Abstract
Purpose
(1) To demonstrate the capability of ultra high resolution anterior segment optical coherence tomography (UHR-OCT) to image Descemet's membrane (DM) and measure its thickness in vivo. (2) To evaluate the use of DM characteristics and thickness in the diagnosis of Fuchs' dystrophy.
Design
Case-Control Study
Participants
20 eyes of 12 Fuchs' dystrophy patients, 20 eyes of 13 young normal and 20 eyes of 15 elderly normal subjects.
Methods
Subjects were imaged using novel custom-built UHR-OCT. Images were used to describe the characteristics of DM. Custom-made software was used to measure DM thickness and central corneal thickness (CCT). Specimens of DM obtained from Fuchs' dystrophy patients who underwent endothelial keratoplasty (EK) were histopathologically examined. Regression analyses were used to assess the correlation of DM thickness measured by UHR-OCT in vivo and by light microscopy and to determine the intergroup correlations between age, CCT and DM thickness.
Main Outcome Measures
DM characteristics and thickness, CCT and age.
Results
Using UHR-OCT, DM appeared in normal young subjects as a single opaque smooth line and in normal elderly subjects as a band of two smooth opaque lines with a translucent space in between. In Fuchs' dystrophy, DM appeared as a thickened band of two opaque lines; the anterior line was smooth while the posterior line had a wavy and irregular appearance with areas of localized thickenings. DM thickness measured in vivo by UHR-OCT correlated significantly to that measured by light microscopy in five Fuchs' dystrophy eyes that underwent EK. The average central thicknesses of DM in normal young, in normal elderly and in Fuchs' dystrophy eyes were 10 ± 3 μm, 16 ± 2 μm, and 34 ± 11 μm respectively (P < 0.001). There was a significant correlation between age and DM thickness only in normal groups. In Fuchs' dystrophy patients, there was a significant correlation between CCT and DM thickness which was non-significant for normal groups.
Conclusions
UHR-OCT is an innovative technique for in vivo imaging of DM. Determining DM characteristics and thickness by UHR-OCT could be a new approach for the diagnosis of Fuchs' dystrophy.
Introduction
Fuchs' dystrophy is characterized by cornea guttata and an accelerated loss of endothelial cells resulting in corneal edema, ocular discomfort, and loss of visual acuity. The disease is clinically diagnosed by slit-lamp examination and confirmed by corneal thickness measurements that can detect corneal edema. Additionally, endothelial cells may be evaluated by specular microscopy or by confocal microscopy. Although those methods provide valuable clinical information, they cannot detect the natural history of the disease or predict its progression, especially after cataract surgery. The wide range of normal corneal thickness complicates its use to diagnose mild to moderate corneal edema. The threshold number of endothelial cells, especially in presence of guttata, necessary to maintain corneal deturgescence is unknown and could be variable. It is also worth mentioning that endothelial cell abnormalities in Fuchs' dystrophy could result in sampling errors during specular microscopy and render the measurements inaccurate.1–3 Thus, a search is needed for new diagnostic criteria to describe the disease progression and natural history.
Histologically, the endothelium in Fuchs' dystrophy produces excessive amounts of an abnormal basement membrane material resulting in a thickened Descemet's membrane. Extreme accumulations of this material result in mushroom-like formations, guttae, projecting into the anterior chamber. Thinning and degeneration of endothelial cells lead to endothelial dysfunction and consequent corneal edema.3–5 Thus, studying the characteristics and the thickness of Descemet's membrane in vivo could be essential to understand better the role it plays in the pathogenesis and progression of Fuchs' dystrophy. However, the inability to adequately resolve Descemet's membrane in vivo has limited study to histopathological analysis.6 Despite the highly magnified images of the cornea that can be obtained by anterior segment imaging devices that have recently been introduced to ophthalmology, detection of Descemet's membrane and measurement of its thickness are still not possible due to the relatively low resolving power. These include instruments for confocal scanning laser microscopy and commercially available time-domain anterior segment optical coherence tomography (AS OCT). The commercially available time-domain AS OCT instruments have an axial resolution of 11 – 18 μm,7;8 which is insufficient to study Descemet's membrane in vivo. The new generation of confocal scanning laser microscopes has an axial resolution of ~4 μm; however, it cannot provide cross-sectional views of Descemet's membrane or measure its thickness.9 Prototypes of spectral domain OCT devices have shown the potential to image the fine details of the cornea that are otherwise undetectable, 10;11 however, those prototypes did not have enough resolution to resolve the Descemet's membrane or measure its thickness in vivo. The obvious limitations of these devices necessitate the search for imaging technology with higher resolution.
Our group has developed a novel custom–built, ultra high resolution, spectral domain anterior segment OCT (UHR-OCT) with an axial resolution of ~3 μm. This ultra high resolution could give our machine an edge over the aforementioned instruments and could help to provide a new in vivo insight into corneal pathologies. We demonstrate in this study the capability of UHR-OCT prototype to detect the characteristics of Descemet's membrane and to measure its thickness in vivo. Furthermore, we demonstrated that UHR-OCT could provide a non-invasive in vivo histological analysis of Fuchs' dystrophy and might provide valuable information on Descemet's membrane characteristics and thickness that can be used as diagnostic criteria for the disease.
Subjects and Methods
Study Population
The study included 20 eyes of normal young subjects (<45 years old), 20 eyes of normal elderly subjects (≥ than 60 years old), and 20 eyes of Fuchs' dystrophy patients. Written informed consent, approved by University of Miami Institutional Review Board (IRB), was obtained from all patients. Fuchs' dystrophy was diagnosed on slit-lamp examination by the presence of areas of confluent corneal guttata. Diagnosis was confirmed with follow-up measurements of central corneal thickness by ultrasound pachymetry (DGH-500 Pachette; DGH Technologies, Exton, Pennsylvania, USA) or UHR-OCT, endothelial cell count evaluation using specular microscopy (Konan Non-contact Specular Microscope; Noncon Robo-CA; Konan Medical, Inc, Hyogo, Japan) or pathological examination of Descemet's membrane specimen obtained from Descemet's stripping automated endothelial keratoplasty (DSAEK) for either eye of the patient.
Instrumentation
A novel custom-built UHR-OCT was used for this study. The device used a three-module superluminescent diode (SLD) light source (Broadlighter, T840-HP, Superlumdiodes Ltd, Moscow, Russia) with a center wavelength of 840 nm and a full width at half maximum bandwidth of 100 nm. The low coherence light, after passing through a fiber pig-tailed isolator, was coupled into a fiber based Michelson interferometer. A 2×2 3dB fiber coupler was used to split the light into the reference arm and the sample arm. The sample light was connected to a light delivery system with a telecentric design for imaging the cornea. The scanning system consisted of an X-Y galvanometer scanner and the optics for delivering the sample light and collecting the back-reflected sample light from the eye. The power of the sample light was lowered to 750 μW by adjusting the source power with a fiber-based pigtail style attenuator to not exceed a safe level of exposure to the eye.12 In the detection arm, a spectrometer with a collimating lens (f = 50 mm), a 1200 line/mm transmission grating, and an achromatic imaging lens (f = 180 mm) were used with a linear scan CCD camera (Aviiva-M2-CL-2014, 2048 pixels with 14 μm pixel size operating in 12-bit mode, Atmel, San Jose, CA, USA). The calculated spectral resolution was 0.055 nm, which corresponds to a detectable depth range of 3.1 mm in air.
Images were passed through an acquisition board and transferred to a computer workstation (IBM IntelliStation Z Pro, dual 3.6 GHz processor, 3 GB memory) for signal processing and image display. The A-line (depth scan) rate of the OCT system was set to 24 kHz. At these operating conditions, the measured sensitivity was about 95 dB. The calibrated axial resolution of the system was ~4 μm in the air and ~3 μm in the water or tissue with a refractive index of ~1.39.13
Image Acquisition and Processing
UHR-OCT radial images of the central horizontal 12 mm of the cornea, centered on the vertex, were captured at 32 frames/scan and used to calculate the thickness of Descemet's membrane and describe its structural characteristics. Custom built software processed the raw data. Scan points derived from the OCT raw data were analyzed and averaged to create an OCT reflectivity profile for the central 2 mm of the cornea. The peaks in the OCT reflectivity profiles were identified and correlated to the corresponding interfaces of the cornea. Descemet's membrane thickness was measured as the distance between the last two peaks in the OCT reflectivity profile.
Histological Analysis
Descemet membrane specimens were obtained from five Fuchs' dystrophy patients who underwent DSAEK and were studied using light microscopy. Specimens were fixed in 10% buffered formalyn, dehydrated, and embedded in paraffin. Slides, sectioned at 5 μm, were stained with periodic acid–Schiff (PAS) and analyzed using a microscope (Olympus Optical Co., Tokyo, Japan). The mean thickness of Descemet membrane was determined by averaging thickness measurements that included and excluded guttae in three representative randomly selected central high power field per slide (original magnification X 400)
Statistical Analysis
Statistical analyses with SPSS software version 17.0 (SPSS, Chicago, IL, USA) were used to calculate descriptive statistics for all eyes of all participants; however, if a subject contributed measurements from both eyes to the study, p-values were obtained after averaging data from the left and right eyes. Means were compared between groups with one-way analysis of variance, and Tamhane post-hoc comparisons were performed to account for differences in variance between groups. Linear regression analysis was used to assess the correlation of Descemet's membrane thickness measured by UHR-OCT in vivo and by light microscopy. Linear regression, multiple linear regression, and non-linear regression analyses were used to assess the effect of age and central corneal thickness on Descemet's membrane thickness. Because of the asymmetrical nature of the disease between two eyes of the same patient (Table 1), all Fuchs' dystrophy eyes were included to determine the correlation between central corneal thickness and Descemet's membrane thickness. P-values less than 0.05 were considered statistically significant. Values are presented as means ± standard deviation.
Table 1.
Fuchs' dystrophy group
| Eyes (n) | Patients (n) | Eye | Age (years) | Gender | Confirmation of Diagnosis | DM Thickness (μm) | CCT (μm) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | OS | 80 | M | SM | 24 | 639 |
| 2 | 2 | OD | 88 | F | Pach | 26 | 593 |
| 3 | OS | 88 | F | Pach | 17 | 574 | |
| 4 | 3 | OD | 56 | M | SM | 46 | 634 |
| 5 | OS | 56 | M | SM | 46 | 653 | |
| 6 | 4 | OD | 68 | M | Pach | 29 | 651 |
| 7 | OS | 68 | M | Pach | 30 | 667 | |
| 8 | 5 | OD | 73 | F | Path | 55 | 582 |
| 9 | OS | 73 | F | Path | 41 | 600 | |
| 10 | 6 | OD | 58 | M | SM | 34 | 618 |
| 11 | OS | 58 | M | SM | 33 | 600 | |
| 12 | 7 | OD | 68 | F | SM | 17 | 532 |
| 13 | OS | 68 | F | SM | 23 | 529 | |
| 14 | 8 | OD | 68 | M | Path | 29 | 619 |
| 15 | 9 | OD | 80 | F | Path other | 36 | 765 |
| 16 | 10 | OD | 65 | M | SM | 53 | 711 |
| 17 | OS | 65 | M | SM | 34 | 610 | |
| 18 | 11 | OS | 61 | F | Path | 37 | 610 |
| 19 | 12 | OD | 65 | M | Path other | 38 | 741 |
| 20 | OS | 65 | M | Path | 36 | 719 |
OD: Right eye
OS: Left eye
DM: Descemet's membrane
CCT: Central corneal thickness
M: Male
F: Female
SM: Specular microscopy
Pach : Pacymetry follow up
Path: Pathological examination of specimen obtained by Descemet's stripping automated endothelial keratoplasty (DSAEK).
Path other: Pathological examination was done for a specimen obtained from the fellow eye
Results
This study included 20 eyes of 13 young (age range: 19–44 years) normal subjects, 20 eyes of 15 elderly (age range: 60–86 years) normal subjects, and 20 eyes of 12 Fuchs' patients (age range: 56–88 years) (Table 2). The clinical diagnosis of Fuchs' dystrophy was made by slit-lamp examination. The diagnosis was confirmed in seven eyes by histopathological examination of Descemet's membrane specimens obtained by DSAEK, including 2 cases from the fellow eye. In 9 eyes the diagnosis was confirmed by specular microscopy and in 4 eyes by follow-up of central corneal thickness by ultrasound pachymetry or UHR-OCT showing progression of corneal edema (Table 1).
Table 2.
Population analysis
| Group | Eyes number | Patients number | Age (years)* | Central corneal thickness (μm)** | Descemet's membrane thickness (μm)*** |
|---|---|---|---|---|---|
| Young normal (Y) | 20 | 13 | 29 ± 6 [19–44]† | 551 ± 26 [500–592] | 10 ± 3 [6–16] |
| Elderly normal (E) | 20 | 15 | 74 ± 8 [60–86] | 537 ± 35 [494–594] | 16 ± 2 [13–20] |
| Fuchs' dystrophy (F) | 20 | 12 | 69 ± 10 [56–88] | 632 ± 64 [529–765] | 34 ± 11 [17–55] |
P-value <0.001; Post-hoc comparison results Y < E and F
P-value <0.001; Post-hoc comparison results Y and E < F
P-value <0.001; Post-hoc comparison results Y < E < F
Range of values
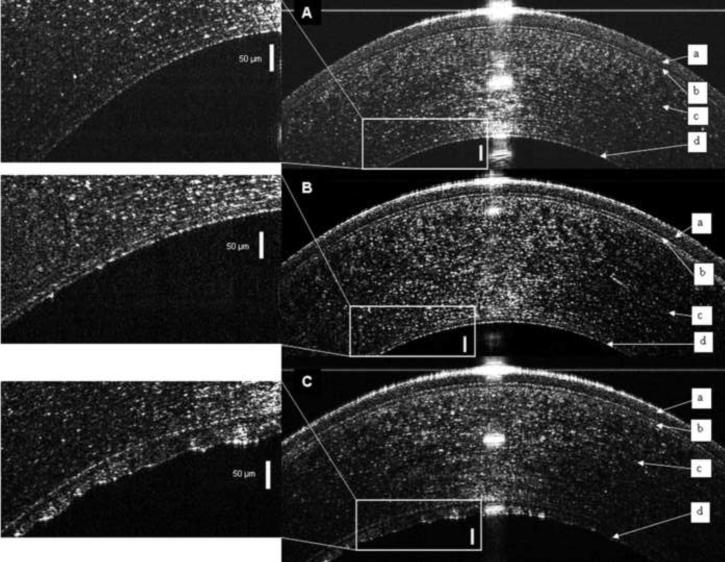
Images of Descemet's membrane were produced by UHR-OCT of normal corneas and those with Fuchs' dystrophy. In normal young subjects, Descemet's membrane was visualized as a single smooth line (Fig. 1A). In normal elderly subjects, the UHR-OCT images showed a band formed by two smooth opaque lines with a translucent space in between (Fig. 1B). In contrast, UHR-OCT images of Fuchs' dystrophy patients appeared as a thickened band formed by two opaque lines; the anterior line was smooth while the posterior line had a wavy irregular appearance with areas of localized thickenings (Fig. 1C). The corresponding OCT reflectivity profiles were obtained using the custom made software, different peaks were correlated to the corresponding corneal layers and were used to measure central corneal and Descemet's membrane thicknesses (Fig. 2). Thus, we were able to develop standardized quantitative method to process UHR-OCT raw data.
Figure 1. Ultra high resolution optical coherence tomography (UHR-OCT) images of the central cornea.
The epithelium (a), Bowman's layer (b), and stroma (c) are readily seen in each image and Descemet's membrane (d) is enlarged in the inset. (A) Descemet's membrane of a normal young subject appeared as a single smooth opaque line on the back surface of the cornea. (B) Descemet's membrane in a normal elderly subject appeared as a band formed of two smooth opaque lines with a translucent space in between. (C) In UHR-OCT image of the right eye of Fuchs' dystrophy patient number 5, Descemet's membrane consisted of a thickened band composed of two opaque lines separated by a translucent space. The anterior line was smooth while the posterior line had a wavy irregular appearance with areas of localized thickenings. Bars are 50 μm.
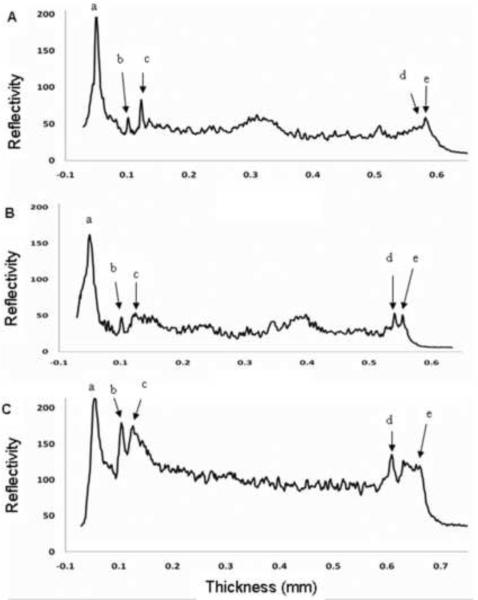
Figure 2. Reflectivity profiles of ultra high resolution optical coherence tomography (UHR-OCT) images of young normal, elderly normal and Fuchs' dystrophy corneas.
Each profile was created by averaging the UHR-OCT A-scan data for the central 2 mm of the corneas shown in Figure 1. Peaks were identified and correlated to the different corneal layers. The interval from (a) to (b) represents epithelium, while from (b) to (c) represents Bowman's layer. The stroma is represented by the interval from (c) to (d). The thickness of Descemet's membrane was calculated as the distance from (d) to (e). The measured thicknesses of Descemet's membrane were 9 μm for the normal young subject (A), 17 μm for the normal elderly subject (B), and 55 μm for the right eye of Fuchs' dystrophy patient number 5 (C). For the Fuchs' dystrophy patient (C), the reflectivity profile of the corneal stroma was greater than that in the normal subjects (A and B), probably due to corneal edema. The posterior line of the Descemet's membrane in (C) had multiple small spikes that correlated with its irregular appearance on the UHR-OCT image.
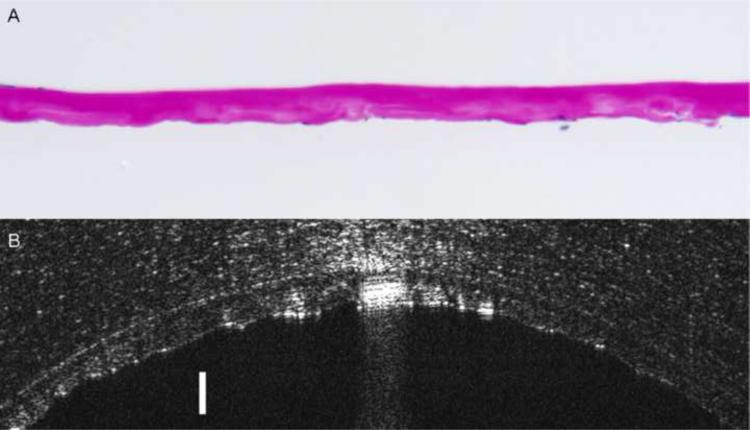
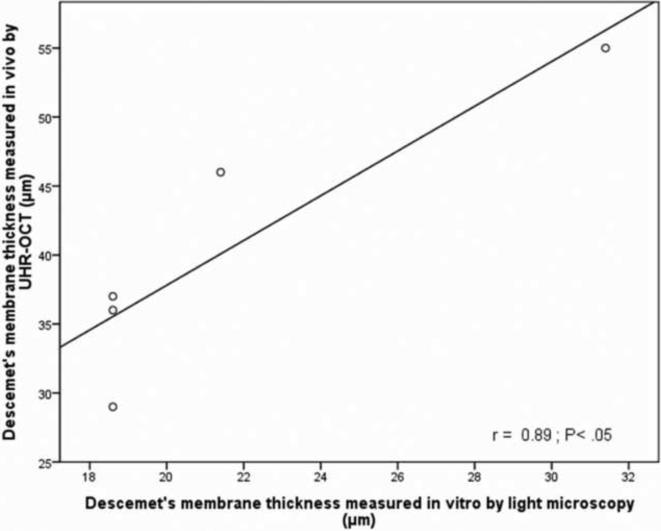
In order to correlate in vivo Descemet's membrane thickness measurements obtained by UHR-OCT to histopathological light microscopic measurements, Descemet's membrane from five Fuchs' dystrophy patients imaged preoperatively by UHR-OCT were obtained by DSAEK surgery and submitted for histopathological evaluation (Fig. 3). Examination disclosed an abnormally thickened Descemet's membrane with prominent posterior nodular excrescences (guttata), consistent with the diagnosis of Fuchs' dystrophy. The mean membrane thickness measured by light microscopy was 21.7 ± 5.5 μm; however, the preoperative UHR-OCT in vivo mean measurement was 40.6 ± 10 μm for the five cases. While the thickness measured by light microscopy was significantly less than that measured by UHR-OCT (P < 0.001), there was a significant correlation between the two methods (r = 0.89, P < 0.05, Fig 4).
Figure 3. Comparison of corneal histological analysis and ultra high resolution optical coherence tomography (UHR-OCT) images of a Fuchs' dystrophy patient.
Image (A) shows the photomicrograph of the pathology section of the same Descemet's membrane in image (B) obtained by Descemet's stripping automated endothelial keratoplasty (DSAEK) of the right eye of Fuchs' dystrophy patient number 5. Image (A) discloses thickened Descemet's membrane with areas of nodular excrescences correlating with cornea guttae. The average central thickness of the Descemet's membrane measured histopathologically by light microscope was 31μm (Periodic acid-Schiff stain; original magnification X400). UHR- OCT image (B) of the same Descemet's membrane appears as a thickened band formed of two opaque lines. The anterior line is smooth while the posterior line has a wavy irregular appearance with areas of localized thickenings that can be correlated to cornea guttae. In vivo UHR-OCT measurements of this Descemet's membrane was 55 μm. Bar is 50 μm.
Figure 4. Fuchs' dystrophy Descemet's membrane thickness measured by ultra high resolution optical coherence tomography (UHR-OCT) was significantly correlated with light microscopic histopathological measurements.
For Fuchs' dystrophy corneas that underwent Descemet's stripping automated endothelial keratoplasty (DSAEK), preoperative UHR-OCT measured thicknesses showed a significant correlation with that measured by light microscopic histopathological examination. In Vivo Descemet's membrane thickness of the central 2 mm of the cornea was measured using custom-made software that created a reflectivity profile form UHR-OCT raw images. Different peaks of the reflectivity profile were correlated to corresponding layers of the cornea and the distance between the last two peaks were used to calculate the thickness of Descemet's membrane. Light microscopy mean thicknesses of Descemet's membrane were determined by averaging thickness measurements that included and excluded guttae in one representative randomly selected central high power field per slide.
To determine if the thickness of Descemet's membrane in normal subjects differ with age, we measured the central thicknesses of Descemet's membrane in normal young subjects and in normal elderly subjects. Average central Descemet's membrane thicknesses were 10 ± 3 μm and 16 ± 2 μm in the young and elderly normal groups, respectively. The measured thicknesses were significantly different from each other (P < 0.001, Table 2). However, in the Fuchs' dystrophy group, Descemet's membrane was significantly thicker than both the young and elderly normal groups. Average central thickness in Fuchs' dystrophy patients was 34 ± 11 μm (P < 0.001, Table 2), 18 μm thicker than that of the elderly normal subjects (Tamhane's post-hoc multiple comparison test, P < 0.001, Table 2). The central corneal thickness in the eyes of Fuchs' patients was also significantly thicker than in normal subjects of either age group (Tamhane's post-hoc multiple comparison test, P < 0.001, Table 2). The age of the elderly normal subjects and the Fuchs' dystrophy patients were approximately the same (Table 2). After adjusting for central corneal thickness and age, Descemet's membrane still averaged 11.4 μm ±3.2 thicker in Fuchs' patients than in the older normal subjects (multiple linear regression, P = 0.002). Moreover, in Fuchs' dystrophy patients with pachymetry ≤ 600 μm, Descemet's membrane had similar characteristics as advanced Fuchs' dystrophy cases with pachymetry > 600 μm and a mean membrane thickness significantly different from the elderly age matched group (two sample t test, P<0.05).
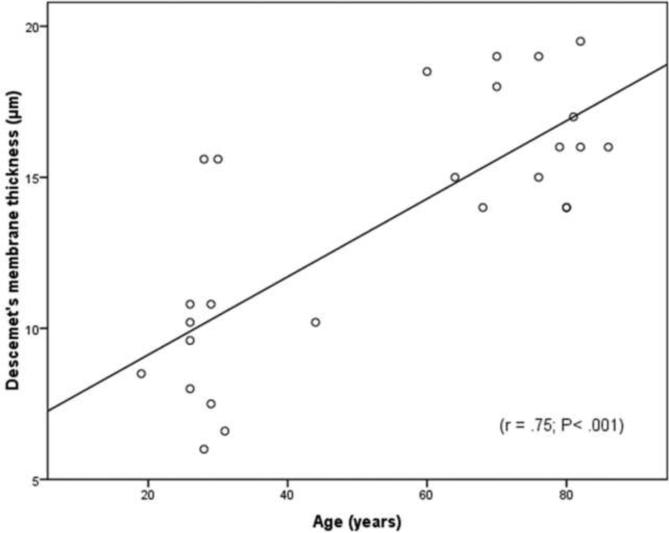
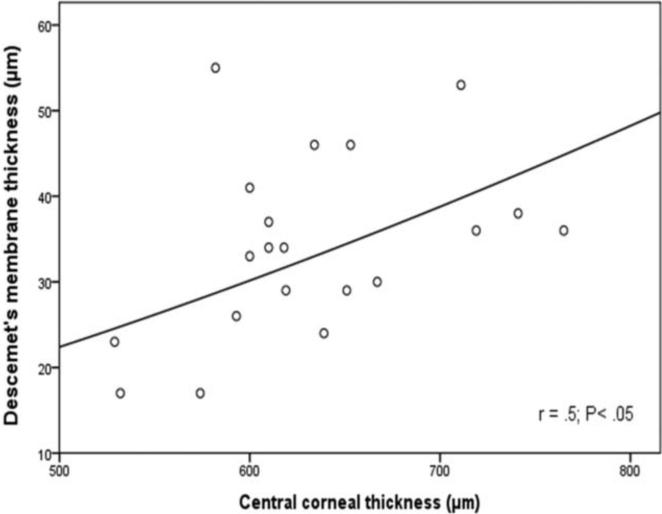
To understand better the impact of age on the thickness of Descemet's membrane in normal and in Fuchs' dystrophy corneas, regression analyses were done. In normal subjects of both age groups, Descemet's membrane thickness was highly correlated with age (r = 0.75, P < 0.001, Fig. 5) and increased by 1.3 ±0.2μm/decade. However, for the Fuchs' dystrophy patients, there was non-statistically significant correlation between age and membrane thickness (P=0.052). This data indicates that age has a strong impact on Descemet's membrane thickness in normal subjects. On the contrary, in Fuchs' dystrophy patients, age does not have a strong impact and there must be other factors that have a greater impact on Descemet's membrane thickness other than age. On the other hand, studying the impact of central corneal thickness on the membrane thickness revealed that the membrane thickness was not significantly correlated with central corneal thickness (r < 0.2, P > 0.35) in the young and elderly normal groups. However, non-linear power regression analysis demonstrated a significant correlation (r = 0.5; P < 0.05) between central corneal thickness and the membrane thickness for the Fuchs' dystrophy group (Fig. 6). This data indicates that normal subjects with thicker corneas does not necessarily have thicker Descemet's membrane, however, Fuchs' dystrophy patients with thicker corneas tend to have thicker Descemet's membrane.
Figure 5. In normal subjects, Descemet's membrane tends to become thicker with age.
There was a highly significant positive correlation (r = 0.75; P< 0.001) between the thickness of Descemet's membrane and age in the healthy young and elderly groups.
Figure 6. In Fuchs' dystrophy, Descemet's membrane thickness correlates significantly with the central cornea thickness.
There was a significant non-linear power correlation (r = 0.5; P < 0.05) correlation between the thickness of Descemet's membrane and the central corneal thickness in Fuchs' dystrophy patients.
Discussion
The use of this novel imaging technique of the cornea allowed us to perform in vivo non-invasive histological analysis of the cornea and to describe the characteristics of a living Descemet's membrane. To our knowledge this is the first study in the literature to report in vivo Descemet's membrane thickness in normal and in Fuchs' dystrophy patients and to demonstrate its possible use as a diagnostic criterion for Fuchs' dystrophy.
This study demonstrated the ability of UHR-OCT to image and measure Descemet's membrane thickness in vivo and that gives it an edge over other high resolution, anterior segment imaging devices. Although confocal laser scanning microscopy can visualize the endothelium at the cellular level, it cannot generate cross-sectional images for clinical analysis.14 Commercially available time domain AS OCT instruments and high resolution spectral domain AS OCT prototypes have limited ability to depict Descemet's membrane and cannot measure its thickness.15 Akiba et al16 imaged eye bank corneas with a full-field UHR-OCT prototype and reported its capability to study Descemet's membrane. However, this modality requires immersion of the examined eye in a coupling fluid, which is inconvenient for in vivo imaging of patients. Christopoulos et al 10and Kalzuny et al 11 reported the use of non-contact spectral domain AS OCT prototypes to image eyes with different corneal pathologies. However, these prototypes could not measure the thickness of Descemet's membrane.
Descemet's membrane appeared as a single opaque line in all normal young subjects between the ages of 19 and 44 years. It was not possible to visualize the interface between the Descemet's membrane and corneal stroma in the young normal group using the UHR-OCT. However, using the reflectivity profile, which was created by averaging the central 2 mm of the cornea, the peaks of this interface were, to some extent, magnified. Nevertheless, the peaks were still not as evident as in elderly and Fuchs' dystrophy subjects. However, measuring the distance between the last two peaks on the reflectivity profiles of the young normal group lead to Descemet's membrane thickness measurements with standard deviation of only 3 μm and consistent with Murphy et al 6 histopathological measurements. The difficult visualization of this interface on the UHR-OCT images is most likely due to masking of the Descemet's membrane stromal surface peaks by other stromal peaks on the OCT reflectivity profile. By imaging the membrane obliquely, the high reflectivity of the central stroma can be avoided, allowing for better visualization of the central stromal face. However, this oblique view could affect the accuracy of the thickness measurement. In elderly normal subjects, Descemet's membrane was visualized as a band of two opaque lines, the stromal and endothelial faces, and it was significantly thicker than in young normal subjects. In Fuchs' dystrophy patients, Descemet's membrane was visualized as a thickened band of two opaque lines. The anterior line was smooth and represented the stromal face of the Descemet's membrane, while the posterior line had an irregular wavy appearance with local thickenings that represented the endothelial face of Descemet's membrane with cornea guttata typical of Fuchs' dystrophy.
The average central thickness of Descemet's membrane measured by light microscopy for the five post-DSAEK cases included in our study is in agreement with Heindl et al. 17 The in vivo measurements using UHR-OCT for the same patients were significantly thicker than the histological measurements; however the presence of a statistically significant high correlation between them indicates that this discrepancy could be due to differences in thickness between hydrated in vivo Descemet's membranes in edematous corneas versus dehydrated fixed tissue processed for histological analysis.
Our study demonstrated a highly significant thickening of Descemet's membrane in Fuchs' dystrophy compared to normal subjects. The large standard deviation of the membrane thickness in Fuchs' dystrophy patients is most likely due to sampling from different stages of the disease. Fayol et al18 correlated time-domain AS OCT images of Fuchs' dystrophy with typical clinical and confocal microscopy findings. However; they could not demonstrate thickening of the Descemet's membrane. Kalzuny et al 19 reported the use of spectral domain AS OCT prototype to image three Fuchs' dystrophy patients. They reported the thickness of Descemet's membrane and demonstrated its abnormal thickening in only one of the reported cases. Our UHR-OCT images are similar, but with higher resolution that are able to resolve the thickened membranes in all the examined eyes and in normal subjects. Furthermore, the Descemet's membrane characteristics in mild Fuchs' dystrophy patients had similar characteristics as advanced cases and mean thickness significantly thicker than the elderly age matched group. This observation suggests that UHR-OCT findings might be helpful in the diagnosis of the early form as well as the advanced forms of the disease.
Our data also showed a strong positive correlation between age and Descemet's membrane thickness in normal subjects, suggesting that the membrane increases in thickness with age. Using electron and light microscopic examination of fixed and processed corneas, Murphy et al 6 reported the thickness of Descemet's membrane in prenatal corneas and in young and elderly subjects. Their results are in agreement with ours regarding the impact of age on membrane thickness. In Fuchs' dystrophy patients, age was not correlated with Descemet's membrane thickness. This is probably due to the pathological thickening of Descemet's membrane associated with the disease which could have a stronger impact on Descemet's membrane thickness than age. However, it should be noted that Fuchs' dystrophy patients recruited for our study were all elderly. Thus, the correlation between age and Descemet's membrane thickness in the Fuchs' dystrophy group might be limited to this age group and not necessarily representative of younger patients.
The effect of central corneal thickness on Descemet's membrane thickness was not statistically significant in normal subjects. In a normal subject, a thick cornea does not necessarily leads to a thicker Descemet's membrane. However, in the Fuchs' dystrophy group, the central corneal thickness had a significant non-linear power correlation with Descemet's membrane thickness. This non-linear power correlation suggests that the impact of central corneal thickness on Descemet's membrane thickness increases with the increase of central corneal thickness. This correlation, which is absent in normal subjects, could be an indirect indicator of the progression of the disease. However, further studies with larger sample size are needed to confirm these results.
One of the limitations of the UHR-OCT is its inability to resolve the endothelial cell layer. This is most probably because the refractive index of the endothelial cell layer is very close to either the Descemet's membrane or the aqueous humor and thus backscattered light by this layer interfaces is subtle enough to make it undetectable. Accordingly, in our study, it was not possible to determine if endothelial cell layer was undifferentiated from the Descemet's membrane and thus incorporated in membrane thickness measurements or undifferentiated from the aqueous humor and thus not incorporated in the membrane thickness measurements. This is also a limitation in correlating the thickness measured histopathogically by light microscopy to that measured in vivo by UHR-OCT. Another limitation is that it was not possible to visualize the two interfaces of Descemet's membrane in normal young subjects. However, we were able to calculate its thickness in all cases based on the average UHR-OCT reflectivity profile in the central 2 mm of the cornea.
Refractive index of the cornea, 1.39, was used in this study for the calculation of Descemet's membrane thickness.13 This is a source of minor calculation error because the refractive index of the corneal tissue differs with different pathological conditions such as corneal edema.20 In fact, the exact refractive index of the Descemet's membrane is still unknown; therefore, we used the group refractive index instead.
Further studies with larger sample sizes and a greater diversity of pathological endothelial conditions are necessary to confirm and extend our results. These studies should explore the use of Descemet's membrane characteristics and thickness as a means to determine the prognosis and natural history of the disease and to differentiate Fuchs' dystrophy from other corneal pathological conditions.
In conclusion, we demonstrated the capability of the UHR-OCT to non-invasively image Descemet's membrane and measure its thickness in vivo. This capability will help ophthalmologists better understand its structure and role in the diagnosis and treatment of different pathological conditions. In this study, we were also able to shine a light on the living Descemet's membrane in Fuchs' dystrophy patients by describing its characteristics and thickness compared to the normal Descemet's membrane. This data could provide a new insight into the disease and a novel way to start exploring the natural history of the disease and generating a treatment algorithm based on better ability to predict the prognosis of the disease.
PRÉCIS.
Ultra-high resolution optical coherence tomography is capable of in vivo imaging Descemet's membrane in normal and Fuchs' dystrophy eyes. Descemet's membrane characteristics and thickness is a novel technique to confirm the diagnosis of Fuchs' dystrophy.
Acknowledgments
Financial Support: This study was supported by a NEI core center grant to the University of Miami (P30 EY014801) and Research to Prevent Blindness (RPB).
The funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chiou AG, Kaufman SC, Beuerman RW, et al. Confocal microscopy in cornea guttata and Fuchs' endothelial dystrophy. Br J Ophthalmol. 1999;83:185–9. doi: 10.1136/bjo.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs' corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology. 2005;112:441–6. doi: 10.1016/j.ophtha.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Seitzman GD. Cataract surgery in Fuchs' dystrophy. Curr Opin Ophthalmol. 2005;16:241–5. doi: 10.1097/01.icu.0000172828.39608.7c. [DOI] [PubMed] [Google Scholar]
- 4.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–68. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues MM, Krachmer JH, Hackett J, et al. Fuchs' corneal dystrophy: a clinicopathologic study of the variation in corneal edema. Ophthalmology. 1986;93:789–96. [PubMed] [Google Scholar]
- 6.Murphy C, Alvarado J, Juster R. Prenatal and postnatal growth of the human Descemet's membrane. Invest Ophthalmol Vis Sci. 1984;25:1402–15. [PubMed] [Google Scholar]
- 7.Labbe A, Hamard P, Iordanidou V, et al. Utility of the Visante OCT in the follow-up of glaucoma surgery [in French] J Fr Ophtalmol. 2007;30:225–31. doi: 10.1016/s0181-5512(07)89582-9. [DOI] [PubMed] [Google Scholar]
- 8.Muller M, Hoerauf H, Geerling G, et al. Filtering bleb evaluation with slit-lamp-adapted 1310-nm optical coherence tomography. Curr Eye Res. 2006;31:909–15. doi: 10.1080/02713680600910528. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi A, Mawatari Y, Yokogawa H, Sugiyama K. In vivo laser confocal microscopy after Descemet stripping with automated endothelial keratoplasty. Am J Ophthalmol. 2008;145:977–85. doi: 10.1016/j.ajo.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Christopoulos V, Kagemann L, Wollstein G, et al. In vivo corneal high-speed, ultra-high-resolution optical coherence tomography. Arch Ophthalmol. 2007;125:1027–35. doi: 10.1001/archopht.125.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaluzny BJ, Kaluzny JJ, Szkulmowska A, et al. Spectral optical coherence tomography: a novel technique for cornea imaging. Cornea. 2006;25:960–5. doi: 10.1097/01.ico.0000224644.81719.59. [DOI] [PubMed] [Google Scholar]
- 12.Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–14. doi: 10.1167/iovs.06-0815. [DOI] [PubMed] [Google Scholar]
- 13.Lin RC, Shure MA, Rollins AM, et al. Group index of the human cornea at 1.3-microm wavelength obtained in vitro by optical coherence domain reflectometry. Opt Lett. 2004;29:83–5. doi: 10.1364/ol.29.000083. [DOI] [PubMed] [Google Scholar]
- 14.Avetisov SE, Egorova GB, Fedorov AA, Bobrovskikh NV. Confocal microscopy of the cornea. Communication 1. The normal morphological pattern [in Russian] Vestn Oftalmol. 2008;124:3–5. [PubMed] [Google Scholar]
- 15.Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004;138:412–9. doi: 10.1016/j.ajo.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Akiba M, Maeda N, Yumikake K, et al. Ultrahigh-resolution imaging of human donor cornea using full-field optical coherence tomography. J Biomed Opt. 2007;12:041202. doi: 10.1117/1.2764461. [DOI] [PubMed] [Google Scholar]
- 17.Heindl LM, Hofmann-Rummelt C, Schlotzer-Schrehardt U, et al. Histologic analysis of Descemet stripping in posterior lamellar keratoplasty. Arch Ophthalmol. 2008;126:461–4. doi: 10.1001/archophthalmol.2007.75. [DOI] [PubMed] [Google Scholar]
- 18.Fayol N, Labbe A, Dupont-Monod S, et al. Contribution of confocal microscopy and anterior chamber OCT to the study of corneal endothelial pathologies [in French] J Fr Ophtalmol. 2007;30:348–56. doi: 10.1016/s0181-5512(07)89604-5. [DOI] [PubMed] [Google Scholar]
- 19.Kaluzny BJ, Szkulmowska A, Szkulmowski M, et al. Fuchs' endothelial dystrophy in 830-nm spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2009;40:198–200. doi: 10.3928/15428877-20090301-11. [DOI] [PubMed] [Google Scholar]
- 20.Wang JH, Simpson TL, Fonn D. Objective measurements of corneal light-backscatter during corneal swelling, by optical coherence tomography. Invest Ophthalmol Vis Sci. 2004;45:3493–8. doi: 10.1167/iovs.04-0096. [DOI] [PubMed] [Google Scholar]