Abstract
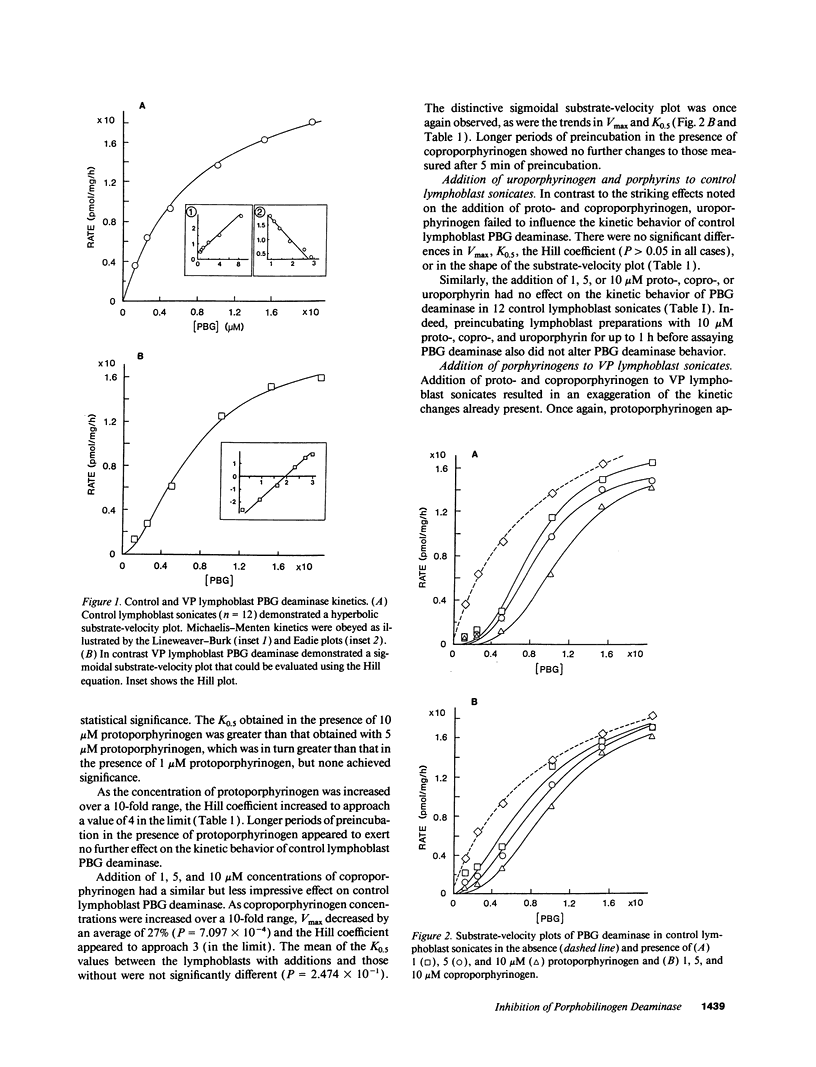
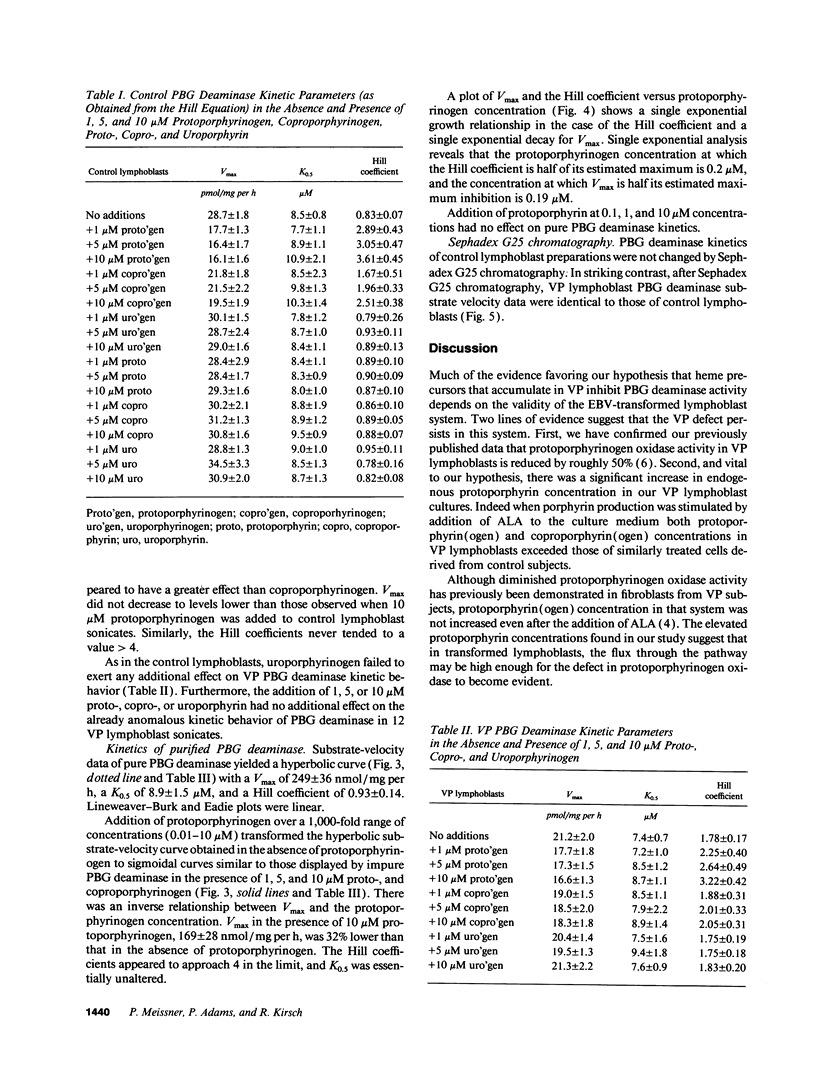
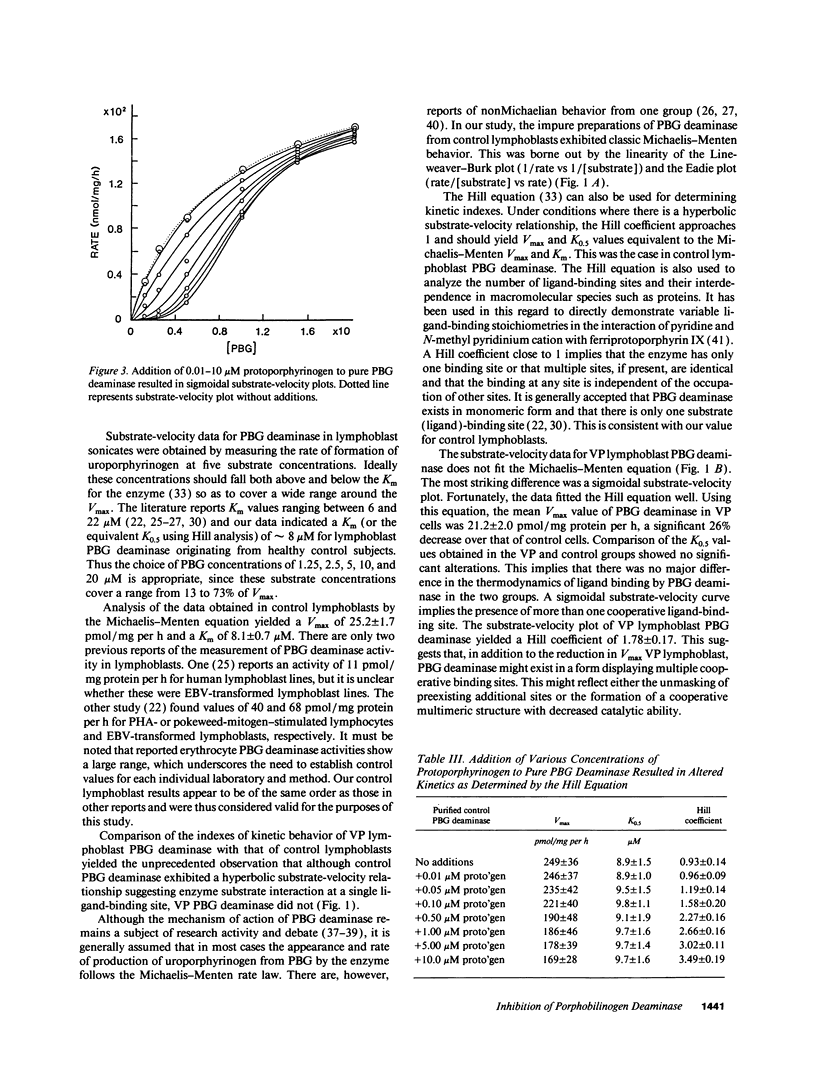
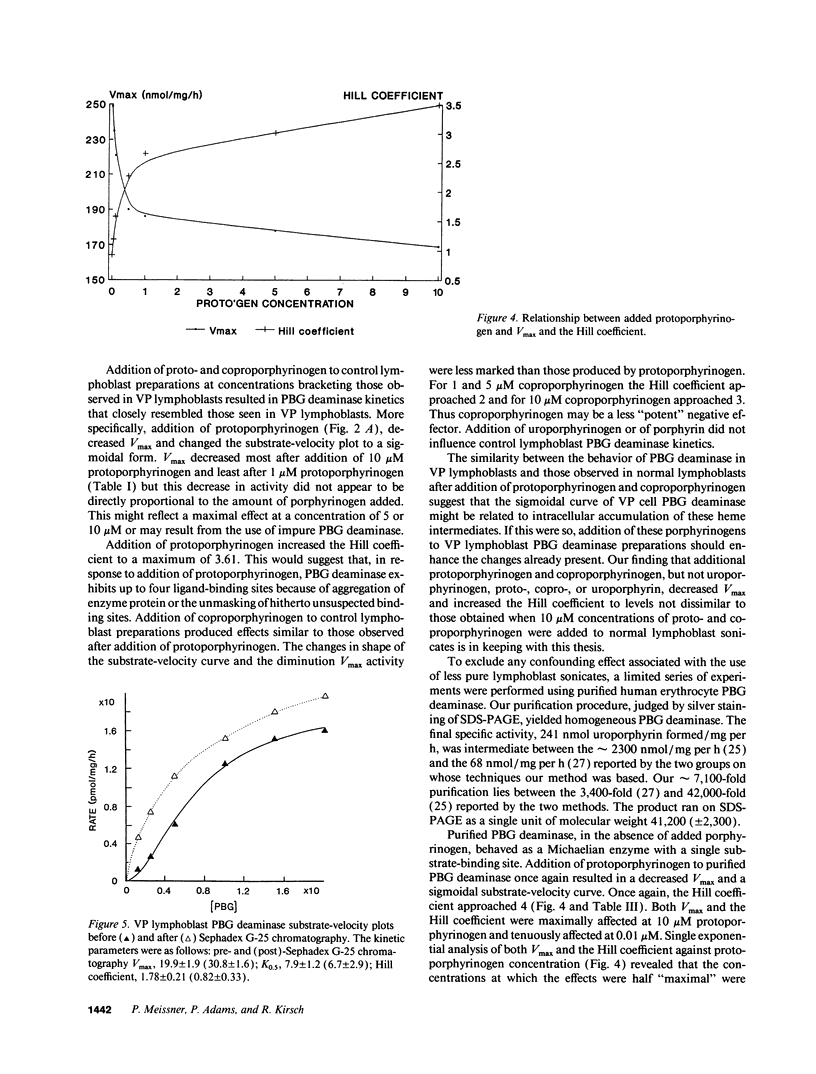
Variegate porphyria (VP) is characterized by photocutaneous lesions and acute neuropsychiatric attacks. Decreased protoporphyrinogen oxidase activity results in accumulation of protoporphyrin (ogen) IX and coproporphyrin (ogen) III. During acute attacks delta-aminolevulinic acid and porphobilinogen also increase, suggesting that porphobilinogen deaminase (PBG-D) may be rate limiting. We have examined the effects of porphyrinogens accumulating in VP on PBG-D activity in Epstein-Barr virus-transformed lymphoblast sonicates from 12 VP and 12 control subjects. Protoporphyrinogen oxidase activity was decreased and protoporphyrin increased in VP lymphoblasts. PBG-D in control lymphoblasts obeyed Michaelis-Menten kinetics (Vmax 28.7 +/- 1.8 pmol/mg per h, Hill coefficient 0.83 +/- 0.07). VP sonicates yielded sigmoidal substrate-velocity curves that did not obey Michaelis-Menten kinetics. Vmax was decreased (21.2 +/- 2.0 pmol/mg per h) and the Hill coefficient was 1.78 +/- 0.17. Addition of protoporphyrinogen IX and coproporphyrinogen III to control sonicates yielded sigmoidal PBG-D substrate-velocity curves and decreased PBG-D Vmax. Addition of porphyrins or uroporphyrinogen III did not affect PBG-D activity. Removal of endogenous porphyrin (ogens) from VP sonicates restored normal PBG-D kinetics. Purified human erythrocyte PBG-D obeyed Michaelis-Menten kinetics (Vmax 249 +/- 36 nmol/mg per h, Km 8.9 +/- 1.5 microM, Hill coefficient 0.93 +/- 0.14). Addition of protoporphyrinogen yielded a sigmoidal curve with decreased Vmax. The Hill coefficient approached 4. These findings provide a rational explanation for the increased delta-aminolevulinic acid and porphobilinogen during acute attacks of VP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Porphobilinogen deaminase: methods and principles of the enzymatic assay. Enzyme. 1982;28(2-3):146–157. doi: 10.1159/000459098. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- Battersby A. R. Biosynthesis of the pigments of life. J Nat Prod. 1988 Jul-Aug;51(4):629–642. doi: 10.1021/np50058a001. [DOI] [PubMed] [Google Scholar]
- Bonnett R. Oxygen activation and tetrapyrroles. Essays Biochem. 1981;17:1–51. [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. A fluorometric assay for measurement of protoporphyrinogen oxidase activity in mammalian tissue. Clin Chim Acta. 1980 Jan 31;100(3):259–266. doi: 10.1016/0009-8981(80)90275-2. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. The enzymatic defect in variegate prophyria. Studies with human cultured skin fibroblasts. N Engl J Med. 1980 Apr 3;302(14):765–769. doi: 10.1056/NEJM198004033021401. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., Moore M. R., Thompson G. G., Campbell B. C., Goldberg A. Is porphobilinogen deaminase activity a secondary control mechanism in haem biosynthesis in humans? [proceedings]. Biochem Soc Trans. 1977;5(5):1466–1468. doi: 10.1042/bst0051466. [DOI] [PubMed] [Google Scholar]
- Corrigall A. V., Meissner P. N., Kirsch R. E. Purification of human erythrocyte porphobilinogen deaminase. S Afr Med J. 1991 Sep 21;80(6):294–296. [PubMed] [Google Scholar]
- Day R. S., Pimstone N. R., Eales L. The diagnostic value of blood plasma porphyrin methyl ester profiles produced by quantitative TLC. Int J Biochem. 1978;9(12):897–904. doi: 10.1016/0020-711x(78)90067-8. [DOI] [PubMed] [Google Scholar]
- Day R. S., de Salamanca R. E., Eales L. Quantitation of red cell porphyrins by fluorescence scanning after thin-layer chromatography. Clin Chim Acta. 1978 Oct 2;89(1):25–33. doi: 10.1016/0009-8981(78)90358-3. [DOI] [PubMed] [Google Scholar]
- Deybach J. C., de Verneuil H., Nordmann Y. The inherited enzymatic defect in porphyria variegata. Hum Genet. 1981;58(4):425–428. doi: 10.1007/BF00282829. [DOI] [PubMed] [Google Scholar]
- Eales L., Day R. S., Blekkenhorst G. H. The clinical and biochemical features of variegate porphyria: an analysis of 300 cases studied at Groote Schuur Hospital, Cape Town. Int J Biochem. 1980;12(5-6):837–853. doi: 10.1016/0020-711x(80)90173-1. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Thomas N. The primary enzyme defect in hereditary coproporphyria. Lancet. 1976 Dec 4;2(7997):1217–1219. doi: 10.1016/s0140-6736(76)91143-0. [DOI] [PubMed] [Google Scholar]
- Ford R. E., Ou C. N., Ellefson R. D. Assay for erythrocyte uroporphyrinogen I synthase activity, with porphobilinogen as substrate. Clin Chem. 1980 Jul;26(8):1182–1185. [PubMed] [Google Scholar]
- Fumagalli S. A., Kotler M. L., Rossetti M. V., Batlle A. M. Human red cell porphobilinogen deaminase. A simpler method of purification and some unusual properties. Int J Biochem. 1985;17(4):485–494. doi: 10.1016/0020-711x(85)90144-2. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Decreased lymphocyte coproporphyrinogen III oxidase activity in hereditary coproporphyria. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1089–1095. doi: 10.1016/0006-291x(77)91630-8. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Warren M. J. Evidence for a dipyrromethane cofactor at the catalytic site of E. coli porphobilinogen deaminase. FEBS Lett. 1987 Dec 10;225(1-2):87–92. doi: 10.1016/0014-5793(87)81136-5. [DOI] [PubMed] [Google Scholar]
- Llambías E. B., del Batlle A. M. Porphyrin biosynthesis in soybean callus. V. The porphobilinogen deaminase-uroporphyrinogen cosynthetase system. Kinetic studies. Biochim Biophys Acta. 1970 Dec 16;220(3):552–559. doi: 10.1016/0005-2744(70)90285-8. [DOI] [PubMed] [Google Scholar]
- Meissner P. N., Day R. S., Moore M. R., Disler P. B., Harley E. Protoporphyrinogen oxidase and porphobilinogen deaminase in variegate porphyria. Eur J Clin Invest. 1986 Jun;16(3):257–261. doi: 10.1111/j.1365-2362.1986.tb01339.x. [DOI] [PubMed] [Google Scholar]
- Meissner P. N., Meissner D. M., Sturrock E. D., Davidson B., Kirsch R. E. Porphyria--the UCT experience. S Afr Med J. 1987 Dec 5;72(11):755–761. [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepkorn M. W., Hamernyik P., Labbé R. F. Modified erythrocyte uroporphyrinogen I synthase assay, and its clinical interpretation. Clin Chem. 1978 Oct;24(10):1751–1754. [PubMed] [Google Scholar]
- Pimstone N. R. Porphyria cutanea tarda. Semin Liver Dis. 1982 May;2(2):132–142. doi: 10.1055/s-2008-1040703. [DOI] [PubMed] [Google Scholar]
- Sancovich H. A., Battle A. M., Grinstein M. Porphyrin biosynthesis. VI. Separation and purification of porphobilinogen deaminase and uroporphyrinogen isomerase from cow liver. Porphobilinogenase an allosteric enzyme. Biochim Biophys Acta. 1969 Sep 30;191(1):130–143. doi: 10.1016/0005-2744(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Zalar G. L., Kappas A. Studies in porphyria. VII. Induction of uroporphyrinogen-I synthase and expression of the gene defect of acute intermittent porphyria in mitogen-stimulated human lymphocytes. J Clin Invest. 1978 Feb;61(2):499–508. doi: 10.1172/JCI108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn L. M., Harley E. H. Intracellular activity of HPRT Cape Town: purine uptake and growth of cultured cells in selective media. J Inherit Metab Dis. 1985;8(4):198–203. doi: 10.1007/BF01805435. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen D. J., Cummins R., Alexopoulos J., Kramer S. Protoporphyrinogen oxidase and ferrochelatase in porphyria variegata. Eur J Clin Invest. 1983 Aug;13(4):283–287. doi: 10.1111/j.1365-2362.1983.tb00102.x. [DOI] [PubMed] [Google Scholar]


