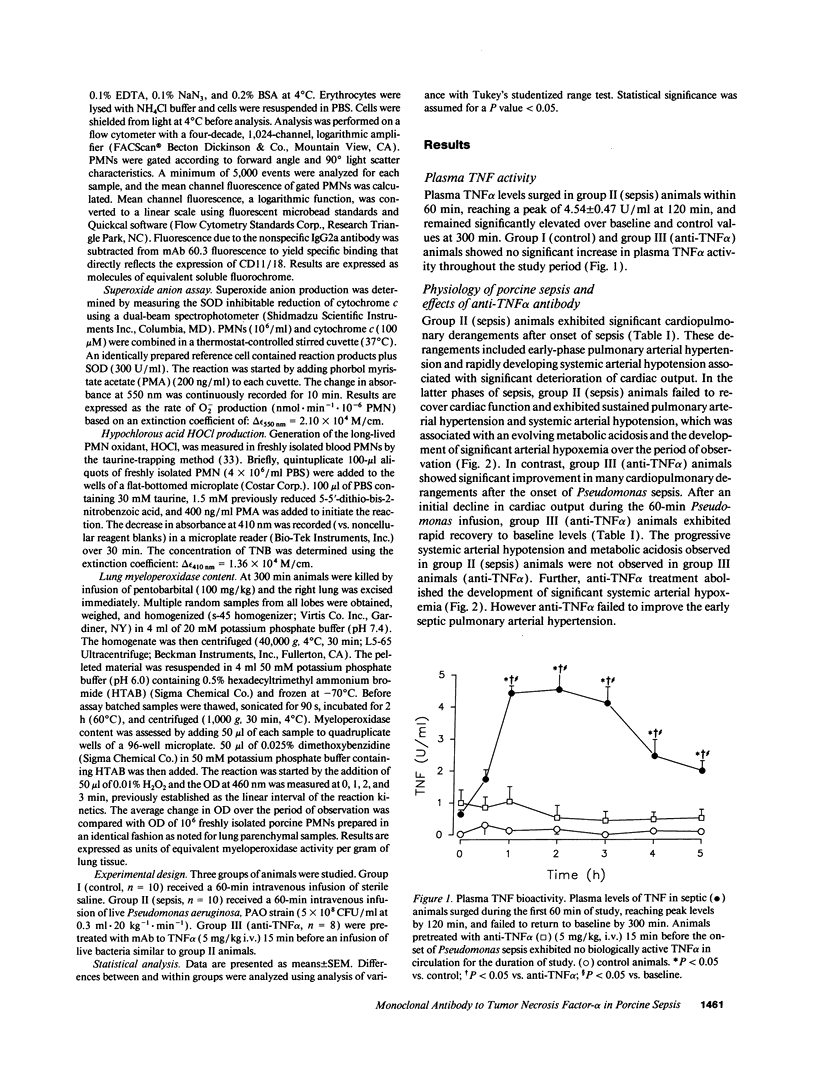
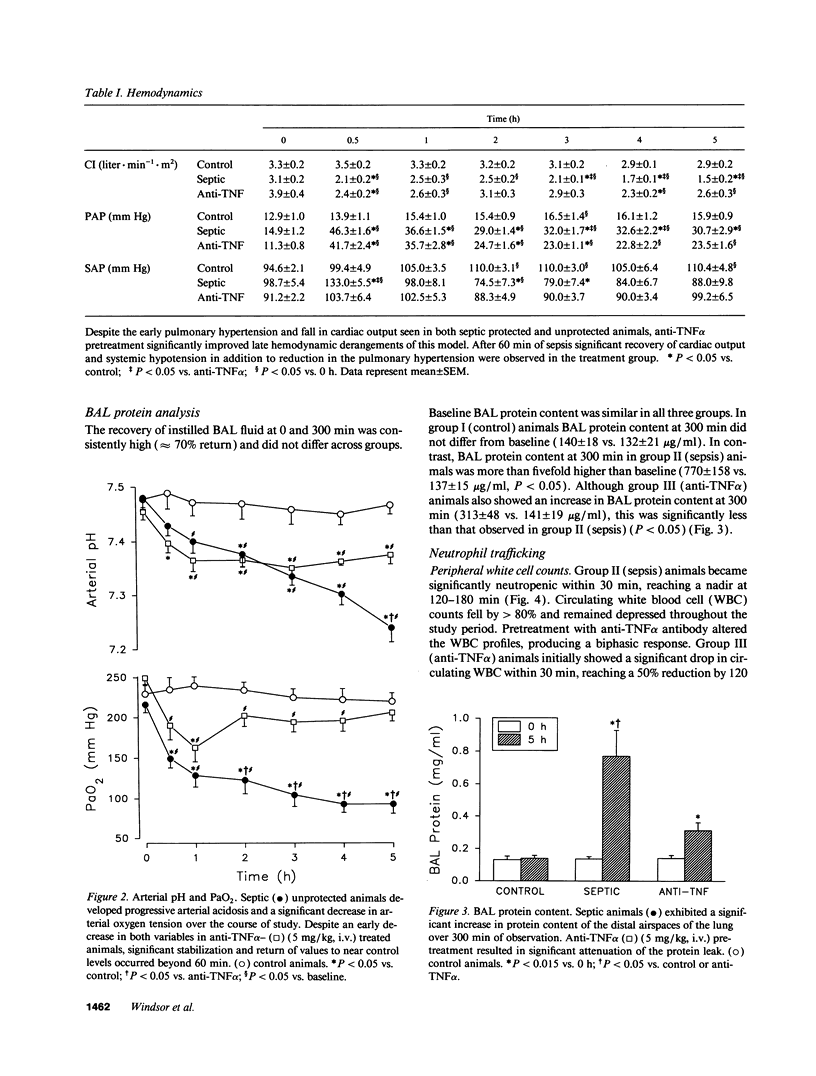
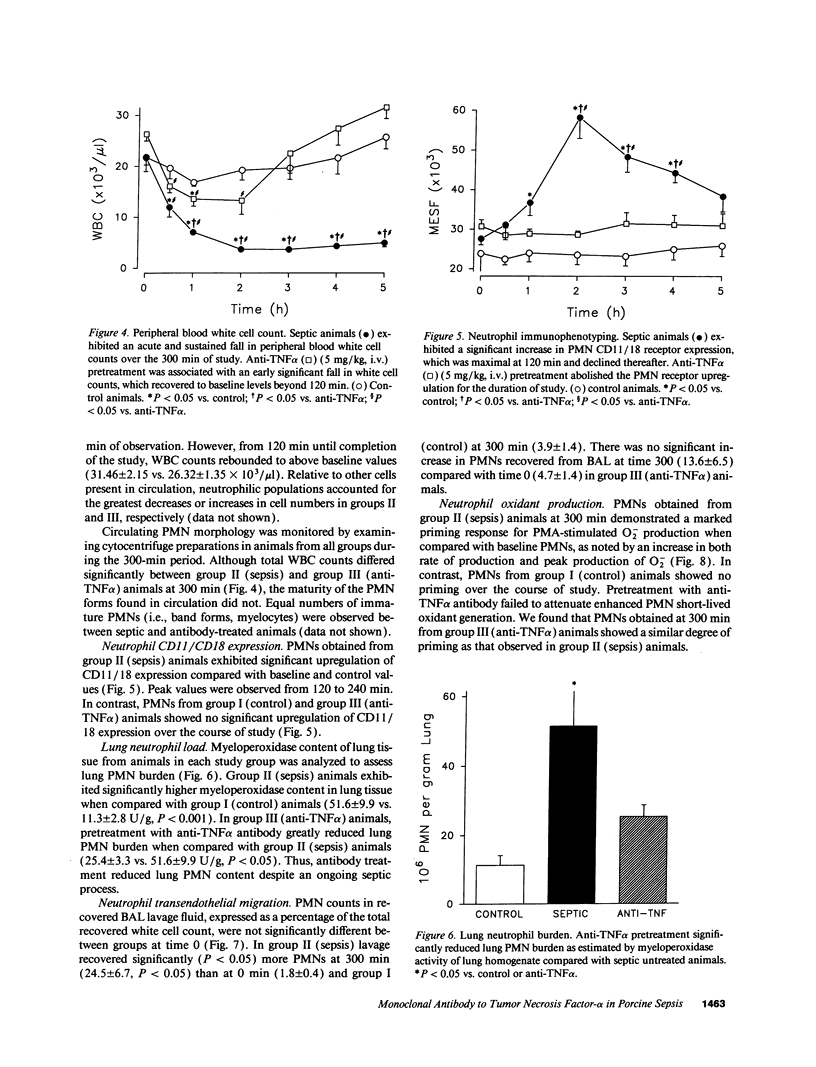
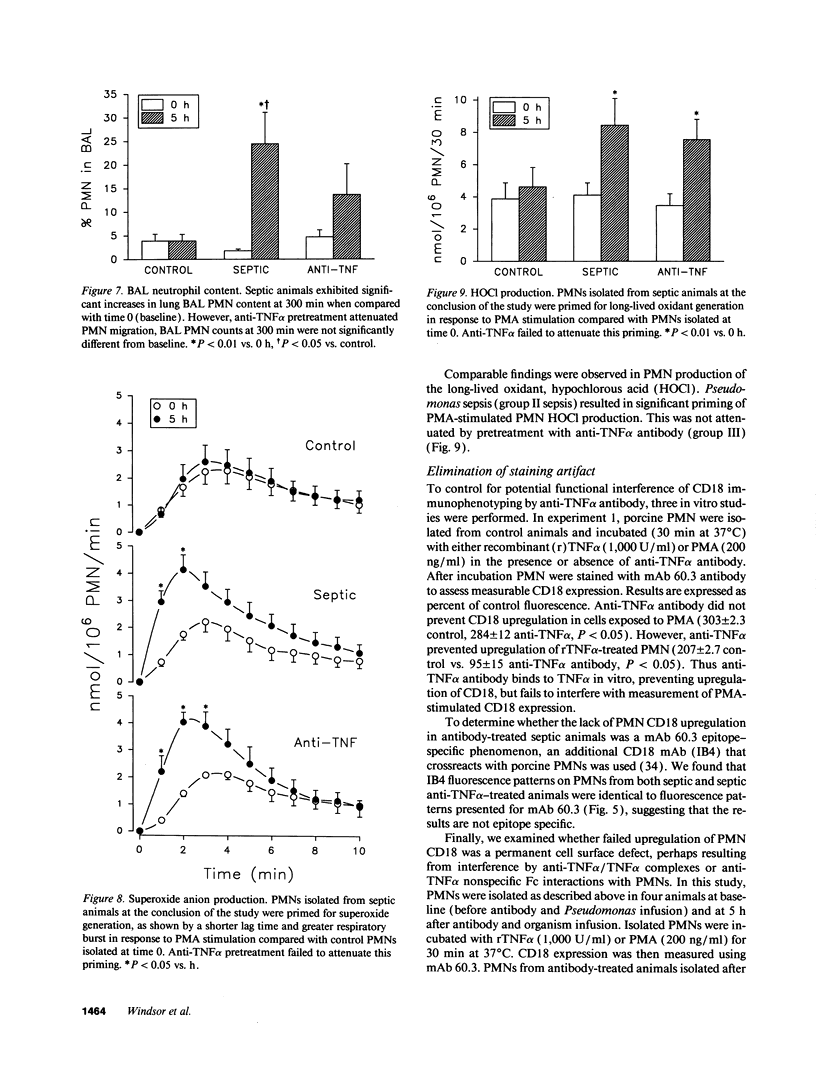
Abstract
Tumor necrosis factor (TNF alpha), both by direct action and by trafficking cells of the immune system, is implicated in cardiopulmonary derangements and PMN-mediated microvascular injury associated with gram-negative sepsis. We examined the effects of pretreatment with a monoclonal antibody to TNF alpha on PMN function, hemodynamic derangements, and alveolar capillary membrane damage in a septic porcine model. Anti-TNF alpha profoundly improved hemodynamic consequences in this model. Reduction in PMN CD11/18 receptor expression, lung myeloperoxidase activity, and attenuation of peripheral neutropenia (all P < 0.05) indicate that pretreatment significantly reduced lung sequestration of PMNs seen in septic controls. In contrast, PMN oxygen radical (O2-) generation was not significantly different from unprotected septic animals. Despite the presence of circulating PMNs primed for O2- burst, alveolar capillary membrane damage, assessed by bronchoalveolar lavage protein content and arterial PO2 was markedly attenuated in the treatment group (P < 0.05). We conclude that anti-TNF alpha suppresses systemic hemodynamic actions of TNF alpha. Further, it prevents upregulation of PMN adhesion receptors inhibiting PMN/endothelial cell interaction. This prevents formation of a "microenvironment," protected from circulating oxidant scavengers, into which sepsis-activated PMNs release their toxic products. Pretreatment with anti-TNF alpha monoclonal antibody thus affords global protection in porcine Gram-negative sepsis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Anderson B. O., Brown J. M., Harken A. H. Mechanisms of neutrophil-mediated tissue injury. J Surg Res. 1991 Aug;51(2):170–179. doi: 10.1016/0022-4804(91)90090-9. [DOI] [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977 Oct;116(4):589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Brigham K. L., Woolverton W. C., Blake L. H., Staub N. C. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974 Oct;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Grosso M. A., Harken A. H. Cytokines, sepsis and the surgeon. Surg Gynecol Obstet. 1989 Dec;169(6):568–575. [PubMed] [Google Scholar]
- Brown R. E., Jarvis K. L., Hyland K. J. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989 Jul;180(1):136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Byrne K., Carey P. D., Sugerman H. J. Adult respiratory distress syndrome. Acute Care. 1987;13(4):206–234. [PubMed] [Google Scholar]
- Byrne K., Sielaff T. D., Michna B., Carey P. D., Blocher C. R., Vasquez A., Sugerman H. J. Increased survival time after delayed histamine and prostaglandin blockade in a porcine model of severe sepsis-induced lung injury. Crit Care Med. 1990 Mar;18(3):303–308. doi: 10.1097/00003246-199003000-00012. [DOI] [PubMed] [Google Scholar]
- Byrne K., Sugerman H. J. Experimental and clinical assessment of lung injury by measurement of extravascular lung water and transcapillary protein flux in ARDS: a review of current techniques. J Surg Res. 1988 Feb;44(2):185–203. doi: 10.1016/0022-4804(88)90048-0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carey P. D., Byrne K., Jenkins J. K., Sielaff T. D., Walsh C. J., Fowler A. A., 3rd, Sugerman H. J. Ibuprofen attenuates hypochlorous acid production from neutrophils in porcine acute lung injury. J Surg Res. 1990 Sep;49(3):262–270. doi: 10.1016/0022-4804(90)90131-k. [DOI] [PubMed] [Google Scholar]
- Carey P. D., Leeper-Woodford S. K., Walsh C. J., Byrne K., Fowler A. A., Sugerman H. J. Delayed cyclo-oxygenase blockade reduces the neutrophil respiratory burst and plasma tumor necrosis factor levels in sepsis-induced acute lung injury. J Trauma. 1991 Jun;31(6):733–741. doi: 10.1097/00005373-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Kelly N., Bernton E., Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989 Jun 1;169(6):2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P., Reuter A., Gysen P., Demonty J., Lamy M., Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med. 1989 Oct;17(10):975–978. doi: 10.1097/00003246-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Flick M. R., Perel A., Staub N. C. Leukocytes are required for increased lung microvascular permeability after microembolization in sheep. Circ Res. 1981 Mar;48(3):344–351. doi: 10.1161/01.res.48.3.344. [DOI] [PubMed] [Google Scholar]
- Fowler A. A., Hyers T. M., Fisher B. J., Bechard D. E., Centor R. M., Webster R. O. The adult respiratory distress syndrome. Cell populations and soluble mediators in the air spaces of patients at high risk. Am Rev Respir Dis. 1987 Nov;136(5):1225–1231. doi: 10.1164/ajrccm/136.5.1225. [DOI] [PubMed] [Google Scholar]
- Glauser M. P., Zanetti G., Baumgartner J. D., Cohen J. Septic shock: pathogenesis. Lancet. 1991 Sep 21;338(8769):732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Harlan J. M. Neutrophil-mediated vascular injury. Acta Med Scand Suppl. 1987;715:123–129. doi: 10.1111/j.0954-6820.1987.tb09912.x. [DOI] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Hinson J. M., Jr, Hutchison A. A., Ogletree M. L., Brigham K. L., Snapper J. R. Effect of granulocyte depletion on altered lung mechanics after endotoxemia in sheep. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jul;55(1 Pt 1):92–99. doi: 10.1152/jappl.1983.55.1.92. [DOI] [PubMed] [Google Scholar]
- Ismail G., Morganroth M. L., Todd R. F., 3rd, Boxer L. A. Prevention of pulmonary injury in isolated perfused rat lungs by activated human neutrophils preincubated with anti-Mo1 monoclonal antibody. Blood. 1987 Apr;69(4):1167–1174. [PubMed] [Google Scholar]
- Jenkins J. K., Carey P. D., Byrne K., Sugerman H. J., Fowler A. A., 3rd Sepsis-induced lung injury and the effects of ibuprofen pretreatment. Analysis of early alveolar events via repetitive bronchoalveolar lavage. Am Rev Respir Dis. 1991 Jan;143(1):155–161. doi: 10.1164/ajrccm/143.1.155. [DOI] [PubMed] [Google Scholar]
- Johnson A., Malik A. B. Effect of granulocytopenia on extravascular lung water content after microembolization. Am Rev Respir Dis. 1980 Oct;122(4):561–566. doi: 10.1164/arrd.1980.122.4.561. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990 Mar;141(3):765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Warnock R. A., Jutila M. A., Butcher E. C., Lane C., Anderson D. C., Smith C. W. Antibodies against human neutrophil LECAM-1 (LAM-1/Leu-8/DREG-56 antigen) and endothelial cell ELAM-1 inhibit a common CD18-independent adhesion pathway in vitro. Blood. 1991 Aug 1;78(3):805–811. [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Graham D., Toy K., Lin L. S., Senyk G., Fendly B. M. Recombinant tumor necrosis factor causes activation of human granulocytes. Blood. 1987 Feb;69(2):640–644. [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Laurent F., Benoliel A. M., Capo C., Bongrand P. Oxidative metabolism of polymorphonuclear leukocytes: modulation by adhesive stimuli. J Leukoc Biol. 1991 Mar;49(3):217–226. doi: 10.1002/jlb.49.3.217. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Livingston D. H., Appel S. H., Sonnenfeld G., Malangoni M. A. The effect of tumor necrosis factor-alpha and interferon-gamma on neutrophil function. J Surg Res. 1989 Apr;46(4):322–326. doi: 10.1016/0022-4804(89)90195-9. [DOI] [PubMed] [Google Scholar]
- Marano M. A., Fong Y., Moldawer L. L., Wei H., Calvano S. E., Tracey K. J., Barie P. S., Manogue K., Cerami A., Shires G. T. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg Gynecol Obstet. 1990 Jan;170(1):32–38. [PubMed] [Google Scholar]
- Matsubara N., Fuchimoto S., Orita K. Tumour necrosis factor-alpha induces translocation of protein kinase C in tumour necrosis factor-sensitive cell lines. Immunology. 1991 Aug;73(4):457–459. [PMC free article] [PubMed] [Google Scholar]
- McGuire W. W., Spragg R. G., Cohen A. B., Cochrane C. G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982 Mar;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Acute effects of Escherichia coli endotoxin on the pulmonary microcirculation of anesthetized sheep structure:function relationships. Lab Invest. 1983 Apr;48(4):458–470. [PubMed] [Google Scholar]
- Michie H. R., Guillou P. J., Wilmore D. W. Tumour necrosis factor and bacterial sepsis. Br J Surg. 1989 Jul;76(7):670–671. doi: 10.1002/bjs.1800760706. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Wilmore D. W. Sepsis and tumor necrosis factor--bedfellows that cannot be ignored. Ann Surg. 1990 Dec;212(6):653–654. doi: 10.1097/00000658-199012000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orell S. R. Lung pathology in respiratory distress following shock in the adult. Acta Pathol Microbiol Scand A. 1971;79(1):65–76. doi: 10.1111/j.1699-0463.1971.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Riede U. N., Joachim H., Hassenstein J., Costabel U., Sandritter W., Augustin P., Mittermayer C. The pulmonary air-blood barrier of human shock lungs (a clinical, ultrastructural and morphometric study). Pathol Res Pract. 1978 May;162(1):41–72. doi: 10.1016/S0344-0338(78)80130-7. [DOI] [PubMed] [Google Scholar]
- Rinaldo J. E., Christman J. W. Mechanisms and mediators of the adult respiratory distress syndrome. Clin Chest Med. 1990 Dec;11(4):621–632. [PubMed] [Google Scholar]
- Rock C. S., Lowry S. F. Tumor necrosis factor-alpha. J Surg Res. 1991 Nov;51(5):434–445. doi: 10.1016/0022-4804(91)90146-d. [DOI] [PubMed] [Google Scholar]
- Seeds M. C., Parce J. W., Szejda P., Bass D. A. Independent stimulation of membrane potential changes and the oxidative metabolic burst in polymorphonuclear leukocytes. Blood. 1985 Jan;65(1):233–240. [PubMed] [Google Scholar]
- Shalaby M. R., Palladino M. A., Jr, Hirabayashi S. E., Eessalu T. E., Lewis G. D., Shepard H. M., Aggarwal B. B. Receptor binding and activation of polymorphonuclear neutrophils by tumor necrosis factor-alpha. J Leukoc Biol. 1987 Mar;41(3):196–204. doi: 10.1002/jlb.41.3.196. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L. Cytokine receptors of the lung. Am J Respir Cell Mol Biol. 1991 Nov;5(5):403–410. doi: 10.1165/ajrcmb/5.5.403. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Lasky L. A. Cell adhesion. Sticky sugars for selectins. Nature. 1991 Jan 17;349(6306):196–197. doi: 10.1038/349196a0. [DOI] [PubMed] [Google Scholar]
- Stahl G. L., Fletcher M. P., Amsterdam E. A., Longhurst J. C. Role of granulocytes and C5a in myocardial response to zymosan-activated serum. Am J Physiol. 1991 Jul;261(1 Pt 2):H29–H37. doi: 10.1152/ajpheart.1991.261.1.H29. [DOI] [PubMed] [Google Scholar]
- Stephens K. E., Ishizaka A., Wu Z. H., Larrick J. W., Raffin T. A. Granulocyte depletion prevents tumor necrosis factor-mediated acute lung injury in guinea pigs. Am Rev Respir Dis. 1988 Nov;138(5):1300–1307. doi: 10.1164/ajrccm/138.5.1300. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Guo K., del Castillo J. Endotoxin-induced cytokine gene expression in vivo. I. Expression of tumor necrosis factor mRNA in visceral organs under physiologic conditions and during endotoxemia. Am J Pathol. 1989 Jan;134(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Walsh C. J., Carey P. D., Cook D. J., Bechard D. E., Fowler A. A., Sugerman H. J. Anti-CD18 antibody attenuates neutropenia and alveolar capillary-membrane injury during gram-negative sepsis. Surgery. 1991 Aug;110(2):205–212. [PubMed] [Google Scholar]
- Walsh C. J., Leeper-Woodford S. K., Carey P. D., Cook D. J., Bechard D. E., Fowler A. A., Sugerman H. J. CD18 adhesion receptors, tumor necrosis factor, and neutropenia during septic lung injury. J Surg Res. 1991 Apr;50(4):323–329. doi: 10.1016/0022-4804(91)90198-u. [DOI] [PubMed] [Google Scholar]
- Walsh C. J., Sugerman H. J., Mullen P. G., Carey P. D., Leeper-Woodford S. K., Jesmok G. J., Ellis E. F., Fowler A. A. Monoclonal antibody to tumor necrosis factor alpha attenuates cardiopulmonary dysfunction in porcine gram-negative sepsis. Arch Surg. 1992 Feb;127(2):138–145. doi: 10.1001/archsurg.1992.01420020020003. [DOI] [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Cohen H. J. Dissociation between aggregation and superoxide production in human granulocytes. J Immunol. 1985 Feb;134(2):1206–1211. [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]


