Abstract
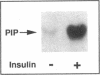
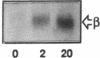
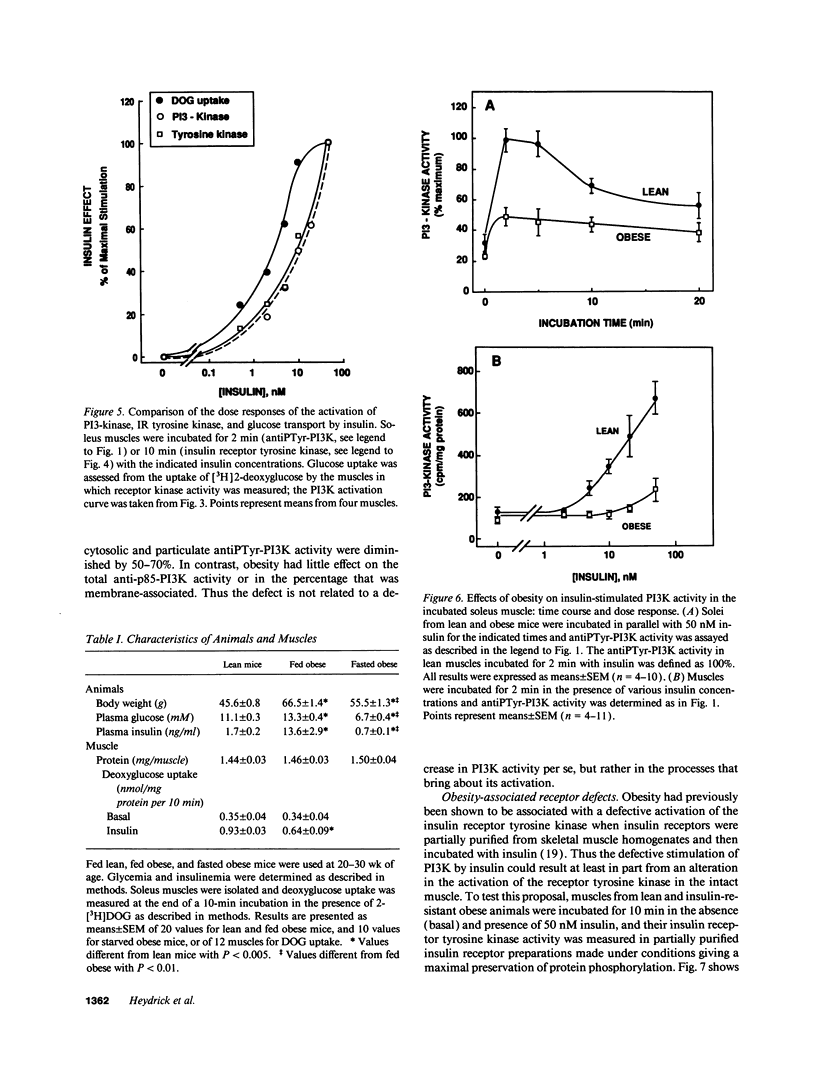
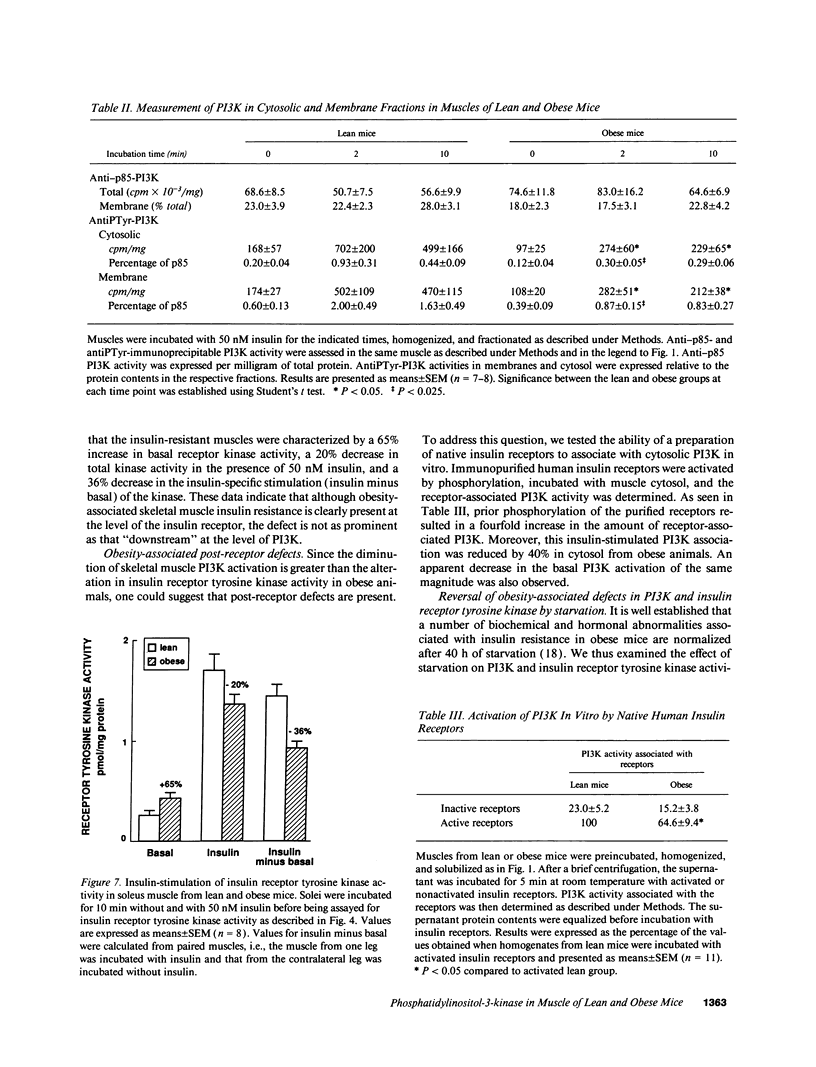
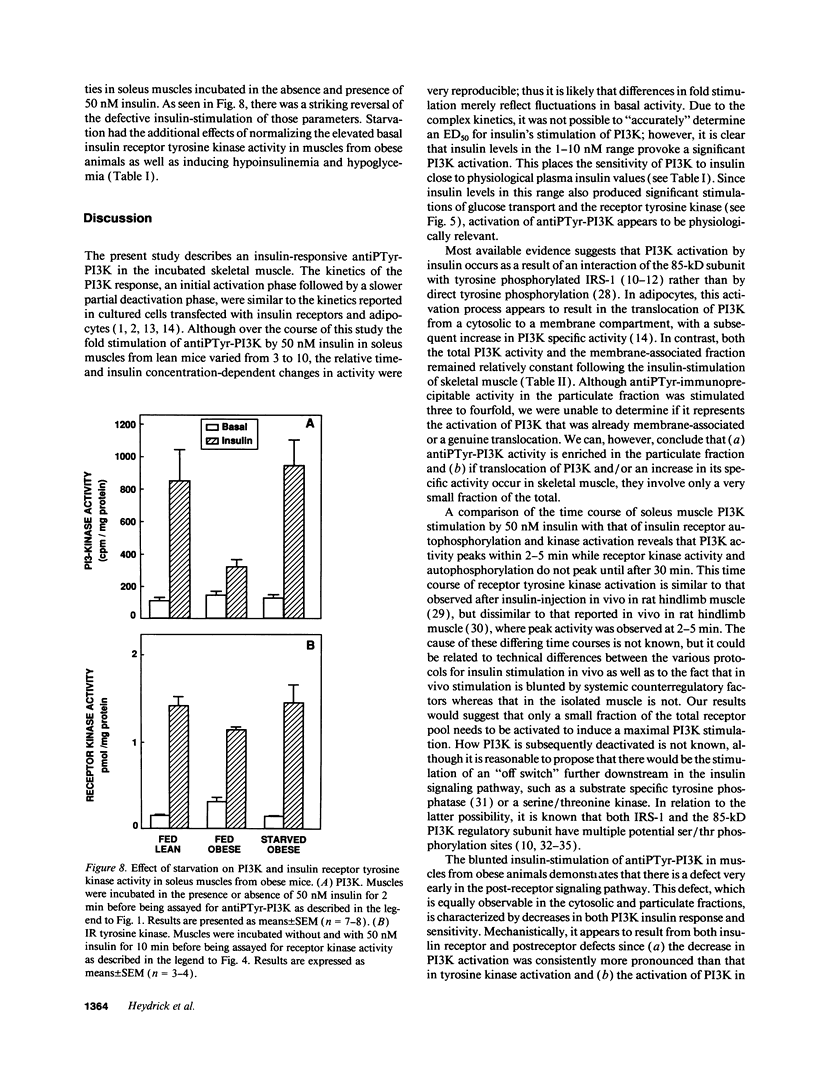
Activation of phosphatidylinositol-3-kinase (PI3K) is one of the earliest postreceptor events in the insulin signaling pathway. Incubation of soleus muscles from lean mice with 50 nM insulin caused a 3-10-fold increase in antiphosphotyrosine-immunoprecipitable PI3K (antiPTyr-PI3K) activity within 2 min in muscle homogenates as well as both the cytosolic and membrane fractions. Insulin did not affect total PI3K activity. Both the antiPTyr-PI3K stimulation and activation of insulin receptor tyrosine kinase were dependent on hormone concentration. In muscles from obese, insulin-resistant mice, there was a 40-60% decrease in antiPTyr-PI3K activity after 2 min of insulin that was present equally in the cytosolic and membrane fractions. A significant reduction in insulin sensitivity was also observed. The defect appears to result from alterations in both insulin receptor and postreceptor signaling. Starvation of obese mice for 48 h, which is known to reverse insulin resistance, normalized the insulin response of both PI3K and the receptor tyrosine kinase. The results demonstrate that: (a) antiPTyr-PI3K activity is responsive to insulin in mouse skeletal muscle, (b) both the insulin responsiveness and sensitivity of this activity are blunted in insulin-resistant muscles from obese mice, (c) these alterations result from a combination of insulin receptor and postreceptor defects, and (d) starvation restores normal insulin responses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989 Apr 7;57(1):167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Schroeder G. G., Kahn C. R., Myers M. G., Jr, Wilden P. A., Cahill D. A., White M. F. Insulin stimulation of phosphatidylinositol 3-kinase activity maps to insulin receptor regions required for endogenous substrate phosphorylation. J Biol Chem. 1992 Jan 15;267(2):1367–1374. [PubMed] [Google Scholar]
- Ballotti R., Scimeca J. C., Kowalski A., Van Obberghen E. Antiphosphotyrosine antibodies modulate insulin receptor kinase activity and insulin action. Cell Signal. 1989;1(2):195–204. doi: 10.1016/0898-6568(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. In vitro and in vivo activation of the insulin receptor kinase in control and denervated skeletal muscle. J Biol Chem. 1986 Jul 5;261(19):8985–8993. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Cantley L. C. Phosphoinositide kinases. Biochemistry. 1990 Dec 25;29(51):11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Choudhury G. G., Wang L. M., Pierce J., Harvey S. A., Sakaguchi A. Y. A mutational analysis of phosphatidylinositol-3-kinase activation by human colony-stimulating factor-1 receptor. J Biol Chem. 1991 May 5;266(13):8068–8072. [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Carter A. N. Phosphoinositide 3-kinase: a new effector in signal transduction? Cell Signal. 1991;3(6):501–513. doi: 10.1016/0898-6568(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Cormont M., Van Obberghen E. Insulin stimulates phosphatidylinositol-3-kinase activity in rat adipocytes. Eur J Biochem. 1992 Jul 15;207(2):599–606. doi: 10.1111/j.1432-1033.1992.tb17086.x. [DOI] [PubMed] [Google Scholar]
- Giorgino F., Chen J. H., Smith R. J. Changes in tyrosine phosphorylation of insulin receptors and a 170,000 molecular weight nonreceptor protein in vivo in skeletal muscle of streptozotocin-induced diabetic rats: effects of insulin and glucose. Endocrinology. 1992 Mar;130(3):1433–1444. doi: 10.1210/endo.130.3.1531627. [DOI] [PubMed] [Google Scholar]
- Hadari Y. R., Tzahar E., Nadiv O., Rothenberg P., Roberts C. T., Jr, LeRoith D., Yarden Y., Zick Y. Insulin and insulinomimetic agents induce activation of phosphatidylinositol 3'-kinase upon its association with pp185 (IRS-1) in intact rat livers. J Biol Chem. 1992 Sep 5;267(25):17483–17486. [PubMed] [Google Scholar]
- Hayashi H., Miyake N., Kanai F., Shibasaki F., Takenawa T., Ebina Y. Phosphorylation in vitro of the 85 kDa subunit of phosphatidylinositol 3-kinase and its possible activation by insulin receptor tyrosine kinase. Biochem J. 1991 Dec 15;280(Pt 3):769–775. doi: 10.1042/bj2800769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Jeanrenaud B. Insulin and obesity. Diabetologia. 1979 Sep;17(3):133–138. doi: 10.1007/BF01219738. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B., Chen K. S. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992 Feb 15;267(5):3423–3428. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Moutard N., Freychet P. Aminoisobutyric acid transport in soleus muscles of lean and gold thioglucose-obese mice. Am J Physiol. 1982 Jul;243(1):E74–E79. doi: 10.1152/ajpendo.1982.243.1.E74. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Freychet P., Jeanrenaud B. Longitudinal study on the establishment of insulin resistance in hypothalamic obese mice. Endocrinology. 1978 Jan;102(1):74–85. doi: 10.1210/endo-102-1-74. [DOI] [PubMed] [Google Scholar]
- McGuire M. C., Fields R. M., Nyomba B. L., Raz I., Bogardus C., Tonks N. K., Sommercorn J. Abnormal regulation of protein tyrosine phosphatase activities in skeletal muscle of insulin-resistant humans. Diabetes. 1991 Jul;40(7):939–942. doi: 10.2337/diab.40.7.939. [DOI] [PubMed] [Google Scholar]
- Monier S., Le Cam A., Le Marchand-Brustel Y. Insulin and insulin-like growth factor I. Effects on protein synthesis in isolated muscles from lean and goldthioglucose-obese mice. Diabetes. 1983 May;32(5):392–397. doi: 10.2337/diab.32.5.392. [DOI] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Rice K. M., Lienhard G. E., Garner C. W. Regulation of the expression of pp160, a putative insulin receptor signal protein, by insulin, dexamethasone, and 1-methyl-3-isobutylxanthine in 3T3-L1 adipocytes. J Biol Chem. 1992 May 15;267(14):10163–10167. [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serunian L. A., Haber M. T., Fukui T., Kim J. W., Rhee S. G., Lowenstein J. M., Cantley L. C. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. J Biol Chem. 1989 Oct 25;264(30):17809–17815. [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Sultan C., Breton M., Mauco G., Grondin P., Plantavid M., Chap H. The novel inositol lipid phosphatidylinositol 3,4-bisphosphate is produced by human blood platelets upon thrombin stimulation. Biochem J. 1990 Aug 1;269(3):831–834. doi: 10.1042/bj2690831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Tanti J. F., Grémeaux T., Van Obberghen E., Le Marchand-Brustel Y. Effects of okadaic acid, an inhibitor of protein phosphatases-1 and -2A, on glucose transport and metabolism in skeletal muscle. J Biol Chem. 1991 Feb 5;266(4):2099–2103. [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Yonezawa K., Yokono K., Shii K., Ogawa W., Ando A., Hara K., Baba S., Kaburagi Y., Yamamoto-Honda R., Momomura K. In vitro association of phosphatidylinositol 3-kinase activity with the activated insulin receptor tyrosine kinase. J Biol Chem. 1992 Jan 5;267(1):440–446. [PubMed] [Google Scholar]
- le Marchand-Brustel Y., Freychet P. Alteration of glycogen synthase activation by insulin in soleus muscles of obese mice. FEBS Lett. 1980 Nov 3;120(2):205–208. doi: 10.1016/0014-5793(80)80298-5. [DOI] [PubMed] [Google Scholar]