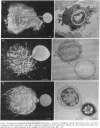
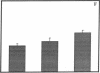
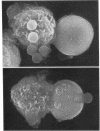
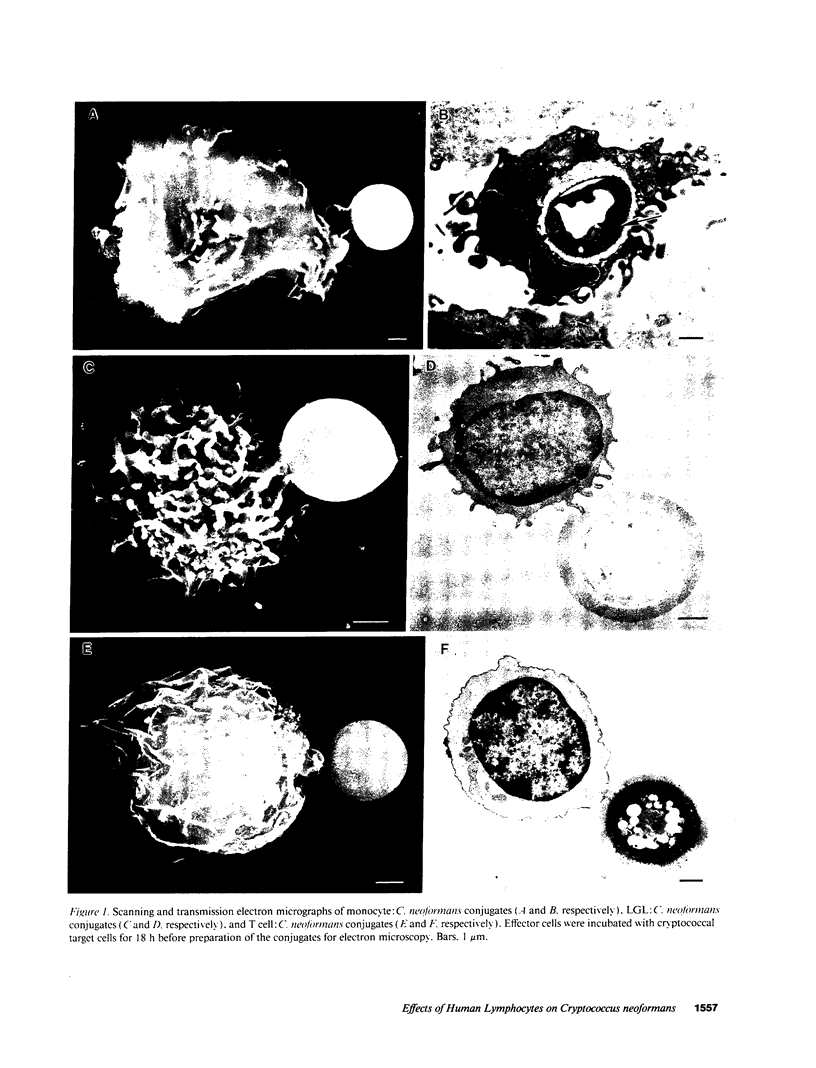
Abstract
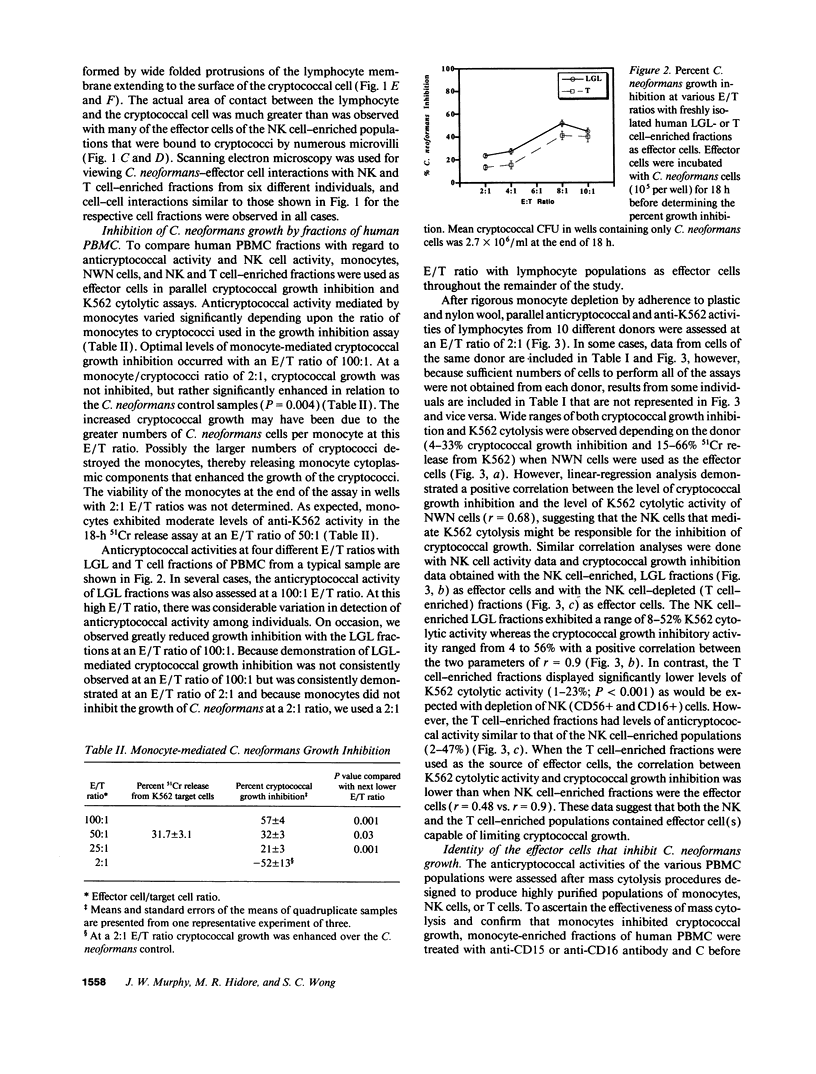
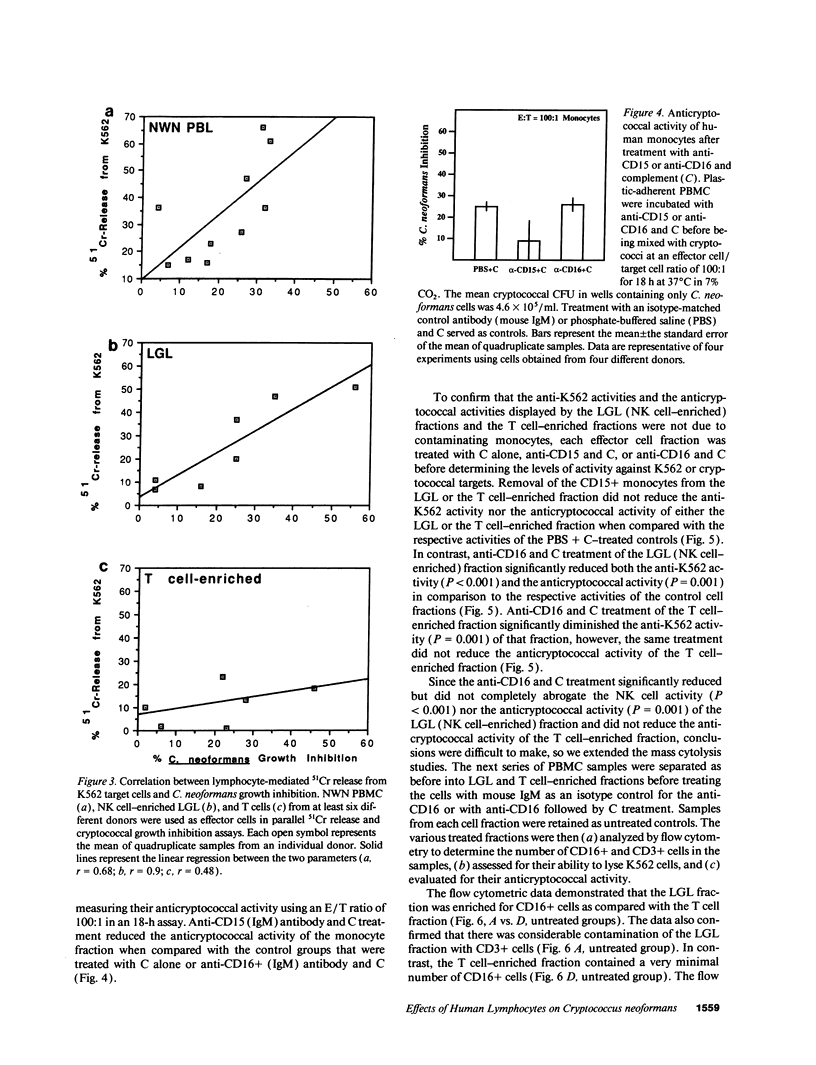
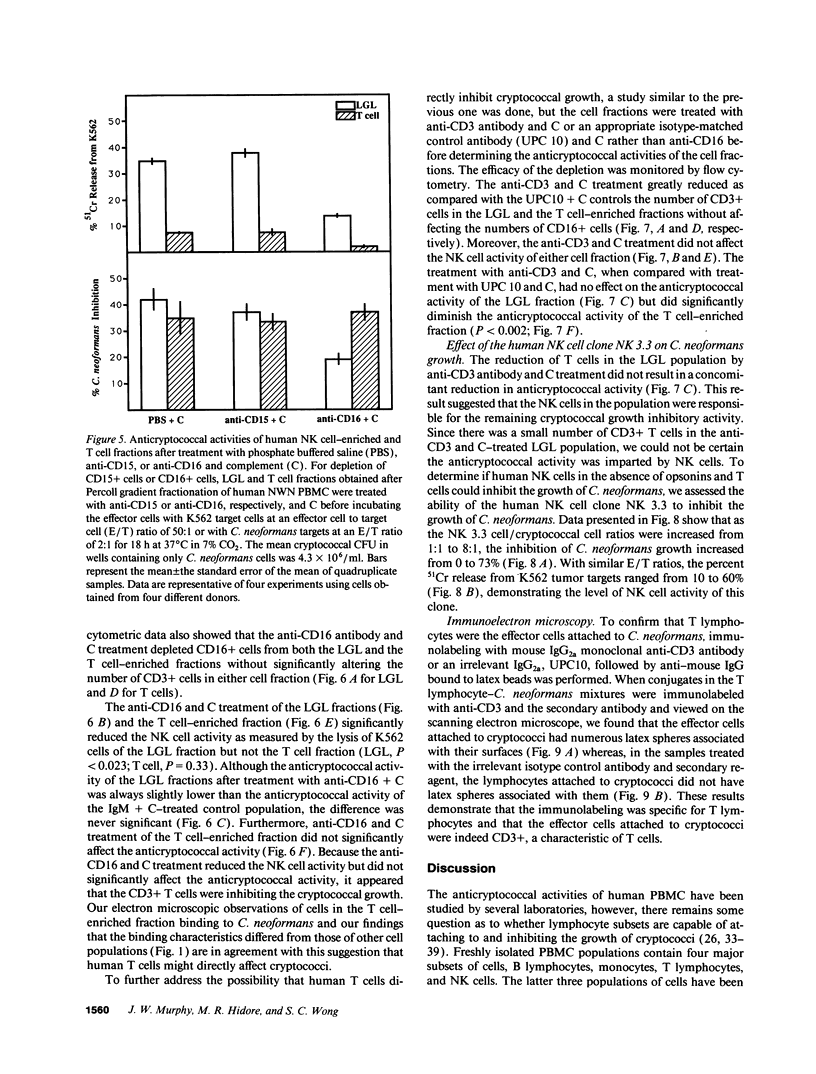
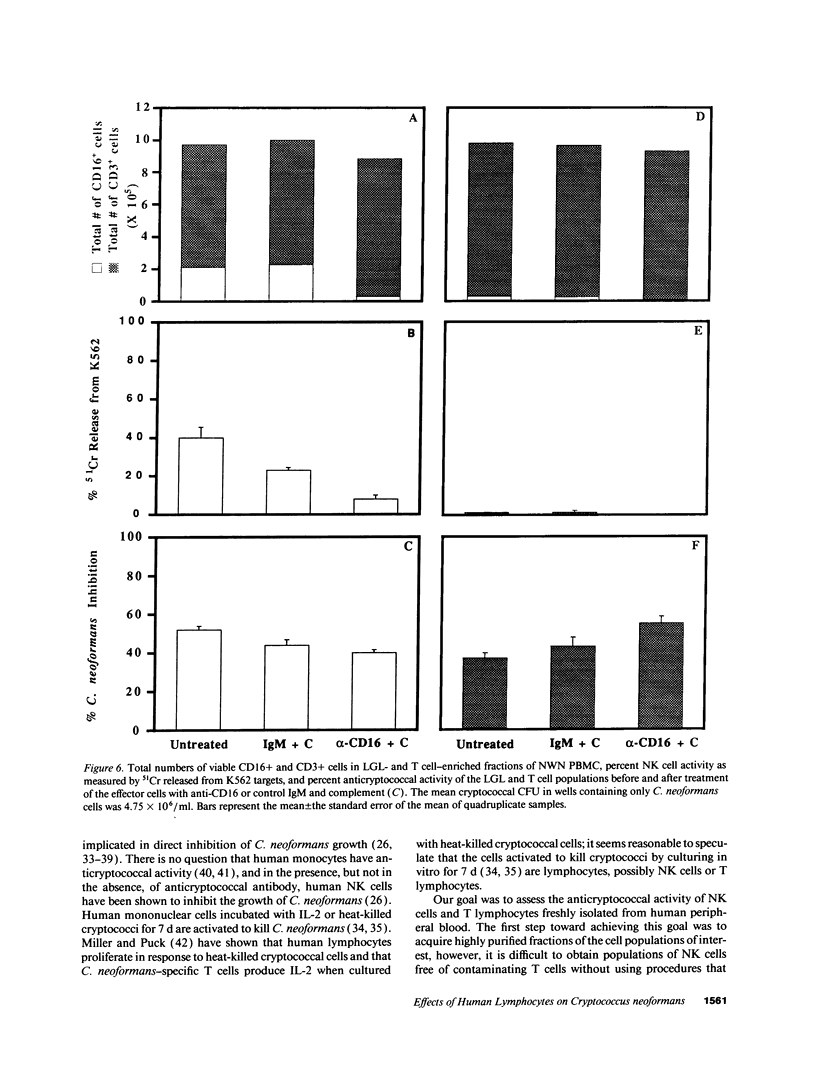
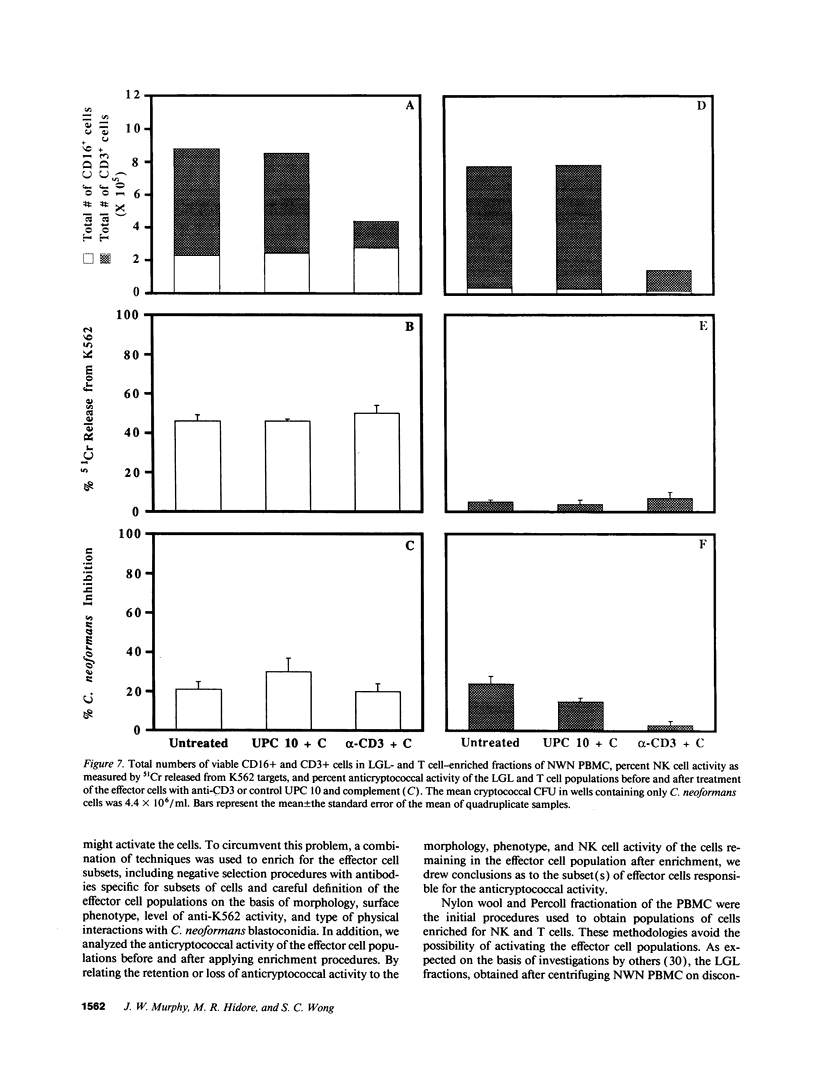
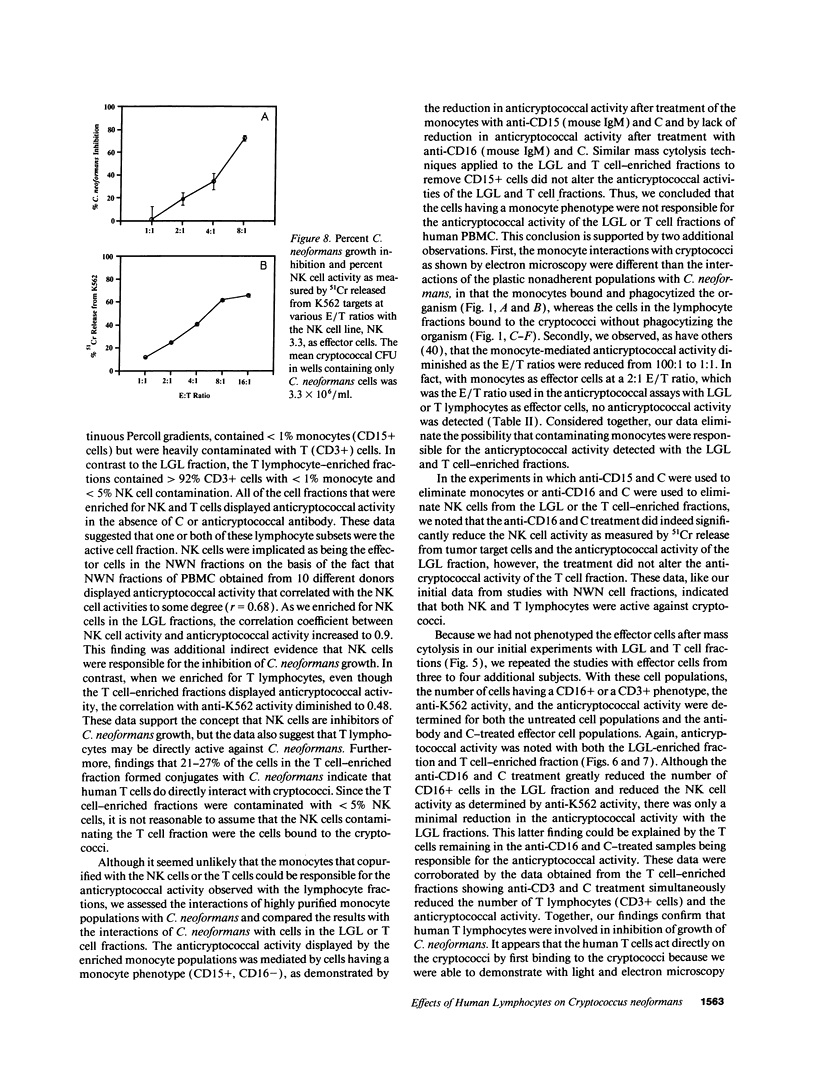
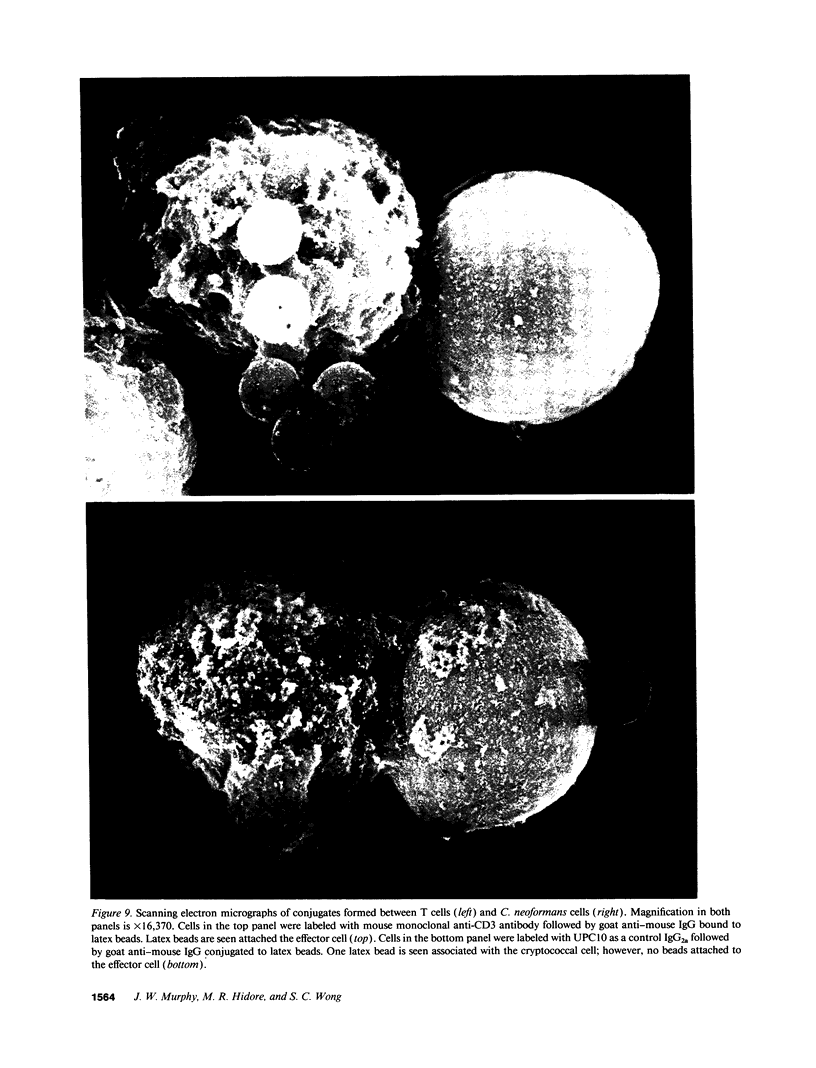
Lymphocytes, especially CD4+ T cells, are essential for clearance of the yeast-like organism Cryptococcus neoformans from the infected host. The mechanism(s) by which the lymphocytes facilitate elimination of cryptococci has not been elucidated. It is generally thought, however, that lymphocytes reactive with C. neoformans indirectly function by production of lymphokines to enhance clearance of the organism by natural effector cells such as macrophages. In the present study, we assessed the ability of freshly isolated human lymphocytes to interact directly with C. neoformans and to limit the growth of the organism in vitro. We found that large granular lymphocytes (LGL) as well as T cells bound to cryptococcal cells when the lymphocytes were mixed with the cryptococcal cells at a 2:1 ratio. The physical binding interactions of the two lymphocyte populations were different. LGL attached to the cryptococcal cells by many microvilli; T lymphocytes associated with the yeast through broad areas of membrane attached to the cryptococcal cell surface. The two types of lymphocyte interactions did not result in phagocytosis but resulted in direct inhibition of cryptococcal growth, making these lymphocyte interactions with cryptococci distinctly different from interactions of monocytes with cryptococci. With the human natural killer (NK) cell line, NK 3.3, we confirmed that NK cells that were present in the LGL population were capable of limiting the growth of C. neoformans. Through immunoelectron microscopy, human CD3+ lymphocytes were seen attached to cryptococcal cells and by mass cytolysis, human CD3+ lymphocytes were shown to be responsible for inhibition of C. neoformans growth. The direct inhibitory interactions of NK cells and T lymphocytes with cryptococcal cells may be important means of host defense against this ubiquitous organism that frequently causes life-threatening disease in AIDS patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. Life-threatening opportunistic fungal infection in patients with the acquired immunodeficiency syndrome. Ann N Y Acad Sci. 1988;544:443–450. doi: 10.1111/j.1749-6632.1988.tb40442.x. [DOI] [PubMed] [Google Scholar]
- Bobak D. A., Washburn R. G., Frank M. M. C1q enhances the phagocytosis of Cryptococcus neoformans blastospores by human monocytes. J Immunol. 1988 Jul 15;141(2):592–597. [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D. Antibody-dependent killing of Cryptococcus neopormans by human peripheral blood mononuclear cells. Nature. 1974 Jan 18;247(5437):148–150. doi: 10.1038/247148a0. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Fung P. Y., Murphy J. W. In vitro interactions of immune lymphocytes and Cryptococcus neoformans. Infect Immun. 1982 Jun;36(3):1128–1138. doi: 10.1128/iai.36.3.1128-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Mislan T. W., Murphy J. W. Responses of murine natural killer cells to binding of the fungal target Cryptococcus neoformans. Infect Immun. 1991 Apr;59(4):1489–1499. doi: 10.1128/iai.59.4.1489-1499.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect Immun. 1989 Jul;57(7):1990–1997. doi: 10.1128/iai.57.7.1990-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Reynolds C. W., Henkart P. A., Murphy J. W. Cytoplasmic components of natural killer cells limit the growth of Cryptococcus neoformans. J Leukoc Biol. 1990 Jul;48(1):15–26. doi: 10.1002/jlb.48.1.15. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Sonleitner F., Murphy J. W. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991 May;59(5):1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992 Jun 1;175(6):1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. O., Harmsen A. G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991 Mar 1;173(3):755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991 Apr 1;173(4):793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth J., Flomenberg N., Dupont B. Cell surface phenotype of a cloned line of human natural killer cells. J Immunol. 1982 Dec;129(6):2831–2837. [PubMed] [Google Scholar]
- Kovacs J. A., Kovacs A. A., Polis M., Wright W. C., Gill V. J., Tuazon C. U., Gelmann E. P., Lane H. C., Longfield R., Overturf G. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Oct;103(4):533–538. doi: 10.7326/0003-4819-103-4-533. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Laroche R., Dupont B., Touze J. E., Taelman H., Bogaerts J., Kadio A., M'Pele P., Latif A., Aubry P., Durbec J. P. Cryptococcal meningitis associated with acquired immunodeficiency syndrome (AIDS) in African patients: treatment with fluconazole. J Med Vet Mycol. 1992;30(1):71–78. doi: 10.1080/02681219280000091. [DOI] [PubMed] [Google Scholar]
- Levitz S. M. Activation of human peripheral blood mononuclear cells by interleukin-2 and granulocyte-macrophage colony-stimulating factor to inhibit Cryptococcus neoformans. Infect Immun. 1991 Oct;59(10):3393–3397. doi: 10.1128/iai.59.10.3393-3397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect Immun. 1990 May;58(5):1201–1209. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P., Maziarz R. T. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J Infect Dis. 1991 May;163(5):1108–1113. doi: 10.1093/infdis/163.5.1108. [DOI] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb M. F., Alvarellos T., Toews G. B., Tompkins R., Evans Z., Koo G., Kumar V. Role of natural killer cells in resistance to Cryptococcus neoformans infections in mice. Am J Pathol. 1987 Aug;128(2):354–361. [PMC free article] [PubMed] [Google Scholar]
- Masur H., Ognibene F. P., Yarchoan R., Shelhamer J. H., Baird B. F., Travis W., Suffredini A. F., Deyton L., Kovacs J. A., Falloon J. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989 Aug 1;111(3):223–231. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- Miller G. P., Puck J. In vitro human lymphocyte responses to Cryptococcus neoformans. Evidence for primary and secondary responses in normals and infected subjects. J Immunol. 1984 Jul;133(1):166–172. [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991 Jan;59(1):24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Murphy J. W., Hidore M. R., Nabavi N. Binding interactions of murine natural killer cells with the fungal target Cryptococcus neoformans. Infect Immun. 1991 Apr;59(4):1476–1488. doi: 10.1128/iai.59.4.1476-1488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E. Pseudomonas aeruginosa immunotherapy. Eur J Clin Microbiol Infect Dis. 1990 Jun;9(6):377–380. doi: 10.1007/BF01979465. [DOI] [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. Role of natural killer cells in resistance to systemic cryptococcosis. J Leukoc Biol. 1991 Aug;50(2):151–159. doi: 10.1002/jlb.50.2.151. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Stewart S. J., Habbersett R. C. Resolving leukocytes using axial light loss. Cytometry. 1989 Jul;10(4):426–432. doi: 10.1002/cyto.990100410. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R. G., Tuazon C. U., Bennett J. E. Phagocytic and fungicidal activity of monocytes from patients with acquired immunodeficiency syndrome. J Infect Dis. 1985 Mar;151(3):565–566. doi: 10.1093/infdis/151.3.565. [DOI] [PubMed] [Google Scholar]