cysteine) proteinase inhibitor, clade E, member 1 | c |
| 102694_at | 26436 | 2.5 | 1.5 | 3.4 | 2.5 ± 0.2 | 0.498 | Psg16 | Pregnancy specific glycoprotein 16 | a, b |
| Miscellaneous classification | |||||||||
| 96515_at | 14204 | 1.9 | 1.9 | 2.5 | 2.1 ± 0.4 | 0.550 | Il4i1 | Interleukin 4 induced 1 | |
| 98774_at | 16365 | 4.0 | 4.2 | 4.1 | 4.1 ± 0.3 | 0.973 | Irg1 | Immunoresponsive gene 1 | a, b, c |
| 98773_s_at | 16365 | 3.4 | 3.1 | 3.3 | 3.3 ± 0.3 | 0.998 | Irg1 | Immunoresponsive gene 1 | a, b |
| 94971_at | 72391 | 2.7 | 1.9 | 2.6 | 2.4 ± 0.4 | 0.851 | Cdkn3 | Cyclin-dependent kinase inhibitor 3 | |
| 162384_f_at | 12457 | 1.9 | 3.4 | 2.8 | 2.7 ± 0.4 | 0.748 | Ccrn4l | CCR4 carbon catabolite repression 4-like | |
| 94389_at | 66373 | 1.9 | 2.4 | 1.9 | 2.1 ± 0.4 | 0.503 | Lsm5 | LSM5 homolog, U6 small nuclear RNA associated (S. cerevi-siae) | |
| 97714_r_at | 54130 | 2.7 | 1.7 | 3.2 | 2.5 ± 0.3 | 0.753 | Actr1a | ARP1 actin-related protein 1 homolog A (yeast) | b |
| 96162_at | 50764 | 1.6 | 3.4 | 2.3 | 2.5 ± 0.2 | 0.492 | Fbxo15 | F-box only protein 15 | b |
| 93869_s_at | 12044 | 2.3 | 2.2 | 2.3 | 2.3 ± 0.2 | 0.627 | Bcl2a1a | B-cell leukemia/lymphoma 2 related protein A1a | b, c |
| 94505_at | 67245 | 1.9 | 1.8 | 2.2 | 2.0 ± 0.1 | 0.512 | Peli1 | Pellino 1 | a, b |
| 104177_at | 58185 | 2.8 | 1.9 | 3.0 | 2.5 ± 0.3 | 0.827 | Rsad2 | Radical S-adenosyl methionine domain containing 2 | |
| 100669_at | 25465 | 2.1 | 2.0 | 2.5 | 2.2 ± 0.6 | 0.583 | — | Interleukin 17 | |
| 161511_f_at | 53606 | 2.1 | 2.1 | 3.1 | 2.4 ± 0.5 | 0.825 | Isg15 | Interferon stimulated gene, ubiquitin-like modifier | |
| 97693_at | 30865 | 5.6 | 5.0 | 5.4 | 5.3 ± 0.2 | 0.965 | C78513 | EST C78513 | |
| 104477_at | 3.0 | 3.3 | 3.8 | 3.4 ± 0.3 | 0.919 | — | Transcribed locus | ||
| 99849_at | 319202 | 2.7 | 2.0 | 2.7 | 2.5 ± 0.2 | 0.908 | 1200016 E24Rik | RIKEN cDNA 1200016E24 gene | |
Signal log ratio = LOG2 (experimental Brucella spp. infected signal/control uninfected signal) ± SEM.
M, N, O indicate Brucella spp. used to infect macrophages B. melitensis, B. neotomae and B. ovis, respectively.
Identified by additional statistical analyses: a, ANOVA P ≥ 0.01; b, SAM delta = 0.05; c, B. abortus microarrays (14).
Genes Identified with Decreased Transcription Following Brucella spp. Infections
Fifty-eight genes were identified with SLR decreases between −2.0 and −3.4 following infection with any Brucella spp. (Table 3). GO categories for genes with repressed transcript levels represented several biological functions, ranging from small GTPase mediated signal transduction and carboxylic acid metabolism to cell proliferation and lysosomal proteins. Small GTPase mediated signaling may be altered in response to the engulfment and association of Brucella spp. with the membrane of macrophages; included among this group are Rab3d, Gna12, Cfl2 and Iqgap1. Small GTPases are key regulators associated with trafficking of Brucella containing vesicles to the endoplasmic reticulum (25), a crucial step in the establishment of a replication niche. In contrast to the inflammatory response genes commonly increased following infection, there were also genes categorized as response to external stimulus with decreased transcription. Mr1, Abhd8 and IL17a may provide insight into mechanisms that the macrophage does not use in the response against this intracellular bacterium. Also, a decrease in prosaposin transcript levels was observed, a lysosomal enzyme that catabolizes glycosphingolipids that may enhance intracellular survival of Brucella spp.
Table 3.
Genes with Decreased Level of Transcript in Brucella spp. Infected as Compared to Uninfected RAW 264.7 Macrophages
| H1: Mφ ≠ Mφ + B. melitensis = Mφ + B. neotomae = Mφ + B. ovis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Probe set | Entrez gene | SLRa |
Average ± SEM | EBarrays probability | Gene symbol | Gene product | Additional statisticsc | ||
| Mb | Nb | Ob | |||||||
| Small GTPase mediated signal transduction | |||||||||
| 97415_at | 19340 | −2.6 | −2.0 | −3.2 | −2.6 ± 0.3 | 0.866 | Rab3d | RAB3D, member RAS oncogene family | b, c |
| 97227_at | 14673 | −2.2 | −2.5 | −1.9 | −2.2 ± 0.3 | 0.807 | Gna12 | Guanine nucleotide binding protein, alpha 12 | a |
| 97549_at | 12632 | −2.1 | −2.2 | −1.9 | −2.1 ± 0.3 | 0.662 | Cfl2 | Cofilin 2, muscle | |
| 93850_at | 29875 | −2.7 | −2.0 | −1.3 | −2.0 ± 0.4 | 0.494 | Iqgap1 | IQ motif containing GTPase activating protein 1 | |
| Cell proliferation | |||||||||
| 161417_r_at | 18109 | −2.5 | −2.8 | −2.5 | −2.6 ± 0.5 | 0.968 | Mycn | V-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian) | |
| 93943_f_at | 12193 | −2.5 | −2.4 | −2.4 | −2.4 ± 0.5 | 0.925 | Zfp36l2 | Zinc finger protein 36, C3H type-like 2 | |
| 93713_at | 20416 | −2.5 | −2.7 | −2.2 | −2.5 ± 0.3 | 0.914 | Shc1 | Src homology 2 domain-containing transforming protein C1 | |
| 92300_at | 17428 | −2.4 | −2.0 | −2.9 | −2.4 ± 0.3 | 0.889 | Mnt | Max binding protein | c |
| 99076_at | 353187 | −2.2 | −1.7 | −2.8 | −2.3 ± 0.3 | 0.786 | Nr1d2 | Nuclear receptor subfamily 1 group D member 2 | c |
| 101571_g_at | 16010 | −2.0 | −1.7 | −3.0 | −2.3 ± 0.2 | 0.685 | Igfbp4 | Insulin-like growth factor binding protein 4 | |
| 99024_at | 17122 | −2.0 | −1.8 | −2.2 | −2.0 ± 0.3 | 0.557 | Mad4 | Max dimerization protein 4 | |
| 100444_at | 12568 | −2.4 | −1.9 | −1.6 | −2.0 ± 0.4 | 0.520 | Cdk5 | Cyclin-dependent kinase 5 | |
| Carboxylic acid metabolism | |||||||||
| 93320_at | 12894 | −3.4 | −1.9 | −3.1 | −2.8 ± 0.4 | 0.663 | Cpt1a | Carnitine palmitoyltransferase 1a, liver | c |
| 96799_at | 30839 | −2.7 | −2.2 | −2.3 | −2.4 ± 0.3 | 0.897 | Fbxw5 | F-box and WD-40 domain protein 5 | |
| 96126_at | 20397 | −2.3 | −2.4 | −2.0 | −2.2 ± 0.2 | 0.704 | Sgpl1 | Sphingosine phosphate lyase 1 | |
| 94405_at | 21366 | −2.7 | −1.5 | −1.7 | −2.0 ± 0.2 | 0.444 | Slc6a6 | Solute carrier family 6 (neurotransmitter transporter, taurine), member 6 | |
| Response to external stimulus | |||||||||
| 101433_at | 15064 | −2.4 | −2.4 | −2.4 | −2.4 ± 0.3 | 0.918 | Mr1 | Major histocompatibility complex, class I-related | |
| 104228_at | 668701 | −2.2 | −2.2 | −2.6 | −2.3 ± 0.4 | 0.865 | EG668701 | Similar to Rap guanine nucleotide exchange factor 2 | |
| 103250_at | 54722 | −2.7 | −2.1 | −2.0 | −2.3 ± 0.2 | 0.838 | Dfna5h | Deafness, autosomal dominant 5 homolog | |
| 104372_at | 64296 | −2.6 | −2.2 | −1.9 | −2.2 ± 0.2 | 0.793 | Abhd8 | Abhydrolase domain containing 8 | |
| 99349_at | 16171 | −1.7 | −2.5 | −2.1 | −2.1 ± 0.3 | 0.681 | Il17a | Interleukin 17A | |
| 93218_at | 20947 | −2.3 | −2.0 | −1.7 | −2.0 ± 0.4 | 0.573 | Swap70 | SWAP complex protein | |
| Protein modification | |||||||||
| 99643_f_at | 12876 | −3.7 | −3.8 | −2.7 | −3.4 ± 0.4 | 0.949 | Cpe | Carboxypeptidase E | b |
| 161848_r_at | 19260 | −2.5 | −2.2 | −2.4 | −2.4 ± 0.4 | 0.870 | Ptpn22 | Protein tyrosine phosphatase, non-receptor type 22 (lymphoid) | |
| 92427_at | 21812 | −2.4 | −1.9 | −2.3 | −2.2 ± 0.4 | 0.729 | Tgfbr1 | Transforming growth factor, beta receptor I | |
| 99642_i_at | 12876 | −3.0 | −2.1 | −1.3 | −2.2 ± 0.7 | 0.618 | Cpe | Carboxypeptidase E | |
| 100427_at | 19277 | −2.0 | −1.4 | −2.7 | −2.0 ± 0.3 | 0.552 | Ptpro | Protein tyrosine phosphatase, receptor type, O | b, c |
| Transcription from Pol II promoter | |||||||||
| 104591_g_at | 17260 | −2.4 | −2.9 | −2.5 | −2.6 ± 0.2 | 0.932 | Mef2c | Myocyte enhancer factor 2C | b, c |
| 104590_at | 17260 | −2.8 | −2.0 | −2.2 | −2.3 ± 0.2 | 0.821 | Mef2c | Myocyte enhancer factor 2C | a, b |
| 96171_at | 54006 | −2.1 | −1.6 | −2.4 | −2.0 ± 0.2 | 0.580 | Deaf1 | Deformed epidermal autoregulatory factor 1 (Drosophila) | b |
| Cytoskeleton organization and biogenesis | |||||||||
| 95705_s_at | 11461 | −2.2 | −3.1 | −2.4 | −2.6 ± 0.5 | 0.887 | Actb | Actin, beta, cytoplasmic | |
| 161981_r_at | 14246 | −2.6 | −1.6 | −2.4 | −2.2 ± 0.4 | 0.693 | Flg | Filaggrin | |
| Metabolism | |||||||||
| 94872_at | 57319 | −2.8 | −2.5 | −2.8 | −2.7 ± 0.4 | 0.934 | Smpdl3a | Sphingomyelin phosphodiesterase, acid-like 3A | |
| 161733_at | 59010 | −2.4 | −2.9 | −2.1 | −2.5 ± 0.3 | 0.919 | Sqrdl | Sulfide quinone reductase-like | |
| 101972_at | 16541 | −2.8 | −2.4 | −2.5 | −2.6 ± 0.3 | 0.915 | Napsa | Napsin A aspartic peptidase | |
| 99667_at | 12862 | −2.3 | −2.2 | −2.5 | −2.3 ± 0.5 | 0.878 | Cox6a2 | Cytochrome c oxidase, subunit VI a, polypeptide 2 | |
| 103538_at | 21386 | −1.8 | −2.1 | −2.5 | −2.1 ± 0.3 | 0.680 | Tbx3 | T-box 3 | |
| 96035_at | 12039 | −1.7 | −2.0 | −2.4 | −2.0 ± 0.4 | 0.606 | Bckdha | Branched chain ketoacid dehydrogenase E1, alpha polypeptide | |
| 97560_at | 19156 | −1.4 | −3.0 | −1.7 | −2.0 ± 0.4 | 0.381 | Psap | Prosaposin | |
| Cell communication | |||||||||
| 160932_at | 17973 | −2.6 | −2.6 | −2.6 | −2.6 ± 0.1 | 0.964 | Nck1 | Non-catalytic region of tyrosine kinase adaptor protein 1 | a, b |
| 97768_at | 13506 | −2.2 | −2.1 | −2.6 | −2.3 ± 0.3 | 0.803 | Dsc2 | Desmocollin 2 | |
| Cell growth and/or maintenance | |||||||||
| 92695_at | 14296 | −2.0 | −1.6 | −2.7 | −2.1 ± 0.3 | 0.658 | Frat1 | Frequently rearranged in advanced T-cell lymphomas | |
| 103534_at | 15130 | −2.8 | −2.0 | −1.5 | −2.1 ± 0.2 | 0.626 | Hbb-b2 | Hemoglobin, beta adult minor chain | b |
| 93736_at | 21452 | −2.0 | −2.0 | −2.3 | −2.1 ± 0.3 | 0.606 | Tcn2 | Transcobalamin 2 | |
| Miscellaneous classification | |||||||||
| 96494_at | 75785 | −2.9 | −2.7 | −2.5 | −2.7 ± 0.2 | 0.959 | Klhl24 | Kelch-like 24 (Drosophila) | |
| 103933_at | 22619 | −2.9 | −1.9 | −2.8 | −2.5 ± 0.2 | 0.895 | Siae | Sialic acid acetylesterase | b |
| 104299_at | 224454 | −3.0 | −1.9 | −2.5 | −2.5 ± 0.3 | 0.852 | Zdhhc14 | Zinc finger, DHHC domain containing 14 | |
| 94299_at | 69654 | −2.5 | −2.0 | −2.2 | −2.2 ± 0.2 | 0.788 | Dctn2 | Dynactin 2 | |
| 162116_r_at | 116891 | −3.0 | −2.7 | −1.7 | −2.5 ± 0.9 | 0.741 | Derl2 | Der1-like domain family, member 2 | |
| 160934_s_at | 73094 | −3.0 | −2.6 | −1.6 | −2.4 ± 0.5 | 0.692 | Sgip1 | SH3-domain GRB2-like (endophilin) interacting protein 1 | |
| 104714_at | 105445 | −2.2 | −1.7 | −2.2 | −2.0 ± 0.2 | 0.611 | Dock9 | Dedicator of cytokinesis 9 | |
| 162075_r_at | 17476 | −2.7 | −1.5 | −3.8 | −2.7 ± 0.4 | 0.551 | Mpeg1 | Macrophage expressed gene 1 | |
| 96464_at | 140570 | −2.1 | −2.2 | −1.9 | −2.1 ± 0.2 | 0.518 | Plxnb2 | Plexin B2 | |
| 160905_s_at | 80515 | −2.2 | −2.2 | −2.1 | −2.1 ± 0.3 | 0.735 | A030009 H04Rik | RIKEN cDNA A030009H04 gene | |
| 97119_at | 99029 | −2.6 | −2.5 | −2.2 | −2.4 ± 0.3 | 0.911 | AI596198 | Expressed sequence AI596198 | |
| 97752_at | 99358 | −2.0 | −2.1 | −2.8 | −2.3 ± 0.3 | 0.858 | E13001 3N09Rik | RIKEN cDNA E130013N09 gene | |
| 103748_at | 74440 | −2.9 | −1.6 | −2.1 | −2.2 ± 0.2 | 0.645 | 4933407 C03Rik | RIKEN cDNA 4933407C03 gene | |
| 94069_r_at | 67163 | −3.4 | −2.3 | −2.2 | −2.6 ± 0.3 | 0.946 | 2610204 L23Rik | RIKEN cDNA 2610204L23 gene | |
Signal log ratio = LOG2 (experimental Brucella spp. infected signal/control uninfected signal) ± SEM.
M, N, O indicate Brucella spp. used to infect macrophages B. melitensis, B. neotomae and B. ovis, respectively.
Identified by additional statistical analyses: a, ANOVA P ≥ 0.01; b, SAM delta = 0.05; c, B. abortus microarrays (14).
Genes Identified with Altered Transcription Among Brucella spp. Infections
Thirty-three genes (Table 4) were identified under hypotheses that transcription among Brucella spp. infections was different from each other and uninfected macrophages (H2–4). Of the 33 genes with altered expression among Brucella spp. infections, 21 genes had decreased transcript levels, 10 genes had increased transcript levels and, for two genes, the direction of change in expression was dependent on the species used for infection. Generally, the direction of change in transcription was uniform across the species tested. Twelve genes were altered based on the virulence descriptions of Brucella spp. (Table 4, part A), as B. melitensis was compared to B. neotomae and B. ovis infections (H2). GO biological and molecular function categorization unveiled genes altered according to H2 are involved in cell growth and maintenance, endopeptidase inhibitor activity, response to external stimuli and G-protein mediated signaling. When the infections were grouped according to LPS phenotype of the Brucella, i.e., smooth B. melitensis and B. neotomae versus rough B. ovis, eleven genes were identified with altered transcription (H3) (Table 4, part B). Although smooth Brucella enter macrophages via lipid rafts (26), the mechanism of B. ovis entry is unknown. If different entry mechanisms occur for B. ovis, a distinct group of host genes may be altered compared to smooth Brucella spp. However, few macrophage genes were altered between rough and smooth Brucella spp. infections, and there was little GO commonality. Ten genes had altered transcript levels when infection with B. melitensis and B. ovis were considered similar and distinct from B. neotomae infection (H4) (Table 4, part C). All three Brucella spp. infections increased interleukin 1 alpha gene expression, but levels were higher in B. melitensis and B. ovis infections. Genes identified according to this pattern (H4) were categorized in GO biological functions of cell growth and maintenance and signal transduction. Lastly, no genes were identified with each of the four conditions, uninfected and three Brucella spp. infections, having distinct transcription (H5).
Table 4A.
Differences in Macrophage Transcript Expression when Infected by Different Brucella Species
| A. B. ovis and B. neotomae Infected Macrophages Express Transcripts Different from B. melitensis or Uninfected Macrophages | ||||||||
|---|---|---|---|---|---|---|---|---|
| H2: Mφ ≠ Mφ + B. melitensis ≠ Mφ + B. neotomae= Mφ + B. ovis | ||||||||
| Probe set | Entrez gene | SLRa |
EBarrays probability | Gene symbol | Gene product | Additional statisticsb | ||
| B. melitensis | B. neotomae | B. ovis | ||||||
| 94337_at | 14453 | −4.2 ± 0.7 | −1.1 ± 0.4 | −1.3 ± 0.5 | 1.000 | Gas2 | Growth arrest specific 2 | |
| 162240_r_at | 13010 | 0.8 ± 0.3 | 3.4 ± 0.2 | 3.9 ± 0.1 | 0.999 | Cst3 | Cystatin C | a, b |
| 101793_at | 14129 | −3.1 ± 0.1 | −1.4 ± 0.3 | −0.5 ± 0.3 | 0.903 | Fcgr1 | Fc receptor, IgG, high affinity I (FcγRI) | a, b, c |
| 102794_at | 12767 | −3.6 ± 0.5 | −1.4 ± 0.7 | −1.7 ± 0.3 | 0.898 | Cxcr4 | Chemokine (C-X-C motif) receptor 4 | c |
| 101070_at | 54484 | −3.6 ± 0.5 | −1.6 ± 0.6 | −1.7 ± 0.3 | 0.885 | Mkrn1 | Makorin, ring finger protein, 1 | |
| 161878_r_at | 52250 | −2.2 ± 0.8 | 0.5 ± 0.1 | 0.3 ± 0.2 | 0.852 | Reep1 | Receptor accessory protein 1 | |
| 93569_f_at | 545005 | 0.6 ± 0.3 | 2.6 ± 0.4 | 3.3 ± 0.7 | 0.829 | LOC544988 | Similar to Spetex-2F protein | |
| 99377_at | 18249 | 2.7 ± 0.6 | 0.5 ± 0.9 | −0.6 ± 0.6 | 0.781 | Obp1a | Odorant binding protein Ia | |
| 94240_i_at | 19944 | 1.7 ± 0.9 | −0.8 ± 1.3 | −0.8 ± 1.0 | 0.734 | Rpl29 | Ribosomal protein L29 | |
| 104697_at | 80837 | −3.0 ± 0.7 | −1.2 ± 0.9 | −0.8 ± 0.4 | 0.622 | Rhoj | ras homolog gene family, member J | |
| 92857_at | 19934 | −3.3 ± 0.7 | −1.2 ± 0.7 | −2.0 ± 0.9 | 0.571 | Rpl22 | Ribosomal protein L22 | |
| 102706_i_at | 16625 | −2.7 ± 0.9 | −0.6 ± 0.4 | −0.1 ± 0.3 | 0.566 | Serpina3c | Serine (or cysteine) proteinase inhibitor, clade A, member 3C | |
Table 4B.
| B. B. melitensis and B. neotomae Infected Macrophages Express Transcripts Different from B. ovis or Uninfected Macrophages | |||||||
|---|---|---|---|---|---|---|---|
| H3: Mφ ≠ Mφ + B. melitensis = Mφ + B. neotomae ≠ Mφ + B. ovis | |||||||
| Probe set | Entrez gene | SLRa |
EBarrays probability | Gene symbol | Gene product | ||
| B. melitensis | B. neotomae | B. ovis | |||||
| 161420_r_at | 13478 | −0.3 ± 0.7 | −0.2 ± 1.3 | −3.2 ± 0.5 | 0.993 | Dpagt1 | Dolichyl-phosphate acetylgluco-saminephosphotransferase 1 (GlcNAc-1-P transferase) |
| 160797_r_at | 12412 | −2.1 ± 0.4 | −2.0 ± 0.4 | 0.6 ± 0.2 | 0.975 | Cbx1 | Chromobox homolog 1 (Drosophila HP1 beta) |
| 92600_f_at | 13117 | −2.4 ± 0.6 | −2.4 ± 0.7 | 0.0 ± 0.1 | 0.953 | Cyp4a10 | Cytochrome P450, family 4, subfamily a, polypeptide 10 |
| 161128_r_at | 544834 | 0.5 ± 1.3 | 1.8 ± 0.9 | 3.7 ± 0.8 | 0.9361 | 6030426L16Rik | Similar to Hippocalcin-like protein 1, Visinin-like protein 3 |
| 99885_at | 16019 | 0.6 ± 0.9 | 1.0 ± 0.3 | 3.1 ± 0.6 | 0.738 | Igh-6 | Immunoglobulin heavy chain 6 (heavy chain of IgM) |
| 93566_at | 74246 | −1.5 ± 0.2 | −2.6 ± 0.6 | 0.1 ± 0.3 | 0.711 | Gale | Galactose-4-epimerase, UDP |
| 162076_r_at | 14800 | −2.2 ± 0.7 | −3.0 ± 0.5 | −0.5 ± 0.4 | 0.681 | Gria2 | Glutamate receptor, ionotropic, AMPA2 (alpha 2) |
| 160728_r_at | 73945 | −2.1 ± 1.1 | −0.4 ± 0.6 | −4.4 ± 0.6 | 0.6579 | Otud4 | OTU domain containing 4 |
| AFFX-BioDn-3_st | 1037747 | −1.6 ± 0.3 | −0.4 ± 0.1 | 1.4 ± 0.1 | 0.6294 | — | Biotin synthesis protein bioC |
| 161131_r_at | 228889 | −0.6 ± 0.4 | −0.7 ± 0.1 | −2.7 ± 0.1 | 0.5167 | — | Dead (asp-glu-ala-asp) box polypeptide 27 |
| 102299_at | 18750 | −1.8 ± 0.7 | −1.2 ± 0.5 | −3.3 ± 0.4 | 0.508 | Prkca | Protein kinase C, alpha |
Table 4C.
| C. B. melitensis and B. ovis Infected Macrophages Express Transcripts Different from B. neotomae or Uninfected Macrophages | ||||||||
|---|---|---|---|---|---|---|---|---|
| H4: Mφ ≠ Mφ + B. melitensis = Mφ + B. ovis ≠ Mφ + B. neotomae | ||||||||
| Probe set | Entrez gene | SLRa |
EBarrays probability | Gene symbol | Gene product | Additional statisticsb | ||
| B. melitensis | B. neotomae | B. ovis | ||||||
| 94051_at | 13612 | 3.0 ± 0.4 | 0.5 ± 0.4 | 3.2 ± 0.3 | 0.998 | Edil3 | EGF-like repeats and discordin I-like domains 3 | a, b |
| 96785_at | 80880 | −3.2 ± 0.6 | −0.5 ± 1.1 | −2.7 ± 0.7 | 0.994 | Ankrd47 | Ankyrin repeat domain 47 | |
| 92913_at | 26874 | −4.1 ± 0.3 | −2.0 ± 0.4 | −3.9 ± 0.3 | 0.920 | Abcd2 | ATP-binding cassette, sub-family D (ALD), member 2 | a, b |
| 103467_g_at | 54151 | −2.3 ± 0.3 | −0.6 ± 0.3 | −2.9 ± 0.3 | 0.897 | Cyhr1 | Cysteine and histidine rich 1 | |
| 94755_at | 16175 | 3.9 ± 0.4 | 1.7 ± 0.7 | 3.1 ± 0.4 | 0.693 | Il1a | Interleukin 1 alpha | b, c |
| 93568_i_at | 1.4 ± 0.0 | −1.1 ± 0.7 | 1.2 ± 0.5 | 0.556 | LOC544988 | Similar to Spetex-2F protein | ||
| 92504_at | 15574 | 2.1 ± 0.6 | −0.1 ± 0.4 | 1.8 ± 0.7 | 0.501 | Hus1 | Hus1 homolog (S. pombe) | |
| 104592_i_at | 17260 | −3.7 ± 0.2 | −1.9 ± 0.3 | −3.3 ± 0.2 | 0.473 | Mef2c | Myocyte enhancer factor 2C | a, b |
| 102798_at | 11535 | 0.9 ± 0.6 | 2.4 ± 0.6 | −0.3 ± 0.7 | 0.444 | Adm | Adrenomedullin | |
| 102374_at | 53902 | −2.9 ± 0.3 | −1.0 ± 0.4 | −2.2 ± 0.5 | 0.425 | Dscr1l2 | Down syndrome critical region gene 1-like 2 | a, b |
Signal log ratio = LOG2 (experimental Brucella spp. infected signal/control uninfected signal) ± SEM.
Identified by additional statistical analyses: a, ANOVA P ≥ 0.01; b, = SAM delta = 0.05; c, B. abortus microarrays (14).
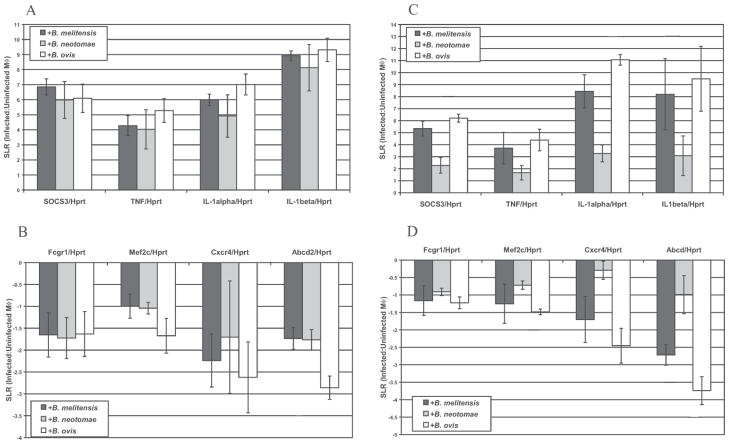
Confirmation of Microarray Data by Quantitative Real Time-PCR (qRT-PCR)
To verify the micro-array changes in transcript levels, eight genes were analyzed by qRT-PCR using the infection conditions as for microarray analysis. Housekeeping genes, Hprt and Tbp, identified via this microarray analysis and previously tested (23, 27, 28) were used to normalize cDNA levels and resulted in similar patterns and levels of gene transcription (data not shown). qRT-PCR confirmed that the eight genes tested (Socs3, Tnf, IL-1α, IL-1β, FcγRI, Mef2c, Cxcr4 and Abcd2) had altered expression in RAW 264.7 macrophages following Brucella spp. infection in the same direction (increased or decreased) indicated by microarray analysis (Fig. 2, panels A and B). Further, J774A.1 and RAW 264.7 macrophage cell lines were used in parallel to verify that qRT-PCR results found in RAW 264.7 were applicable to J774A.1 cells. RAW 264.7 gene transcription altered among Brucella spp. infections was similarly changed (increased or decreased) during J774A.1 infection (Fig. 2, panels C and D) with the direction of altered transcription paralleling microarray results.
Figure 2.
Transcript level changes in select gens following Brucella spp. infection of RAW 264.7 (Panel A) or J774A.1 (Panel B) macrophages. RT-PCR results confirm genes with increased A or C or decreased B or D transcript levels following infection of RAW 264.7 or J774A.1 cells, respectively. Each RNA sample was normalized using Hprt transcript levels. Signal log ratios (SLR), a comparison of each infected sample to uninfected control RAW 264.7 samples, were averaged from four independent RNA isolations and error bars represent the SEM.
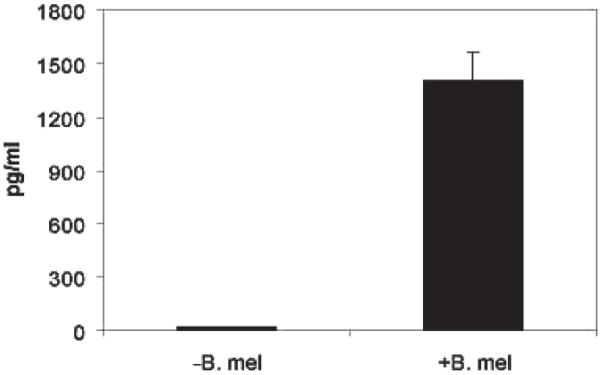
TNFα Production by Macrophages
To confirm the microarray change in TNFα transcription, bone marrow derived macrophages were infected with B. melitensis and supernatant analyzed by ELISA. Figure 3 illustrates that infected macrophages produced greater than 15-fold more TNFα than non-infected macrophages. These results together with the microarray findings suggest that B. melitensis activates TNFα production by macrophages.
Figure 3.
Induction of TNFα by the B. melitensis in BMDMs from C57BL/6 mice. Cytokine levels were assayed from supernatants of B. melitensis 16M infected or medium alone bone-marrow derived macrophages (BMDMs). BMDMs were cultured 5–7 days pre-infection. Cytokines were measured in pg/mL by ELISA at 12 hr post-infection. Data are from 7 mice/experiment.
Discussion
Murine macrophages infected with Brucella strains were profiled by microarray analysis. Interestingly, B. melitensis causes severe human infection, but neither B. ovis nor B. neotomae cause human pathogenesis. However, in vitro growth of B. melitensis and B. neotomae is similar in murine macrophages in contrast to in vivo human infection where B. neotomae is unable to initiate or maintain infection (29, 30). The IRF-1−/− mouse model can distinguish degrees of Brucella virulence (5), where B. melitensis and B. neotomae induce death by 9–12 days (6), while B. ovis does not (E. Petersen, Personal Communication). The differing responses between rough (B. ovis) and smooth (B. melitensis and B. neotomae) Brucella in mice led us to explore transcription profiles of murine macrophages following infection by these three Brucella strains. A comparison of host response among infections by differing Brucella spp. may identify common responses to infection, as well as distinguish genes and pathways specific to each Brucella spp. infection. Identifying alterations in the macrophage transcriptome may provide greater understanding of host mechanisms involved in pathogen killing and bacterial regulation.
Common Macrophage Transcriptional Response by B. melitensis, B. neotomae and B. ovis Infections
At 4 hr, low numbers of intracellular smooth B. melitensis and B. neotomae were observed, as reported by others, prior to Brucella reaching its replicative niche (22, 31, 32). However, RAW 264.7 macrophage uptake of rough B. ovis after 4 hr of infection was almost two logarithmic units higher than smooth B. melitensis or B. neotomae, perhaps from non-lipid raft mediated phagocytosis, although this is untested for rough B. ovis (22, 31, 32). Yet, microarray analysis of rough B. ovis-infected macrophages was qualitatively similar to those from smooth B. melitensis and B. neotomae infections. This similarity suggests that macrophages sense and respond to different Brucella species by a common profile of transcriptional changes.
The majority of these common response genes were inflammatory related suggesting similar activating and/or regulating effects of Brucella on the macrophage regardless of the Brucella species. Host response to Brucella spp. encompassed pro-inflammation independent of infection differences between rough and smooth Brucella spp. In comparing the common macrophage transcription profile to differing Brucella spp., we hypothesized that a large number of host genes with overlapping gene ontology function would be identified similar to that observed in wild-type and mutant Salmonella (33). Others have reported that LPS and CpG DNA can activate TLR signaling activating NFκB and AP-1 pathways inducing innate immune mechanisms (34). Adaptive immune mechanisms may be activated by Schlafen 2 (Slfn2) expression that regulates T cell activity as well as innate immunity (34). Pro-inflammatory gene expression may reflect a host protective response as many genes were chemotaxis related, potentially recruiting additional macrophages and immune cells to the site of Brucella spp. infected macrophages.
Genes that could serve to subdue or counteract inflammatory response genes, however, were also observed as upregulated in macrophages. For example, increased expression of Socs3 was observed. Socs3 participates in a negative feedback loop of cytokine signaling limiting the extent of cytokine in innate and adaptive immune responses (35). Also, IL-13R and Gadd45b were increased in transcription suggesting regulation of activated macrophages. Transcription of Bcl2, Bat4 and Phlda1 that participate in blocking apoptotic mechanisms was upregulated. In agreement with this finding, smooth Brucella spp. can protect against apoptosis (36), while engineered rough Brucella spp. can induce apoptosis (37). Consequently, the upregulation of genes that block apoptotic mechanisms may oppose upregulated inflammatory genes to camouflage immune recognition and produce a protective environment for Brucella replication. Also, decreased transcript levels of Psap (lysosomal catabolic enzyme), Sgip3 (clathrin mediated endocytosis) and Mpeg1 (a perforin like protein) would suggest that Brucella induces evasion strategies to protect its intracellular niche. Others have suggested evasion strategies such as Brucella altering host cell responses by shielding PAMPs from Toll-like receptors (38) and LPS masking of MHC molecules (39).
Of the macrophage genes commonly upregulated by all Brucella species tested, first, eicosanoids are important in mammalian repair (40). In macrophages infected with Brucella we observed induction of eiconosoid Ptgs2, also termed Cox2, encoding the cyclooxygenase 2 enzyme. Eicosanoids are considered important in antigen presentation and in macrophage effector functions, and play an important role in contributing to inflammation (40).
A second common inflammatory signal elicited by Brucella infection of macrophages is Gadd45. Gadd45A is an 18 kDa acidic nuclear protein involved in maintenance of genomic stability, DNA repair, cell cycle checkpoints and suppression of cell growth. Gadd45 is also a known stress response gene with the ability to regulate MAP-kinase signaling (41). Regulation of MAP-kinase signaling by Gadd45 can affect response to extracellular stimuli and regulate gene expression, mitosis, differentiation and cell survival/apoptosis. GADD45A may therefore be expressed as a stress response protein to inhibit macrophage growth during Brucella infection.
A third common inflammatory signal elicited by Brucella infection is an odorant binding protein. Odorant binding proteins (OBPs) belong to the lipocalin family of proteins involved in extracellular transport of hydrophobic ligands. OBPs were originally identified in nasal mucus and mucosa of insects and were proposed as molecular shuttles between the air mucus interface and the olfactory receptor binding sites. Their function in mammals is unknown, but OBPs can bind odorants of diverse chemical structures with a higher affinity for aldehydes and large fatty acids (42). In macrophages, OBPs may serve as chaperones for signaling molecules.
Macrophage Transcriptome Response to B. melitensis Differ from B. neotomae and B. ovis
Macrophages infected with B. melitensis elicited a specific response with altered transcription in twelve genes compared to B. neotomae and B. ovis (H2) (Table 4, part A). These genes provide insight into macrophage mechanisms used by the various Brucella species to establish a replicative niche. For example, two genes with established function during inflammation, FcγRI (43) and Cxcr4 (44), have decreased expression in B. melitensis infected macrophages compared to B. neotomae and B. ovis infections. FcγRI (CD64) may be involved in regulating antigen presentation by limiting additional phagocytosis and committing the cell to present antigens of engulfed pathogens (45). Decreased levels of FcγRI may be advantageous for bacteria by reducing downstream signaling initiation, including antibody dependent cell mediated cytotoxicity (ADCC) (46), production of reactive oxygen and nitrogen species (47, 48), and increased phagocytosis and cytokine secretion (49). Downregulation of Cxcr4 transcription in combination with Socs3 upregulation may reduce macrophage chemotaxis, adhesion and other inflammatory responses (50, 51). Suppressing several immune pathways initiated at the cell membrane, virulent Brucella may secure an intracellular replicative niche.
Three other genes with decreased expression in macrophages infected with B. melitensis compared to B. neotomae and B. ovis (H2 hypothesis) are endopeptidase inhibitors, cystatin C and serpina3c and the actin associated protein encoded by Gas2. These natural peptidase inhibitors may influence cell migration, chemotaxis, proliferation, phagocytosis and respiratory burst (52–55). Interestingly, the treatment of mice harboring Leishmania with cystatin and a sub-optimal dose of IFN-γ led to parasite clearance and shifted the non-productive Th2 (type 2, humoral) response towards a Th1 response (56). Nitric oxide (NO) production (57) and phagosomal processing (55) are mechanisms where these endopeptidase inhibitors act and modulate intracellular infection. These same mechanisms are vital to progression of Brucella spp. infection (58–60) and exogenous modulation may provide the immunologic boost necessary for pathogen clearance. Brucella spp. may specifically alter the protease-inhibitor balance by bacterial production of extracellular proteases and cleavage of host endopeptidase inhibitors, similar to how Staphylococcus aureus functions in accessing nutrients and facilitating dissemination (61). Lastly, Gas2 was specifically down-regulated in B. melitensis. GAS2-like protein 1 is an actin-associated protein expressed at high levels in growth-arrested macrophages (62). Inhibition of Gas2 transcription may increase macrophage survival and growth, an advantage for Brucella spp. survival within an intracellular niche.
Eleven genes were altered in expression (Table 4, part B) by B. melitensis and B. neotomae but not by B. ovis (H3). These genes could not be grouped according to function, but did categorize the Brucella spp. according to LPS type, rough or smooth. While Brucella spp. uptake based on smooth or rough phenotype did not affect transcript levels of many inflammatory-related genes in our study (H1), LPS may subtly affect transcription when comparing B. melitensis and B. neotomae to B. ovis. Microarray analyses of macrophages responding to various bacteria indicate that an early response to infection parallels the response to LPS alone and is conserved across bacterial genera (63–65). For example, macrophage response to Salmonella versus purified Salmonella LPS is similar (64). However, Brucella LPS induces a 100-fold less stimulation of macrophages than enterobacterial LPS (2, 66), and may have less effect on macrophage transcription.
Cytochrome P450 4a10 (Cyp4a10) and protein kinase C-α (Prkca) may provide insight into intracellular pathways that differ between Brucella species (H3) (Table 4, part B). A wide variety of bacterial and parasitic infections depress expression of cytochrome P450 (67), a gene important in detoxification (68). Cyp4a10 can enhance oxidative stress, through production of reactive oxygen species and reactive nitrogen species (69); therefore, decreased transcription induced by bacteria with smooth LPS could protect intracellular Brucella from innate killing mechanisms. Another gene with oxidative related functions, Prkca, has decreased transcript levels in the infected macrophage. Prkca is important in regulating phagosome-lysosome fusion (70), intracellular vesicle regulation (71) and generation of reactive oxygen species (72), events that may be regulated differently by diverse Brucella spp. Constitutive knock down of Pkrca during intracellular infection with Legionella, Leishmania and Salmonella (48, 73) leads to increased pathogen survival, likely through suppression of host respiratory burst. Downregulation of Cyp4a10 and Prkca pathways may prevent additional oxidative stress in the macrophage during smooth Brucella spp. infection, while Brucella spp. production of Cu, Zn superoxide dismutase (SodC) protects Brucella from oxidative damage (74). These results suggest that down-regulation of Cyp4a10 and Pkrca may shift the macrophage toward a less hostile intracellular environment for Brucella.
Finally altered transcription was identified when B. melitensis and B. ovis were considered dissimilar from B. neotomae (H4). Nine of ten genes had decreased transcript levels in B. neotomae infection compared to the other Brucella spp., the most important examples being IL-1a, Abcd2 and mef2c. B. neotomae may moderate the IL-1α transcription earlier or through alternative stimulation. Abcd2 knock down has been correlated with oxidative stress or tissue damage (75) and abnormalities in mitochondria, Golgi and endoplasmic reticulum of host cells (76). The importance of Abcd transporters is reinforced by decreased transcript levels of other Abcd family members during Brucella infection (9, 77). Lastly, the alteration of mef2c expression suggests that B. neotomae infection may render RAW 264.7 macrophages more susceptible to activation-induced cell death (78) than B. melitensis or B. ovis infections. When compared to B. melitensis and B. ovis, B. neotomae infected macrophages appeared to have decreased immuno-stimulatory capability resulting in reduced oxidative stress and increased susceptibility to cell death.
Brucella infection of RAW 264.7 and J774A.1 macrophage cell lines resulted in similar results. While RAW 264.7 and J774A.1 cells are macrophage-like in lysozyme secretion (79), phagocytosis of particles, response to LPS stimulus (80) and activation state (81), differences do exist in the abilities of these cells to mediate cytolytic activity (81), synthesize nitrite and nitrate (80), and potentially other macrophage trafficking and defense mechanisms. Our microarray results were supported by similar transcription profiles between these Brucella infected macrophage cell lines. However, internalization of smooth Brucella by phagocytic macrophages occurs faster than by nonphagocytic cells such as trophoblasts (82) and may contribute to differences in microarray results between phagocytic and nonphagocytic cells. Ultimately, individual genes of interest must be further investigated for influence on Brucella spp. infections. In conclusion, the present study evaluated the macrophage transcriptome altered during B. melitensis, B. neotomae and B. ovis infections. Common changes in gene expression were observed among Brucella spp. infected macrophages suggesting similar innate immune mechanisms by macrophages when responding to different Brucella species. These changes were related to increased chemokine, alteration in cytokine signals and defense responses. Also, these three Brucella spp. induced decreased transcription of select genes, suggesting that repressed transcription may result from pathogen-specific manipulations. Further, a subset of macrophage genes was differentially altered based on a particular Brucella species that may reflect the differing intracellular survival abilities of each Brucella species. Microarray analysis of host transcription provides a foundation to understand variations in Brucella spp. infection of mice. Future studies will reveal variations in the intracellular survival strategies used by different Brucella spp. by evaluating the contribution of individual host genes during infection.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge membership within and support from the Region V Great Lakes RCE (NIH award 1-U54-AI-057153), R21 AI070229, and BARD US-3829–06.
The authors would like to thank S. Splinter and W. Nelson at the Genome Center of Wisconsin for GeneChip® hybridization and scanning, C. Kendziorski for assistance with EBarrays statistical analysis and E. Petersen for personal communications.
References
- 1.Woods JB, editor. USAMRIID’s Medical Management of Biological Casualties Handbook. 6. Fort Detrick, MD: U.S. Army Medical Research Institute of Infectious Diseases; 2005. [Google Scholar]
- 2.Baldwin CL, Goenka R. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit Rev Immunol. 2006;26:407–442. doi: 10.1615/critrevimmunol.v26.i5.30. [DOI] [PubMed] [Google Scholar]
- 3.Halling SM, Peterson-Burch BD, Bricker BJ, Zuerner RL, Qing Z, Li LL, Kapur V, Alt DP, Olsen SC. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J Bacteriol. 2005;187:2715–2726. doi: 10.1128/JB.187.8.2715-2726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajashekara G, Glasner JD, Glover DA, Splitter GA. Comparative whole-genome hybridization reveals genomic islands in Brucella species. J Bacteriol. 2004;186:5040–5051. doi: 10.1128/JB.186.15.5040-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko J, Gendron-Fitzpatrick A, Ficht TA, Splitter GA. Virulence criteria for Brucella abortus strains as determined by interferon regulatory factor 1-deficient mice. Infect Immun. 2002;70:7004–7012. doi: 10.1128/IAI.70.12.7004-7012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek SH, Rajashekara G, Splitter GA, Shapleigh JP. Denitrification genes regulate Brucella virulence in mice. J Bacteriol. 2004;186:6025–6031. doi: 10.1128/JB.186.18.6025-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskra L, Mathison A, Splitter G. Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect Immun. 2003;71:1125–1133. doi: 10.1128/IAI.71.3.1125-1133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Reichow S, Ramamoorthy S, Ding X, Lathigra R, Craig JC, Sobral BW, Schurig GG, Sriranganathan N, Boyle SM. Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infect Immun. 2006;74:5035–5046. doi: 10.1128/IAI.01998-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caron E, Liautard JP, Kohler S. Differentiated U937 cells exhibit increased bactericidal activity upon LPS activation and discriminate between virulent and avirulent Listeria and Brucella species. J Leukoc Biol. 1994;56:174–181. doi: 10.1002/jlb.56.2.174. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Baldwin CL. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rittig MG, Alvarez-Martinez MT, Porte F, Liautard JP, Rouot B. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect Immun. 2001;69:3995–4006. doi: 10.1128/IAI.69.6.3995-4006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskra L, Canavessi A, Carey M, Splitter G. Brucella abortus genes identified following constitutive growth and macrophage infection. Infect Immun. 2001;69:7736–7742. doi: 10.1128/IAI.69.12.7736-7742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton MA, Kendziorski CM. Parametric empirical Bayes methods for microarrays. In: Parmigiani G, Garrett ES, Irizarray RA, Zeger SL, editors. The Analysis of Gene Expression Data: Methods and Software. New York: Springer; 2003. pp. 259–276. [Google Scholar]
- 15.Kendziorski CM, Newton MA, Lan H, Gould MN. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat Med. 2003;22:3899–3914. doi: 10.1002/sim.1548. [DOI] [PubMed] [Google Scholar]
- 16.Zar JH. Biostatistical Analysis. New Jersey: Prentice Hall; 1999. [Google Scholar]
- 17.Chu G, Narasimhan B, Tibshirani R, Tusher V. SAM “Significance Analysis of Microarrays” Users Guide and Technical Document. 2002 Available at: http://www-stat.stanford.edu/~tib/SAM/index.html.
- 18.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 20.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 21.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez de Bagues MP, Terraza A, Gross A, Dornand J. Different responses of macrophages to smooth and rough Brucella spp.: relationship to virulence. Infect Immun. 2004;72:2429–2433. doi: 10.1128/IAI.72.4.2429-2433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3:281–294. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- 25.Chaves-Olarte E, Guzman-Verri C, Meresse S, Desjardins M, Pizarro-Cerda J, Badilla J, Gorvel JP, Moreno E. Activation of Rho and Rab GTPases dissociates Brucella abortus internalization from intracellular trafficking. Cell Microbiol. 2002;4:663–676. doi: 10.1046/j.1462-5822.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 26.Naroeni A, Porte F. Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect Immun. 2002;70:1640–1644. doi: 10.1128/IAI.70.3.1640-1644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 28.Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–114. 116, 118–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- 29.Stoenner HG. The behavior of Brucella neotomae and Brucella suis in reciprocal superinfection experiments in mice and guinea pigs. Am J Vet Res. 1963;24:376–380. [PubMed] [Google Scholar]
- 30.Stoenner HG, Lackman DB. A new species of Brucella isolated from the desert wood rat, Neotoma lepida Thomas. Am J Vet Res. 1957;18:947–951. [PubMed] [Google Scholar]
- 31.Rittig MG, Kaufmann A, Robins A, Shaw B, Sprenger H, Gemsa D, Foulongne V, Rouot B, Dornand J. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J Leukoc Biol. 2003;74:1045–1055. doi: 10.1189/jlb.0103015. [DOI] [PubMed] [Google Scholar]
- 32.Porte F, Naroeni A, Ouahrani-Bettache S, Liautard JP. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect Immun. 2003;71:1481–1490. doi: 10.1128/IAI.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detweiler CS, Cunanan DB, Falkow S. Host microarray analysis reveals a role for the Salmonella response regulator phoP in human macrophage cell death. Proc Natl Acad Sci U S A. 2001;98:5850–5855. doi: 10.1073/pnas.091110098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn WJ, Kim D, Lee KW, Kim MS, Kwon S, Lee Y, Kim DS, Kwon HJ. Novel transcriptional regulation of the schlafen-2 gene in macrophages in response to TLR-triggered stimulation. Mol Immunol. 2007;44:3273–3282. doi: 10.1016/j.molimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Dimitriou ID, Clemenza L, Scotter AJ, Chen G, Guerra FM, Rottapel R. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol Rev. 2008;224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 36.Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun. 2000;68:342–351. doi: 10.1128/iai.68.1.342-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Prada CM, Zelazowska EB, Nikolich M, Hadfield TL, Roop RM, 2nd, Robertson GL, Hoover DL. Interactions between Brucella melitensis and human phagocytes: bacterial surface O-Polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect Immun. 2003;71:2110–2119. doi: 10.1128/IAI.71.4.2110-2119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajashekara G, Covert J, Petersen E, Eskra L, Splitter G. Genomic island 2 of Brucella melitensis is a major virulence determinant: functional analyses of genomic islands. J Bacteriol. 2008;190:6243–6252. doi: 10.1128/JB.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapaque N, Forquet F, de Chastellier C, Mishal Z, Jolly G, Moreno E, Moriyon I, Heuser JE, He HT, Gorvel JP. Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts. Cell Microbiol. 2006;8:197–206. doi: 10.1111/j.1462-5822.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 40.Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens. 2005;65:507–514. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhan Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res. 2005;569:133–143. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 42.Briand L, Eloit C, Nespoulous C, Bezirard V, Huet JC, Henry C, Blon F, Trotier D, Pernollet JC. Evidence of an odorant-binding protein in the human olfactory mucus: location, structural characterization, and odorant-binding properties. Biochemistry. 2002;41:7241–7252. doi: 10.1021/bi015916c. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura K, Malykhin A, Coggeshall KM. The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcgamma receptor-mediated phagocytosis through immunoreceptor tyrosine-based activation motif-bearing phagocytic receptors. Blood. 2002;100:3374–3382. doi: 10.1182/blood-2002-03-0787. [DOI] [PubMed] [Google Scholar]
- 44.Wragg A, Mellad JA, Beltran LE, Konoplyannikov M, San H, Boozer S, Deans RJ, Mathur A, Lederman RJ, Kovacic JC, Boehm M. VEGFR1/CXCR4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a SDF-1-dependent mechanism. J Mol Med. 2008;86:1221–1232. doi: 10.1007/s00109-008-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, Belcher CE, Botstein D, Staudt LM, Brown PO, Relman DA. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci U S A. 2002;99:972–977. doi: 10.1073/pnas.231625398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng L, Zomerdijk TP, Aarnoudse C, van Furth R, Nibbering PH. Role of protein kinase C isozymes in Fc gamma receptor-mediated intracellular killing of Staphylococcus aureus by human monocytes. J Immunol. 1995;155:776–784. [PubMed] [Google Scholar]
- 47.Joshi T, Butchar JP, Tridandapani S. Fcgamma receptor signaling in phagocytes. Int J Hematol. 2006;84:210–216. doi: 10.1532/IJH97.06140. [DOI] [PubMed] [Google Scholar]
- 48.Dasgupta D, Chakraborty P, Basu MK. Ligation of Fc receptor of macrophages stimulates protein kinase C and anti-leishmanial activity. Mol Cell Biochem. 2000;209:1–8. doi: 10.1023/a:1007051413280. [DOI] [PubMed] [Google Scholar]
- 49.Loegering DJ, Lennartz MR. Signaling pathways for Fc gamma receptor-stimulated tumor necrosis factor-alpha secretion and respiratory burst in RAW 264.7 macrophages. Inflammation. 2004;28:23–31. doi: 10.1023/b:ifla.0000014708.87440.45. [DOI] [PubMed] [Google Scholar]
- 50.Paranavitana CM, Zelazowska E, Das R, Izadjoo M, Jett M, Hoover D. Identification of novel genes in the memory response to Brucella infection by cDNA arrays. Mol Cell Probes. 2005;19:341–348. doi: 10.1016/j.mcp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Soriano SF, Hernanz-Falcon P, Rodriguez-Frade JM, De Ana AM, Garzon R, Carvalho-Pinto C, Vila-Coro AJ, Zaballos A, Balomenos D, Martinez AC, Mellado M. Functional inactivation of CXC chemokine receptor 4-mediated responses through SOCS3 up-regulation. J Exp Med. 2002;196:311–321. doi: 10.1084/jem.20012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiemstra PS. Novel roles of protease inhibitors in infection and inflammation. Biochem Soc Trans. 2002;30:116–120. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 53.Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci. 2002;59:1503–1512. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forsyth S, Horvath A, Coughlin P. A review and comparison of the murine alpha1-antitrypsin and alpha1-antichymotrypsin multigene clusters with the human clade A serpins. Genomics. 2003;81:336–345. doi: 10.1016/s0888-7543(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 55.Lefebvre C, Cocquerelle C, Vandenbulcke F, Hot D, Huot L, Lemoine Y, Salzet M. Transcriptomic analysis in the leech Theromyzon tessulatum: involvement of cystatin B in innate immunity. Biochem J. 2004;380:617–625. doi: 10.1042/BJ20040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das L, Datta N, Bandyopadhyay S, Das PK. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J Immunol. 2001;166:4020–4028. doi: 10.4049/jimmunol.166.6.4020. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee S, Ukil A, Das PK. Immunomodulatory peptide from cystatin, a natural cysteine protease inhibitor, against leishmaniasis as a model macrophage disease. Antimicrob Agents Chemother. 2007;51:1700–1707. doi: 10.1128/AAC.01555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko J, Gendron-Fitzpatrick A, Splitter GA. Susceptibility of IFN regulatory factor-1 and IFN consensus sequence binding protein-deficient mice to brucellosis. J Immunol. 2002;168:2433–2440. doi: 10.4049/jimmunol.168.5.2433. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Qureshi N, Soeurt N, Splitter G. High levels of nitric oxide production decrease early but increase late survival of Brucella abortus in macrophages. Microb Pathog. 2001;31:221–230. doi: 10.1006/mpat.2001.0463. [DOI] [PubMed] [Google Scholar]
- 60.Porte F, Liautard JP, Kohler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincents B, Onnerfjord P, Gruca M, Potempa J, Abrahamson M. Down-regulation of human extracellular cysteine protease inhibitors by the secreted staphylococcal cysteine proteases, staphopain A and B. Biol Chem. 2007;388:437–446. doi: 10.1515/BC.2007.042. [DOI] [PubMed] [Google Scholar]
- 62.Stramer B, Winfield M, Shaw T, Millard TH, Woolner S, Martin P. Gene induction following wounding of wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 2008;9:465–471. doi: 10.1038/embor.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci U S A. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberger CM, Scott MG, Gold MR, Hancock RE, Finlay BB. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J Immunol. 2000;164:5894–5904. doi: 10.4049/jimmunol.164.11.5894. [DOI] [PubMed] [Google Scholar]
- 65.Huang H, Park CK, Ryu JY, Chang EJ, Lee Y, Kang SS, Kim HH. Expression profiling of lipopolysaccharide target genes in RAW264.7 cells by oligonucleotide microarray analyses. Arch Pharm Res. 2006;29:890–897. doi: 10.1007/BF02973911. [DOI] [PubMed] [Google Scholar]
- 66.Duenas AI, Orduna A, Crespo MS, Garcia-Rodriguez C. Interaction of endotoxins with Toll-like receptor 4 correlates with their endotoxic potential and may explain the proinflammatory effect of Brucella spp. LPS. Int Immunol. 2004;16:1467–1475. doi: 10.1093/intimm/dxh148. [DOI] [PubMed] [Google Scholar]
- 67.Renton KW. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab. 2004;5:235–243. doi: 10.2174/1389200043335559. [DOI] [PubMed] [Google Scholar]
- 68.Hossain H, Tchatalbachev S, Chakraborty T. Host gene expression profiling in pathogen-host interactions. Curr Opin Immunol. 2006;18:422–429. doi: 10.1016/j.coi.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Morgan ET. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207–212. [PubMed] [Google Scholar]
- 70.Ng Yan Hing JD, Desjardins M, Descoteaux A. Proteomic analysis reveals a role for protein kinase C-alpha in phagosome maturation. Biochem Biophys Res Commun. 2004;319:810–816. doi: 10.1016/j.bbrc.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 71.Chen YW, Lang ML, Wade WF. Protein kinase C-alpha and -delta are required for FcalphaR (CD89) trafficking to MHC class II compartments and FcalphaR-mediated antigen presentation. Traffic. 2004;5:577–594. doi: 10.1111/j.1600-0854.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 72.von Knethen A, Tautenhahn A, Link H, Lindemann D, Brune B. Activation-induced depletion of protein kinase C alpha provokes desensitization of monocytes/macrophages in sepsis. J Immunol. 2005;174:4960–4965. doi: 10.4049/jimmunol.174.8.4960. [DOI] [PubMed] [Google Scholar]
- 73.St-Denis A, Caouras V, Gervais F, Descoteaux A. Role of protein kinase C-alpha in the control of infection by intracellular pathogens in macrophages. J Immunol. 1999;163:5505–5511. [PubMed] [Google Scholar]
- 74.Gee JM, Valderas MW, Kovach ME, Grippe VK, Robertson GT, Ng WL, Richardson JM, Winkler ME, Roop RM., 2nd The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect Immun. 2005;73:2873–2880. doi: 10.1128/IAI.73.5.2873-2880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu JF, Barron-Casella E, Deering R, Heinzer AK, Moser AB, deMesy Bentley KL, Wand GS, McGuinness MC, Pei Z, Watkins PA, Pujol A, Smith KD, Powers JM. The role of peroxisomal ABC transporters in the mouse adrenal gland: the loss of Abcd2 (ALDR), Not Abcd1 (ALD), causes oxidative damage. Lab Invest. 2007;87:261–272. doi: 10.1038/labinvest.3700512. [DOI] [PubMed] [Google Scholar]
- 76.Wanders RJ, Visser WF, van Roermund CW, Kemp S, Waterham HR. The peroxisomal ABC transporter family. Pflugers Arch. 2007;453:719–734. doi: 10.1007/s00424-006-0142-x. [DOI] [PubMed] [Google Scholar]
- 77.Roux CM, Rolan HG, Santos RL, Beremand PD, Thomas TL, Adams LG, Tsolis RM. Brucella requires a functional Type IV secretion system to elicit innate immune responses in mice. Cell Microbiol. 2007;9:1851–1869. doi: 10.1111/j.1462-5822.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 78.Fu W, Wei J, Gu J. MEF2C mediates the activation induced cell death (AICD) of macrophages. Cell Res. 2006;16:559–565. doi: 10.1038/sj.cr.7310073. [DOI] [PubMed] [Google Scholar]
- 79.Ralph P, Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J Immunol. 1977;119:950–954. [PubMed] [Google Scholar]
- 80.Stuehr DJ, Marletta MA. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987;47:5590–5594. [PubMed] [Google Scholar]
- 81.Taniyama T, Holden HT. Cytolytic activity against tumor cells by macrophage cell lines and augmentation by macrophage stimulants. Int J Cancer. 1980;26:61–69. doi: 10.1002/ijc.2910260110. [DOI] [PubMed] [Google Scholar]
- 82.Carvalho Neta AV, Stynen AP, Paixao TA, Miranda KL, Silva FL, Roux CM, Tsolis RM, Everts RE, Lewin HA, Adams LG, Carvalho AF, Lage AP, Santos RL. Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infect Immun. 2008;76:1897–1907. doi: 10.1128/IAI.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]