Abstract
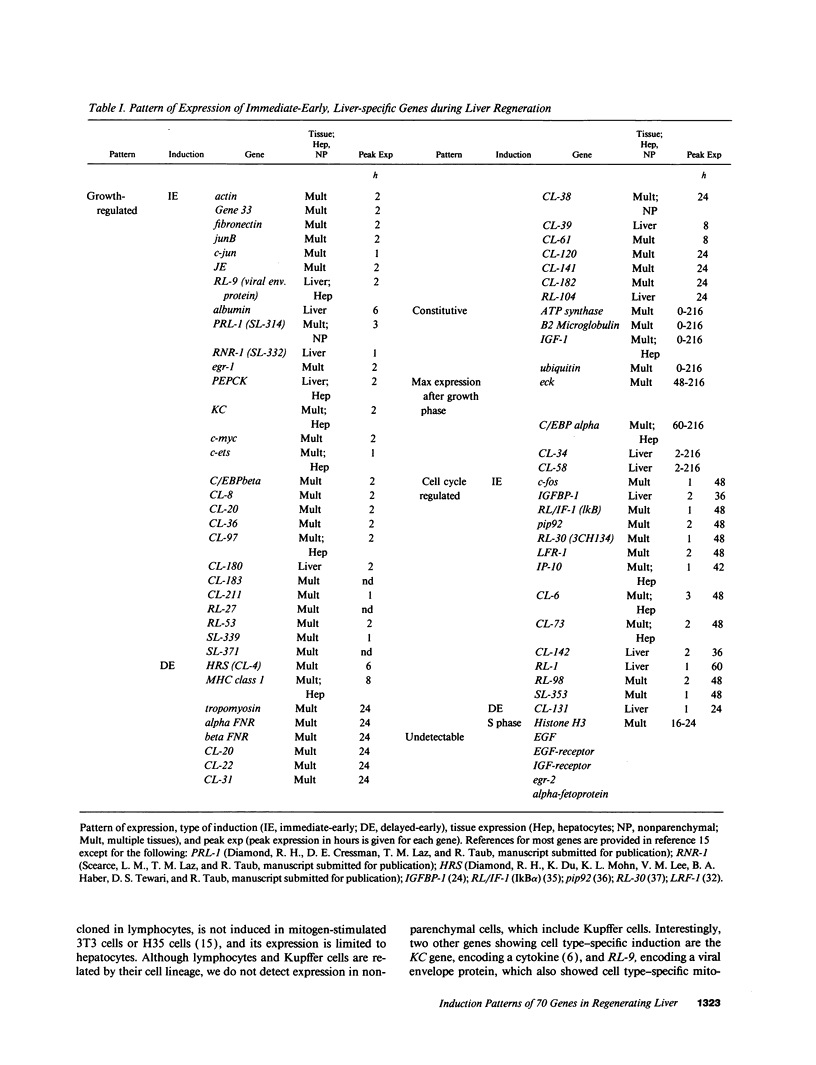
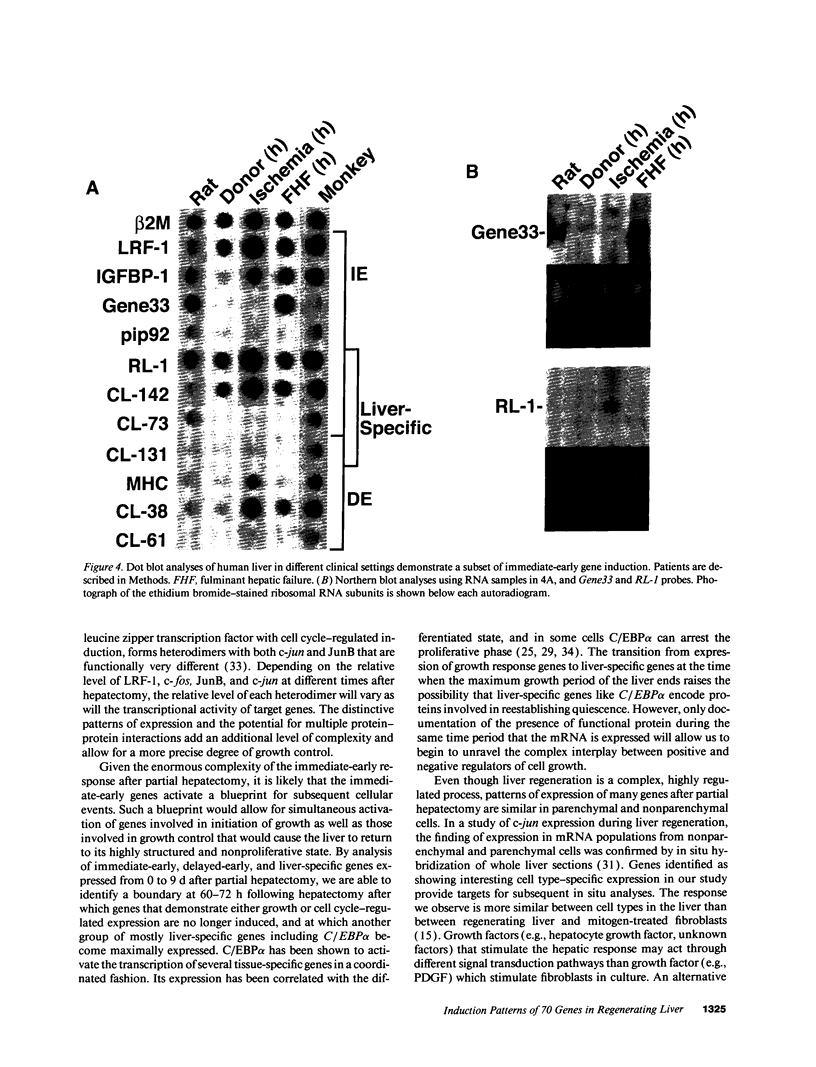
Liver regeneration is an important process that allows for recovery from hepatic injuries caused by viruses, toxins, ischemia, surgery, and transplantation. Previously, we identified > 70 immediate-early genes induced in regenerating liver after hepatectomy, 41 of which were novel. While it is expected that the proteins encoded by these genes may have important roles in regulating progression through the G1 phase of the cell cycle during regeneration, we were surprised to note that many of these "early" genes are expressed for extended periods during the hepatic growth response. Here we define several patterns of expression of immediate-early, delayed-early, and liver-specific genes during the 9-d period after hepatectomy. One pattern of induction parallels the major growth period of the liver that ends at 60-72 h after hepatectomy. A second pattern has two peaks coincident with the first and second G1 phases of the two hepatic cell cycles. A third group, which includes liver-specific genes such as C/EBP alpha, shows maximal expression after the growth period. Although the peak in DNA synthesis in nonparenchymal cells occur 24 h later than in hepatocytes, most of the genes studied demonstrate similar induction in both cell types. This finding suggests that the G0/G1 transition occurs simultaneously in all cells in the liver, but that the G1 phase of nonparenchymal cells may be relatively prolonged. Finally, we examined the expression of > 70 genes in clinical settings that could induce liver regeneration, including after perfusion in a donor liver, hepatic ischemia, and fulminant hepatic failure. We found that a small number of early and liver-specific genes were selectively activated in human livers under these conditions, and we thereby provide a potential means of measuring the caliber of the regenerative response in clinical situations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorn J. A., Feitelberg S. P., Brenner D. A. Transient induction of c-jun during hepatic regeneration. Hepatology. 1990 Jun;11(6):909–915. doi: 10.1002/hep.1840110602. [DOI] [PubMed] [Google Scholar]
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Bucher N. L., Swaffield M. N. Synergistic action of glucagon and insulin in regulation of hepatic regeneration. Adv Enzyme Regul. 1975;13:281–293. [PubMed] [Google Scholar]
- Charles C. H., Abler A. S., Lau L. F. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992 Jan;7(1):187–190. [PubMed] [Google Scholar]
- Charles C. H., Simske J. S., O'Brien T. P., Lau L. F. Pip92: a short-lived, growth factor-inducible protein in BALB/c 3T3 and PC12 cells. Mol Cell Biol. 1990 Dec;10(12):6769–6774. doi: 10.1128/mcb.10.12.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Kaestner K. H., Geiman D. E., Lane M. D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Fausto N., Mead J. E. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989 Jan;60(1):4–13. [PubMed] [Google Scholar]
- GRISHAM J. W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962 Aug;22:842–849. [PubMed] [Google Scholar]
- Gores G. J., Kost L. J., LaRusso N. F. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 1986 May-Jun;6(3):511–517. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- Hendriks H. F., Brouwer A., Knook D. L. Isolation, purification, and characterization of liver cell types. Methods Enzymol. 1990;190:49–58. doi: 10.1016/0076-6879(90)90008-o. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hsu J. C., Bravo R., Taub R. Interactions among LRF-1, JunB, c-Jun, and c-Fos define a regulatory program in the G1 phase of liver regeneration. Mol Cell Biol. 1992 Oct;12(10):4654–4665. doi: 10.1128/mcb.12.10.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. C., Laz T., Mohn K. L., Taub R. Identification of LRF-1, a leucine-zipper protein that is rapidly and highly induced in regenerating liver. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3511–3515. doi: 10.1073/pnas.88.9.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson N. V. Review article: improved preservation of the liver for transplantation. Aliment Pharmacol Ther. 1991 Apr;5(2):91–104. doi: 10.1111/j.1365-2036.1991.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Blansjaar N., Sleyster E. C. Isolation and characterization of Kupffer and endothelial cells from the rat liver. Exp Cell Res. 1977 Oct 15;109(2):317–329. doi: 10.1016/0014-4827(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Skelly H., Botteri F., van der Putten H., Barber J. R., Verma I. M., Leffert H. L. Proto-oncogene expression in regenerating liver is simulated in cultures of primary adult rat hepatocytes. J Biol Chem. 1986 Jun 15;261(17):7929–7933. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., Herschman H. R. Cloning of tetradecanoyl phorbol ester-induced 'primary response' sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1(3):263–270. [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Mischoulon D., Rana B., Bucher N. L., Farmer S. R. Growth-dependent inhibition of CCAAT enhancer-binding protein (C/EBP alpha) gene expression during hepatocyte proliferation in the regenerating liver and in culture. Mol Cell Biol. 1992 Jun;12(6):2553–2560. doi: 10.1128/mcb.12.6.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Hsu J. C., Melby A. E., Bravo R., Taub R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol. 1991 Jan;11(1):381–390. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Melby A. E., Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990 Dec 15;265(35):21914–21921. [PubMed] [Google Scholar]
- Mohn K. L., Melby A. E., Tewari D. S., Laz T. M., Taub R. The gene encoding rat insulinlike growth factor-binding protein 1 is rapidly and highly induced in regenerating liver. Mol Cell Biol. 1991 Mar;11(3):1393–1401. doi: 10.1128/mcb.11.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Kartha S., Toback F. G., Taub R., Hoover R. G., Tsai-Morris C. H. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res. 1987 Sep-Oct;1(4):343–355. [PubMed] [Google Scholar]
- Taub R., Roy A., Dieter R., Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987 Aug 5;262(22):10893–10897. [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Johansen K. L., Goeser T. The isolated perfused rat liver: preparation and application. Anal Biochem. 1987 Nov 15;167(1):1–14. doi: 10.1016/0003-2697(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]